Comparison of Echocardiography and Myocardial Scintigraphy to Detect Cancer Therapy-Related Cardiovascular Toxicity in Breast Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Tl/BMIPP Dual-Isotope Myocardial Scintigraphy
3.2. Echocardiography
3.3. Morbidity and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Berger-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Munoz, D.R.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar]
- Campia, U.; Moslehi, J.J.; Amiri-Kordestani, L.; Barac, A.; Beckman, J.A.; Chism, D.D.; Cohen, P.; Groarke, J.D.; Herrmann, J.; Reilly, C.M.; et al. Cardio-Oncology: Vascular and metabolic perspectives: A scientific statement from the American Heart Association. Circulation 2019, 139, e579–e602. [Google Scholar] [CrossRef]
- Jokar, N.; Amini, A.; Ravanbod, M.; Barekat, M.; Shooli, H.; Gholamrezanezhad, A.; Assadi, M. State-of-the-art modalities in cardio-oncology: Insight from a nuclear medicine approach. Nucl. Med. Rev. 2021, 24, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, H.; Abe, M.; Abe, T.; Nagai, Y.; Ibukiyama, C. Serial change of TL/BMIPP dual SPECT myocardial scintigram in patients with acute myocardial infarction; meaning of chronic mismatch phenomenon. Kaku Igaku. 1999, 36, 349–355. (In Japanese) [Google Scholar]
- Biswas, S.K.; Sarai, M.; Hishida, H.; Ozaki, Y. 123I-BMIPP fatty acid analogue imaging is a novel diagnostic and prognostic approach following acute myocardial infarction. Singap. Med. J. 2009, 50, 943–948. [Google Scholar] [PubMed]
- Fukushima, M.; Seino, Y.; Kumita, S.; Nakajo, H.; Cho, K.; Takano, T. Dual-isotope myocardial SPECT in patients with redefined myocardial infarction. Int. J. Cardiol. 2005, 104, 204–212. [Google Scholar] [CrossRef]
- Takeishi, Y.; Suekawa, H.; Sakurai, T.; Saito, H.; Nishimura, S.; Shibu, T.; Sasaki, Y.; Tomoike, H. Noninvasive identification of anthracycline cardiotoxicity: Comparison of 123I-MIBG and 123I-BMIPP imaging. Ann. Nucl. Med. 1994, 8, 177–182. [Google Scholar] [CrossRef]
- Harada, Y.; Shimada, K.; Harada, S.J.; Sato, T.; Kubota, Y.; Yamashita, M. Iodine-123 β-methyl-P-iodophenyl-pentadecanoic acid (123I-BMIPP) myocardial scintigraphy for breast cancer patients and possible early signs of cancer-therapeutics-related cardiac dysfunction (CTRCD). J. Imaging 2022, 8, 296. [Google Scholar] [CrossRef]
- De Geus-Oei, L.F.; Mavinkurve-Groothuis, A.M.; Bellersen, L.; Gotthardt, M.; Oyen, W.J.; Kapusta, L.; van Laarhoven, H.W. Scintigraphic techniques for early detection of cancer treatment-induced cardiotoxicity. J. Nucl. Med. Technol. 2013, 41, 170–181. [Google Scholar] [CrossRef]
- Kossaify, A.; Nasr, M. Diastolic disfunction and the new recommendations for echocardiographic assessment of left ventricular diastolic function: Summary of guidelines and novelties in diagnosis and grading. J. Diagn. Med. Sonogr. 2019, 35, 317–325. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelista, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 107–133. [Google Scholar] [CrossRef]
- Iwata, K.; Committee of Clinical Practice Guidelines. The Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer 2018; The Japanese Breast Cancer Society: Kanahara-Shuppan: Tokyo, Japan, 2018. [Google Scholar]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. America Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar]
- Nakajima, K.; Okuda, K.; Kawano, S.; Slomka, P.; Germano, G.; Kinuya, S. The importance of population-specific normal database for quantification of myocardial ischemia; comparison between Japanese 360 and 180-degree databases and a US database. J. Nucl. Cardiol. 2009, 16, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kumita, S.; Ishida, Y.; Momose, M.; Hashimoto, J.; Morita, K.; Taki, J.; Yamashita, S.; Maruno, H.; Ogawa, M.; et al. Creation and characterization of Japanese standards for myocardial perfusion SPECT: Database from the Japanese Society of Nuclear Medicine Working Group. Ann. Nucl. Med. 2007, 21, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Matsuki, T.; Hashimoto, A.; Tsukamoto, K.; Nakata, T.; Tamaki, N. Validation of automated quantification of myocardial perfusion and fatty acid metabolism abnormalities on SPECT images. Circ. J. 2011, 75, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Garver, P.R.; Wasnich, R.D.; Shibuya, A.M.; Yeh, F. Appearance of breast attenuation artifacts with thallium myocardial SPECT imaging. Clin. Nucl. Med. 1985, 10, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.; Schechter, D.; Kieun, M.; Marciano, R.; Rozenman, Y.; Chisin, R. SPECT attenuation artifacts in normal and overweight persons: Insights from a retrospective comparison of Rb-82 positron emission tomography and Tl-201 SPECT myocardial perfusion imaging. Clin. Nucl. Med. 2000, 25, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakata, T.; Oka, T.; Ogawa, T.; Okamoto, F.; Kusaka, Y.; Sohmiya, K.; Shimamoto, K.; Itakura, K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J. Lipid Res. 2001, 42, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Ikeda, Y.; Sugimura, K.; Sakata, Y. Cardiomyocyte steatosis and defective washout of iodine-123-β-methyl iodophenyl-pentadecanoic acid in genetic deficiency of adipose triglyceride lipase. Eur. Heart J. 2015, 36, 580. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Takeda, K.; Okamoto, S.; Okamoto, R.; Makino, K.; Tameda, Y.; Nomura, Y.; Maeda, H.; Ichihara, T.; Nakano, T. Detection of doxorubicin cardiology by using iodine-123 BMIPP early dynamic SPECT: Quantitative evaluation of early abnormality of fatty acid metabolism with the Rutland method. J. Nucl. Cardiol. 2000, 7, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Takeda, K.; Imanaka-Yoshida, K.; Imai, H.; Sekine, T.; Kamikura, Y. Assessment of fatty acid metabolism in taxan-induced myocardial damage with iodine-123 BMIPP SPECT: Comparative study with myocardial perfusion, left ventricular function, and histopathological findings. Ann. Nucl. Med. 2003, 17, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Dilsizian, V. Challenging nuclear cardiology research: Stimulating discovery, validation, and clinical relevance. J. Nucl. Med. 2018, 59, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Sueda, S. Clinical usefulness of myocardial scintigraphy in patients with vasospastic angina. J. Cardiol. 2020, 75, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Teragawa, H.; Oshita, C.; Orita, Y. Perfusion-metabolic mismatch in myocardial scintigraphy in patients with vasospastic angina: What does it mean clinically? Eur. Jeart J. 2020, 41, ehaa946.0278. [Google Scholar] [CrossRef]
- National Cancer Center Japan. Ganjoho. Available online: https://ganjoho.jp/reg_stat/statistics/stat/cancer/14_breast.html (accessed on 23 November 2023).
- World Health Organization. WHO Mortality Database. Available online: https://platform.who.int/mortality/themes/theme-details/topics/indicator-groups/indicator-group-details/MDB/breast-cancer (accessed on 23 November 2023).
- Laufer-Perl, M.; Mor, L.; Milwidsky, A.; Derakhshesh, M.; Amrami, N.; Moshkovits, Y.; Arnold, J.; Topilsky, Y.; Arbel, Y.; Rozenbaum, Z. Caner therapeutics-related cardiac dysfunction among patients with active breast cancer: A cardio-oncology registry. Isr. Med. Assoc. J. 2020, 22, 564–568. [Google Scholar]
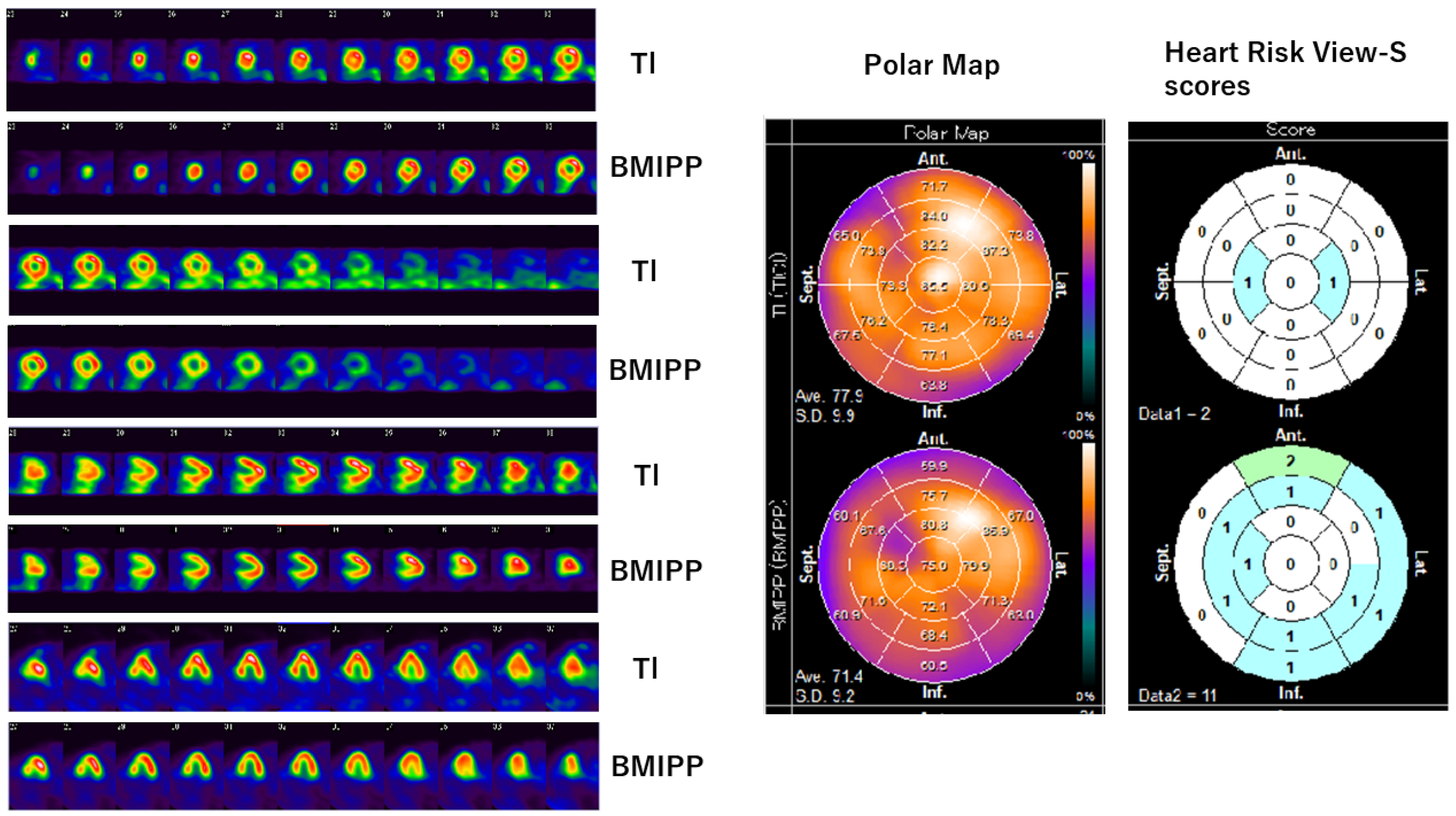
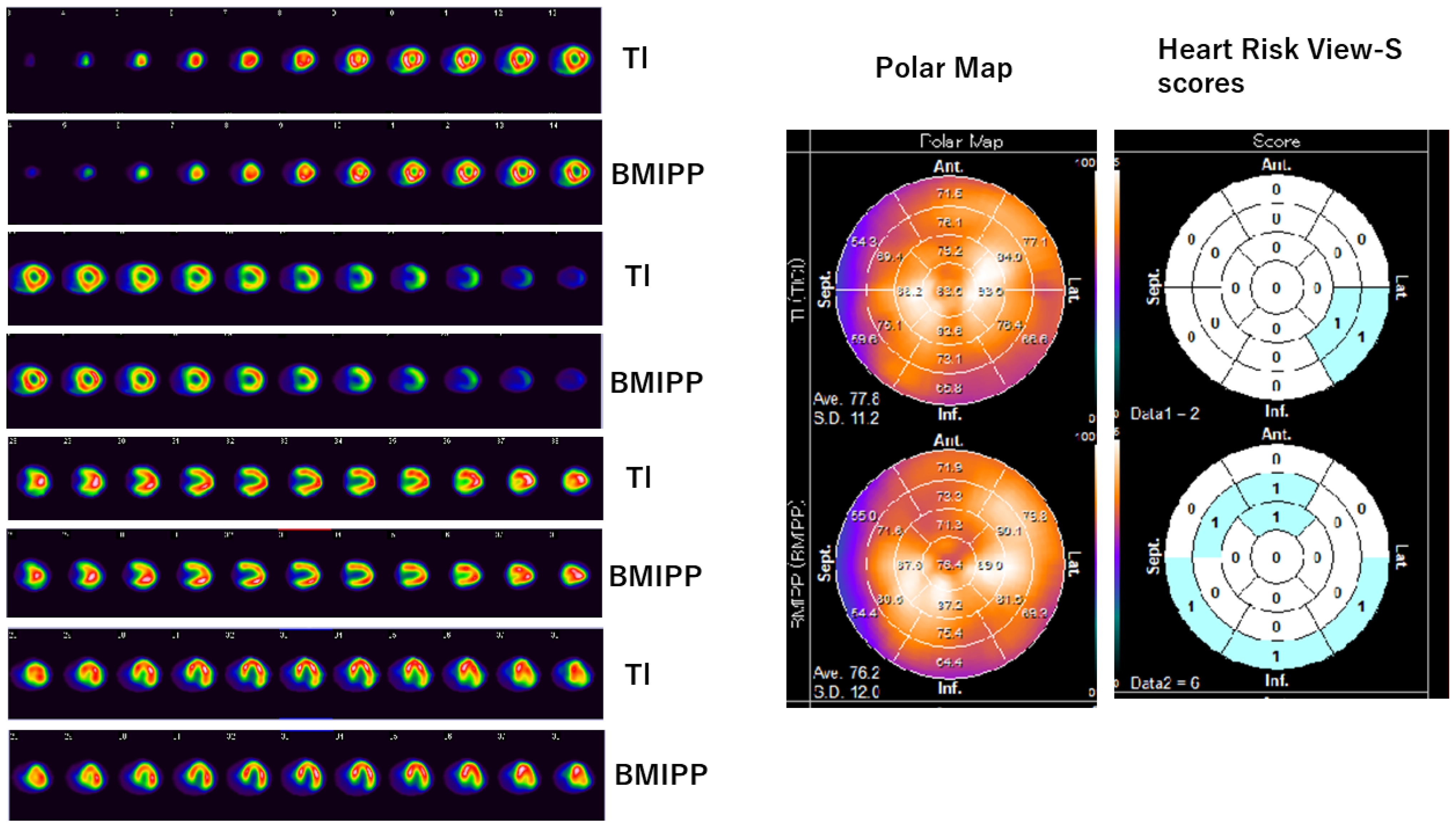
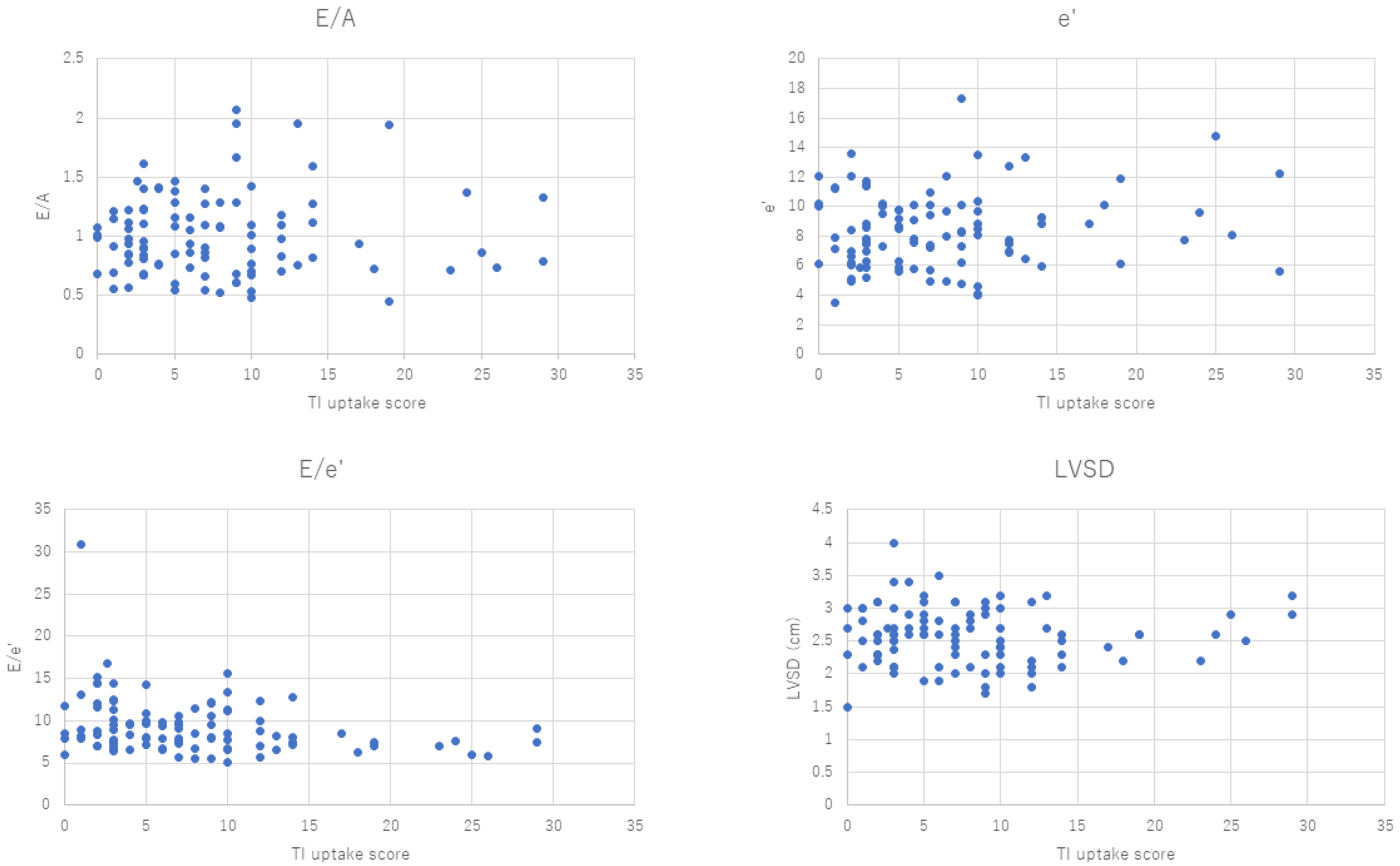

| Isotope | E/A | e’ | E/e’ | LVSD (cm) |
| 201Thallium | 0.045024 | 0.128372 | −0.27713 | −0.01576 |
| 123II-BMIPP | 0.19986 | 0.121109 | −0.1199 | −0.08403 |
| Patient | Age | 201Tl Score | 123I-BMIPP Score | Duration to Death (Months) | CVR-CVT | Cause of Death |
| A | 40 | 2 | 1 | 20 | - | Multiple organ failure |
| B | 52 | 10 | 5 | 6 | - | Multiple organ failure |
| C | 55 | 9 | 22 | 3 | Possible CTRCD patient by scintigraphy | Interstitial pneumonia |
| D | 58 | 3 | 8 | 5 | - | Multiple organ failure |
| E | 59 | 2 | 11 | 11 | Possible CTRCD patient by scintigraphy | Multiple organ failure |
| F | 68 | 12 | 7 | 10 | - | Multiple organ failure |
| G | 72 | 3 | 68 | 6 | CTRCD patient by LVEF criteria | Multiple organ failure |
| H | 75 | 2 | 6 | 3 | - | Interstitial pneumonia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harada, Y.; Shimada, K.; Harada, S.J.; Sato, T.; Kubota, Y.; Yamashita, M. Comparison of Echocardiography and Myocardial Scintigraphy to Detect Cancer Therapy-Related Cardiovascular Toxicity in Breast Cancer Patients. J. Imaging 2024, 10, 54. https://doi.org/10.3390/jimaging10030054
Harada Y, Shimada K, Harada SJ, Sato T, Kubota Y, Yamashita M. Comparison of Echocardiography and Myocardial Scintigraphy to Detect Cancer Therapy-Related Cardiovascular Toxicity in Breast Cancer Patients. Journal of Imaging. 2024; 10(3):54. https://doi.org/10.3390/jimaging10030054
Chicago/Turabian StyleHarada, Yuko, Kyosuke Shimada, Satoshi John Harada, Tomomi Sato, Yukino Kubota, and Miyoko Yamashita. 2024. "Comparison of Echocardiography and Myocardial Scintigraphy to Detect Cancer Therapy-Related Cardiovascular Toxicity in Breast Cancer Patients" Journal of Imaging 10, no. 3: 54. https://doi.org/10.3390/jimaging10030054
APA StyleHarada, Y., Shimada, K., Harada, S. J., Sato, T., Kubota, Y., & Yamashita, M. (2024). Comparison of Echocardiography and Myocardial Scintigraphy to Detect Cancer Therapy-Related Cardiovascular Toxicity in Breast Cancer Patients. Journal of Imaging, 10(3), 54. https://doi.org/10.3390/jimaging10030054





