Decolorization with Warmth–Coolness Adjustment in an Opponent and Complementary Color System
Abstract
1. Introduction
2. Related Works
2.1. Color Representation in the Retina
2.2. Color Representation Systems
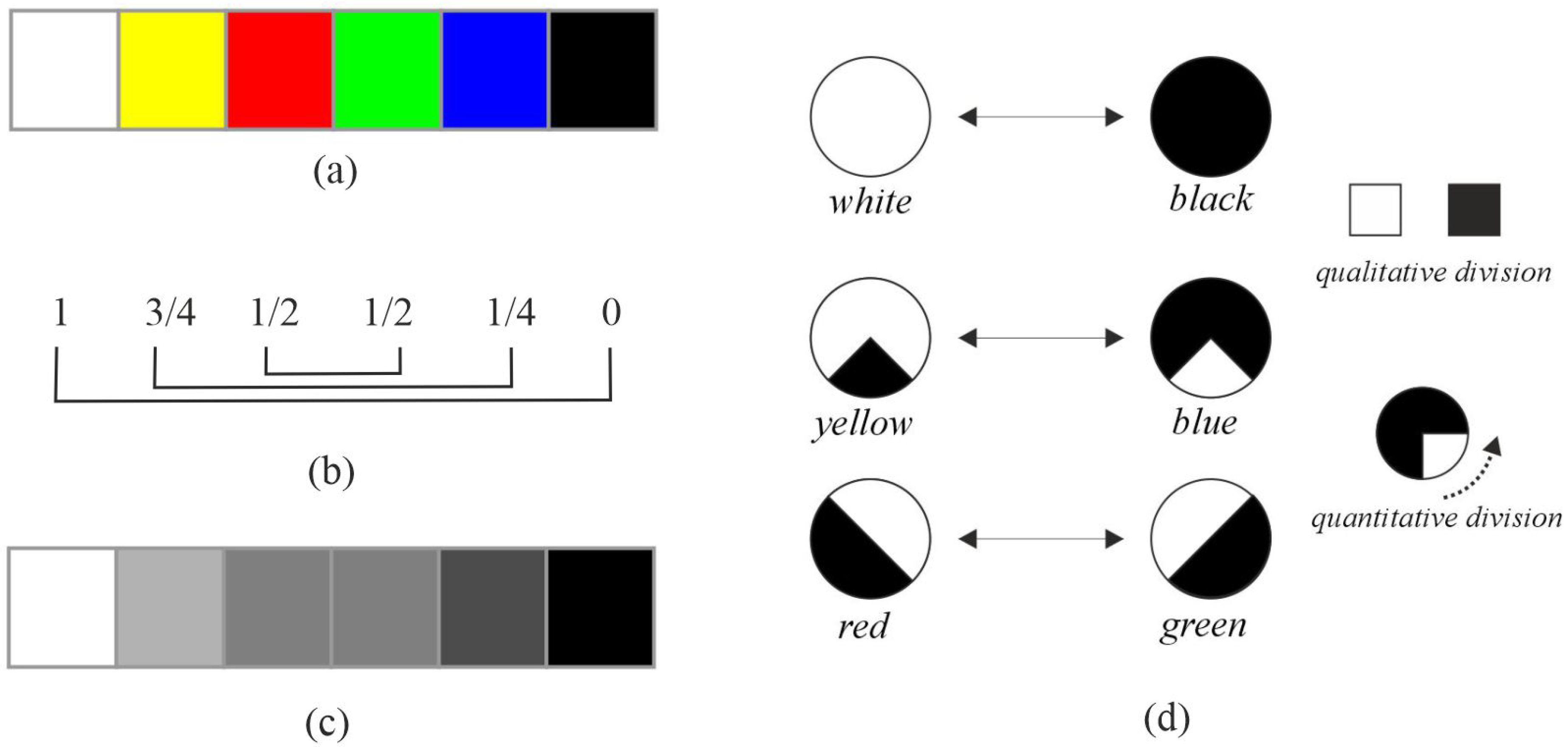
- Retinal activity and color processing: he argued that retinal activity is intrinsically linked to color perception, countering the prevailing theory that colors are solely generated by light decomposition;
- Qualitative and quantitative division of activity: he further proposed that retinal activity can be divided both qualitatively and quantitatively.
2.3. Color Contrast
2.4. Decolorization Methods
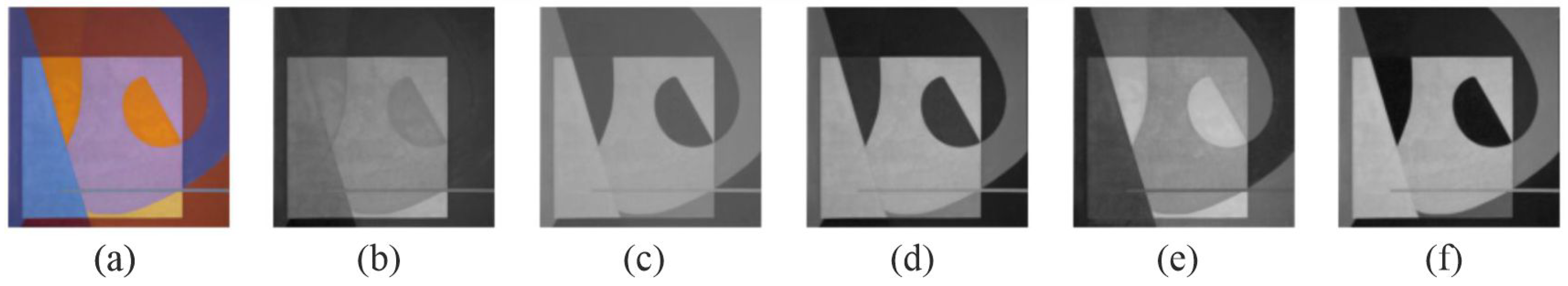
- Global approaches (nonlinear): Kim et al. [33] processed luminance, chroma, and tone; Ancuti et al. [42] incorporated three RGB channels and an additional image to preserve color contrast; Ancuti et al. [43] mixed saturation and hue channels for salience preservation; Liu et al. [44] employed gradient correlation with a nonlinear global mapping in the RGB space; and Song et al. [45] used a probabilistic graphical model to minimize an integral, preserving visual key elements.
2.5. Evaluation of Color Contrast in the Decolorization
3. Methods
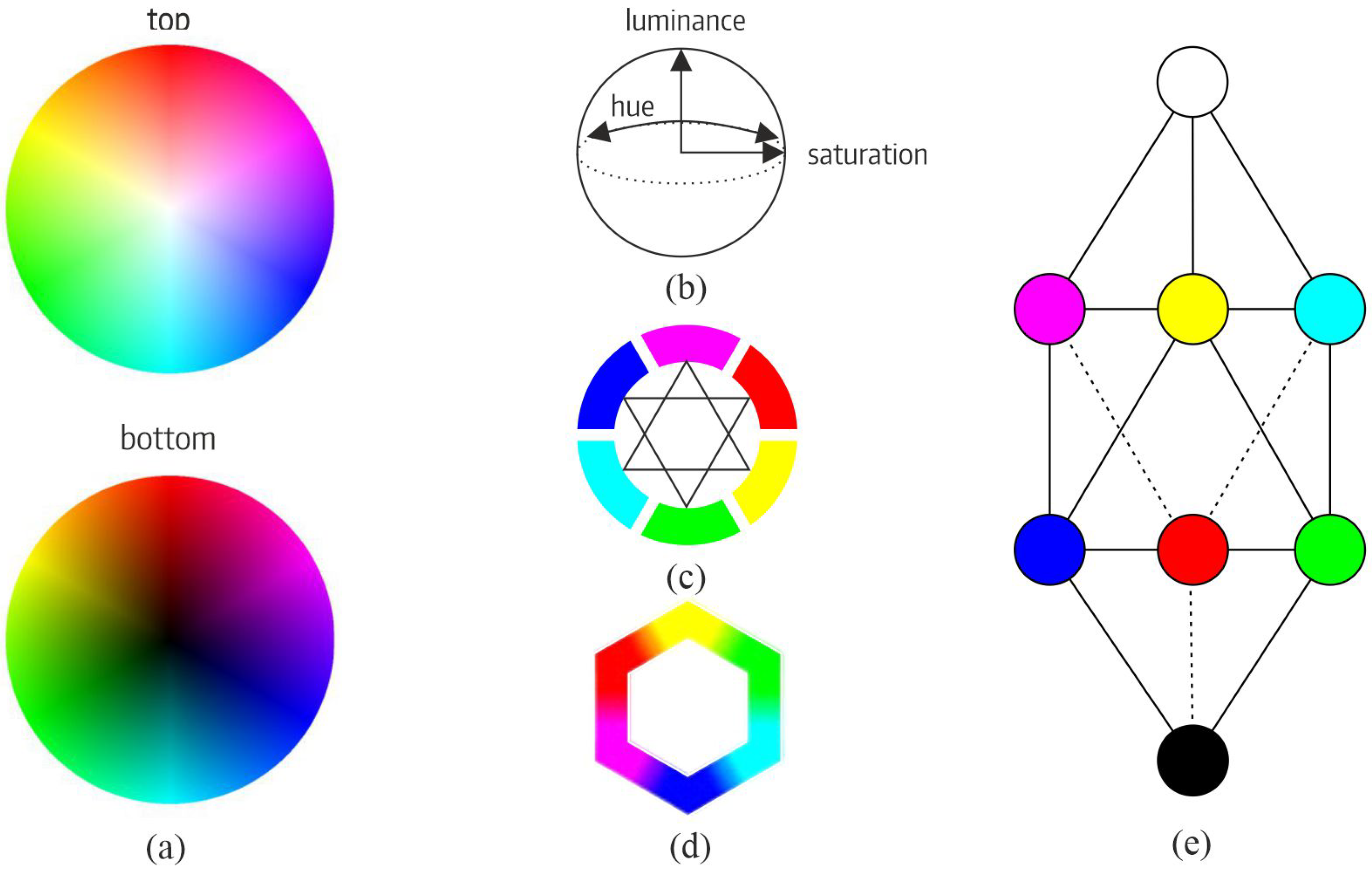
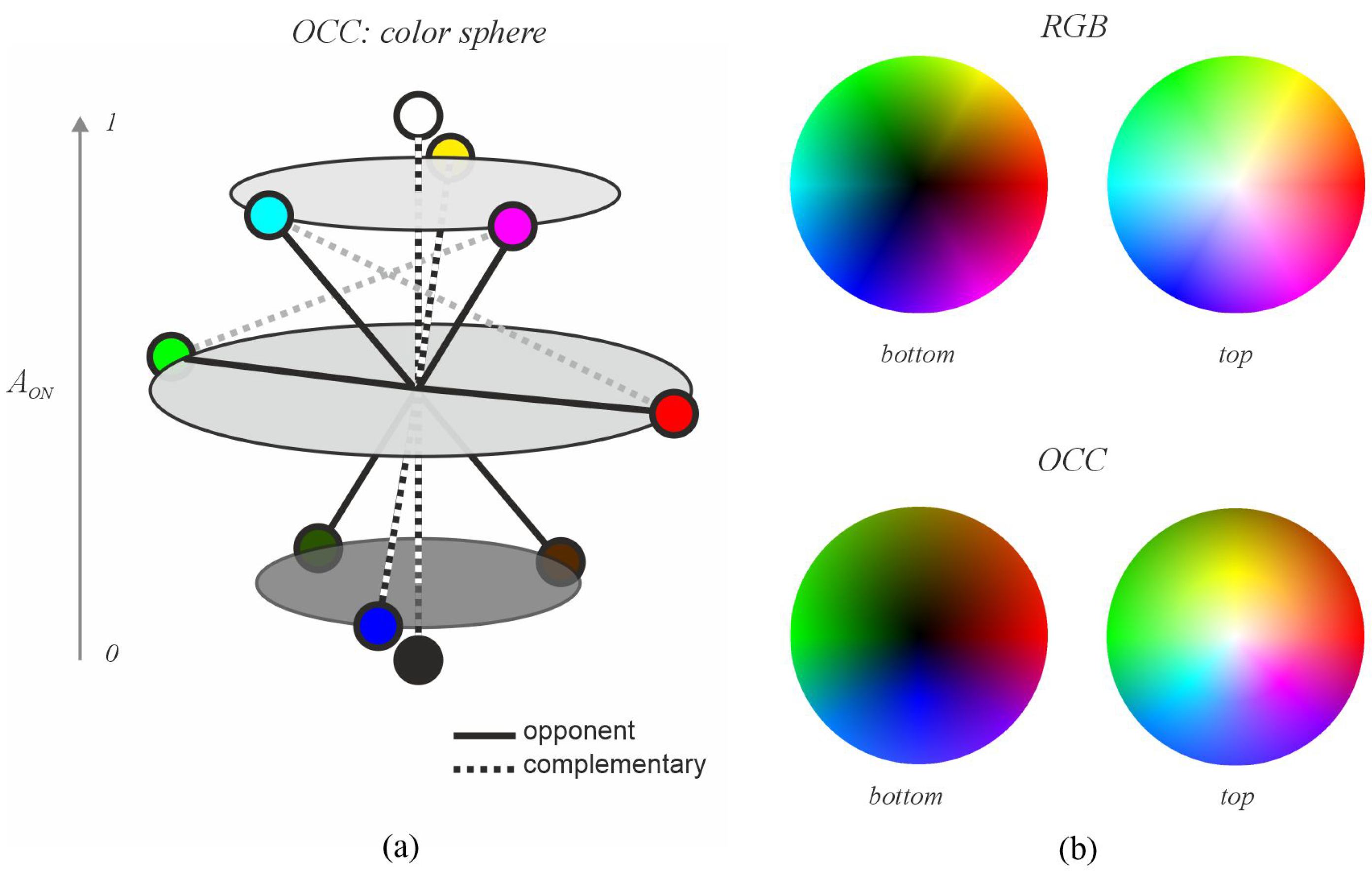
3.1. Opponent and Complementary Color System (OCC)
3.1.1. Relationship Between Color and Retinal Neural Activity
3.1.2. Definition of Warm and Cool Categories
3.2. Decolorization with Warmth–Coolness Adjustment (DWCA)
3.2.1. Converting Color to Grayscale
3.2.2. Adjustment of Grayscale Position Based on Warmth or Coolness
4. Experimental Benchmarking
4.1. Datasets
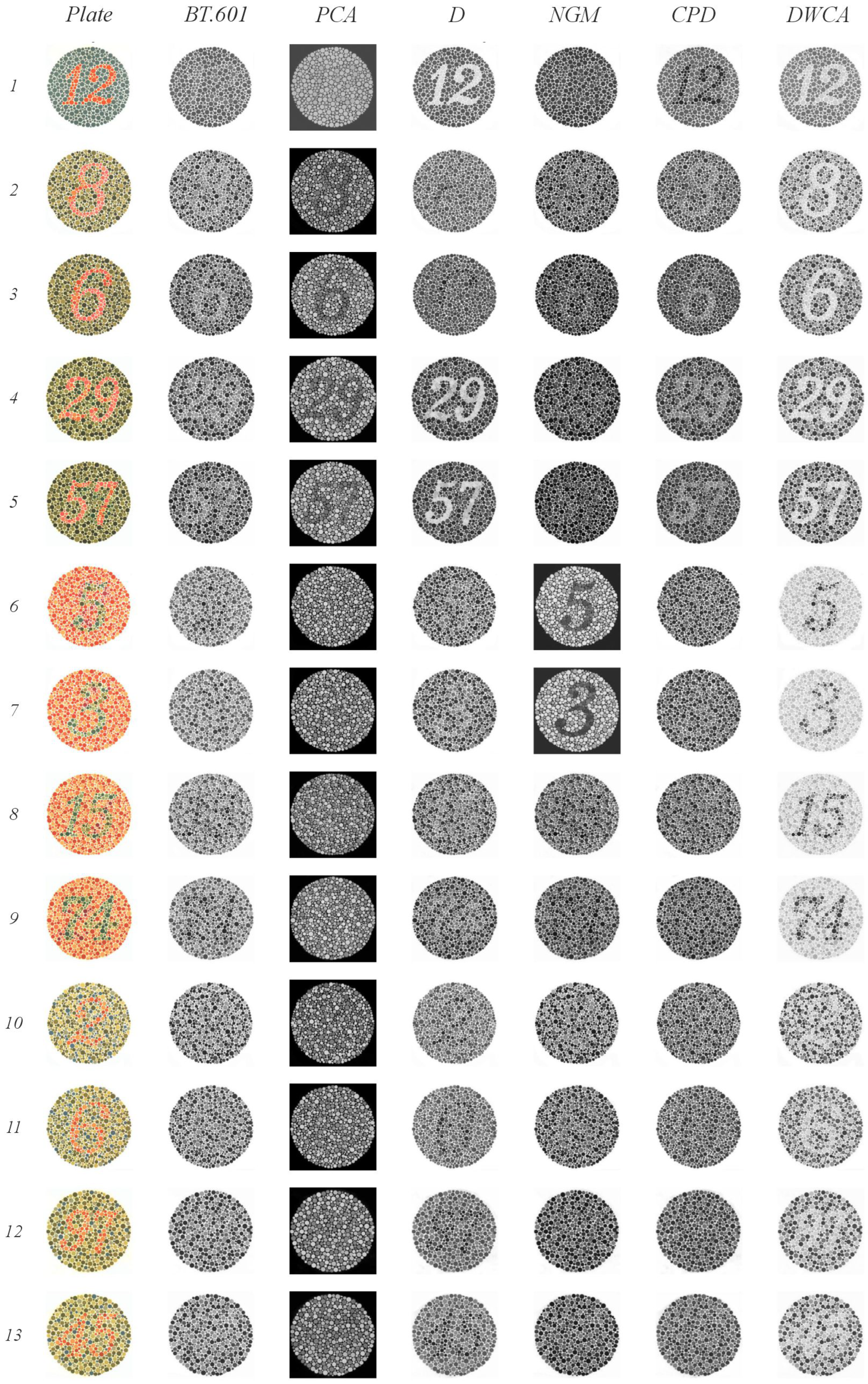
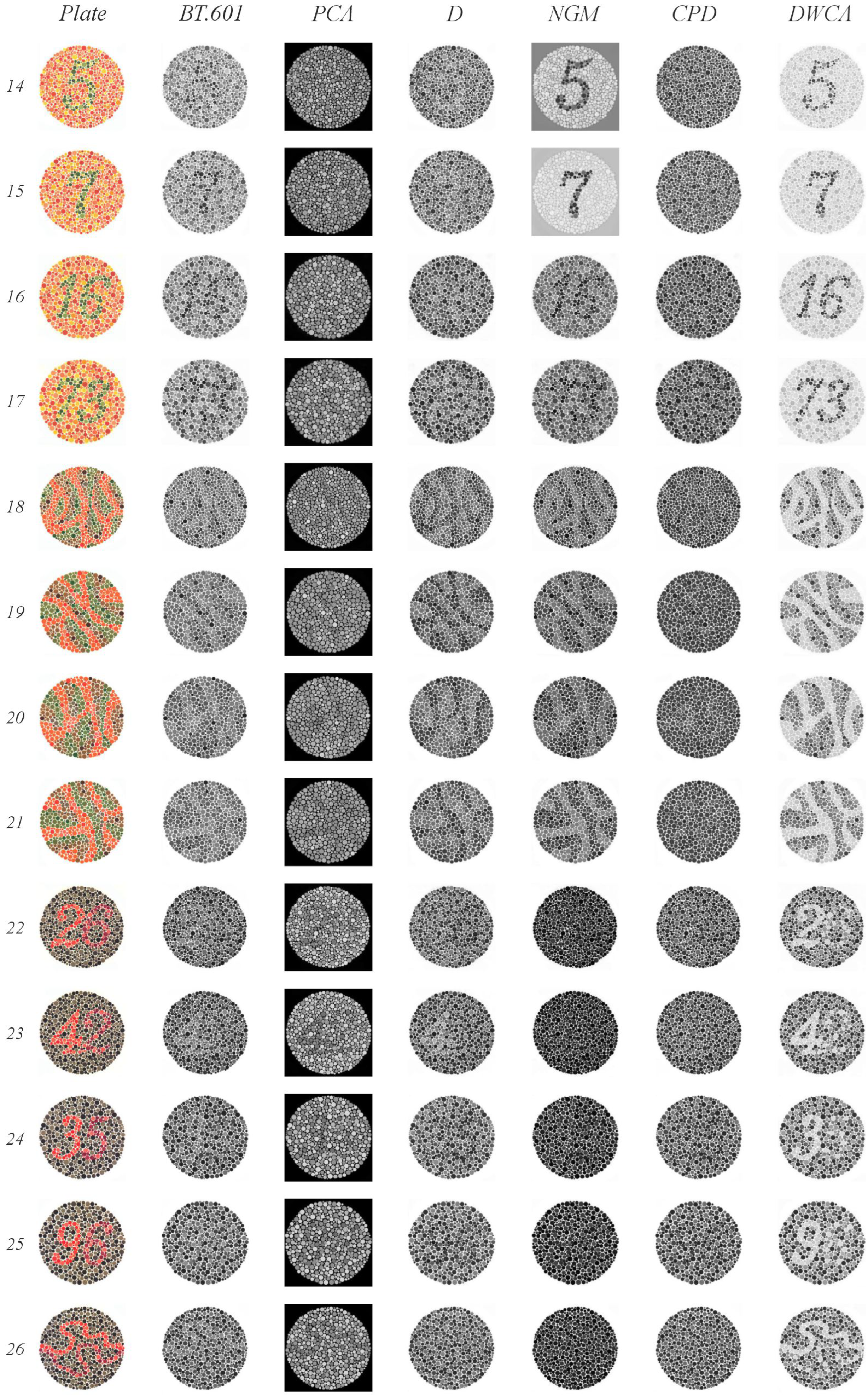
- Plates 1 and 38: Numbers should be clearly visible to all individuals with normal color vision.
- Plates 2–9: A number is visible to individuals with normal color vision but appears different or invisible to those with red or green deficiencies.
- Plates 10–17: A number is visible to individuals with normal color vision and is not visible to those with red or green deficiencies.
- Plates 18–21: Numbers are not visible to individuals with normal color vision but appear to those with red or green deficiencies.
- Plates 22–25: A two-digit number is visible to individuals with normal color vision; the first digit appears to those with red deficiencies and the second digit to those with green deficiencies.
- Plates 26 and 27: Lines are visible to individuals with normal color vision, with only the top portion visible to those with red deficiencies and the bottom portion to those with green deficiencies.
- Plates 28 and 29: Lines are visible only to individuals with red or green deficiencies.
- Plates 31–33: Numbers are visible only to individuals with normal color vision.
- Plates 34 and 35: Normal vision detects a green line, while those with red and green deficiencies detect a violet line.
- Plates 36 and 37: Normal vision detects an orange line, while those with red and green deficiencies detect a violet line.
4.2. Setup
- Weighted channels: BT.601 recommendation (ITU);
- Channel analysis: Principal Component Analysis (PCA);
- Global linear contrast: Decolorization (D) with global contrast using a linear function to adjust chroma and intensity, as described by Grundland and Dodgson [32];
- Global nonlinear contrast: Nonlinear Global Map (NGM), derived from luminance, chroma, and hue functions, proposed by Kim et al. [33];
- Local contrast: Contrast Preserving Decolorization (CPD), employing a nonlinear real-time function to preserve local contrast, as detailed by Lu et al. [34].
4.2.1. Parameter Configuration
- Quantitative evaluation and Ishihara test: , , and (a fixed, low value). This configuration was chosen to minimize alterations to the original color palette while still achieving decolorization.
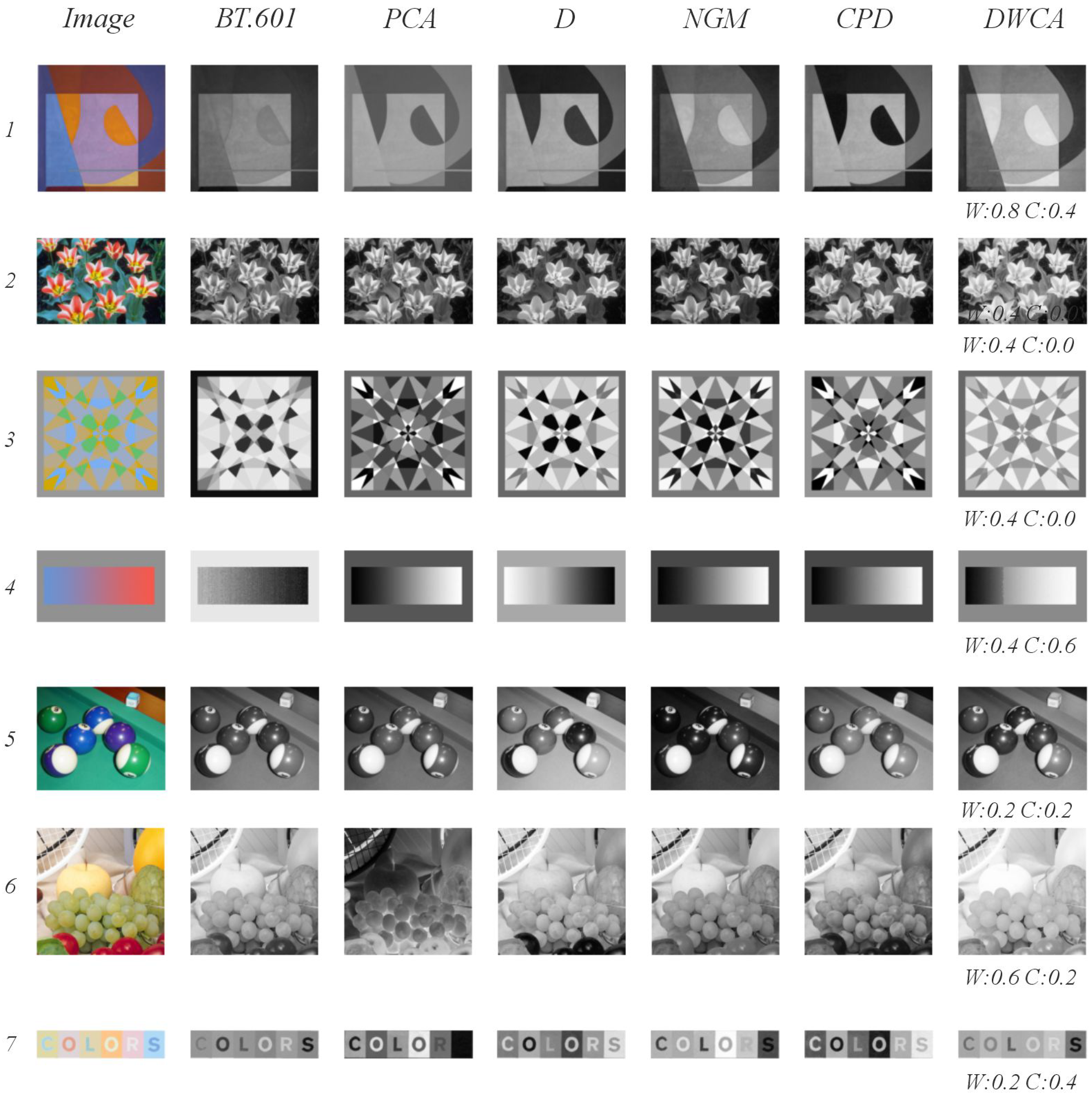
- Qualitative evaluation: The values of W, C, and s were adjusted on a per-image basis to optimize visual results. The default value for s was 0.3; when a different value was used, this is explicitly stated in the results section. We prioritized maintaining subtle color differences during this evaluation.
4.2.2. Evaluation
5. Experimental Results
5.1. Quantitative Analysis
5.1.1. Color Contrast
5.1.2. Structural Preservation Analysis
5.1.3. Ishihara Test
- BT.601: It detected four plates with orange figures on a green background (plates 2–5) and two, albeit faintly, with green figures on an orange background (plates 8 and 9). It also correctly identified three plates with green figures and orange backgrounds (plates 15, 16, and 17), as well as two lines (plates 30 and 31). The normal gaze line was successfully detected in plates 36 and 37.
- PCA: It detected four plates with orange figures on a green background (plates 2–5). Notably, it identified numbers in plates 22 through 25 that were only visible when red or green range detection failed. It struggled to detect lines in plates 26 and 27 when there were issues with green range detection; however, the line was detected correctly in plates 36 and 37.
- D: It detected plates 1 and 38 (expected to be visible by all methods) and two plates with orange figures on a green background (plates 4 and 5). In plates 18 through 21, it identified lines. One plate from the groups of 22 through 25 and 27 was detected. Plates 32 and 33, which had a green background, were correctly identified. Finally, despite some blurring, it successfully detected the orange line in plate 37 (normal vision), which featured a green background.
- NGM: It detected plates 4 and 5 (green figures on orange backgrounds); from groups 10–17, it detected 14 and 15 clearly, and plate 16 was slightly blurry. It identified the lines in plates 18–21. Plates 30 and 31 were detected within groups 30–33, and plate 34 was detected from blocks 34–35.
- GPD: It detected plates 1 and 38. From groups 2–9, it detected only the plates with a green background (plates 2–5). In plate 20, instead of detecting lines, it incorrectly displayed the number 45, which is typically only visible when red or green range detection fails. It also correctly identified plates 36 and 37.
- DWCA: Successfully detected all plates without any issues.
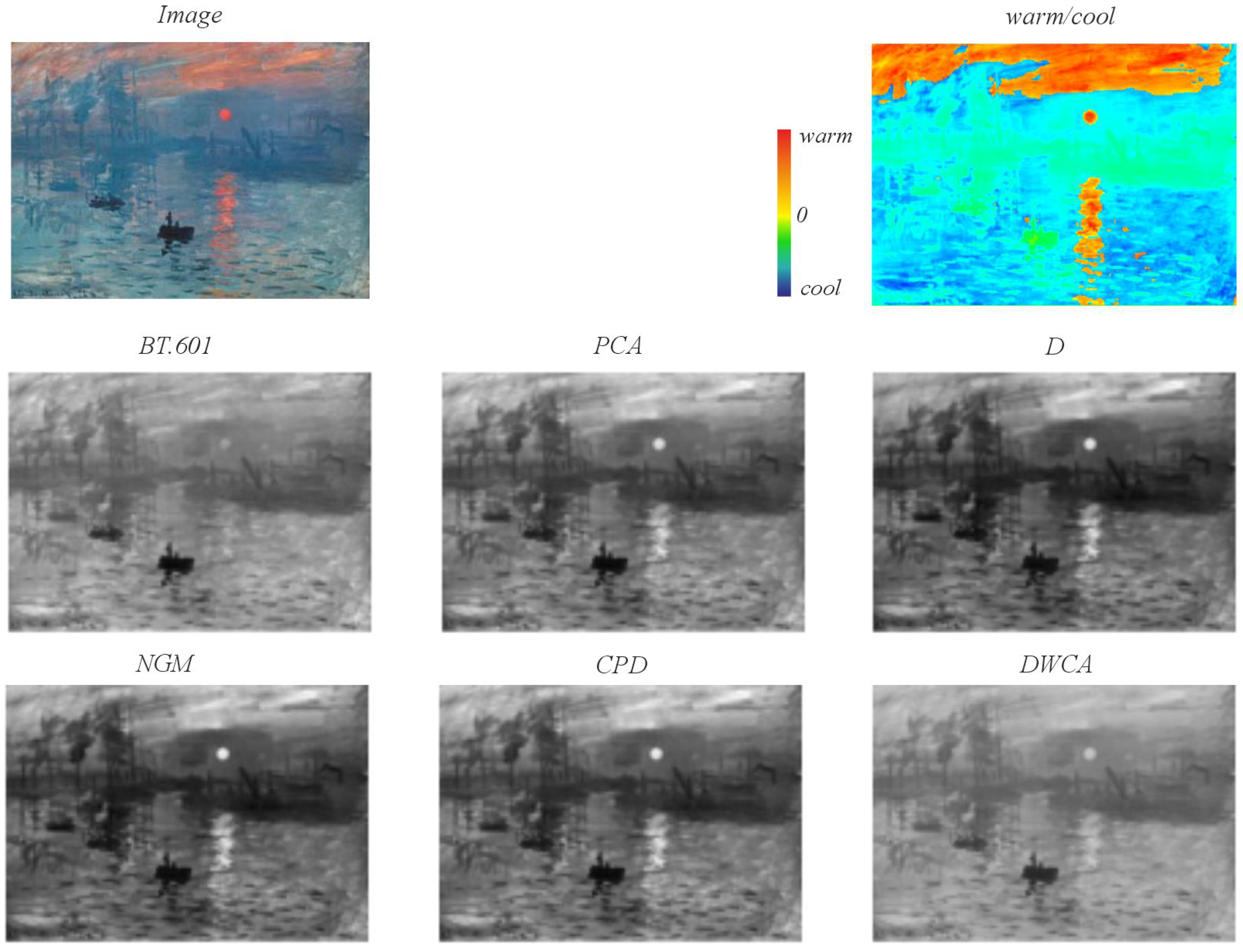
5.2. Qualitative Analysis
6. Discussion
- Quantitatively, DWCA achieves higher CCPR and CCFR ratios than methods that do not analyze contrast (BT.601 and PCA), slightly surpassing those performing global analysis (D and NGM) and marginally lower compared to those employing local analysis (CPD);
- Qualitatively, the grayscale conversion maintains hue relationships at a high level without distorting the visual meaning of the image;
- As a method that does not perform contrast analysis but relies on the OCC system representing each color and its properties, DWCA yields results independent of local or global circumstances within each image; therefore, the resulting gray value is consistent for each color;
- As a pixel-by-pixel method, it significantly reduces computational cost;
- The warmth/coolness adjustment facilitates grayscale conversion control for both automated processes and human calibration.
7. Conclusions and Future Works
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S. Two decades of colorization and decolorization for images and videos. arXiv 2022, arXiv:2204.13322. [Google Scholar]
- Teller, D. Vision and the Visual System; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Schiller, P.H.; Sandell, J.H.; Maunsell, J.H. Functions of the ON and OFF channels of the visual system. Nature 1986, 322, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D. Eye, Brain and Vision. Scientific American Libarary; Scientific American Libarary: New York, NY, USA, 1988. [Google Scholar]
- Newton, I. Optics: Or a Treatise of the Refections, Refractions, Inflections and Colours of Light; William Inny: London, UK, 1730. [Google Scholar]
- Young, R.A. On the Theory of Light and Colours. Philos. Trans. R. Soc. Lond. 1802, 92, 12–48. [Google Scholar]
- Von Helmholtz, H. Handbuch der Physiologischen Optik; Voss, L., Ed.; Hamburg und Leipzig: Leipzig, Germany, 1867; Volume 9. [Google Scholar]
- Goethe, J.W. Theory of Colours; Mit Press: Cambridge, MA, USA, 1840. [Google Scholar]
- Schopenhauer, A. On Vision and Colors; Pricenton Architectural Press: Pricenton, NJ, USA, 2010. [Google Scholar]
- Hering, E. Ueber Individuelle Verschiedenheiten des Farbensinnes; Druck von Heinr: Prague, Czech Republic, 1885. [Google Scholar]
- Munsell, A.H. Atlas of the Munsell Color System; Wadsworth, Howland & Company, Incorporated, Printers: Boston, MA, USA, 1915. [Google Scholar]
- Runge, P.O. Farben-Kugel; Verlag Nicht Ermittelbar: Munchen, Germany, 1810. [Google Scholar]
- Itten, J. The Art of Color: The Subjective Experience and Objective Rationale of Color; Van Nostrand Reinhold Company: New York, NY, USA, 1961. [Google Scholar]
- Klee, P. Paul Klee: The Thinking Eye: The Notebooks of Paul Klee; Wittenborn, G., Ed.; George Wittenborn, Inc.: New York, NY, USA, 1961; Volume 15. [Google Scholar]
- On Illumination (CIE), I.C. CIE 1976 L*a*b* Color Space. 1976. Available online: https://en.wikipedia.org/wiki/CIELAB_color_space (accessed on 5 June 2025).
- Sharma, G.; Wu, W.; Dalal, E.N. The CIEDE2000 color-difference formula: Implementation notes, supplementary test data, and mathematical observations. Color Res. Appl. 2005, 30, 21–30. [Google Scholar] [CrossRef]
- Pawlik, J. Theorie der Farbe; DuMont: Köln, Germany, 1976. [Google Scholar]
- Puhalla, D.M. Perceiving hierarchy through intrinsic color structure. Vis. Commun. 2008, 7, 199–228. [Google Scholar] [CrossRef]
- Loreto, V.; Mukherjee, A.; Tria, F. On the origin of the hierarchy of color names. Proc. Natl. Acad. Sci. USA 2012, 109, 6819–6824. [Google Scholar] [CrossRef]
- Gravesen, J. The metric of colour space. Graph. Model. 2015, 82, 77–86. [Google Scholar] [CrossRef]
- Kueppers, H. The Basic Law of Color Theory; Barrons Educational Series Incorporated: New York, NY, USA, 1982. [Google Scholar]
- Manzotti, R. A Perception-Based Model of Complementary Afterimages. SAGE Open 2017, 7, 2158244016682478. [Google Scholar] [CrossRef]
- Zeki, S.; Cheadle, S.; Pepper, J.; Mylonas, D. The Constancy of Colored After-Images. Front. Hum. Neurosci. 2017, 11, 229. [Google Scholar] [CrossRef]
- Pridmore, R.W. Chromatic induction: Opponent color or complementary color process? Color Res. Appl. 2008, 33, 77–81. [Google Scholar] [CrossRef]
- Arnheim, R. Art and Visual Perception: A Psychology of the Creative Eye; University of California Press: Oakland, CA, USA, 1956. [Google Scholar]
- Berlin, B.; Kay, P. Basic Color Terms: Their Universality and Evolution; University of California Press: Oakland, CA, USA, 1991. [Google Scholar]
- Rosch, E. The nature of mental codes for color categories. J. Exp. Psychol. Hum. Percept. Perform. 1975, 1, 303. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, Y.J.; Jang, C.H. Method of Converting Color Image into Grayscale Image and Recording Medium Storing Program for Performing the Same. U.S. Patent US8526719B2, 03 September 2013. [Google Scholar]
- Bala, R.; Eschbach, R. Color to Grayscale Conversion Method and Apparatus. U.S. Patent US7382915B2, 3 June 2008. [Google Scholar]
- Majewicz, P.; Smith, K.K. Method and Apparatus for Converting a Color Image to Grayscale. U.S. Patent US8594419B2, 26 November 2013. [Google Scholar]
- Ng, M.K.P. Method and Device for Use in Converting a Colour Image into a Grayscale Image. U.S. Patent US8355566B2, 15 January 2013. [Google Scholar]
- Grundland, M.; Dodgson, N.A. Decolorize: Fast, contrast enhancing, color to grayscale conversion. Pattern Recognit. 2007, 40, 2891–2896. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, C.; Demouth, J.; Lee, S. Robust color-to-gray via nonlinear global mapping. In ACM SIGGRAPH Asia 2009 Papers; Association for Computing Machinery: New York, NY, USA, 2009; pp. 1–4. [Google Scholar]
- Lu, C.; Xu, L.; Jia, J. Contrast preserving decolorization with perception-based quality metrics. Int. J. Comput. Vis. 2014, 110, 222–239. [Google Scholar] [CrossRef]
- Seo, J.W.; Kim, S.D. Novel pca-based color-to-gray image conversion. In Proceedings of the 2013 IEEE International Conference on Image Processing, Melbourne, Australia, 15–18 September 2013; pp. 2279–2283. [Google Scholar]
- Sommer, R.; Wischer, H. Method for Converting a Video Signal into a Black/White Signal. U.S. Patent US4257070A, 18 November 1980. [Google Scholar]
- Bala, R.; Eschbach, R. Color to Grayscale Conversion Method and Apparatus. U.S. Patent US20080181491A1, 20 July 2010. [Google Scholar]
- Neumann, L.; Čadík, M.; Nemcsics, A. An efficient perception-based adaptive color to gray transformation. In Proceedings of the 3rd Eurographics Conference on Computational Aesthetics in Graphics, Visualization and Imaging, Banff, AB, Canada, 20–22 June 2007; pp. 73–80. [Google Scholar]
- Lu, C.; Xu, L.; Jia, J. Real-time contrast preserving decolorization. In SIGGRAPH Asia 2012 Technical Briefs; Association for Computing Machinery: New York, NY, USA, 2012; pp. 1–4. [Google Scholar]
- Zhang, H.; Liu, S. Efficient decolorization via perceptual group difference enhancement. In International Conference on Image and Graphics; Springer: Cham, Switzerland, 2017; pp. 560–569. [Google Scholar]
- Gooch, A.A.; Olsen, S.C.; Tumblin, J.; Gooch, B. Color2gray: Salience-preserving color removal. ACM Trans. Graph. (TOG) 2005, 24, 634–639. [Google Scholar] [CrossRef]
- Ancuti, C.O.; Ancuti, C.; Hermans, C.; Bekaert, P. Image and video decolorization by fusion. In Asian Conference on Computer Vision; Springer: Berlin/Heidelberg, Germany, 2010; pp. 79–92. [Google Scholar]
- Ancuti, C.O.; Ancuti, C.; Bekaert, P. Enhancing by saliency-guided decolorization. In Proceedings of the CVPR 2011, Colorado Springs, CO, USA, 20–25 June 2011; pp. 257–264. [Google Scholar]
- Liu, Q.; Liu, P.X.; Xie, W.; Wang, Y.; Liang, D. GcsDecolor: Gradient correlation similarity for efficient contrast preserving decolorization. IEEE Trans. Image Process. 2015, 24, 2889–2904. [Google Scholar]
- Song, M.; Tao, D.; Chen, C.; Li, X.; Chen, C.W. Color to gray: Visual cue preservation. IEEE Trans. Pattern Anal. Mach. Intell. 2010, 32, 1537–1552. [Google Scholar] [CrossRef]
- Smith, K.; Landes, P.E.; Thollot, J.; Myszkowski, K. Apparent grayscale: A simple and fast conversion to perceptually accurate images and video. Comput. Graph. Forum 2008, 27, 193–200. [Google Scholar] [CrossRef]
- Kuk, J.G.; Ahn, J.H.; Cho, N.I. A color to grayscale conversion considering local and global contrast. In Asian Conference on Computer Vision; Springer: Berlin/Heidelberg, Germany, 2010; pp. 513–524. [Google Scholar]
- Lin, Z.; Zhang, W.; Tang, X. Learning Partial Differential Equations for Computer Vision. Peking Univ. Chin. Univ. of Hong Kong 2008. [Google Scholar]
- Hou, X.; Duan, J.; Qiu, G. Deep feature consistent deep image transformations: Downscaling, decolorization and HDR tone mapping. arXiv 2017, arXiv:1707.09482. [Google Scholar]
- Liu, S.; Zhang, X. Image decolorization combining local features and exposure features. IEEE Trans. Multimed. 2019, 21, 2461–2472. [Google Scholar] [CrossRef]
- Cai, B.; Xu, X.; Xing, X. Perception preserving decolorization. In Proceedings of the 2018 25th IEEE International Conference on Image Processing (ICIP), Athens, Greece, 7–10 October 2018; pp. 2810–2814. [Google Scholar]
- Ĉadík, M. Perceptual evaluation of color-to-grayscale image conversions. In Computer Graphics Forum; Wiley Online Library: New York, NY, USA, 2008; Volume 27, pp. 1745–1754. [Google Scholar]
- Ma, K.; Zhao, T.; Zeng, K.; Wang, Z. Objective quality assessment for color-to-gray image conversion. IEEE Trans. Image Process. 2015, 24, 4673–4685. [Google Scholar] [CrossRef] [PubMed]
- Ayunts, H.; Agaian, S. No-Reference Quality Metrics for Image Decolorization. IEEE Trans. Consum. Electron. 2023, 69, 1177–1185. [Google Scholar] [CrossRef]
- Clark, J. The Ishihara test for color blindness. Am. J. Physiol. Opt. 1924, 5, 269–276. [Google Scholar]





| Category | Colors | Dominant Channels |
|---|---|---|
| warm | red | L |
| cool | green and blue | M y S |
| BT.601 | PCA | D | NGM | CPD | DWCA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ave | Ave | Ave | Ave | Ave | Ave | |||||||
| CCPR | 0.86 | 0.11 | 0.81 | 0.13 | 0.87 | 0.11 | 0.84 | 0.12 | 0.88 | 0.10 | 0.87 | 0.12 |
| CCFR | 0.99 | 0.00 | 0.99 | 0.00 | 0.99 | 0.01 | 0.99 | 0.01 | 0.99 | 0.01 | 0.99 | 0.01 |
| Escore | 0.92 | 0.07 | 0.89 | 0.08 | 0.92 | 0.07 | 0.90 | 0.08 | 0.93 | 0.06 | 0.92 | 0.09 |
| CCPR | 0.76 | 0.16 | 0.69 | 0.17 | 0.78 | 0.16 | 0.75 | 0.16 | 0.80 | 0.15 | 0.78 | 0.17 |
| CCFR | 0.99 | 0.01 | 1.00 | 0.00 | 0.99 | 0.01 | 0.99 | 0.02 | 0.99 | 0.01 | 0.99 | 0.03 |
| Escore | 0.85 | 0.11 | 0.80 | 0.13 | 0.86 | 0.11 | 0.84 | 0.11 | 0.87 | 0.10 | 0.85 | 0.12 |
| CCPR | 0.64 | 0.24 | 0.54 | 0.23 | 0.68 | 0.22 | 0.65 | 0.21 | 0.72 | 0.20 | 0.66 | 0.25 |
| CCFR | 0.99 | 0.00 | 1.00 | 0.00 | 0.99 | 0.04 | 0.98 | 0.04 | 0.99 | 0.02 | 0.97 | 0.05 |
| Escore | 0.74 | 0.20 | 0.67 | 0.21 | 0.78 | 0.18 | 0.76 | 0.17 | 0.80 | 0.16 | 0.74 | 0.21 |
| BT.601 | PCA | D | NGM | CPD | DWCA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ave | Ave | Ave | Ave | Ave | Ave | |||||||
| C2G-SSIM | 0.96 | 0.01 | 0.54 | 0.43 | 0.89 | 0.05 | 0.74 | 0.15 | 0.93 | 0.04 | 0.91 | 0.04 |
| Plates | BT.601 | PCA | D | NGM | GPD | DWCA |
|---|---|---|---|---|---|---|
| 1 & 38 | 0/2 | 0/2 | 2/2 | 0/2 | 2/2 | 2/2 |
| 2–9 | 6/8 | 4/8 | 2/8 | 2/8 | 4/8 | 8/8 |
| 10–17 | 3/8 | 0/8 | 4/8 | 3/8 | 0/8 | 8/8 |
| 18–21 | 0/4 | 0/4 | 4/4 | 4/4 | 0/4 | 4/4 |
| 22–25 | 0/4 | 0/4 | 0/4 | 1/4 | 0/4 | 4/4 |
| 26 & 27 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 |
| 28 & 29 | 2/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 |
| 30–33 | 2/4 | 0/4 | 2/4 | 2/4 | 0/4 | 4/4 |
| 34 & 35 | 0/2 | 0/2 | 0/2 | 1/2 | 0/2 | 2/2 |
| 36 & 37 | 2/2 | 2/2 | 1/2 | 0/2 | 2/2 | 2/2 |
| Total | 15/38 | 6/38 | 17/38 | 12/38 | 8/38 | 38/38 |
| % | 39% | 15% | 44% | 31% | 21% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Cesteros, O.; Rincon, M. Decolorization with Warmth–Coolness Adjustment in an Opponent and Complementary Color System. J. Imaging 2025, 11, 199. https://doi.org/10.3390/jimaging11060199
Sanchez-Cesteros O, Rincon M. Decolorization with Warmth–Coolness Adjustment in an Opponent and Complementary Color System. Journal of Imaging. 2025; 11(6):199. https://doi.org/10.3390/jimaging11060199
Chicago/Turabian StyleSanchez-Cesteros, Oscar, and Mariano Rincon. 2025. "Decolorization with Warmth–Coolness Adjustment in an Opponent and Complementary Color System" Journal of Imaging 11, no. 6: 199. https://doi.org/10.3390/jimaging11060199
APA StyleSanchez-Cesteros, O., & Rincon, M. (2025). Decolorization with Warmth–Coolness Adjustment in an Opponent and Complementary Color System. Journal of Imaging, 11(6), 199. https://doi.org/10.3390/jimaging11060199








