Quantitative Analyses of the Left Ventricle Volume and Cardiac Function in Normal and Infarcted Yucatan Minipigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Myocardial Infarction
2.3. Cardiac Magnetic Resonance Imaging (CMRI)
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Volumetric LV Analysis in the Normal Pigs
3.2. Volumetric LV Analysis in the Infarcted Pigs
3.3. Comparison of LV Mass Assessment In Vivo and Ex Vivo
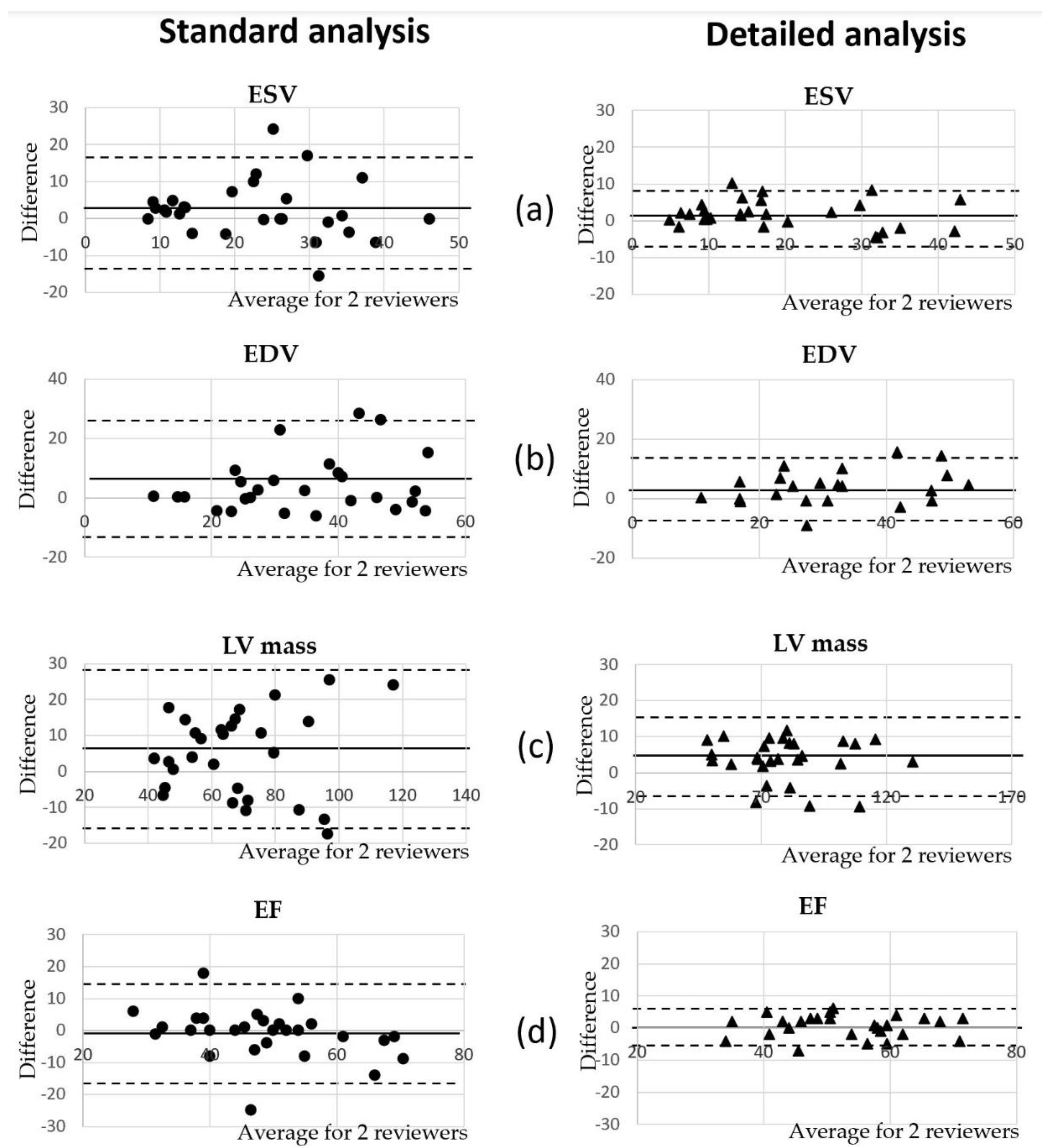
3.4. Comparison of Volumetric Analysis between Reviewers
4. Discussion
5. Conclusions
- 1.
- The standard clinical CMRI approach of endocardial tracing that excludes the papillary muscles and the trabeculae from LV mass underestimates ventricular mass and the LV volume in pigs due to the relatively large papillary muscles.
- 2.
- The detailed method of endocardium tracing accounts for the large volume of the papillary muscles in the pig heart, and despite the minor mismatch with the actual LV mass measured ex vivo, the detailed method might be preferable for the analysis of LV mass.
- 3.
- The papillary muscles add approximately 21% to LV mass in normal and infarcted hearts of Yucatan minipigs.
- 4.
- The detailed method of tracing all papillary muscles as part of LV mass requires ~30% more time for analysis in comparison with the standard method but provides better accuracy.
- 5.
- The detailed method of endocardium tracing showed better interobserver consistency in the assessment of LV mass and the ejection fraction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maceira, A.M.; Prasad, S.K.; Khan, M.; Pennell, D.J. Normalized Left Ventricular Systolic and Diastolic Function by Steady State Free Precession Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reason. 2006, 8, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Camastra, G.; Monti, L.; Lombardi, M.; Pepe, A.; Castelletti, S.; Maestrini, V.; Todiere, G.; Masci, P.G.; Di Giovine, G.; et al. Reference values of cardiac volumes, dimensions, and new functional parameters by MR: A multicenter, multivendor study. J. Magn. Reson. Imaging 2017, 45, 1055–1067. [Google Scholar] [CrossRef]
- Lee, V.S.; Resnick, D.; Bundy, J.M.; Simonetti, O.P.; Lee, P.; Weinreb, J.C. Cardiac function: MR evaluation in one breath hold with real-time true fast imaging with steady-state precession. Radiology 2002, 222, 835–842. [Google Scholar] [CrossRef]
- François, C.J.; Fieno, D.S.; Shors, S.M.; Finn, J.P. Left ventricular mass: Manual and automatic segmentation of true FISP and FLASH cine MR images in dogs and pigs. Radiology 2004, 230, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Gilson, W.D.; Kraitchman, D.L. Cardiac magnetic resonance imaging in small rodents using clinical 1.5 T and 3.0 T scanners. Methods 2007, 43, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Estes, E.H., Jr.; Dalton, F.M.; Entman, M.L.; Dixon, H.B., 2nd; Hackel, D.B. The anatomy and blood supply of the papillary muscles of the left ventricle. Am. Heart J. 1966, 71, 356–362. [Google Scholar] [CrossRef]
- Roberts, W.C.; Cohen, L.S. Left ventricular papillary muscles. Description of the normal and a survey of conditions causing them to be abnormal. Circulation 1972, 46, 138–154. [Google Scholar] [CrossRef] [Green Version]
- Vogel-Claussen, J.; Finn, J.P.; Gomes, A.S.; Hundley, G.W.; Jerosch-Herold, M.; Pearson, G.; Sinha, S.; João, A.C.; Bluemke, D.A. Left ventricular papillary muscle mass: Relationship to left ventricular mass and volumes by magnetic resonance imaging. J. Comput. Assist. Tomogr. 2006, 30, 426–432. [Google Scholar] [CrossRef]
- Harrigan, C.J.; Appelbaum, E.; Maron, B.J.; Buros, J.L.; Gibson, C.M.; Lesser, J.R.; Udelson, J.E.; Manning, W.J.; Maron, M.S. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am. J. Cardiol. 2008, 101, 668–673. [Google Scholar] [CrossRef]
- Janik, M.; Cham, M.D.; Ross, M.I.; Wang, Y.; Codella, N.; Min, J.K.; Prince, M.; Manoushagian, S.; Okin, P.M.; Devereux, R.B.; et al. Effects of papillary muscles and trabeculae on left ventricular quantification: Increased impact of methodological variability in patients with left ventricular hypertrophy. J. Hypertens. 2008, 26, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, S.; Aben, J.P.; Baks, T.; Moelker, A.; Gruszczynska, K.; Krestin, G.P.; van der Giessen, W.J.; Duncker, D.J.; de Feyter, P.J.; van Geuns, R.-J.M. Accurate automatic papillary muscle identification for quantitative left ventricle mass measurements in cardiac magnetic resonance imaging. Acad. Radiol. 2008, 15, 1227–1233. [Google Scholar] [CrossRef]
- Peters, D.C.; Ennis, D.B.; McVeigh, E.R. High-resolution MRI of cardiac function with projection reconstruction and steady-state free precession. Magn. Reson. Med. 2002, 48, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Mineoi, K.; Shigematsu, Y.; Ochi, T.; Hiwada, K. Left ventricular mass and atrial volume determined by cine magnetic resonance imaging in essential hypertension. Am. J. Hypertens. 2000, 13, 1103–1109. [Google Scholar] [CrossRef] [Green Version]
- Strohm, O.; Schulz-Menger, J.; Pilz, B.; Osterziel, K.J.; Dietz, R.; Friedrich, M.G. Measurement of left ventricular dimensions and function in patients with dilated cardiomyopathy. J. Magn. Reason. Imaging 2001, 13, 367–371. [Google Scholar] [CrossRef]
- Han, Y.; Osborn, E.A.; Maron, M.S.; Manning, W.J.; Yeon, S.B. Impact of papillary and trabecular muscles on quantitative analyses of cardiac function in hypertrophic cardiomyopathy. J. Magn. Reson. Imaging 2009, 30, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- McCall, F.C.; Telukuntla, K.S.; Karantalis, V.; Suncion, V.Y.; Heldman, A.W.; Mushtaq, M.; Williams, A.R.; Hare, J.M. Myocardial infarction and intramyocardial injection models in swine. Nat. Protoc. 2012, 7, 1479–1496. [Google Scholar] [CrossRef] [Green Version]
- Kellman, P.; Arai, A.E.; McVeigh, E.R.; Aletras, A.H. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn. Reson. Med. 2002, 47, 372–383. [Google Scholar] [CrossRef] [Green Version]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Gho, J.M.I.H.; van Es, R.; van Slochteren, F.J.; Jansen of Lorkeers, S.J.; Hauer, A.J.; van Oorschot, J.W.M.; Doevendans, P.A.; Leiner, T.; Vink, A.; Asselbergs, F.W.; et al. A systematic comparison of cardiovascular magnetic resonance and high resolution histological fibrosis quantification in a chronic porcine infarct model. Int. J. Cardiovasc. Imaging 2017, 3, 1797–1807. [Google Scholar] [CrossRef] [Green Version]
- Natsumeda, M.; Florea, V.; Rieger, A.C.; Tompkins, B.A.; Banerjee, M.N.; Golpanian, S.; Fritsch, J.; Landin, A.M.; Kashikar, N.D.; Karantalis, V.; et al. A Combination of Allogeneic Stem Cells Promotes Cardiac Regeneration. J. Am. Coll. Cardiol. 2017, 70, 2504–2515. [Google Scholar] [CrossRef]
- Lopez, D.; Pan, J.A.; Pollak, P.M.; Clarke, S.; Kramer, C.M.; Yeager, M.; Salerno, M. Multiparametric CMR imaging of infarct remodeling in a percutaneous reperfused Yucatan mini-pig model. NMR Biomed. 2017, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitaker, J.; Tschabrunn, C.M.; Jang, J.; Leshem, E.; O’Neill, M.; Manning, W.J.; Anter, E.; Nezafat, R. Cardiac MR Characterization of left ventricular remodeling in a swine model of infarct followed by reperfusion. J. Magn. Reson. Imaging 2018, 48, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Crisostomo, V.; Baez, C.; Abad, J.L.; Sanchez, B.; Alvarez, V.; Rosado, R.; Gómez-Mauricio, G.; Gheysens, O.; Blanco-Blazquez, V.; Blazquez, R.; et al. Dose-dependent improvement of cardiac function in a swine model of acute myocardial infarction after intracoronary administration of allogeneic heart-derived cells. Stem. Cell Res. Ther. 2019, 10, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobi, J.; Solanes, N.; Fernández-Jiménez, R.; Galán-Arriola, C.; Dantas, A.P.; Fernández-Friera, L.; Gálvez-Montón, C.; Rigol-Monzó, E.; Agüero, J.; Ramírez, J.; et al. Intracoronary Administration of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells Improves Myocardial Perfusion but Not Left Ventricle Function, in a Translational Model of Acute Myocardial Infarction. J. Am. Heart Assoc. 2017, 6, e005771. [Google Scholar] [CrossRef] [Green Version]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016, 134, e282–e293. [Google Scholar] [PubMed]

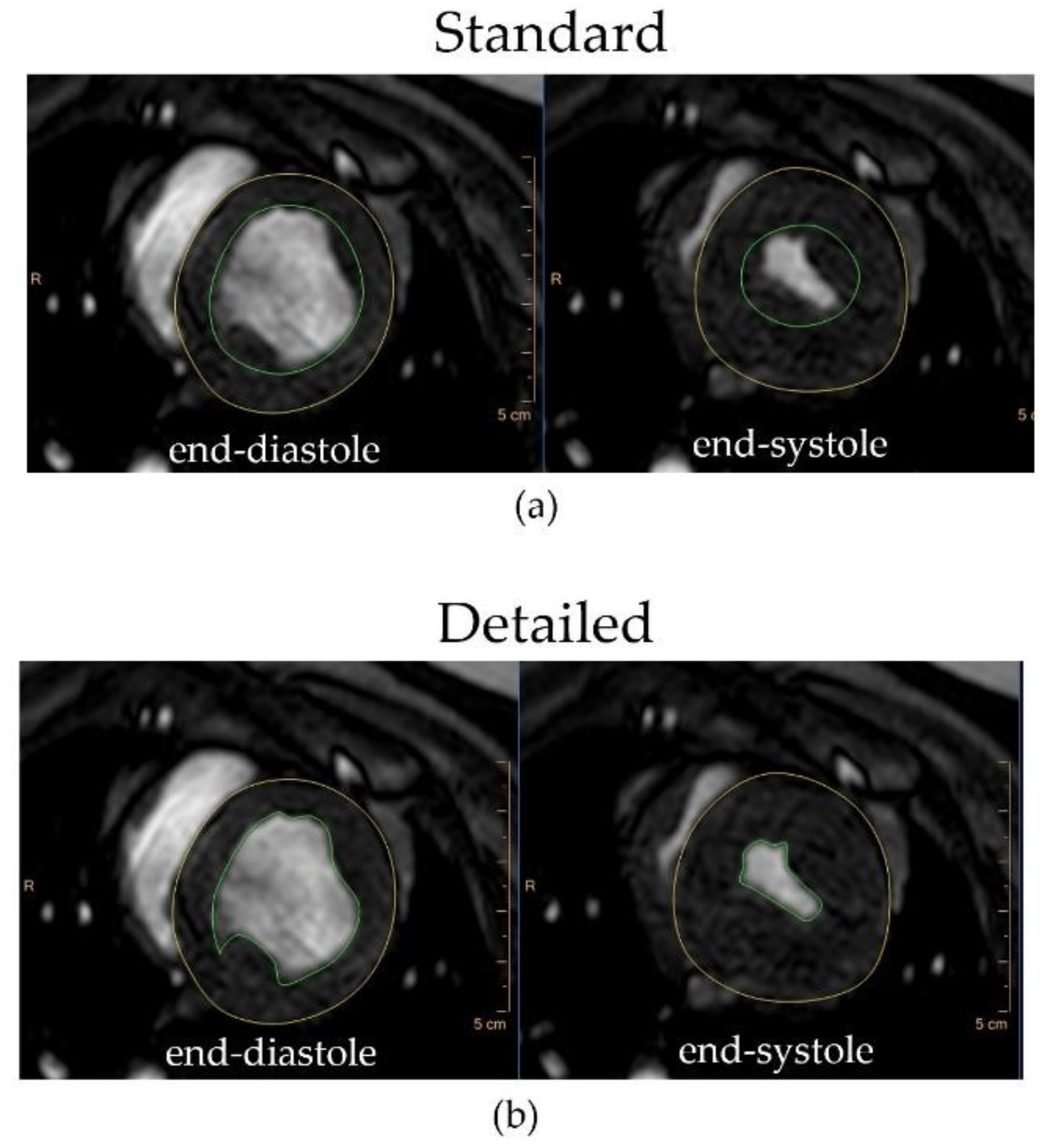
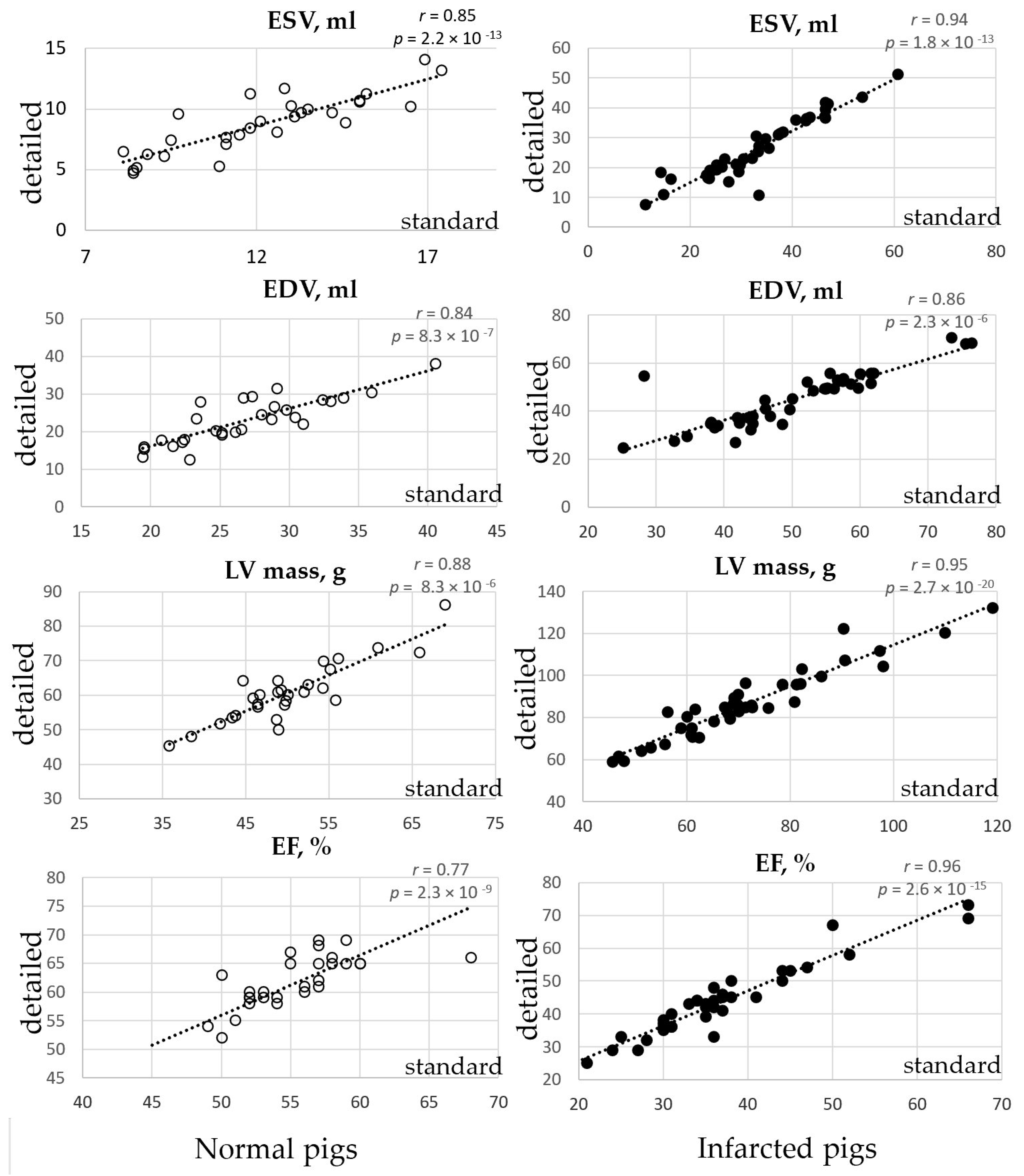

| Measurement | Standard Method | Detailed Method | Mean Relative Difference | p Value | Pearson Correlation Coefficient |
|---|---|---|---|---|---|
| ESV, ml | 12.2 ± 2.7 | 8.8 ± 2.4 | −28% | 2.18 × 10−13 | 0.85 |
| EDV, ml | 26.8 ± 5.3 | 22.9 ± 6.2 | −15% | 8.34 × 10−7 | 0.84 |
| SV, ml | 14.8 ± 3.1 | 14.2 ± 4.4 | −4% | 0.19 | 0.84 |
| LV mass, g | 50.1 ± 7.3 | 60.7 ± 8.7 | +21% | 8.33 × 10−6 | 0.88 |
| CO, L/min | 1.3 ± 0.3 | 1.2 ± 0.4 | −8% | 0.04 | 0.81 |
| EF, % | 55.0 ± 4.1 | 61.2 ± 6.0 | +11% | 2.28 × 10−9 | 0.77 |
| LV mass/Body weight | 0.0013 ± 0.0002 | 0.0016 ± 0.0002 | +23% | 1.79 × 10−8 | 0.7 |
| LV mass/ESV | 3.5 ± 1.0 | 6.2 ± 1.8 | +77% | 5.39 × 10−13 | 0.86 |
| LV mass/EDV | 1.6 ± 0.4 | 2.4 ± 0.7 | +50% | 6.52 × 10−10 | 0.79 |
| Measurement | Standard Method | Detailed Method | Mean Relative Difference | p Value | Pearson Correlation Coefficient |
|---|---|---|---|---|---|
| ESV, ml | 33.3 ± 11.1 | 26.2 ± 10.2 | −21% | 1.80 × 10−13 | 0.94 |
| EDV, ml | 50.2 ± 11.8 | 44.8 ± 11.5 | −1% | 2.32 × 10−6 | 0.86 |
| SV, ml | 17.2 ± 5.1 | 18.4 ± 5.8 | +7% | 0.05 | 0.75 |
| LV mass, g | 71.5 ± 16.4 | 86.5 ± 17.0 | +21% | 2.74 × 10−2 | 0.95 |
| CO, L/min | 1.4 ± 0.6 | 1.5 ± 0.5 | +7% | 0.08 | 0.86 |
| EF, % | 35.5 ± 10.7 | 42.1 ± 11.9 | +19% | 2.59 × 10−15 | 0.96 |
| LV mass/Body weight | 0.0017 ± 0.0005 | 0.002 ± 0.0005 | +18% | 1.53 × 10−9 | 0.94 |
| LV mass/ESV | 2.1 ± 0.9 | 3.3 ± 2.0 | +57% | 6.11 × 10−8 | 0.9 |
| LV mass/EDV | 1.2 ± 0.3 | 1.7 ± 0.6 | +42% | 3.52 × 10−9 | 0.9 |
| Infarct size, % to LV | 9 ± 4 | 10 ± 4 | +11% | 0.19 | 0.98 |
| Measurement | 2 Weeks (n = 16) | 4 Weeks (n = 7) | 8 Weeks (n = 7) | 12 Weeks (n = 6) | 16 Weeks (n = 4) |
|---|---|---|---|---|---|
| Standard Method | |||||
| ESV, ml | 26.9 ± 8.3 | 31.8 ± 11.9 | 31.6 ± 9.8 | 45.0 ± 11.4 | 39.7 ± 7.9 |
| EDV, ml | 45.0 ± 10.3 | 47.1 ± 9.6 | 48.3 ± 10.4 | 63.9 ± 12.8 | 55.5 ± 7.4 |
| SV, ml | 18.1 ± 3.9 | 15.3 ± 6.8 | 16.7 ± 6.6 | 18.8 ± 5.7 | 15.7 ± 1.2 |
| LV mass, g | 66.9 ± 11.8 | 86.2 ± 17.1 | 81.4 ± 26.3 | 71.1 ± 6.0 | 83.6 ± 11.9 |
| CO, L/min | 1.5 ± 0.5 | 1.3 ± 0.7 | 1.5 ± 0.6 | 1.5 ± 0.6 | 1.3 ± 0.5 |
| EF, % | 40.9 ± 8.5 | 33.6 ± 15.4 | 35.6 ± 11.7 | 30.0 ± 8.2 | 28.8 ± 5.2 |
| Detailed Method | |||||
| ESV, ml | 21.8 ± 7.6 | 25.3 ± 12.1 | 25.3 ± 7.8 | 36.8 ± 11.3 | 26.9 ± 13.6 |
| EDV, ml | 41.1 ± 10.4 | 39.8 ± 11.0 | 45.7 ± 8.3 | 57.2 ± 13.6 | 45.5 ± 9.7 |
| SV, ml | 19.2 ± 4.5 | 14.5 ± 5.1 | 20.4 ± 8.8 | 20.3 ± 6.1 | 16.1 ± 2.1 |
| LV mass, g | 81.6 ± 13.0 | 83.2 ± 18.6 | 96.5 ± 27.8 | 86.2 ± 10.0 | 97.2 ± 6.3 |
| CO, L/min | 1.5 ± 0.5 | 1.2 ± 0.6 | 1.8 ± 0.5 | 1.6 ± 0.7 | 1.3 ± 0.5 |
| EF, % | 47.5 ± 9.7 | 38.6 ± 16.4 | 44.4 ± 14.1 | 36.0 ± 9.5 | 36.3 ± 6.4 |
| Measured Parameters | Main Relative Difference between Reviewers Using the Standard Method | Main Relative Difference between Reviewers Using the Detailed Method | Standard Method Pearson Interclass Correlation Correlation | Detailed Method Pearson Interclass Correlation Correlation | ||
|---|---|---|---|---|---|---|
| ESV | 9% * | 8% | 0.76 | 0.65 | 0.95 | 0.85 |
| EDV | 11% ** | 9% * | 0.83 | 0.77 | 0.93 | 0.90 |
| LV mass | 7% ** | 5% * | 0.83 | 0.83 | 0.96 | 0.94 |
| EF | 2% | 1% | 0.81 | 0.81 | 0.94 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naumova, A.V.; Kicska, G.; Pimentel, K.; Neidig, L.E.; Tsuchida, H.; Nakamura, K.; Murry, C.E. Quantitative Analyses of the Left Ventricle Volume and Cardiac Function in Normal and Infarcted Yucatan Minipigs. J. Imaging 2021, 7, 107. https://doi.org/10.3390/jimaging7070107
Naumova AV, Kicska G, Pimentel K, Neidig LE, Tsuchida H, Nakamura K, Murry CE. Quantitative Analyses of the Left Ventricle Volume and Cardiac Function in Normal and Infarcted Yucatan Minipigs. Journal of Imaging. 2021; 7(7):107. https://doi.org/10.3390/jimaging7070107
Chicago/Turabian StyleNaumova, Anna V., Gregory Kicska, Kiana Pimentel, Lauren E. Neidig, Hiroshi Tsuchida, Kenta Nakamura, and Charles E. Murry. 2021. "Quantitative Analyses of the Left Ventricle Volume and Cardiac Function in Normal and Infarcted Yucatan Minipigs" Journal of Imaging 7, no. 7: 107. https://doi.org/10.3390/jimaging7070107
APA StyleNaumova, A. V., Kicska, G., Pimentel, K., Neidig, L. E., Tsuchida, H., Nakamura, K., & Murry, C. E. (2021). Quantitative Analyses of the Left Ventricle Volume and Cardiac Function in Normal and Infarcted Yucatan Minipigs. Journal of Imaging, 7(7), 107. https://doi.org/10.3390/jimaging7070107






