Peripheral Non-Contrast MR Angiography Using FBI: Scan Time and T2 Blurring Reduction with 2D Parallel Imaging
Abstract
1. Introduction
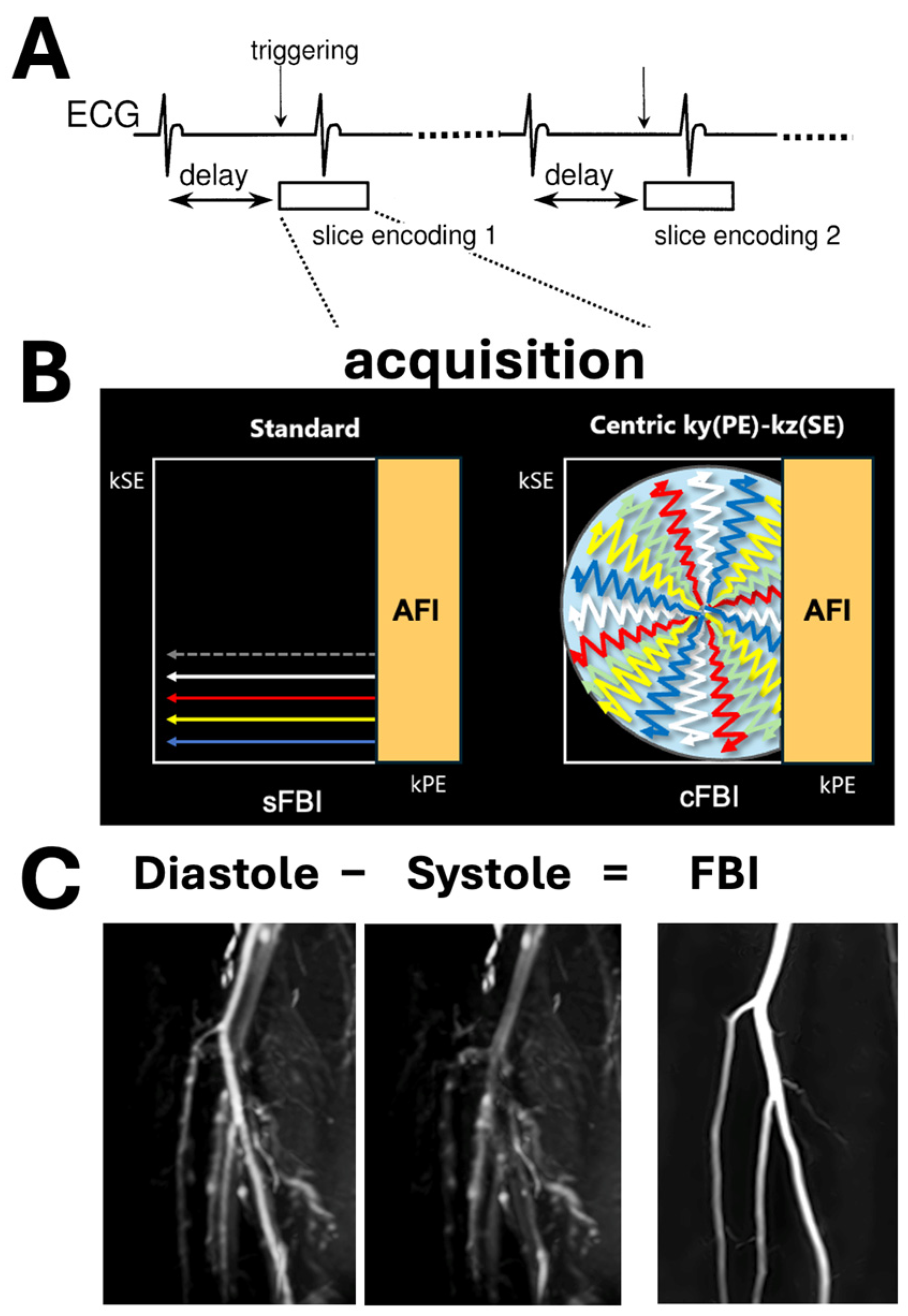
2. Materials and Methods
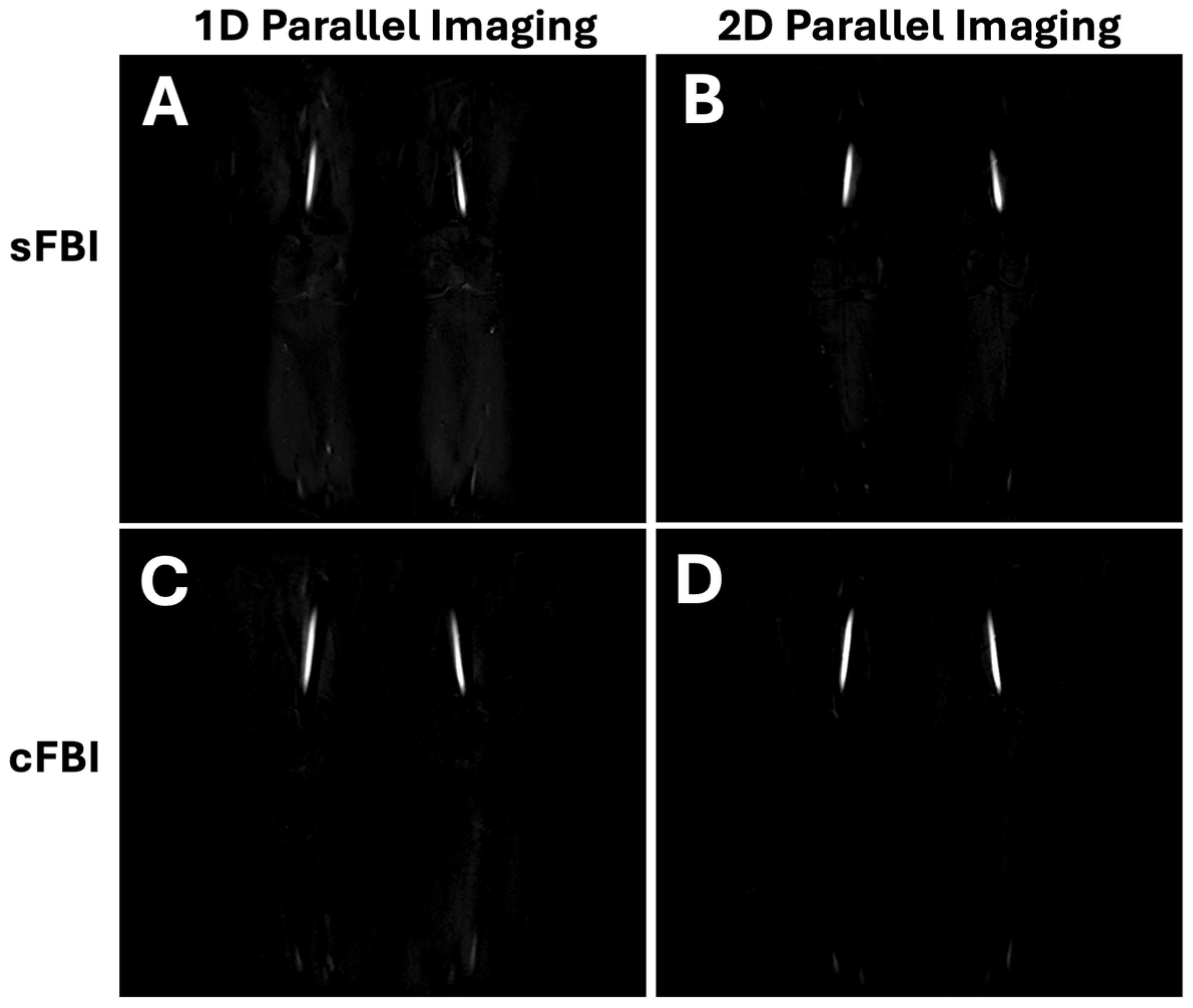
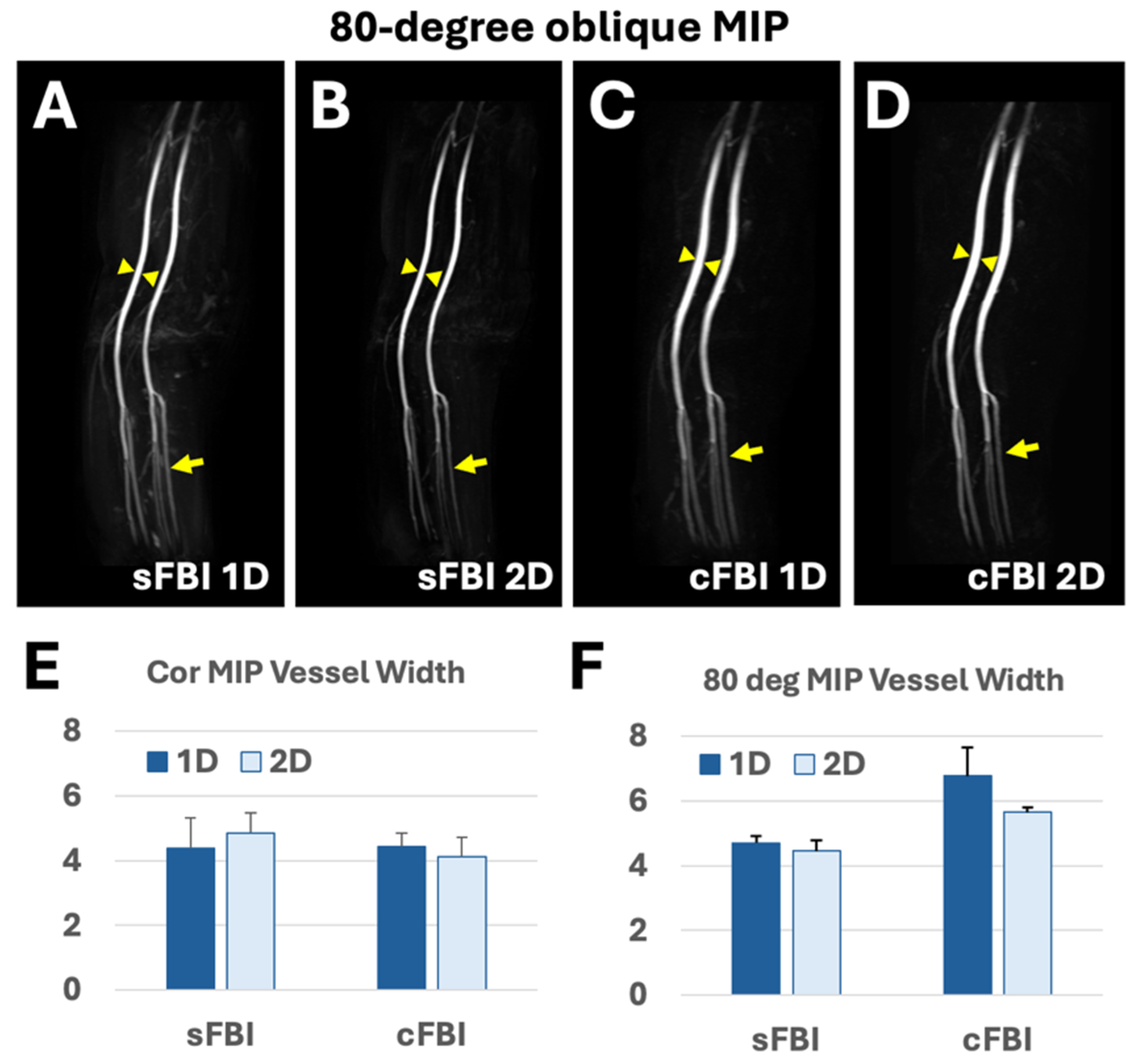
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selvin, E.; Erlinger, T.P. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004, 110, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, R.; Santoro, M.; Marano, R.; Di Stasi, C.; Dattesi, R.; Kirchin, M.; Tinelli, G.; Snider, F.; Bonomo, L. Low-dose multidetector CT angiography in the evaluation of infrarenal aorta and peripheral arterial occlusive disease. Radiology 2012, 263, 287–298. [Google Scholar] [CrossRef]
- Nadolski, G.J.; Stavropoulos, S.W. Contrast alternatives for iodinated contrast allergy and renal dysfunction: Options and limitations. J. Vasc. Surg. 2013, 57, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, D.; Hallett, R.L.; Rubin, G.D. CT angiography of peripheral arterial disease. J. Vasc. Interv. Radiol. 2006, 17, 3–26. [Google Scholar] [CrossRef]
- DeLoach, S.S.; Mohler, E.R., 3rd. Peripheral arterial disease: A guide for nephrologists. Clin. J. Am. Soc. Nephrol. 2007, 2, 839–846. [Google Scholar] [CrossRef]
- Leung, D.A.; Debatin, J.F. Three-dimensional contrast-enhanced magnetic resonance angiography of the thoracic vasculature. Eur. Radiol. 1997, 7, 981–989. [Google Scholar] [CrossRef]
- Ho, K.Y.; Leiner, T.; de Haan, M.W.; Kessels, A.G.; Kitslaar, P.J.; van Engelshoven, J.M. Peripheral vascular tree stenoses: Evaluation with moving-bed infusion-tracking MR angiography. Radiology 1998, 206, 683–692. [Google Scholar] [CrossRef]
- Lim, R.P.; Koktzoglou, I. Noncontrast magnetic resonance angiography: Concepts and clinical applications. Radiol. Clin. N. Am. 2015, 53, 457–476. [Google Scholar] [CrossRef]
- Aime, S.; Caravan, P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J. Magn. Reson. Imaging 2009, 30, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Ishii, K.; Kawaguchi, H.; Kitajima, K.; Takenaka, D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014, 270, 834–841. [Google Scholar] [CrossRef]
- Kanda, T.; Osawa, M.; Oba, H.; Toyoda, K.; Kotoku, J.; Haruyama, T.; Takeshita, K.; Furui, S. High Signal Intensity in Dentate Nucleus on Unenhanced T1-weighted MR Images: Association with Linear versus Macrocyclic Gadolinium Chelate Administration. Radiology 2015, 275, 803–809. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef]
- Miyazaki, M.; Sugiura, S.; Tateishi, F.; Wada, H.; Kassai, Y.; Abe, H. Non-contrast-enhanced MR angiography using 3D ECG-synchronized half-Fourier fast spin echo. J. Magn. Reson. Imaging 2000, 12, 776–783. [Google Scholar] [CrossRef]
- Miyazaki, M.; Takai, H.; Sugiura, S.; Wada, H.; Kuwahara, R.; Urata, J. Peripheral MR angiography: Separation of arteries from veins with flow-spoiled gradient pulses in electrocardiography-triggered three-dimensional half-Fourier fast spin-echo imaging. Radiology 2003, 227, 890–896. [Google Scholar] [CrossRef]
- Edelman, R.R.; Sheehan, J.J.; Dunkle, E.; Schindler, N.; Carr, J.; Koktzoglou, I. Quiescent-interval single-shot unenhanced magnetic resonance angiography of peripheral vascular disease: Technical considerations and clinical feasibility. Magn. Reson. Med. 2010, 63, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Sheehan, J.; Bi, X.; Liu, X.; Carr, J.; Li, D. 3D noncontrast MR angiography of the distal lower extremities using flow-sensitive dephasing (FSD)-prepared balanced SSFP. Magn. Reson. Med. 2009, 62, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhou, X.; Bi, X.; Dharmakumar, R.; Carr, J.C.; Li, D. Determination of the optimal first-order gradient moment for flow-sensitive dephasing magnetization-prepared 3D noncontrast MR angiography. Magn. Reson. Med. 2011, 65, 964–972. [Google Scholar] [CrossRef]
- Shin, T.; Hu, B.S.; Nishimura, D.G. Off-resonance-robust velocity-selective magnetization preparation for non-contrast-enhanced peripheral MR angiography. Magn. Reson. Med. 2013, 70, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.; Qin, Q.; Park, J.Y.; Crawford, R.S.; Rajagopalan, S. Identification and reduction of image artifacts in non-contrast-enhanced velocity-selective peripheral angiography at 3T. Magn. Reson. Med. 2016, 76, 466–477. [Google Scholar] [CrossRef]
- Wu, G.; Yang, J.; Zhang, T.; Morelli, J.N.; Giri, S.; Li, X.; Tang, W. The diagnostic value of non-contrast enhanced quiescent interval single shot (QISS) magnetic resonance angiography at 3T for lower extremity peripheral arterial disease, in comparison to CT angiography. J. Cardiovasc. Magn. Reson. 2016, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Malis, V.; Vucevic, D.; Bae, W.C.; Yamamoto, A.; Kassai, Y.; Lane, J.; Hsiao, A.; Nakamura, K.; Miyazaki, M. Fast Non-Contrast MR Angiography using Zigzag Centric ky-kz k-space Trajectory and exponential refocusing flip angles with restoration of longitudinal magnetization. Magn. Reson. Med. Sci. 2024. [CrossRef]
- Ota, H.; Morita, Y.; Vucevic, D.; Higuchi, S.; Takagi, H.; Kutsuna, H.; Yamashita, Y.; Kim, P.; Miyazaki, M. Motion robust coronary MR angiography using zigzag centric ky-kz trajectory and high-resolution deep learning reconstruction. Magn. Reson. Mater. Phys. Biol. Med. 2024; in press. [Google Scholar] [CrossRef]
- Miyazaki, M.; Akahane, M. Non-contrast enhanced MR angiography: Established techniques. J. Magn. Reson. Imaging 2012, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Lee, V.S. Nonenhanced MR angiography. Radiology 2008, 248, 20–43. [Google Scholar] [CrossRef]
- Constable, R.T.; Gore, J.C. The loss of small objects in variable TE imaging: Implications for FSE, RARE, and EPI. Magn. Reson. Med. 1992, 28, 9–24. [Google Scholar] [CrossRef]
- Miyazaki, M.; Ichinose, N.; Sugiura, S.; Kassai, Y.; Kanazawa, H.; Machida, Y. A novel MR angiography technique: SPEED acquisition using half-Fourier RARE. J. Magn. Reson. Imaging 1998, 8, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q. Point spread functions of the T2 decay in k-space trajectories with long echo train. Magn. Reson. Imaging 2012, 30, 1134–1142. [Google Scholar] [CrossRef]
- Lu, H.; Xu, F.; Grgac, K.; Liu, P.; Qin, Q.; van Zijl, P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn. Reson. Med. 2012, 67, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Boujan, T.; Neuberger, U.; Pfaff, J.; Nagel, S.; Herweh, C.; Bendszus, M.; Mohlenbruch, M.A. Value of Contrast-Enhanced MRA versus Time-of-Flight MRA in Acute Ischemic Stroke MRI. AJNR Am. J. Neuroradiol. 2018, 39, 1710–1716. [Google Scholar] [CrossRef]
- Krishnam, M.S.; Tomasian, A.; Deshpande, V.; Tran, L.; Laub, G.; Finn, J.P.; Ruehm, S.G. Noncontrast 3D steady-state free-precession magnetic resonance angiography of the whole chest using nonselective radiofrequency excitation over a large field of view: Comparison with single-phase 3D contrast-enhanced magnetic resonance angiography. Investig. Radiol. 2008, 43, 411–420. [Google Scholar] [CrossRef]
- Team, J. JASP, Version 0.18.3. Computer software. 2024.
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Nielsen, Y.W.; Thomsen, H.S. Contrast-enhanced peripheral MRA: Technique and contrast agents. Acta Radiol. 2012, 53, 769–777. [Google Scholar] [CrossRef]
- Shin, T.; Menon, R.G.; Thomas, R.B.; Cavallo, A.U.; Sarkar, R.; Crawford, R.S.; Rajagopalan, S. Unenhanced Velocity-Selective MR Angiography (VS-MRA): Initial Clinical Evaluation in Patients with Peripheral Artery Disease. J. Magn. Reson. Imaging 2019, 49, 744–751. [Google Scholar] [CrossRef]
- Shimada, K.; Isoda, H.; Okada, T.; Kamae, T.; Arizono, S.; Hirokawa, Y.; Shibata, T.; Togashi, K. Non-contrast-enhanced hepatic MR angiography: Do two-dimensional parallel imaging and short tau inversion recovery methods shorten acquisition time without image quality deterioration? Eur. J. Radiol. 2011, 77, 137–142. [Google Scholar] [CrossRef]



| Subject # | Age (years) | Sex | HR (bpm) | BMI (kg/m2) |
|---|---|---|---|---|
| 1 | 50 | M | 73 | 26.0 |
| 2 | 23 | F | 54 | 29.3 |
| 3 | 26 | F | 53 | 18.6 |
| 4 | 32 | M | 63 | 25.2 |
| mean | 32.8 | 60.8 | 24.8 | |
| S.D. | 12.1 | 9.3 | 4.5 |
| 1D PIF (s) | 2D PIF (s) | |
|---|---|---|
| sFBI | 205.5 (37.7) | 183.5 (30.9) |
| cFBI | 97.8 (15.0) | 162.0 (37.8) |
| 1D PIF (mm) | 2D PIF (mm) | |
|---|---|---|
| sFBI | 4.7 (0.22) | 4.5 (0.33) |
| cFBI | 6.8 (0.86) | 5.6 (0.15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, W.C.; Hahn, L.; Malis, V.; Mesa, A.; Vucevic, D.; Miyazaki, M. Peripheral Non-Contrast MR Angiography Using FBI: Scan Time and T2 Blurring Reduction with 2D Parallel Imaging. J. Imaging 2024, 10, 223. https://doi.org/10.3390/jimaging10090223
Bae WC, Hahn L, Malis V, Mesa A, Vucevic D, Miyazaki M. Peripheral Non-Contrast MR Angiography Using FBI: Scan Time and T2 Blurring Reduction with 2D Parallel Imaging. Journal of Imaging. 2024; 10(9):223. https://doi.org/10.3390/jimaging10090223
Chicago/Turabian StyleBae, Won C., Lewis Hahn, Vadim Malis, Anya Mesa, Diana Vucevic, and Mitsue Miyazaki. 2024. "Peripheral Non-Contrast MR Angiography Using FBI: Scan Time and T2 Blurring Reduction with 2D Parallel Imaging" Journal of Imaging 10, no. 9: 223. https://doi.org/10.3390/jimaging10090223
APA StyleBae, W. C., Hahn, L., Malis, V., Mesa, A., Vucevic, D., & Miyazaki, M. (2024). Peripheral Non-Contrast MR Angiography Using FBI: Scan Time and T2 Blurring Reduction with 2D Parallel Imaging. Journal of Imaging, 10(9), 223. https://doi.org/10.3390/jimaging10090223






