Effects of Motion in Ultrashort Echo Time Quantitative Susceptibility Mapping for Musculoskeletal Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
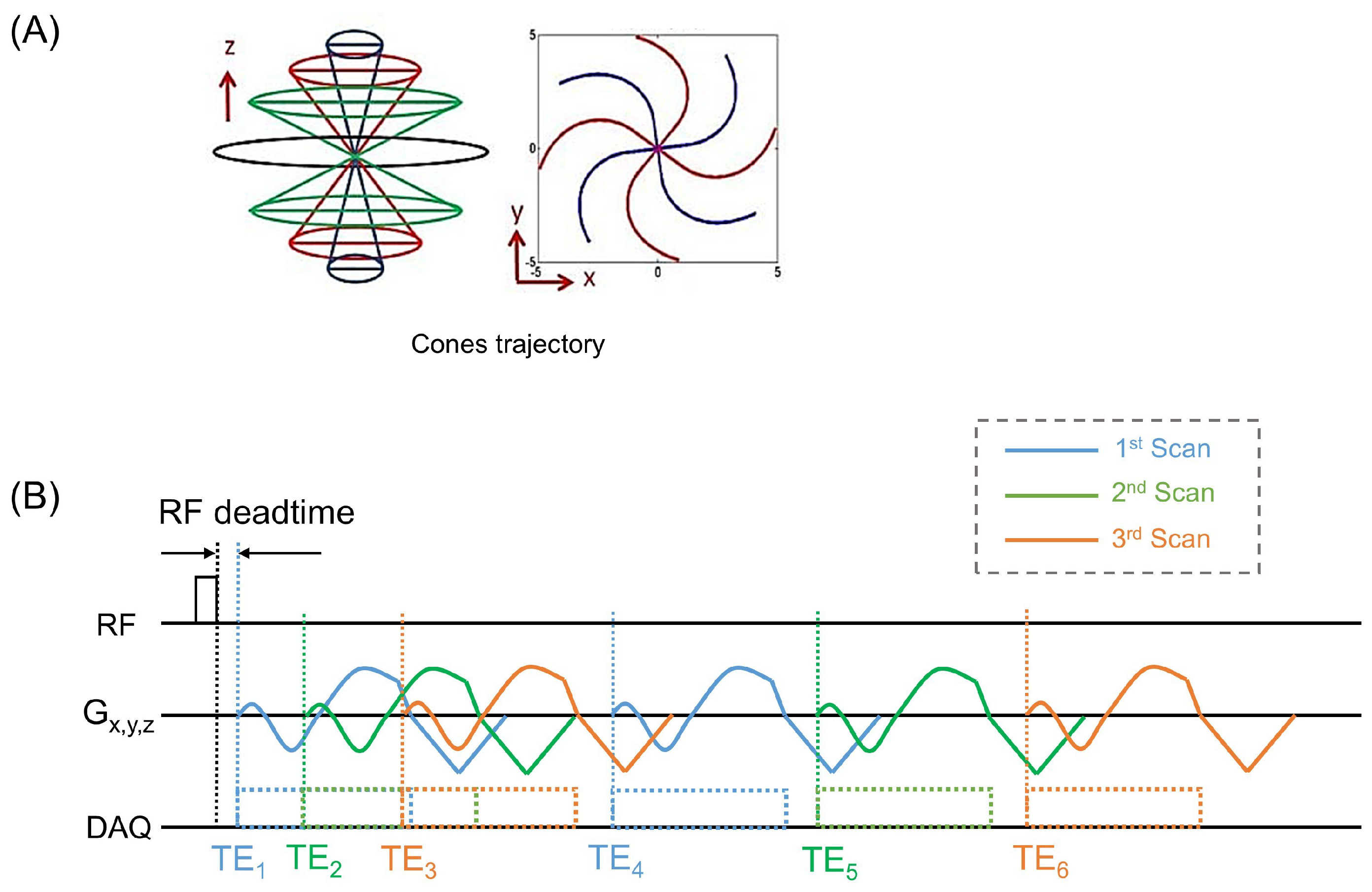
2.2. UTE Sequence
2.3. MRI Protocol
2.4. UTE-QSM Process
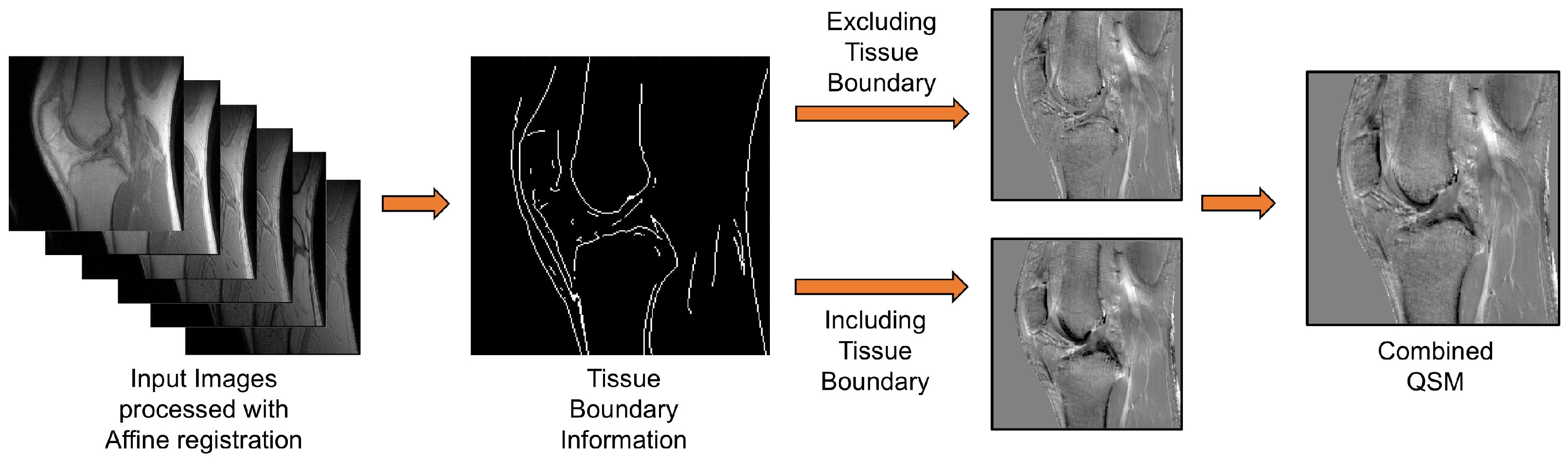
2.5. Two-Step UTE-QSM Using Tissue Boundary Information
2.6. Data Processing
3. Results
3.1. Baseline Data
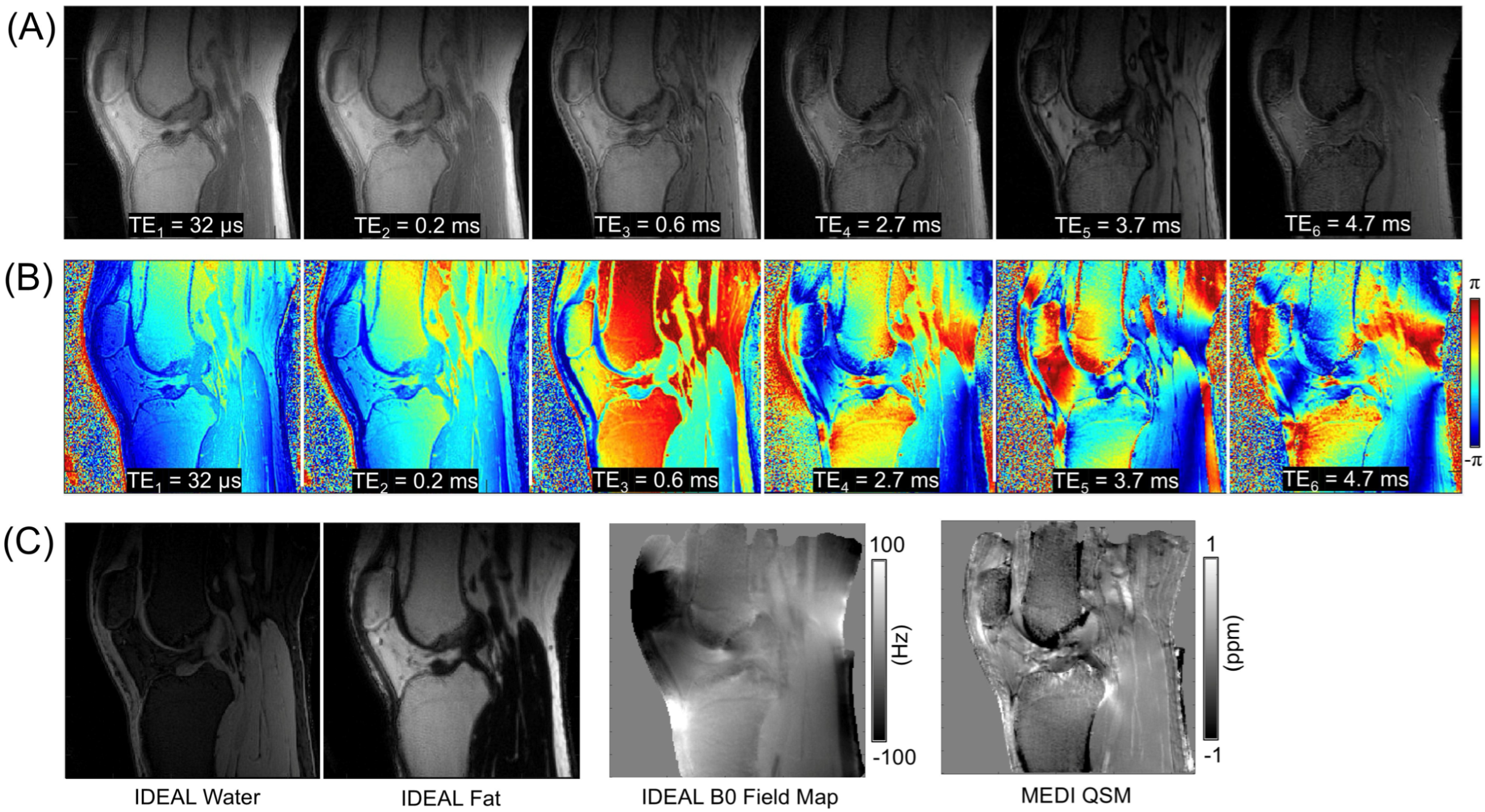
3.2. UTE-QSM with IDEAL
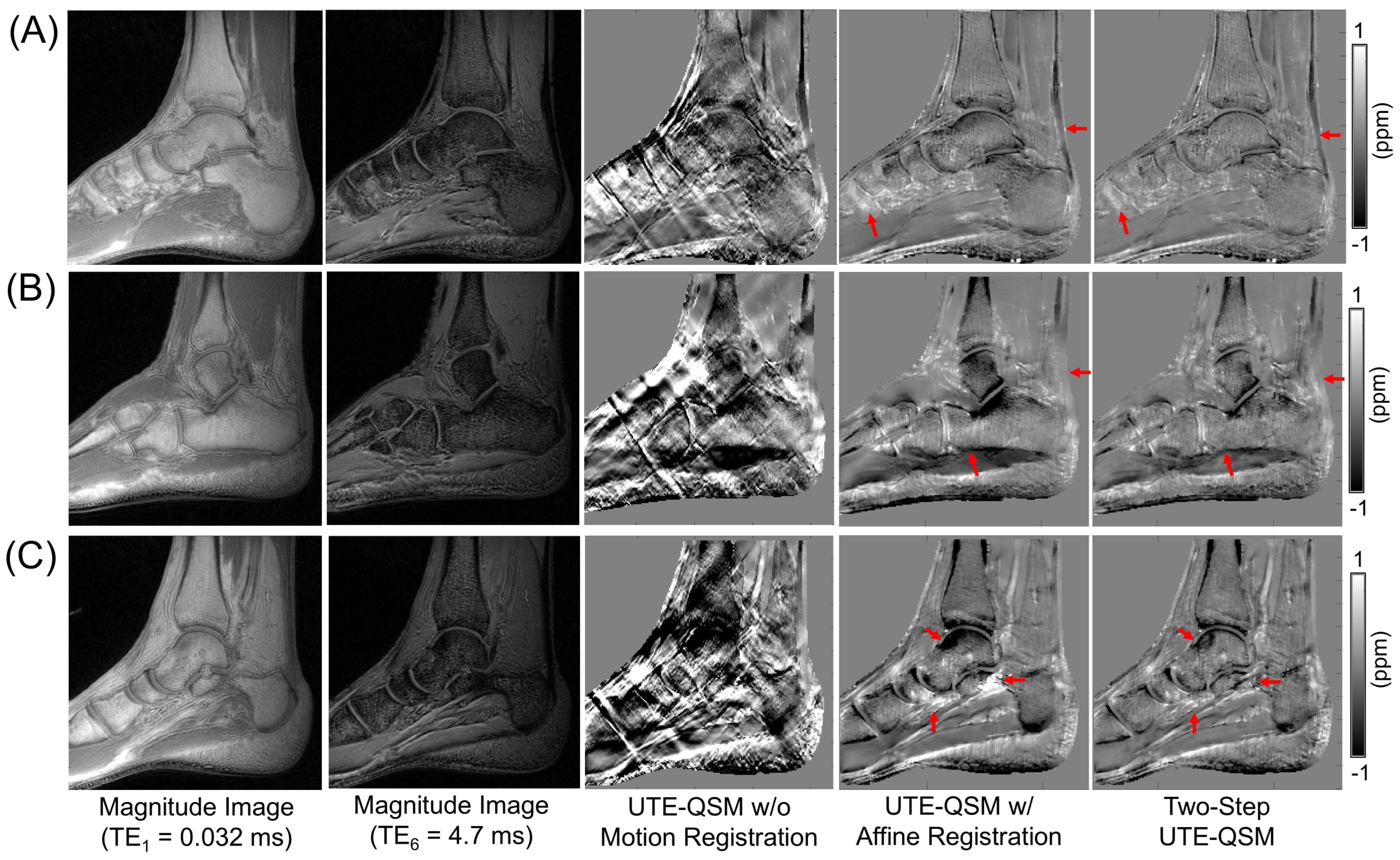
3.3. Effect of Motion and Motion Correction
3.4. UTE-QSM on the Knee and Ankle Joints
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UTE | Ultrashort Echo Time |
| QSM | Quantitative Susceptibility Mapping |
| MRI | Magnetic Resonance Imaging |
| TEs | Echo Times |
| UTE-QSM | UTE-based QSM |
| RF | Radiofrequency |
| MSK | Musculoskeletal |
| IDEAL | Iterative Decomposition of Water and Fat with Echo Asymmetry and Least-squares Estimation |
| MEDI | Morphology Enabled Dipole Inversion |
| PCL | Posterior Cruciate Ligament |
| SNR | Signal-to-Noise Ratio |
| ROI | Region of Interest |
| Projection onto Dipole Fields |
References
- Parigi, G.; Ravera, E.; Luchinat, C. Magnetic Susceptibility and Paramagnetism-Based NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 114–115, 211–236. [Google Scholar] [CrossRef]
- Broersma, S. The Magnetic Susceptibility of Organic Compounds. J. Chem. Phys. 1949, 17, 873–882. [Google Scholar] [CrossRef]
- Farrell, D.E.; Allen, C.J.; Whilden, M.W.; Kidane, T.K.; Baig, T.N.; Tripp, J.H.; Brown, R.W.; Sheth, S.; Brittenham, G.M. A New Instrument Designed to Measure the Magnetic Susceptibility of Human Liver Tissue In Vivo. IEEE Trans. Magn. 2007, 43, 3543–3554. [Google Scholar] [CrossRef]
- Treit, S.; Naji, N.; Seres, P.; Rickard, J.; Stolz, E.; Wilman, A.H.; Beaulieu, C. R2* and Quantitative Susceptibility Mapping in Deep Gray Matter of 498 Healthy Controls from 5 to 90 Years. Hum. Brain Mapp. 2021, 42, 4597–4610. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.H.O.; Santos, A.C.; Tumas, V.; Liu, M.; Zheng, W.; Haacke, E.M.; Salmon, C.E.G. Quantifying Brain Iron Deposition in Patients with Parkinson’s Disease Using Quantitative Susceptibility Mapping, R2 and R2*. Magn. Reson. Imaging 2015, 33, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Cao, H.; Wei, X.; Li, Y.; Li, W. Iron Deposition Is Positively Related to Cognitive Impairment in Patients with Chronic Mild Traumatic Brain Injury: Assessment with Susceptibility Weighted Imaging. BioMed Res. Int. 2015, 2015, 470676. [Google Scholar] [CrossRef]
- Dimov, A.V.; Li, J.; Nguyen, T.D.; Roberts, A.G.; Spincemaille, P.; Straub, S.; Zun, Z.; Prince, M.R.; Wang, Y. QSM throughout the Body. J. Magn. Reson. Imaging 2023, 57, 1621–1640. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T. Quantitative Susceptibility Mapping (QSM): Decoding MRI Data for a Tissue Magnetic Biomarker. Magn. Reson. Med. 2015, 73, 82–101. [Google Scholar] [CrossRef]
- Langkammer, C.; Liu, T.; Khalil, M.; Enzinger, C.; Jehna, M.; Fuchs, S.; Fazekas, F.; Wang, Y.; Ropele, S. Quantitative Susceptibility Mapping in Multiple Sclerosis. Radiology 2013, 267, 551–559. [Google Scholar] [CrossRef]
- Acosta-Cabronero, J.; Betts, M.J.; Cardenas-Blanco, A.; Yang, S.; Nestor, P.J. In Vivo MRI Mapping of Brain Iron Deposition across the Adult Lifespan. J. Neurosci. 2016, 36, 364–374. [Google Scholar] [CrossRef]
- Deistung, A.; Schweser, F.; Wiestler, B.; Abello, M.; Roethke, M.; Sahm, F.; Wick, W.; Nagel, A.M.; Heiland, S.; Schlemmer, H.-P.; et al. Quantitative Susceptibility Mapping Differentiates between Blood Depositions and Calcifications in Patients with Glioblastoma. PLoS ONE 2013, 8, e57924. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Zhang, X.; Zuo, P.; Hu, J.; Haacke, E.M.; Ni, H. Quantification of Liver Iron Concentration Using the Apparent Susceptibility of Hepatic Vessels. Quant. Imaging Med. Surg. 2018, 8, 123–134. [Google Scholar] [CrossRef]
- Dimov, A.V.; Liu, Z.; Spincemaille, P.; Prince, M.R.; Du, J.; Wang, Y. Bone Quantitative Susceptibility Mapping Using a Chemical Species-Specific R2* Signal Model with Ultrashort and Conventional Echo Data. Magn. Reson. Med. 2018, 79, 121–128. [Google Scholar] [CrossRef]
- Jerban, S.; Lu, X.; Jang, H.; Ma, Y.; Namiranian, B.; Le, N.; Li, Y.; Chang, E.Y.; Du, J. Significant Correlations between Human Cortical Bone Mineral Density and Quantitative Susceptibility Mapping (QSM) Obtained with 3D Cones Ultrashort Echo Time Magnetic Resonance Imaging (UTE-MRI). Magn. Reson. Imaging 2019, 62, 104–110. [Google Scholar] [CrossRef]
- Lu, X.; Jang, H.; Ma, Y.; Jerban, S.; Chang, E.; Du, J. Ultrashort Echo Time Quantitative Susceptibility Mapping (UTE-QSM) of Highly Concentrated Magnetic Nanoparticles: A Comparison Study about Different Sampling Strategies. Molecules 2019, 24, 1143. [Google Scholar] [CrossRef]
- Jang, H.; Drygalski, A.; Wong, J.; Zhou, J.Y.; Aguero, P.; Lu, X.; Cheng, X.; Ball, S.T.; Ma, Y.; Chang, E.Y.; et al. Ultrashort Echo Time Quantitative Susceptibility Mapping (UTE-QSM) for Detection of Hemosiderin Deposition in Hemophilic Arthropathy: A Feasibility Study. Magn. Reson. Med. 2020, 84, 3246–3255. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, S.; Luck, J.V.; von Drygalski, A.; Fu, E.; Park, J.I.; Gehling, K.G.; Ma, Y.; Ball, S.; Chang, E.Y.; Du, J.; et al. Hemosiderin Quantification in Hemophilic Arthropathy Using Quantitative Magnetic Resonance Imaging. Sci. Rep. 2025, 15, 14353. [Google Scholar] [CrossRef]
- Gurney, P.T.; Hargreaves, B.A.; Nishimura, D.G. Design and Analysis of a Practical 3D Cones Trajectory. Magn. Reson. Med. 2006, 55, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; Pineda, A.R.; Wen, Z.; Shimakawa, A.; Yu, H.; Brittain, J.H.; Gold, G.E.; Beaulieu, C.H.; Pelc, N.J. Iterative Decomposition of Water and Fat with Echo Asymmetry and Least-Squares Estimation (IDEAL): Application with Fast Spin-Echo Imaging. Magn. Reson. Med. 2005, 54, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Khalidov, I.; de Rochefort, L.; Spincemaille, P.; Liu, J.; Tsiouris, A.J.; Wang, Y. A Novel Background Field Removal Method for MRI Using Projection onto Dipole Fields (PDF). NMR Biomed. 2011, 24, 1129–1136. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; De Rochefort, L.; Ledoux, J.; Khalidov, I.; Chen, W.; Tsiouris, A.J.; Wisnieff, C.; Spincemaille, P.; Prince, M.R.; et al. Morphology Enabled Dipole Inversion for Quantitative Susceptibility Mapping Using Structural Consistency between the Magnitude Image and the Susceptibility Map. NeuroImage 2012, 59, 2560–2568. [Google Scholar] [CrossRef]
- Canny, J. A Computational Approach to Edge Detection. IEEE Trans. Pattern Anal. Mach. Intell. 1986, PAMI-8, 679–698. [Google Scholar] [CrossRef]
- Hu, H.H.; Börnert, P.; Hernando, D.; Kellman, P.; Ma, J.; Reeder, S.; Sirlin, C. ISMRM Workshop on Fat-Water Separation: Insights, Applications and Progress in MRI. Magn. Reson. Med. 2012, 68, 378–388. [Google Scholar] [CrossRef]
- Jang, H.; Sedaghat, S.; Athertya, J.S.; Moazamian, D.; Carl, M.; Ma, Y.; Lu, X.; Ji, A.; Chang, E.Y.; Du, J. Feasibility of Ultrashort Echo Time Quantitative Susceptibility Mapping with a 3D Cones Trajectory in the Human Brain. Front. Neurosci. 2022, 16, 1033801. [Google Scholar] [CrossRef]
- Wei, H.; Dibb, R.; Decker, K.; Wang, N.; Zhang, Y.; Zong, X.; Lin, W.; Nissman, D.B.; Liu, C. Investigating Magnetic Susceptibility of Human Knee Joint at 7 Tesla. Magn. Reson. Med. 2017, 78, 1933–1943. [Google Scholar] [CrossRef]
- Newbury, N.; Sedaghat, S.; Athertya, J.S.; Shin, S.H.; Ma, Y.; Jerban, S.; Carl, M.; Silva, M.L.; Chang, E.Y.; Du, J.; et al. Novel Fat Suppression Technique for Ultrashort Echo Time MRI Using Single-Point Dixon Phase Modeling. Quant. Imaging Med. Surg. 2025, 15, 4580591–4584591. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.; Liu, T.; Zhang, Z.; Prince, M.R.; Gillen, K.; Yan, X.; Song, Q.; Hua, T.; Zhao, X.; et al. Quantitative Susceptibility Mapping (QSM) Minimizes Interference from Cellular Pathology in R2* Estimation of Liver Iron Concentration. J. Magn. Reson. Imaging 2018, 48, 1069–1079. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, W.; Kovanlikaya, I.; Kovanlikaya, A.; Liu, T.; Wang, S.; Salustri, C.; Wang, Y. Intracranial Calcifications and Hemorrhages: Characterization with Quantitative Susceptibility Mapping. Radiology 2014, 270, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Maekawa, K.; Yamashita, A.; Yokogami, K.; Enzaki, M.; Khant, Z.A.; Takeshima, H.; Asada, Y.; Wang, Y.; Hirai, T. Characterization of Carotid Plaque Components by Quantitative Susceptibility Mapping. AJNR Am. J. Neuroradiol. 2020, 41, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Straub, S.; Laun, F.B.; Emmerich, J.; Jobke, B.; Hauswald, H.; Katayama, S.; Herfarth, K.; Schlemmer, H.P.; Ladd, M.E.; Ziener, C.H.; et al. Potential of Quantitative Susceptibility Mapping for Detection of Prostatic Calcifications. J. Magn. Reson. Imaging 2017, 45, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Athertya, J.S.; Akers, J.; Sedaghat, S.; Wei, Z.; Moazamian, D.; Dwek, S.; Thu, M.; Jang, H. Detection of Iron Oxide Nanoparticle (IONP)-Labeled Stem Cells Using Quantitative Ultrashort Echo Time Imaging: A Feasibility Study. Quant. Imaging Med. Surg. 2023, 13, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Z.; Wang, H.; Chen, T.; Lu, Y.; Yan, F.; Zhang, Y.; Wei, H. Simultaneous Quantitative Susceptibility Mapping of Articular Cartilage and Cortical Bone of Human Knee Joint Using Ultrashort Echo Time Sequences. Front. Endocrinol. 2022, 13, 844351. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, S.; Jang, H.; Athertya, J.S.; Groezinger, M.; Corey-Bloom, J.; Du, J. The Signal Intensity Variation of Multiple Sclerosis (MS) Lesions on Magnetic Resonance Imaging (MRI) as a Potential Biomarker for Patients’ Disability: A Feasibility Study. Front. Neurosci. 2023, 17, 1145251. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, S.; Jang, H.; Ma, Y.; Afsahi, A.M.; Reichardt, B.; Corey-Bloom, J.; Du, J. Clinical Evaluation of White Matter Lesions on 3D Inversion Recovery Ultrashort Echo Time MRI in Multiple Sclerosis. Quant. Imaging Med. Surg. 2023, 13, 4171180–4174180. [Google Scholar] [CrossRef]
- Klein, S.; Staring, M.; Murphy, K.; Viergever, M.A.; Pluim, J.P.W. Elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans. Med. Imaging 2010, 29, 196–205. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, W.; Wan, L.; Kakos, L.; Li, L.; Jerban, S.; Jang, H.; Chang, E.Y.; Du, J.; Ma, Y. Quantitative Three-dimensional Ultrashort Echo Time Cones Imaging of the Knee Joint with Motion Correction. NMR Biomed. 2020, 33, e4214. [Google Scholar] [CrossRef]

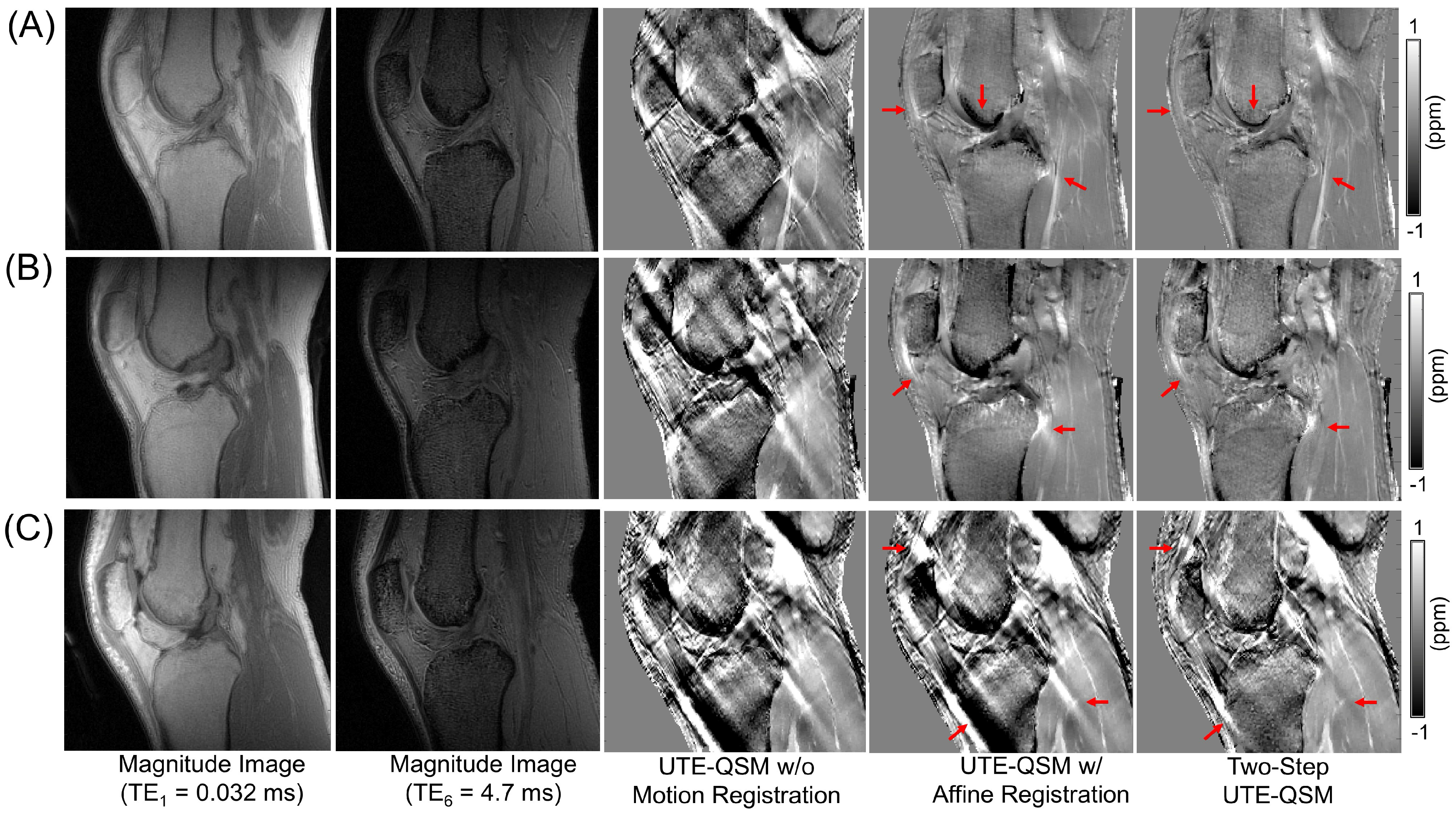
| ROI | QSMA No Registration (ppm) | QSMB Affine Registration (ppm) | QSMC Affine Registration with Two-Step QSM (ppm) | p-Value QSMA vs. QSMB | p-Value QSMB vs. QSMC | p-Value QSMA vs. QSMC | |
|---|---|---|---|---|---|---|---|
| Knee | Patella Tendon | −0.0138 ± 0.2594 | −0.1797 ± 0.1769 | −0.1278 ± 0.1322 | 0.0523 | 0.0387 | 0.1715 |
| PCL | −0.5127 ± 0.2582 | −0.3835 ± 0.2155 | −0.1503 ± 0.1403 | 0.1572 | 0.0026 | 0.0130 | |
| Meniscus | −0.6010 ± 0.2943 | −0.3181 ± 0.1679 | −0.1999 ± 0.1134 | 0.0467 | 0.0315 | 0.0093 | |
| Muscle | 0.1913 ± 0.0857 | 0.1334 ± 0.0976 | 0.0708 ± 0.0933 | 0.0546 | 0.0051 | 0.0003 | |
| Ankle | Achilles Tendon | −0.3660 ± 0.1252 | −0.2262 ± 0.1012 | −0.2567 ± 0.1145 | 0.1280 | 0.1999 | 0.1860 |
| Muscle | −0.0853 ± 0.0599 | −0.0705 ± 0.0555 | −0.0336 ± 0.0285 | 0.5954 | 0.0252 | 0.0673 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedaghat, S.; Park, J.; Fu, E.; Liu, F.; Jung, Y.; Jang, H. Effects of Motion in Ultrashort Echo Time Quantitative Susceptibility Mapping for Musculoskeletal Imaging. J. Imaging 2025, 11, 347. https://doi.org/10.3390/jimaging11100347
Sedaghat S, Park J, Fu E, Liu F, Jung Y, Jang H. Effects of Motion in Ultrashort Echo Time Quantitative Susceptibility Mapping for Musculoskeletal Imaging. Journal of Imaging. 2025; 11(10):347. https://doi.org/10.3390/jimaging11100347
Chicago/Turabian StyleSedaghat, Sam, Jinil Park, Eddie Fu, Fang Liu, Youngkyoo Jung, and Hyungseok Jang. 2025. "Effects of Motion in Ultrashort Echo Time Quantitative Susceptibility Mapping for Musculoskeletal Imaging" Journal of Imaging 11, no. 10: 347. https://doi.org/10.3390/jimaging11100347
APA StyleSedaghat, S., Park, J., Fu, E., Liu, F., Jung, Y., & Jang, H. (2025). Effects of Motion in Ultrashort Echo Time Quantitative Susceptibility Mapping for Musculoskeletal Imaging. Journal of Imaging, 11(10), 347. https://doi.org/10.3390/jimaging11100347






