Examining the Relationship between Semiquantitative Methods Analysing Concentration-Time and Enhancement-Time Curves from Dynamic-Contrast Enhanced Magnetic Resonance Imaging and Cerebrovascular Dysfunction in Small Vessel Disease †
Abstract
:1. Introduction
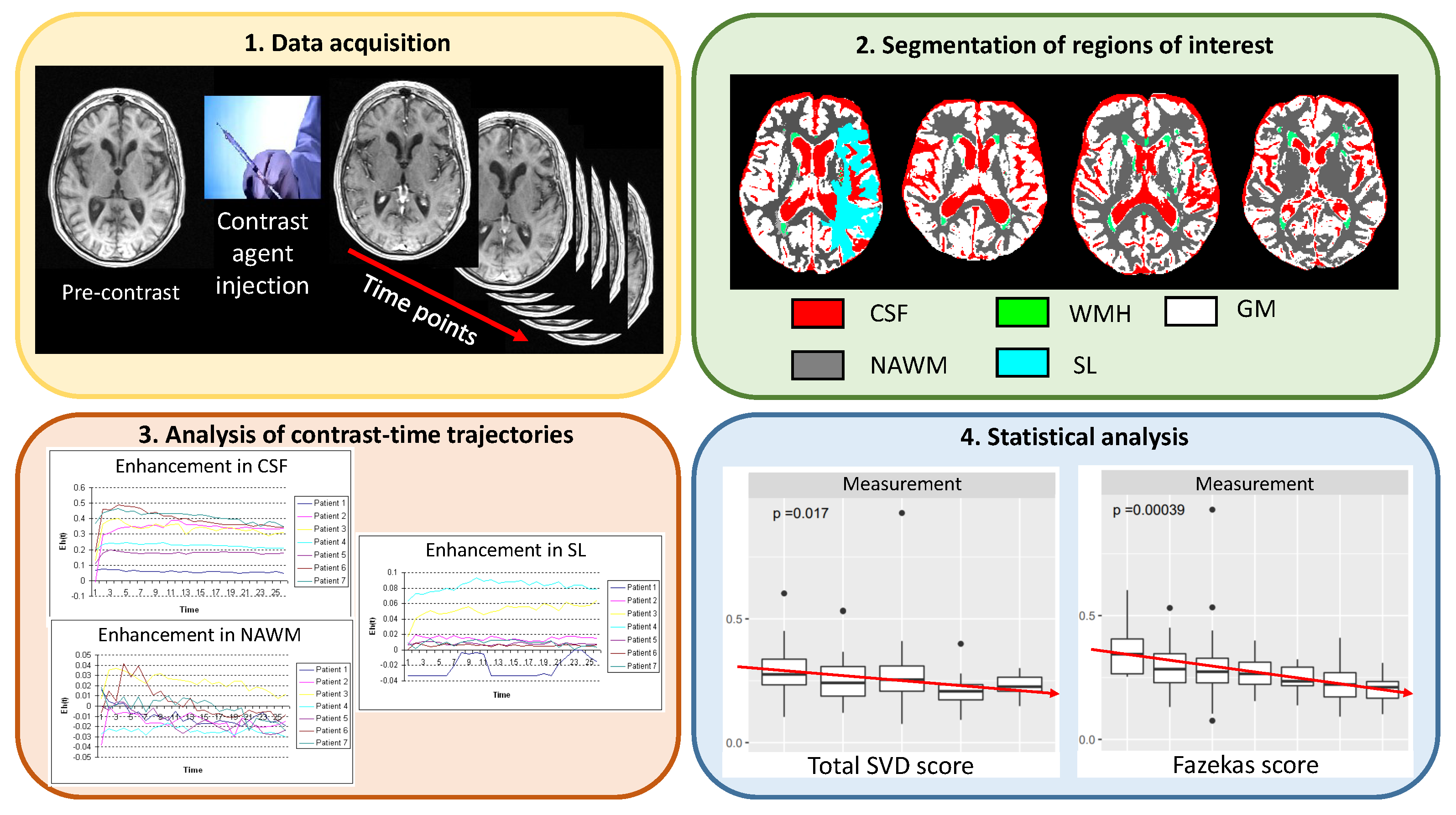
2. Materials and Methods
2.1. Subjects, Imaging, and Clinical Variables
2.2. Segmentation of Regions of Interest
2.3. Methods
2.3.1. Methods Analysing the Enhancement-Time Trajectory
2.3.2. Methods Analysing the Concentration-Time Trajectory
2.3.3. Methods to Analyse Changes in the Radial Power Spectrum
2.4. Validation against Clinical Parameters
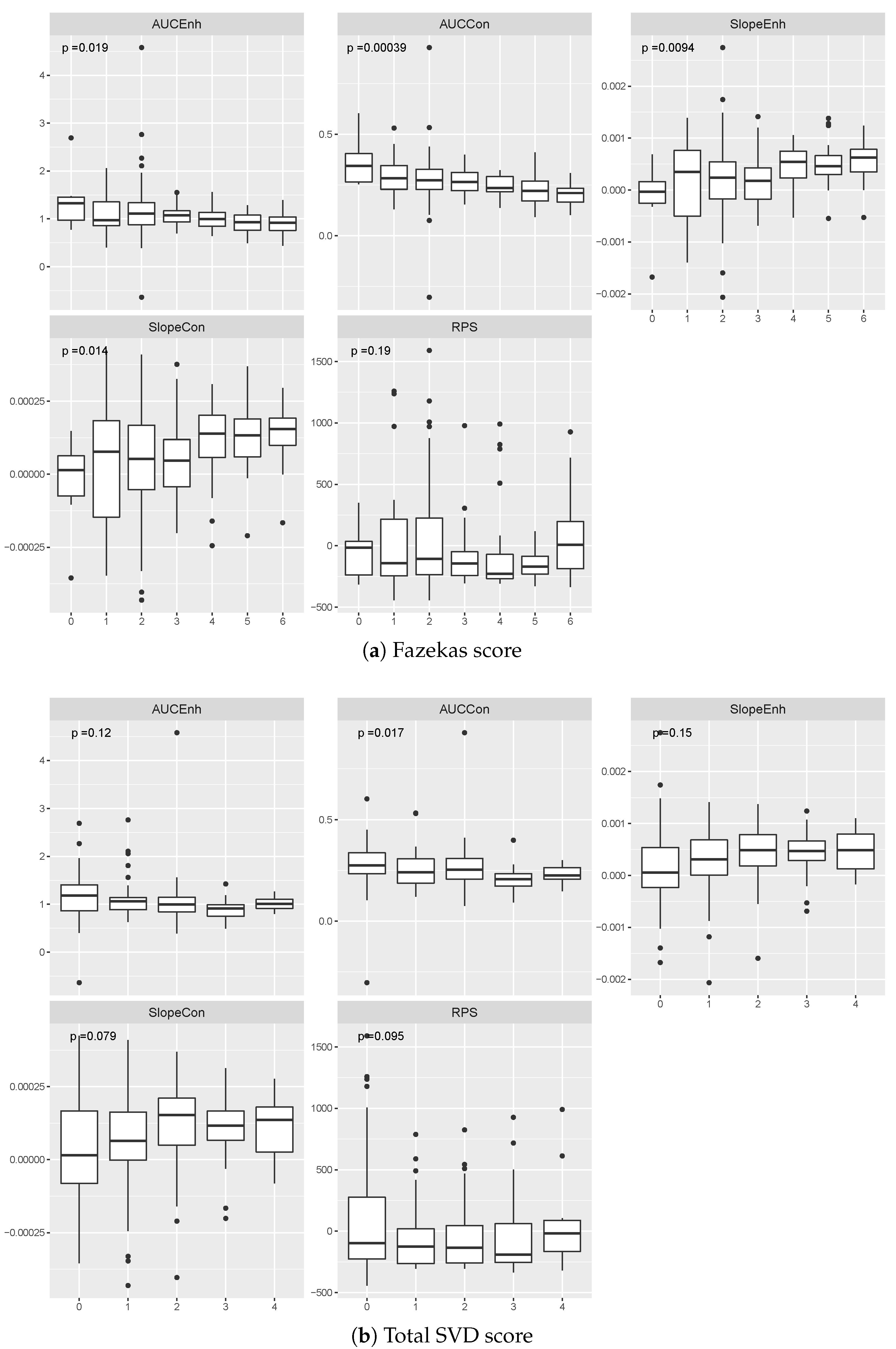
3. Results
3.1. Comparison of Effect Sizes
3.2. Relationship between Contrast Uptake/Washout Measures and Clinical Variables
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AUC | Area under the curve |
| Area under the concentration-time curve | |
| Area under the enhancement-time curve | |
| CI | Confidence interval |
| CSF | Blood-brain barrier |
| DCE-MRI | Dynamic contrast-enhanced magnetic resonance imaging |
| DGM | Deep grey matter |
| MAP | Mean arterial pressure |
| MRI | Magnetic resonance imaging |
| NAWM | Normal-appearing white matter |
| WMH | White matter hyperintensity |
| RPS | Radial power spectrum |
| SL | Stroke lesion |
| Slope of the concentration-time curve | |
| Slope of the enhancement-time curve | |
| SVD | Small vessel disease |
| TE | Echo time |
| TI | Inversion time |
| TR | Repetition time |
Appendix A
| ROI | Method | p-Value | Age | Biological Sex | MAP | Hyperlipidaemia | Smoker | Diabetes | Stroke Type | WMH Volume | SL Volume | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | |||||||||||||
| CSF | 16 | 2.17 × | −4.27 × | 5.02 × | −5.46 × | 1.40 × | −4.41 × | 4.25 × | −1.10 × | 5.17 × | −9.63 × | 5.84 × | −1.40 × | 5.85 × | −4.65 × | 7.88 × | 3.33 × | 3.90 × | 1.07 × | 1.61 × | |
| (−5.89 ×, −2.65 ×) | (−8.78 ×, −2.14 ×) | (−1.53 ×, 6.48 ×) | (−4.44 ×, 2.24 ×) | (−4.42 ×, 2.50 ×) | (−6.44 ×, 3.64 ×) | (−3.88 ×, 2.95 ×) | (−4.30 ×, 1.10 ×) | (−4.32 ×, 2.57 ×) | |||||||||||||
| 36 | 4.44 × | −1.52 × | 3.77 × | −1.40 × | 1.61 × | −9.58 × | 4.23 × | −3.73 × | 3.09 × | 1.18 × | 7.56 × | −1.17 × | 8.32 × | −1.27 × | 7.35 × | 2.46 × | 7.69 × | −8.12 × | 9.61 × | ||
| (−1.87 ×, −1.17 ×) | (−2.12 ×, −6.84 ×) | (−3.31 ×, 1.39 ×) | (−1.10 ×, 3.48 ×) | (−6.30 ×, 8.67 ×) | (−1.21 ×, 9.72 ×) | (−8.65 ×, 6.12 ×) | (−1.40 ×, 1.89 ×) | (−3.33 ×, 3.17 ×) | |||||||||||||
| 10 | 5.92 × | −3.51 × | 1.12 × | −1.50 × | 4.14 × | −6.13 × | 3.07 × | −1.98 × | 9.14 × | −1.32 × | 4.88 × | 3.95 × | 1.56 × | −9.23 × | 6.24 × | −5.99 × | 1.55 × | 6.36 × | 4.43 × | ||
| (−5.27 ×, −1.76 ×) | (−5.10 ×, 2.11 ×) | (−1.79 ×, 5.68 ×) | (−3.82 ×, 3.42 ×) | (−5.08 ×, 2.43 ×) | (−1.52 ×, 9.41 ×) | (−4.63 ×, 2.78 ×) | (−1.43 ×, 2.28 ×) | (−9.95 ×, 2.27 ×) | |||||||||||||
| 33 | 9.97 × | −1.29 × | 2.46 × | −1.19 × | 4.97 × | −1.71 × | 1.20 × | −3.71 × | 9.12 × | 1.58 × | 6.51 × | 6.27 × | 2.19 × | −2.92 × | 3.98 × | −5.49 × | 4.76 × | 1.94 × | 2.03 × | ||
| (−1.61 ×, −9.66 ×) | (−1.85 ×, −5.27 ×) | (−3.88 ×, 4.51 ×) | (−7.01 ×, 6.27 ×) | (−5.31 ×, 8.47 ×) | (−3.76 ×, 1.63 ×) | (−9.72 ×, 3.88 ×) | (−2.07 ×, 9.68 ×) | (−1.05 ×, 4.93 ×) | |||||||||||||
| RPS | 2 | 1.78 × | −5.43 × | 8.97 × | 2.94 × | 7.32 × | −8.39 × | 7.66 × | −2.16 × | 8.02 × | 1.85 × | 3.95 × | −4.23 × | 7.46 × | 3.29 × | 7.10 × | −3.62 × | 6.79 × | −3.53 × | 3.64 × | |
| (−8.80 ×, 7.71 ×) | (−1.40 ×, 1.99 ×) | (−6.39 ×, 4.71 ×) | (−1.92 ×, 1.49 ×) | (8.97 ×, 3.62 ×) | (−2.99 ×, 2.15 ×) | (−1.41 ×, 2.07 ×) | (−7.50 ×, 2.69 ×) | (−1.12 ×, 4.13 ×) | |||||||||||||
| DGM | 3 | 8.11 × | −2.55 × | 2.97 × | 3.37 × | 5.02 × | −8.73 × | 5.95 × | −2.97 × | 5.56 × | −8.02 × | 1.26 × | 1.69 × | 2.75 × | 3.89 × | 4.51 × | 1.99 × | 8.53 × | 3.62 × | 1.12 × | |
| (−7.37 ×, 2.26 ×) | (−6.51 ×, 1.33 ×) | (−4.11 ×, 2.36 ×) | (−1.29 ×, 6.96 ×) | (−1.83 ×, 2.28 ×) | (1.89 ×, 3.19 ×) | (−6.27 ×, 1.41 ×) | (−2.79 ×, 4.26 ×) | (−8.48 ×, 8.09 ×) | |||||||||||||
| 13 | 6.20 × | −2.29 × | 9.30 × | −5.52 × | 5.94 × | −8.96 × | 7.92 × | −1.53 × | 1.41 × | −7.05 × | 5.13 × | 5.37 × | 7.47 × | 5.52 × | 6.04 × | −6.77 × | 7.75 × | −1.08 × | 8.18 × | ||
| (−3.29 ×, −1.30 ×) | (−2.59 ×, 1.49 ×) | (−7.57 ×, 5.78 ×) | (−3.58 ×, 5.13 ×) | (−2.83 ×, 1.42 ×) | (2.28 ×, 8.46 ×) | (−1.54 ×, 2.65 ×) | (−5.35 ×, 4.00 ×) | (−1.03 ×, 8.14 ×) | |||||||||||||
| 3 | 9.77 × | −3.00 × | 1.93 × | −1.27 × | 7.73 × | −2.50 × | 1.07 × | 3.72 × | 4.32 × | 1.83 × | 7.09 × | 3.43 × | 6.31 × | 2.17 × | 6.54 × | −9.17 × | 3.97 × | 8.51 × | 6.90 × | ||
| (−7.52 ×, 1.53 ×) | (−2.20 ×, −3.39 ×) | (−5.54 ×, 5.42 ×) | (−5.60 ×, 1.30 ×) | (−7.84 ×, 1.15 ×) | (−1.06 ×, 1.75 ×) | (−7.37 ×, 1.17 ×) | (−3.05 ×, 1.21 ×) | (−3.35 ×, 5.05 ×) | |||||||||||||
| 8 | 3.59 × | −1.35 × | 1.55 × | −2.94 × | 1.01 × | −4.97 × | 1.81 × | 7.61 × | 5.04 × | 9.31 × | 4.30 × | 1.70 × | 3.23 × | 3.57 × | 7.59 × | −4.05 × | 1.20 × | −8.70 × | 9.86 × | ||
| (−2.43 ×, −2.59 ×) | (−5.17 ×, −7.08 ×) | (−1.23 ×, 2.33 ×) | (−1.48 ×, 3.00 ×) | (−1.39 ×, 3.25 ×) | (−1.68 ×, 5.08 ×) | (−1.94 ×, 2.65 ×) | (−9.17 ×, 1.06 ×) | (−1.02 ×, 1.00 ×) | |||||||||||||
| RPS | 5 | 3.33 × | 1.73 × | 4.89 × | −6.55 × | 2.02 × | −2.14 × | 2.03 × | −2.09 × | 6.84 × | 5.72 × | 2.84 × | −1.25 × | 1.09 × | 1.17 × | 8.24 × | −3.19 × | 7.05 × | −1.72 × | 4.57 × | |
| (−3.18 ×, 6.64 ×) | (−1.66 ×, 3.53 ×) | (−5.44 ×, 1.16 ×) | (−1.22 ×, 8.04 ×) | (−4.78 ×, 1.62 ×) | (−2.77 ×, 2.82 ×) | (−9.19 ×, 1.15 ×) | (−5.51 ×, −8.81 ×) | (−6.28 ×, 2.84 ×) | |||||||||||||
| NAWM | 3 | 1.27 × | −1.58 × | 2.68 × | −3.34 × | 2.54 × | −5.93 × | 5.35 × | −1.65 × | 5.74 × | −6.89 × | 2.45 × | 1.92 × | 6.64 × | −2.25 × | 4.53 × | 1.51 × | 2.47 × | 7.27 × | 5.82 × | |
| (−4.38 ×, 1.23 ×) | (−9.09 ×, 2.42 ×) | (−2.48 ×, 1.29 ×) | (−7.43 ×, 4.13 ×) | (−1.29 ×, −8.95 ×) | (−6.80 ×, 1.06 ×) | (−8.17 ×, 3.66 ×) | (1.95 ×, 2.83 ×) | (−1.88 ×, 3.33 ×) | |||||||||||||
| 6 | 1.07 × | −1.81 × | 1.03 × | −7.85 × | 4.02 × | −4.70 × | 8.78 × | −9.61 × | 3.07 × | −1.48 × | 1.30 × | 1.89 × | 1.83 × | −8.38 × | 3.84 × | 1.07 × | 6.17 × | −4.22 × | 3.19 × | ||
| (−2.70 ×, −9.08 ×) | (−2.63 ×, 1.06 ×) | (−6.50 ×, 5.56 ×) | (−2.81 ×, 8.90 ×) | (−3.40 ×, 4.38 ×) | (−8.99 ×, 4.69 ×) | (−2.73 ×, 1.06 ×) | (−3.15 ×, 5.30 ×) | (−1.25 ×, 4.12 ×) | |||||||||||||
| −1 | 5.94 × | 6.78 × | 7.04 × | −2.42 × | 5.09 × | −1.43 × | 9.90 × | 6.60 × | 7.40 × | 2.84 × | 4.58 × | −5.99 × | 9.14 × | −4.56 × | 2.27 × | −9.58 × | 2.55 × | −1.55 × | 3.50 × | ||
| (−2.84 ×, 4.19 ×) | (−9.64 ×, 4.80 ×) | (−2.38 ×, 2.35 ×) | (−6.48 ×, 1.39 ×) | (−4.68 ×, 1.04 ×) | (−1.15 ×, 1.03 ×) | (−1.20 ×, 2.87 ×) | (−2.61 ×, 6.98 ×) | (−4.81 ×, 1.72 ×) | |||||||||||||
| 2 | 1.43 × | −6.60 × | 2.73 × | −7.77 × | 5.29 × | 2.02 × | 6.16 × | 1.94 × | 1.19 × | 9.82 × | 4.45 × | 1.14 × | 5.44 × | −1.65 × | 1.94 × | −4.56 × | 1.08 × | −8.97 × | 1.09 × | ||
| (−1.84 ×, 5.23 ×) | (−3.21 ×, 1.65 ×) | (−5.93 ×, 9.98 ×) | (−5.01 ×, 4.38 ×) | (−1.55 ×, 3.51 ×) | (−2.55 ×, 4.82 ×) | (−4.15 ×, 8.48 ×) | (−1.01 ×, 1.02 ×) | (−2.00 ×, 2.01 ×) | |||||||||||||
| RPS | −2 | 8.53 × | 2.36 × | 9.57 × | 1.59 × | 7.85 × | 1.39 × | 9.62 × | 1.52 × | 8.67 × | 9.35 × | 3.19 × | 3.26 × | 8.11 × | 5.55 × | 5.49 × | 4.87 × | 8.13 × | −1.74 × | 9.97 × | |
| (−8.40 ×, 8.87 ×) | (−1.83 ×, 3.36 ×) | (−5.67 ×, 5.94 ×) | (−1.63 ×, 1.93 ×) | (−9.12 ×, 2.78 ×) | (−2.36 ×, 3.01 ×) | (−1.27 ×, 2.38 ×) | (−3.58 ×, 4.55 ×) | (−8.04 ×, 8.00 ×) | |||||||||||||
| WMH | 13 | 3.60 × | −6.81 × | 2.04 × | 5.37 × | 3.70 × | 2.29 × | 9.07 × | −1.68 × | 7.80 × | −1.73 × | 7.81 × | 2.72 × | 3.07 × | 1.67 × | 7.86 × | −4.46 × | 1.37 × | 6.38 × | 1.93 × | |
| (−1.26 ×, −1.07 ×) | (−6.42 ×, 1.72 ×) | (−3.63 ×, 4.09 ×) | (−1.35 ×, 1.02 ×) | (−1.40 ×, 1.06 ×) | (9.31 ×, 4.51 ×) | (−1.05 ×, 1.38 ×) | (−7.17 ×, −1.75 ×) | (1.05 ×, 1.17 ×) | |||||||||||||
| 25 | 2.49 × | −2.88 × | 3.38 × | 1.96 × | 1.15 × | 1.20 × | 7.67 × | −1.30 × | 2.95 × | −4.53 × | 7.25 × | 6.13 × | 1.25 × | −1.02 × | 9.94 × | −1.08 × | 1.98 × | −9.75 × | 8.61 × | ||
| (−4.07 ×, −1.69 ×) | (−4.78 ×, 4.39 ×) | (−6.78 ×, 9.17 ×) | (−3.75 ×, 1.14 ×) | (−2.99 ×, 2.08 ×) | (2.44 ×, 9.82 ×) | (−2.51 ×, 2.49 ×) | (−1.63 ×, −5.17 ×) | (−1.20 ×, 1.00 ×) | |||||||||||||
| 4 | 6.11 × | −2.23 × | 4.59 × | −1.61 × | 9.94 × | −1.01 × | 6.18 × | 1.11 × | 7.47 × | 8.52 × | 1.87 × | 5.82 × | 5.35 × | −6.90 × | 2.79 × | 1.19 × | 4.04 × | 4.35 × | 1.21 × | ||
| (−8.17 ×, 3.70 ×) | (−2.83 ×, −3.90 ×) | (−5.00 ×, 2.98 ×) | (−1.12 ×, 2.34 ×) | (−4.18 ×, 2.12 ×) | (−1.27 ×, 2.43 ×) | (−1.94 ×, 5.63 ×) | (−1.61 ×, 3.98 ×) | (−1.16 ×, 9.87 ×) | |||||||||||||
| 4 | 7.23 × | −1.32 × | 6.88 × | −3.76 × | 1.18 × | −2.34 × | 6.30 × | 2.53 × | 9.04 × | 2.03 × | 1.91 × | 2.37 × | 2.92 × | −1.58 × | 3.00 × | 3.35 × | 3.25 × | 1.74 × | 7.95 × | ||
| (−2.74 ×, 1.03 ×) | (−6.68 ×, −8.43 ×) | (−1.19 ×, 7.22 ×) | (−4.03 ×, 5.47 ×) | (−1.02 ×, 5.07 ×) | (−2.06 ×, 6.80 ×) | (−4.58 ×, 1.42 ×) | (−3.35 ×, 1.00 ×) | (−1.15 ×, 1.50 ×) | |||||||||||||
| RPS | −3 | 9.58 × | 1.90 × | 4.82 × | 5.00 × | 3.68 × | 9.04 × | 6.19 × | −2.76 × | 6.21 × | 1.13 × | 8.45 × | −7.28 × | 3.88 × | −4.11 × | 4.72 × | 1.96 × | 8.78 × | −1.27 × | 6.12 × | |
| (−3.43 ×, 7.23 ×) | (−5.94 ×, 1.59 ×) | (−2.68 ×, 4.49 ×) | (−1.37 ×, 8.23 ×) | (−1.03 ×, 1.25 ×) | (−2.39 ×, 9.31 ×) | (−1.54 ×, 7.14 ×) | (−2.31 ×, 2.71 ×) | (−6.22 ×, 3.67 ×) | |||||||||||||
| SL | 33 | 9.90 × | 5.95 × | 5.04 × | 2.28 × | 2.06 × | 2.36 × | 6.82 × | 2.18 × | 2.15 × | 1.40 × | 4.38 × | 1.13 × | 3.43 × | −8.31 × | 1.52 × | −6.66 × | 1.07 × | 2.41 × | 8.69 × | |
| (−1.16 ×, 2.35 ×) | (−1.27 ×, 5.83 ×) | (−9.03 ×, 1.38 ×) | (−1.28 ×, 5.65 ×) | (−2.16 ×, 4.96 ×) | (6.10 ×, 1.65 ×) | (−1.20 ×, −4.65 ×) | (−1.48 ×, 1.45 ×) | (1.01 ×, 3.82 ×) | |||||||||||||
| 28 | 6.33 × | −2.22 × | 2.21 × | 5.17 × | 1.58 × | 6.43 × | 5.82 × | 3.20 × | 3.70 × | 1.98 × | 5.88 × | 3.06 × | 6.73 × | −1.19 × | 1.92 × | −1.47 × | 7.88 × | 2.63 × | 6.87 × | ||
| (−5.78 ×, 1.35 ×) | (−2.02 ×, 1.24 ×) | (−1.67 ×, 2.95 ×) | (−3.83 ×, 1.02 ×) | (−5.23 ×, 9.20 ×) | (2.00 ×, 4.11 ×) | (−1.93 ×, −4.45 ×) | (−3.11 ×, 1.72 ×) | (−2.05 ×, 5.47 ×) | |||||||||||||
| 14 | 6.30 × | 5.02 × | 6.69 × | 1.69 × | 4.77 × | 2.74 × | 7.19 × | 1.45 × | 5.30 × | 2.41 × | 3.12 × | 6.85 × | 5.05 × | −4.62 × | 6.02 × | −7.57 × | 1.63 × | 3.37 × | 4.36 × | ||
| (−1.81 ×, 2.82 ×) | (−2.99 ×, 6.37 ×) | (−1.23 ×, 1.78 ×) | (−3.12 ×, 6.03 ×) | (−2.28 ×, 7.10 ×) | (−1.45 ×, 1.37 ×) | (−9.44 ×, 2.01 ×) | (−1.83 ×, 3.12 ×) | (1.52 ×, 5.22 ×) | |||||||||||||
| 13 | 1.02 × | −2.54 × | 3.41 × | 5.33 × | 3.24 × | 1.43 × | 4.08 × | 3.69 × | 4.84 × | 8.71 × | 8.72 × | 2.60 × | 1.28 × | −1.29 × | 2.17 × | −2.01 × | 1.04 × | 3.87 × | 7.06 × | ||
| (−7.81 ×, 2.73 ×) | (−5.32 ×, 1.60 ×) | (−1.98 ×, 4.85 ×) | (−6.71 ×, 1.41 ×) | (−9.81 ×, 1.15 ×) | (1.04 ×, 4.16 ×) | (−2.38 ×, −1.92 ×) | (−4.44 ×, 4.20 ×) | (−3.30 ×, 8.07 ×) | |||||||||||||
| RPS | 4 | 4.51 × | 1.43 × | 9.52 × | 3.15 × | 5.18 × | 5.64 × | 7.23 × | −2.27 × | 6.42 × | 1.45 × | 7.75 × | −3.59 × | 6.26 × | 2.40 × | 6.31 × | 2.45 × | 2.95 × | 7.16 × | 1.34 × | |
| (−4.53 ×, 4.81 ×) | (−6.44 ×, 1.27 ×) | (−2.58 ×, 3.70 ×) | (−1.19 ×, 7.36 ×) | (−8.54 ×, 1.14 ×) | (−1.81 ×, 1.09 ×) | (−7.46 ×, 1.23 ×) | (2.46 ×, 4.65 ×) | (2.82 ×, 1.15 × ) | |||||||||||||
References
- Wardlaw, J.M.; Doubal, F.; Armitage, P.; Chappell, F.; Carpenter, T.; Muñoz Maniega, S.; Farrall, A.; Sudlow, C.; Dennis, M.; Dhillon, B. Lacunar stroke is associated with diffuse blood–brain barrier dysfunction. Ann. Neurol. 2009, 65, 194–202. [Google Scholar] [CrossRef]
- Kidwell, C.; Burgess, R.; Menon, R.; Warach, S.; Latour, L. Hyperacute injury marker (HARM) in primary hemorrhage: A distinct form of CNS barrier disruption. Neurology 2011, 77, 1725–1728. [Google Scholar] [CrossRef] [PubMed]
- Thrippleton, M.J.; Backes, W.H.; Sourbron, S.; Ingrisch, M.; van Osch, M.J.; Dichgans, M.; Fazekas, F.; Ropele, S.; Frayne, R.; van Oostenbrugge, R.J.; et al. Quantifying blood–brain barrier leakage in small vessel disease: Review and consensus recommendations. Alzheimer’s Dement. 2019, 15, 840–858. [Google Scholar] [CrossRef] [PubMed]
- Topakian, R.; Barrick, T.; Howe, F.; Markus, H. Blood–brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J. Neurol. Neurosurg. Psychiatry 2010, 81, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Maniega, S.; Chappell, F.M.; Valdés Hernández, M.C.; Armitage, P.A.; Makin, S.D.; Heye, A.K.; Thrippleton, M.J.; Sakka, E.; Shuler, K.; Dennis, M.S.; et al. Integrity of normal-appearing white matter: Influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J. Cereb. Blood Flow Metab. 2017, 37, 644–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardlaw, J.M.; Makin, S.J.; Valdés-Hernández, M.C.; Armitage, P.A.; Heye, A.K.; Chappell, F.M.; Munoz-Maniega, S.; Sakka, E.; Shuler, K.; Dennis, M.S.; et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: Evidence from a cohort study. Alzheimer’s Dement. 2017, 13, 634–643. [Google Scholar] [CrossRef] [Green Version]
- Bernal, J.; Valdés-Hernández, M.d.C.; Escudero, J.; Armitage, P.A.; Makin, S.; Touyz, R.M.; Wardlaw, J.M. Analysis of spatial spectral features of dynamic contrast-enhanced brain magnetic resonance images for studying small vessel disease. In Proceedings of the Annual Conference on Medical Image Understanding and Analysis, Liverpool, UK, 24–26 July 2019; pp. 282–293. [Google Scholar]
- Bernal, J.; Valdés-Hernández, M.d.C.; Escudero, J.; Viksne, L.; Heye, A.K.; Armitage, P.A.; Makin, S.; Touyz, R.M.; Wardlaw, J.M. Analysis of dynamic texture and spatial spectral descriptors of dynamic contrast-enhanced brain magnetic resonance images for studying small vessel disease. Magn. Reson. Imaging 2020, 66, 240–247. [Google Scholar] [CrossRef]
- Armitage, P.A.; Farrall, A.J.; Carpenter, T.K.; Doubal, F.N.; Wardlaw, J.M. Use of dynamic contrast-enhanced MRI to measure subtle blood–brain barrier abnormalities. Magn. Reson. Imaging 2011, 29, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Barnes, S.R.; Ng, T.S.C.; Montagne, A.; Law, M.; Zlokovic, B.V.; Jacobs, R.E. Optimal acquisition and modeling parameters for accurate assessment of low Ktrans blood–brain barrier permeability using dynamic contrast-enhanced MRI. Magn. Reson. Med. 2016, 75, 1967–1977. [Google Scholar] [CrossRef] [Green Version]
- Heye, A.K.; Thrippleton, M.J.; Armitage, P.A.; Valdés-Hernández, M.d.C.; Makin, S.D.; Glatz, A.; Sakka, E.; Wardlaw, J.M. Tracer kinetic modelling for DCE-MRI quantification of subtle blood–brain barrier permeability. Neuroimage 2016, 125, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Valdés-Hernández, M.d.C.; Armitage, P.A.; Thrippleton, M.J.; Chappell, F.; Sandeman, E.; Muñoz Maniega, S.; Shuler, K.; Wardlaw, J.M. Rationale, design and methodology of the image analysis protocol for studies of patients with cerebral small vessel disease and mild stroke. Brain Behav. 2015, 5, e00415. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Chappell, F.M.; Valdés-Hernández, M.d.C.; Makin, S.D.; Staals, J.; Shuler, K.; Thrippleton, M.J.; Armitage, P.A.; Muñoz-Maniega, S.; Heye, A.K.; et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology 2017, 89, 1003–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazekas, F.; Niederkorn, K.; Schmidt, R.; Offenbacher, H.; Horner, S.; Bertha, G.; Lechner, H. White matter signal abnormalities in normal individuals: Correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke 1988, 19, 1285–1288. [Google Scholar] [CrossRef] [Green Version]
- Staals, J.; Makin, S.D.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef]
- Valdés-Hernández, M.d.C.; Ferguson, K.J.; Chappell, F.M.; Wardlaw, J.M. New multispectral MRI data fusion technique for white matter lesion segmentation: Method and comparison with thresholding in FLAIR images. Eur. Radiol. 2010, 20, 1684–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapter 3 Power spectrum and its applications. In Analysis of Geophysical Potential Fields; Naidu, P.S.; Mathew, M. (Eds.) Elsevier: Amsterdam, The Netherlands, 1998; Volume 5, pp. 75–143. [Google Scholar]
- Happ, C.; Greven, S. Multivariate functional principal component analysis for data observed on different (dimensional) domains. J. Am. Stat. Assoc. 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bronge, L.; Wahlund, L.O. White matter lesions in dementia: An MRI study on blood-brain barrier dysfunction. Dement. Geriatr. Cogn. Disord. 2000, 11, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, H.; Asano, T.; Tanaka, Y.; Iwamoto, T.; Takasaki, M.; Abe, K. Increased blood-brain barrier permeability in white matter lesions of Binswanger’s disease evaluated by contrast-enhanced MRI. Dement. Geriatr. Cogn. Disord. 2002, 14, 1–6. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Farrall, A.; Armitage, P.A.; Carpenter, T.; Chappell, F.; Doubal, F.; Chowdhury, D.; Cvoro, V.; Dennis, M.S. Changes in background blood–brain barrier integrity between lacunar and cortical ischemic stroke subtypes. Stroke 2008, 39, 1327–1332. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, J.M.; Doubal, F.N.; Valdes-Hernandez, M.; Wang, X.; Chappell, F.M.; Shuler, K.; Armitage, P.A.; Carpenter, T.C.; Dennis, M.S. Blood–brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke 2013, 44, 525–527. [Google Scholar] [CrossRef] [Green Version]
- Starr, J.M.; Farrall, A.J.; Armitage, P.; McGurn, B.; Wardlaw, J. Blood–brain barrier permeability in Alzheimer’s disease: A case–control MRI study. Psychiatry Res. Neuroimaging 2009, 171, 232–241. [Google Scholar] [CrossRef]
- Heye, A.K.; Culling, R.D.; Valdés-Hernández, M.d.C.; Thrippleton, M.J.; Wardlaw, J.M. Assessment of blood–brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. NeuroImage Clin. 2014, 6, 262–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, W.R.; Thore, C.R. Cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol. 2011, 37, 56–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heye, A.K. Measurement of Subtle Blood-Brain Barrier Disruption in Cerebral Small Vessel Disease Using Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Ph.D. Thesis, The University of Edinburgh, Edinburgh, UK, 2015. [Google Scholar]
- Wardlaw, J.M.; Valdés-Hernández, M.C.; Muñoz-Maniega, S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 2015, 4, e001140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Thrippleton, M.J.; Makin, S.D.; Marshall, I.; Geerlings, M.I.; de Craen, A.J.; van Buchem, M.A.; Wardlaw, J.M. Cerebral blood flow in small vessel disease: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2016, 36, 1653–1667. [Google Scholar] [CrossRef] [Green Version]
- Bahrani, A.A.; Powell, D.K.; Yu, G.; Johnson, E.S.; Jicha, G.A.; Smith, C.D. White matter hyperintensity associations with cerebral blood flow in elderly subjects stratified by cerebrovascular risk. J. Stroke Cerebrovasc. Dis. 2017, 26, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Blair, G.W.; Thrippleton, M.J.; Shi, Y.; Hamilton, I.; Stringer, M.; Chappell, F.M.; Dickie, D.A.; Andrews, P.J.; Marshall, I.; Doubal, F.N.; et al. Intracranial functional haemodynamic relationships in patients with cerebral small vessel disease. bioRxiv 2019. [Google Scholar] [CrossRef]
- Biessels, G.J. Cerebral complications of diabetes: Clinical findings and pathogenetic mechanisms. Neth. J. Med. 1999, 54, 35–45. [Google Scholar] [CrossRef]
- Prasad, S.; Sajja, R.K.; Naik, P.; Cucullo, L. Diabetes mellitus and blood-brain barrier dysfunction: An overview. J. Pharmacovigil. 2014, 2, 125. [Google Scholar]
- Xu, R.S. Pathogenesis of diabetic cerebral vascular disease complication. World J. Diabetes 2015, 6, 54. [Google Scholar] [CrossRef] [PubMed]


| Stroke | WMH | SL | ||||||
|---|---|---|---|---|---|---|---|---|
| ROI | Method | p-Value | Age | Diabetes | Type | Volume | Volume | |
| CSF | 16 | 2.17× | 5.02 × | 5.85 × | 7.88× | 3.90 × | 1.61 × | |
| 36 | 4.44 × | 3.77 × | 8.32 × | 7.35 × | 7.69 × | 9.61 × | ||
| 10 | 5.92 × | 1.12 × | 1.56 × | 6.24 × | 1.55 × | 4.43 × | ||
| 33 | 9.97 × | 2.46 × | 2.19 × | 3.98 × | 4.76 × | 2.03 × | ||
| RPS | 2 | 1.78 × | 8.97 × | 7.46 × | 7.10 × | 6.79 × | 3.64 × | |
| DGM | 3 | 8.11 × | 2.97 × | 2.75 × | 4.51 × | 8.53 × | 1.12 × | |
| 13 | 6.20 × | 9.30 × | 7.47 × | 6.04 × | 7.75 × | 8.18 × | ||
| 3 | 9.77 × | 1.93 × | 6.31 × | 6.54 × | 3.97 × | 6.90 × | ||
| 8 | 3.59 × | 1.55 × | 3.23 × | 7.59 × | 1.20 × | 9.86 × | ||
| RPS | 5 | 3.33 × | 4.89 × | 1.09 × | 8.24 × | 7.05 × | 4.57 × | |
| NAWM | 3 | 1.27 × | 2.68 × | 6.64 × | 4.53 × | 2.47 × | 5.82 × | |
| 6 | 1.07 × | 1.03 × | 1.83 × | 3.84 × | 6.17 × | 3.19 × | ||
| −1 | 5.94 × | 7.04 × | 9.14 × | 2.27 × | 2.55 × | 3.50 × | ||
| 2 | 1.43 × | 2.73 × | 5.44 × | 1.94 × | 1.08 × | 1.09 × | ||
| RPS | −2 | 8.53 × | 9.57 × | 8.11 × | 5.49 × | 8.13 × | 9.97 × | |
| WMH | 13 | 3.60 × | 2.04 × | 3.07 × | 7.86 × | 1.37 × | 1.93 × | |
| 25 | 2.49 × | 3.38 × | 1.25 × | 9.94 × | 1.98 × | 8.61 × | ||
| 4 | 6.11 × | 4.59 × | 5.35 × | 2.79 × | 4.04 × | 1.21 × | ||
| 4 | 7.23 × | 6.88 × | 2.92 × | 3.00 × | 3.25 × | 7.95 × | ||
| RPS | −3 | 9.58 × | 4.82 × | 3.88 × | 4.72 × | 8.78 × | 6.12 × | |
| SL | 33 | 9.90 × | 5.04 × | 3.43 × | 1.52 × | 1.07 × | 8.69 × | |
| 28 | 6.33 × | 2.21 × | 6.73 × | 1.92 × | 7.88 × | 6.87 × | ||
| 14 | 6.30 × | 6.69 × | 5.05 × | 6.02 × | 1.63 × | 4.36 × | ||
| 13 | 1.02 × | 3.41 × | 1.28 × | 2.17 × | 1.04 × | 7.06 × | ||
| RPS | 4 | 4.51 × | 9.52 × | 6.26 × | 6.31 × | 2.95 × | 1.34 × |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal, J.; Valdés-Hernández, M.; Escudero, J.; Sakka, E.; Armitage, P.A.; Makin, S.; Touyz, R.M.; Wardlaw, J.M. Examining the Relationship between Semiquantitative Methods Analysing Concentration-Time and Enhancement-Time Curves from Dynamic-Contrast Enhanced Magnetic Resonance Imaging and Cerebrovascular Dysfunction in Small Vessel Disease. J. Imaging 2020, 6, 43. https://doi.org/10.3390/jimaging6060043
Bernal J, Valdés-Hernández M, Escudero J, Sakka E, Armitage PA, Makin S, Touyz RM, Wardlaw JM. Examining the Relationship between Semiquantitative Methods Analysing Concentration-Time and Enhancement-Time Curves from Dynamic-Contrast Enhanced Magnetic Resonance Imaging and Cerebrovascular Dysfunction in Small Vessel Disease. Journal of Imaging. 2020; 6(6):43. https://doi.org/10.3390/jimaging6060043
Chicago/Turabian StyleBernal, Jose, María Valdés-Hernández, Javier Escudero, Eleni Sakka, Paul A. Armitage, Stephen Makin, Rhian M. Touyz, and Joanna M. Wardlaw. 2020. "Examining the Relationship between Semiquantitative Methods Analysing Concentration-Time and Enhancement-Time Curves from Dynamic-Contrast Enhanced Magnetic Resonance Imaging and Cerebrovascular Dysfunction in Small Vessel Disease" Journal of Imaging 6, no. 6: 43. https://doi.org/10.3390/jimaging6060043





