Bone Mineral Density (BMD) Assessment Using Dual-Energy CT with Different Base Material Pairs (BMPs)
Abstract
1. Introduction
1.1. State of the Art
1.2. Aim of the Study
2. Materials and Methods
2.1. Study Population
2.2. Imaging
2.3. Post-Processing
2.4. Data Evaluation and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, 580–592. [Google Scholar] [CrossRef]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Melton, L.J., 3rd; Khaltaev, N. A reference standard for the description of osteoporosis. Bone 2008, 42, 467–475. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Niemi, S.; Parkkari, J.; Palvanen, M.; Vuori, I.; Järvinen, M. Hip fractures in Finland between 1970 and 1997 and predictions for the future. Lancet 1999, 353, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef]
- Curtis, J.R.; Safford, M.M. Management of osteoporosis among the elderly with other chronic medical conditions. Drugs Aging 2012, 29, 549–564. [Google Scholar] [CrossRef]
- Berry, M.E. Using DXA to Identify and Treat Osteoporosis in Pediatric Patients. Radiol. Technol. 2018, 89, 312–317. [Google Scholar] [PubMed]
- Sangondimath, G.; Sen, R.K.; Fazal Rehman, T. DEXA and Imaging in Osteoporosis. Indian J. Orthop. 2023, 57, 82–93. [Google Scholar] [CrossRef]
- El Maghraoui, A.; Roux, C. DXA scanning in clinical practice. QJM Int. J. Med. 2008, 101, 605–617. [Google Scholar] [CrossRef]
- Bolotin, H.H. DXA in vivo BMD methodology: An erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone 2007, 41, 138–154. [Google Scholar] [CrossRef]
- Rajiah, P.; Sundaram, M.; Subhas, N. Dual-Energy CT in Musculoskeletal Imaging: What Is the Role Beyond Gout? Am. J. Roentgenol. 2019, 213, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, B.; Tong, X.; Fan, Y.; Wang, S.; Liu, Y.; Fang, X.; Liu, L. Diagnostic Accuracy of Dual-Energy CT Material Decomposition Technique for Assessing Bone Status Compared with Quantitative Computed Tomography. Diagnostics 2023, 13, 1751. [Google Scholar] [CrossRef] [PubMed]
- Patino, M.; Prochowski, A.; Agrawal, M.D.; Simeone, F.J.; Gupta, R.; Hahn, P.F.; Sahani, D.V. Material Separation Using Dual-Energy CT: Current and Emerging Applications. Radiographics 2016, 36, 1087–1105. [Google Scholar] [CrossRef]
- Foti, G.; Ascenti, G.; Agostini, A.; Longo, C.; Lombardo, F.; Inno, A.; Modena, A.; Gori, S. Dual-Energy CT in Oncologic Imaging. Tomography 2024, 10, 299–319. [Google Scholar] [CrossRef]
- Guerrini, S.; Bagnacci, G.; Perrella, A.; Meglio, N.D.; Sica, C.; Mazzei, M.A. Dual Energy CT in Oncology: Benefits for Both Patients and Radiologists From an Emerging Quantitative and Functional Diagnostic Technique. Semin. Ultrasound CT MR 2023, 44, 205–213. [Google Scholar] [CrossRef]
- Wait, J.M.S.; Cody, D.; Jones, A.K.; Rong, J.; Baladandayuthapani, V.; Kappadath, S.C. Performance Evaluation of Material Decomposition with Rapid-Kilovoltage-Switching Dual-Energy CT and Implications for Assessing Bone Mineral Density. Am. J. Roentgenol. 2015, 204, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- van Hamersvelt, R.W.; Schilham, A.M.R.; Engelke, K.; den Harder, A.M.; de Keizer, B.; Verhaar, H.J.; Leiner, T.; de Jong, P.A.; Willemink, M.J. Accuracy of bone mineral density quantification using dual-layer spectral detector CT: A phantom study. Eur. Radiol. 2017, 27, 4351–4359. [Google Scholar] [CrossRef]
- Touban, B.M.; Sayegh, M.J.; Galina, J.; Pavlesen, S.; Radwan, T.; Anders, M. Computed Tomography Measured Psoas Cross Sectional Area Is Associated with Bone Mineral Density Measured by Dual Energy X-Ray Absorptiometry. J. Clin. Densitom. 2022, 25, 592–598. [Google Scholar] [CrossRef]
- Gruenewald, L.D.; Koch, V.; Martin, S.S.; Yel, I.; Eichler, K.; Gruber-Rouh, T.; Lenga, L.; Wichmann, J.L.; Alizadeh, L.S.; Albrecht, M.H.; et al. Diagnostic accuracy of quantitative dual-energy CT-based volumetric bone mineral density assessment for the prediction of osteoporosis-associated fractures. Eur. Radiol. 2022, 32, 3076–3084. [Google Scholar] [CrossRef]
- Booz, C.; Hofmann, P.C.; Sedlmair, M.; Flohr, T.G.; Schmidt, B.; D’Angelo, T.; Martin, S.S.; Lenga, L.; Leithner, D.; Vogl, T.J.; et al. Evaluation of bone mineral density of the lumbar spine using a novel phantomless dual-energy CT post-processing algorithm in comparison with dual-energy X-ray absorptiometry. Eur. Radiol. Exp. 2017, 1, 11. [Google Scholar] [CrossRef]
- Wichmann, J.L.; Booz, C.; Wesarg, S.; Kafchitsas, K.; Bauer, R.W.; Kerl, J.M.; Lehnert, T.; Vogl, T.J.; Khan, M.F. Dual-energy CT-based phantomless in vivo three-dimensional bone mineral density assessment of the lumbar spine. Radiology 2014, 271, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Lee, S.-W.; In, Y.; Kim, M.S.; Kim, Y.D.; Lee, S.-Y.; Lee, J.-W.; Koh, I.J. Dual-Energy CT-Based Bone Mineral Density Has Practical Value for Osteoporosis Screening around the Knee. Medicina 2022, 58, 1085. [Google Scholar] [CrossRef]
- Gruenewald, L.D.; Booz, C.; Gotta, J.; Reschke, P.; Martin, S.S.; Mahmoudi, S.; Bernatz, S.; Eichler, K.; D’Angelo, T.; Chernyak, V.; et al. Incident fractures of the distal radius: Dual-energy CT-derived metrics for opportunistic risk stratification. Eur. J. Radiol. 2024, 171, 111283. [Google Scholar] [CrossRef]
- Guo, D.M.; Weng, Y.Z.; Yu, Z.H.; Li, S.H.; Qu, W.R.; Liu, X.N.; Qi, H.; Ma, C.; Tang, X.F.; Li, R.Y.; et al. Semi-automatic proximal humeral trabecular bone density assessment tool: Technique application and clinical validation. Osteoporos. Int. 2024, 35, 1049–1059. [Google Scholar] [CrossRef]
- Tong, X.; Fang, X.; Wang, S.; Fan, Y.; Wei, W.; Xiao, Q.; Chen, A.; Liu, Y.; Liu, L. Opportunistic screening for osteoporosis using enhanced images based on dual-energy computed tomography material decomposition: A comparison with quantitative computed tomography. Quant. Imaging Med. Surg. 2024, 14, 352–364. [Google Scholar] [CrossRef]
- Gruenewald, L.D.; Koch, V.; Yel, I.; Eichler, K.; Gruber-Rouh, T.; Alizadeh, L.S.; Mahmoudi, S.; D’Angelo, T.; Wichmann, J.L.; Wesarg, S.; et al. Association of Phantomless Dual-Energy CT-based Volumetric Bone Mineral Density with the Prevalence of Acute Insufficiency Fractures of the Spine. Acad. Radiol. 2023, 30, 2110–2117. [Google Scholar] [CrossRef] [PubMed]
- Waqar, A.; Bazzocchi, A.; Aparisi Gómez, M.P. Phantomless estimation of bone mineral density on computed tomography: A scoping review. Rofo 2025. [Google Scholar] [CrossRef]
- Kang, Y.; Hwang, S.H.; Han, K.; Shin, H.J. Comparison of image quality, contrast administration, and radiation doses in pediatric abdominal dual-layer detector dual-energy CT using propensity score matching analysis. Eur. J. Radiol. 2023, 169, 111177. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Shayan, R.; Oladghaffari, M.; Sajjadian, F.; Fazel Ghaziyani, M. Image Quality and Dose Comparison of Single-Energy CT (SECT) and Dual-Energy CT (DECT). Radiol. Res. Pract. 2020, 2020, 1403957. [Google Scholar] [CrossRef]
- Booz, C.; Noeske, J.; Albrecht, M.H.; Lenga, L.; Martin, S.S.; Yel, I.; Huizinga, N.A.; Vogl, T.J.; Wichmann, J.L. Diagnostic accuracy of quantitative dual-energy CT-based bone mineral density assessment in comparison to Hounsfield unit measurements using dual X-ray absorptiometry as standard of reference. Eur. J. Radiol. 2020, 132, 109321. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Li, J.; Jiao, X.; Jia, X.; Zhang, X.; Fan, G.; Yang, J.; Guo, J. The accuracy of bone mineral density measurement using dual-energy spectral CT and quantitative CT: A comparative phantom study. Clin. Radiol. 2020, 75, 320.e9–320.e15. [Google Scholar] [CrossRef]
- Yue, D.; Fei, S.L.; Jing, C.; Xin, W.R.; Tong, D.R.; Lian, L.A.; Luo, Y.H. The relationship between calcium (water) density and age distribution in adult women with spectral CT: Initial result compared to bone mineral density by dual-energy X-ray absorptiometry. Acta Radiol. 2019, 60, 762–768. [Google Scholar] [CrossRef]
- Warriner, A.H.; Patkar, N.M.; Curtis, J.R.; Delzell, E.; Gary, L.; Kilgore, M.; Saag, K. Which fractures are most attributable to osteoporosis? J. Clin. Epidemiol. 2011, 64, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Slart, R.H.J.A.; Ali, D.S.; Bock, O.; Carey, J.J.; Camacho, P.; Engelke, K.; Erba, P.A.; Harvey, N.C.; Lems, W.F.; et al. Osteoporotic Fractures: Diagnosis, Evaluation, and Significance From the International Working Group on DXA Best Practices. Mayo Clin. Proc. 2024, 99, 1127–1141. [Google Scholar] [CrossRef]
- Stoppino, L.P.; Piscone, S.; Saccone, S.; Ciccarelli, S.A.; Marinelli, L.; Milillo, P.; Gallo, C.; Macarini, L.; Vinci, R. Vertebral and Femoral Bone Mineral Density (BMD) Assessment with Dual-Energy CT versus DXA Scan in Postmenopausal Females. J. Imaging 2024, 10, 104. [Google Scholar] [CrossRef]
- Haworth, C.S.; Selby, P.L.; Webb, A.K.; Dodd, M.E.; Musson, H.; McL Niven, R.; Economou, G.; Horrocks, A.W.; Freemont, A.J.; Mawer, E.B.; et al. Low bone mineral density in adults with cystic fibrosis. Thorax 1999, 54, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Grotz, W.H.; Mundinger, F.A.; Rasenack, J.; Speidel, L.; Olschewski, M.; Exner, V.M.; Schollmeyer, P.J. Bone loss after kidney transplantation: A longitudinal study in 115 graft recipients. Nephrol. Dial. Transplant. 1995, 10, 2096–2100. [Google Scholar] [PubMed]
- Deng, L.; Yao, Y.; Shang, A.L.; Du, T.; Zhang, J.; Yang, Q.; Li, J.; Wang, Q.; Li, X. Opportunistic screening for osteoporosis using hydroxyapatite measurements of the vertebral by thorax dual-energy spectral CT in postmenopausal females. Sci. Rep. 2022, 12, 21642. [Google Scholar] [CrossRef]
- Borggrefe, J.; Neuhaus, V.F.; Le Blanc, M.; Grosse Hokamp, N.; Maus, V.; Mpotsaris, A.; Lennartz, S.; Pinto Dos Santos, D.; Maintz, D.; Abdullayev, N. Accuracy of iodine density thresholds for the separation of vertebral bone metastases from healthy-appearing trabecular bone in spectral detector computed tomography. Eur. Radiol. 2019, 29, 3253–3261. [Google Scholar] [CrossRef]
- Fervers, P.; Fervers, F.; Rinneburger, M.; Weisthoff, M.; Kottlors, J.; Reimer, R.; Zopfs, D.; Celik, E.; Maintz, D.; Große-Hokamp, N.; et al. Physiological iodine uptake of the spine’s bone marrow in dual-energy computed tomography-using artificial intelligence to define reference values based on 678 CT examinations of 189 individuals. Front. Endocrinol. 2023, 14, 1098898. [Google Scholar] [CrossRef]



| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Postmenopausal females | Previous fractures or bone lesions |
| Patients in oncological follow-up | Presence of metal prosthesis |
| Patients with maximum gap of 6 months between DXA and DECT | Post-surgical patients |
| Characteristics | Osteoporosis | Osteopenia | Normal |
|---|---|---|---|
| Mean age (range) | 66.5 ± 11.5 (58–78) | 59 ± 15 (44–74) | 60 ± 16 (44–76) |
| Patients | 10 | 15 | 16 |
| T-score | −3.1 ± 0.6 | −1.65 ± 0.75 | 0.15 ± 0.85 |
| BMD (g/cm2) | 0.586 ± 0.065 | 0.698 ± 0.08 | 0.833 ± 0.07 |
| CaOxMono–water (g/cm3) | 50.93 ± 22.8 | 83.92 ± 47.5 | 167.07 ± 32.2 |
| Calcium–Fat (g/cm3) | 34.68 ± 6.6 | 43.84 ± 12.1 | 65.1 ± 9.2 |
| Calcium–water (g/cm3) | 17.12 ± 9.2 | 26.13 ± 15.0 | 46.7 ± 7.4 |
| HAP–Fat (g/cm3) | 131.4 ± 21.2 | 105.19 ± 32.6 | 82.06 ± 21.3 |
| HAP–water (g/cm3) | 30.58 ± 12.7 | 49.81 ± 28.7 | 94.1 ± 19.7 |
| CaOxMono–Water | Calcium–Fat | Calcium–Water | HAP–Fat | HAP–Water | |
|---|---|---|---|---|---|
| ICC | 0.984 | 0.983 | 0.975 | 0.997 | 0.983 |
| CaOxMono–Water | Calcium–Fat | Calcium–Water | HAP–Fat | HAP–Water | ||
|---|---|---|---|---|---|---|
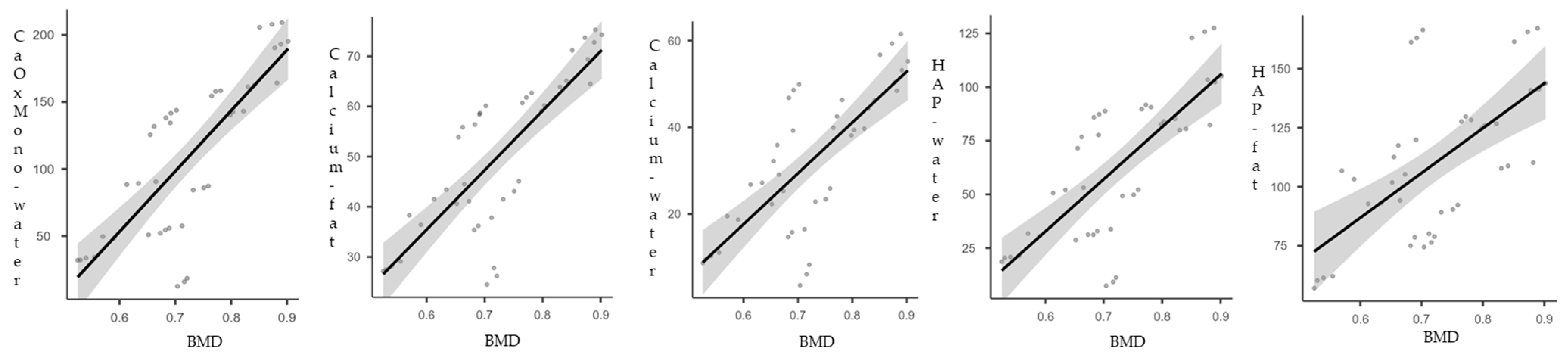
| BMD | Spearman’s rho | 0.783 | 0.797 | 0.702 | 0.616 | 0.725 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piscone, S.; Saccone, S.; Milillo, P.; Schiraldi, G.; Vinci, R.; Macarini, L.; Stoppino, L.P. Bone Mineral Density (BMD) Assessment Using Dual-Energy CT with Different Base Material Pairs (BMPs). J. Imaging 2025, 11, 236. https://doi.org/10.3390/jimaging11070236
Piscone S, Saccone S, Milillo P, Schiraldi G, Vinci R, Macarini L, Stoppino LP. Bone Mineral Density (BMD) Assessment Using Dual-Energy CT with Different Base Material Pairs (BMPs). Journal of Imaging. 2025; 11(7):236. https://doi.org/10.3390/jimaging11070236
Chicago/Turabian StylePiscone, Stefano, Sara Saccone, Paola Milillo, Giorgia Schiraldi, Roberta Vinci, Luca Macarini, and Luca Pio Stoppino. 2025. "Bone Mineral Density (BMD) Assessment Using Dual-Energy CT with Different Base Material Pairs (BMPs)" Journal of Imaging 11, no. 7: 236. https://doi.org/10.3390/jimaging11070236
APA StylePiscone, S., Saccone, S., Milillo, P., Schiraldi, G., Vinci, R., Macarini, L., & Stoppino, L. P. (2025). Bone Mineral Density (BMD) Assessment Using Dual-Energy CT with Different Base Material Pairs (BMPs). Journal of Imaging, 11(7), 236. https://doi.org/10.3390/jimaging11070236






