Enhancing Sewage Sludge Stabilization, Pathogen Removal, and Biomass Production through Indigenous Microalgae Promoting Growth: A Sustainable Approach for Sewage Sludge Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Experimental Setup
2.3. Determination of Biomass Concentration
2.4. Microbiological Analysis
2.5. EPS Extraction and Analysis
2.6. Other Analytical Methods
2.7. Statistical Analysis
3. Results and Discussion
3.1. Biomass Growth
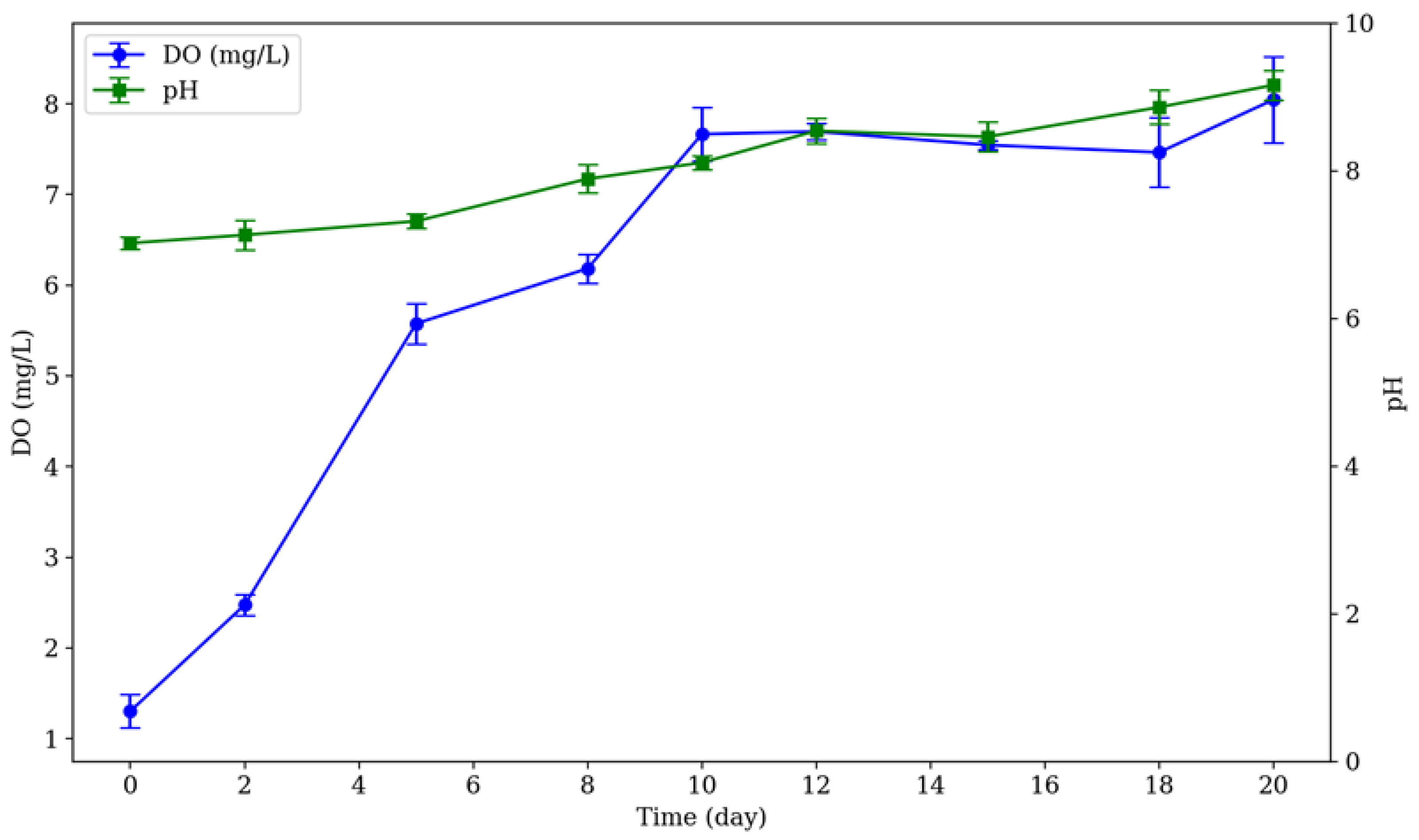
3.2. Dissolved Oxygen and pH Variation
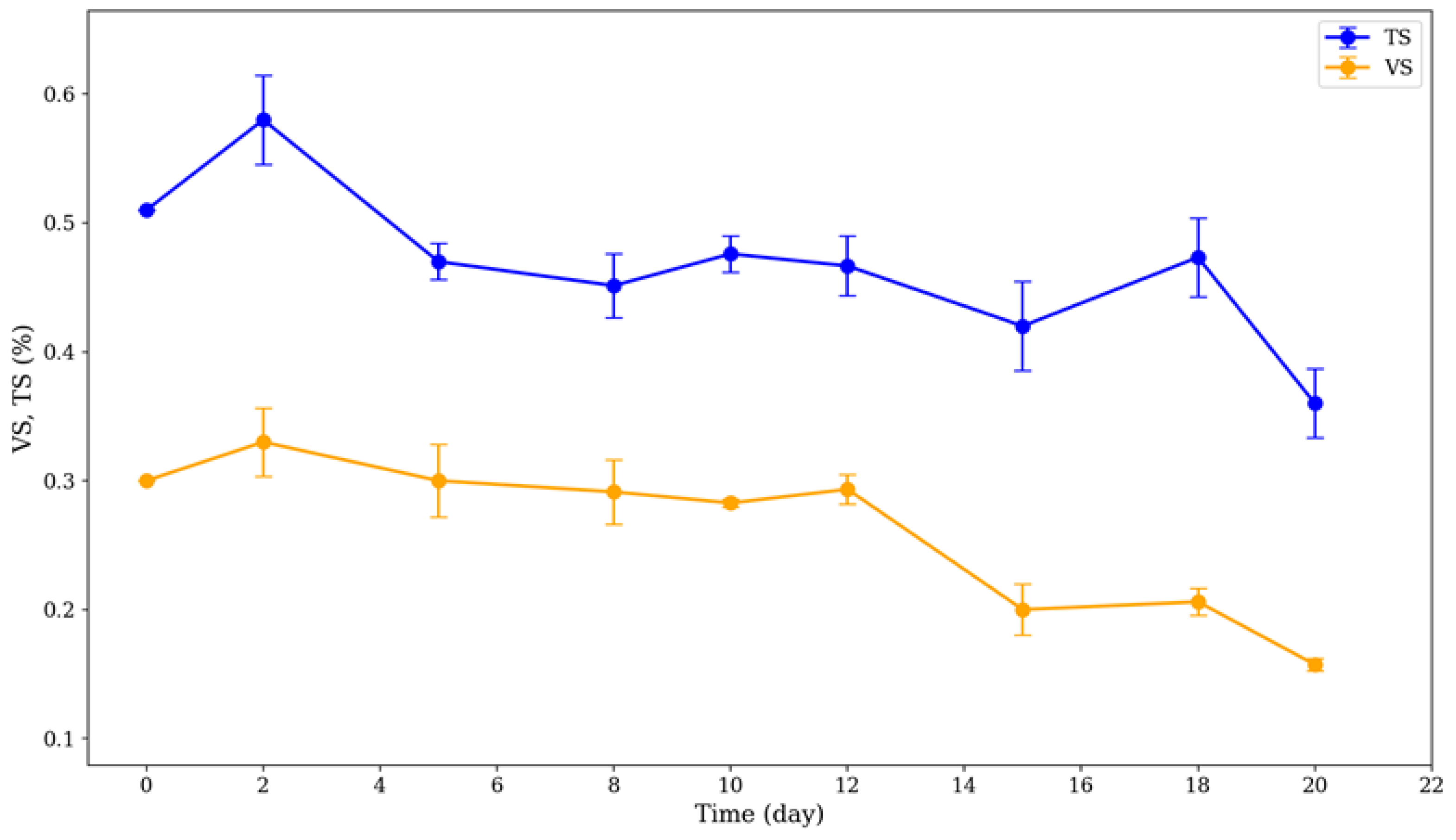
3.3. Volatile Solid and Total Solid Reduction
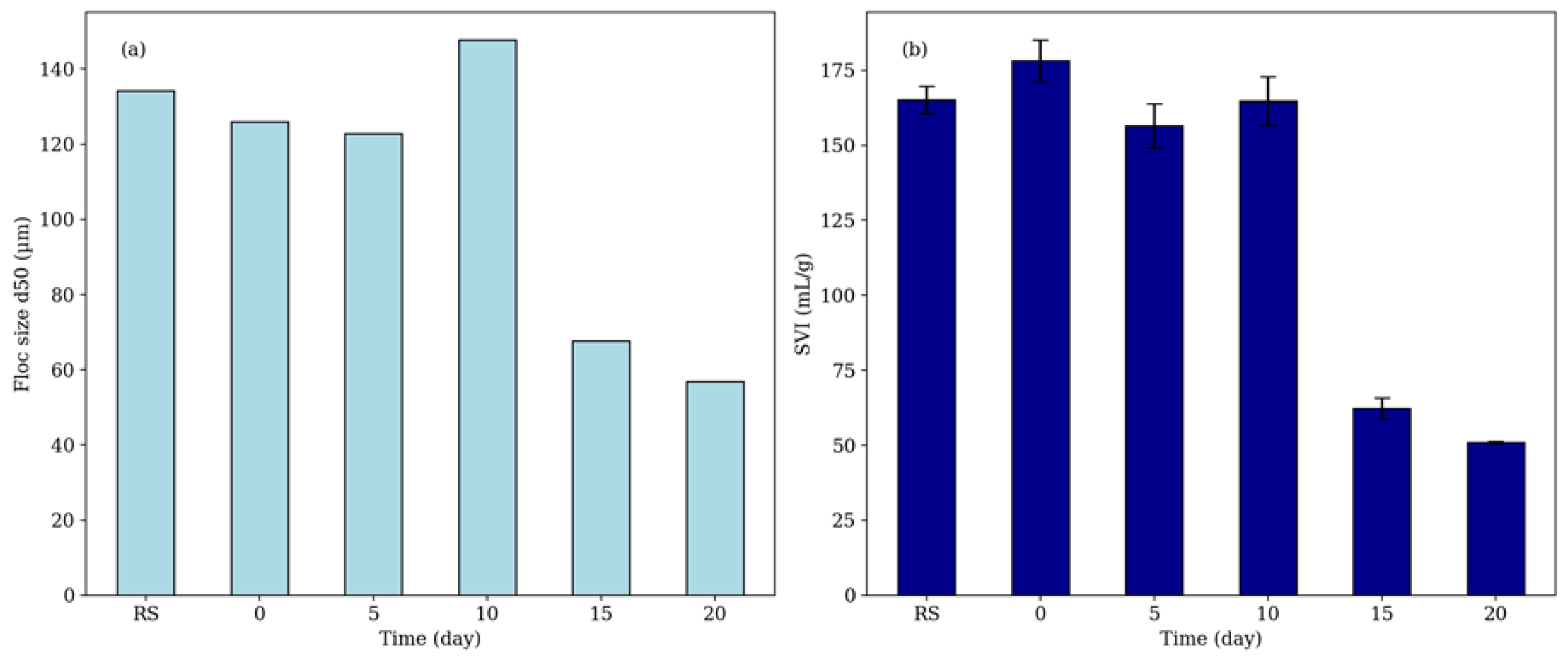
3.4. Effect of Treatment on Floc Size, Settling, and Filtration Properties
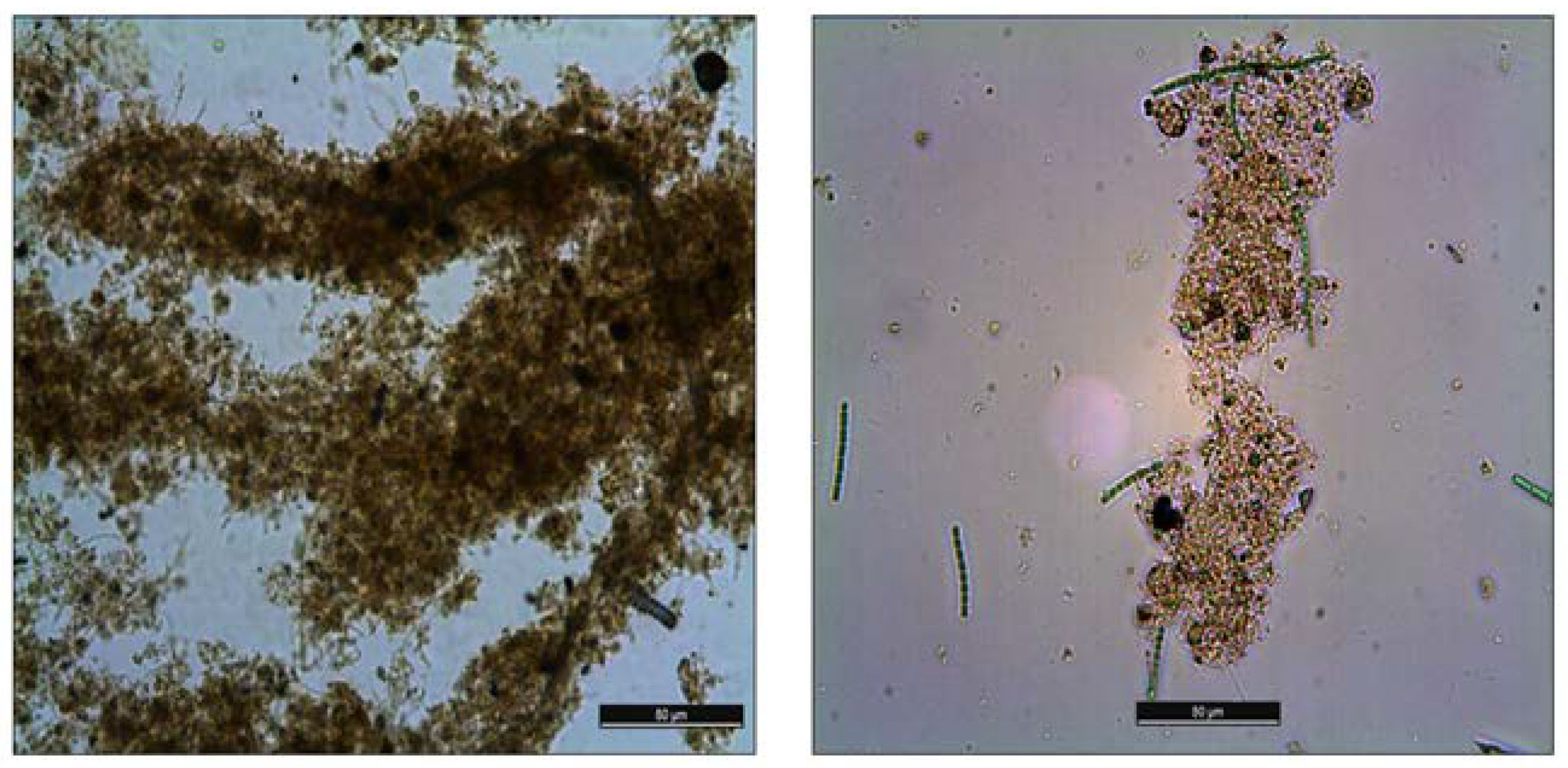
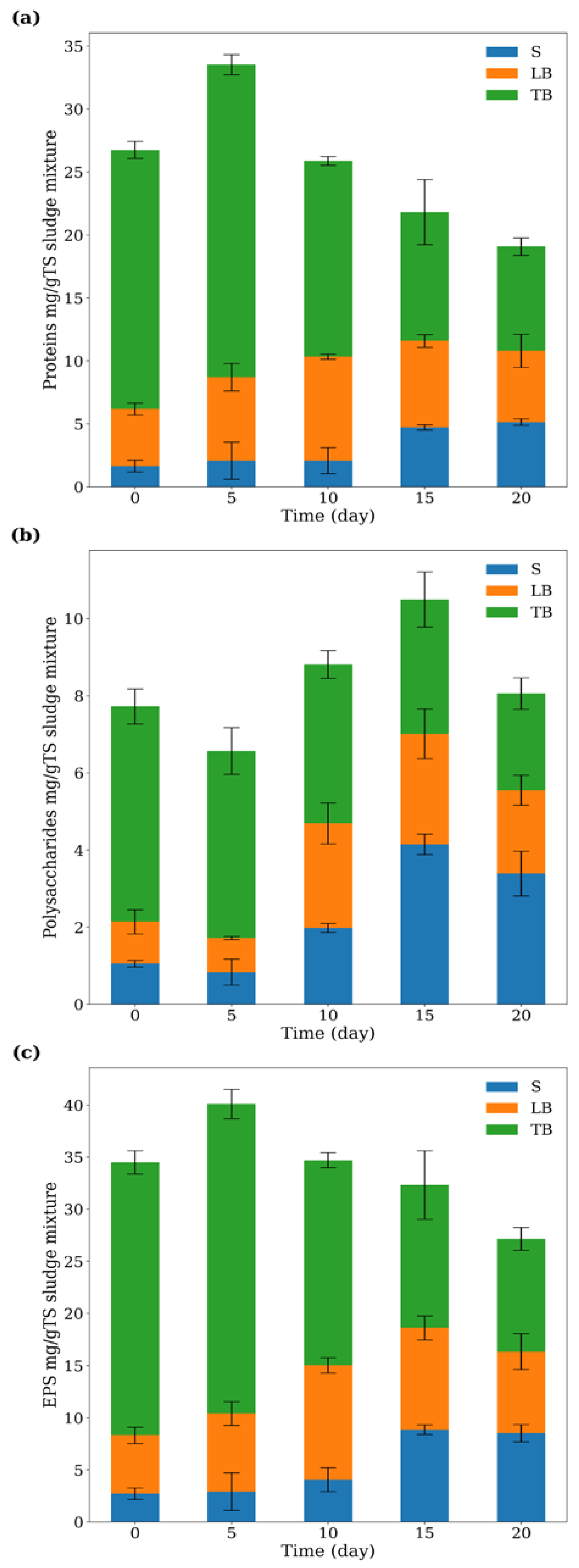
3.5. The Effect of Treatment on EPS Distribution
3.6. Pathogen Removal
| RS | 0 | 5 | 10 | 15 | 20 | LRV | % Reduction | |
|---|---|---|---|---|---|---|---|---|
| E. coli (LS) CFU/gTS | 7.49 × 106 ±3.83 × 105 | 5.23 × 106 ±9.24 × 105 | 9.81 × 105 ±1.67 × 104 | 3.52 × 103 ±3.32 × 102 | 6.46 ±1.29 × 101 | <10 UFC | 6.72 | 99.999 |
| E. coli (DS) CFU/gTS | 4.22 × 106 ± 5.12 × 105 | 3.61 × 106 ±4.47 × 105 | 4.57 × 105 ±2.73 × 104 | 2.04 × 103 ±6.77 × 102 | ND | <10 UFC | 6.56 | 99.999 |
| Fecal. Coliforms (LS) CFU/gTS | 8.67 × 107 ±4.01 × 106 | 2.48 × 107 ±3.57 × 106 | 3.04 × 106 ±1.70 × 105 | 1.63 × 105 ±1.70 × 104 | 1.56 × 105 ±2.94 × 104 | 1.39 × 105 ±7.07 × 104 | 2.25 | 99.44 |
| Fecal Coliforms (DS) CFU/gTS | 5.33 × 107 ±1.57 × 106 | 1.37 × 107 ±1.57 × 106 | 2.17 × 106 ±7.86 × 104 | 1.89 × 105 ±6.29 × 104 | ND | 2.22 × 104 ±1.11 × 104 | 2.79 | 99.84 |
| Treatment Day | LS | DS |
|---|---|---|
| 0 | + | + |
| 5 | + | + |
| 10 | + | + |
| 15 | − | ND |
| 20 | − | − |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kacprzak, M. Sewage sludge as a source of organic to be used as soil improvement. In Water Management and Circular Economy; Elsevier: Amsterdam, The Netherlands, 2023; pp. 303–316. ISBN 978-0-323-95280-4. [Google Scholar] [CrossRef]
- Silva, C.; Saldanha Matos, J.; Rosa, M.J. Performance indicators and indices of sludge management in urban wastewater treatment plants. J. Environ. Manag. 2016, 184, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Rorat, A.; Courtois, P.; Vandenbulcke, F.; Lemiere, S. Sanitary and environmental aspects of sewage sludge management. In Industrial and Municipal Sludge; Elsevier: Amsterdam, The Netherlands, 2019; pp. 155–180. ISBN 978-0-12-815907-1. [Google Scholar] [CrossRef]
- López, A.; Baguer, B.; Goñi, P.; Rubio, E.; Gómez, J.; Mosteo, R.; Ormad, M.P. Assessment of the methodologies used in microbiological control of sewage sludge. Waste Manag. 2019, 96, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Maw, M.M.; Boontanon, N.; Fujii, S.; Boontanon, S.K. Rapid and efficient removal of organic matter from sewage sludge for extraction of microplastics. Sci. Total Environ. 2022, 853, 158642. [Google Scholar] [CrossRef] [PubMed]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge—The current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Garrec, N.; Picard-Bonnaud, F.; Pourcher, A.M. Occurrence of Listeria sp. and L. monocytogenes in sewage sludge used for land application: Effect of dewatering, liming and storage in tank on survival of Listeria species. FEMS Immunol. Med. Microbiol. 2003, 35, 275–283. [Google Scholar] [CrossRef]
- Wéry, N.; Lhoutellier, C.; Ducray, F.; Delgenès, J.-P.; Godon, J.-J. Behaviour of pathogenic and indicator bacteria during urban wastewater treatment and sludge composting, as revealed by quantitative PCR. Water Res. 2008, 42, 53–62. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, T. Pathogenic Bacteria in Sewage Treatment Plants as Revealed by 454 Pyrosequencing. Environ. Sci. Technol. 2011, 45, 7173–7179. [Google Scholar] [CrossRef]
- Osuolale, O.; Okoh, A. Human enteric bacteria and viruses in five wastewater treatment plants in the Eastern Cape, South Africa. J. Infect. Public Health 2017, 10, 541–547. [Google Scholar] [CrossRef]
- Malcheva, B.Z.; Petrov, P.G.; Stefanova, V.V. Microbiological Control in Decontamination of Sludge from Wastewater Treatment Plant. Processes 2022, 10, 406. [Google Scholar] [CrossRef]
- Moynihan, E.L.; Richards, K.G.; Brennan, F.P.; Tyrrel, S.F.; Ritz, K. Enteropathogen survival in soil from different land-uses is predominantly regulated by microbial community composition. Appl. Soil Ecol. 2015, 89, 76–84. [Google Scholar] [CrossRef]
- Buchholz, U.; Bernard, H.; Werber, D.; Böhmer, M.M.; Remschmidt, C.; Wilking, H.; Deleré, Y.; An Der Heiden, M.; Adlhoch, C.; Dreesman, J.; et al. German Outbreak of Escherichia coli O104:H4 Associated with Sprouts. N. Engl. J. Med. 2011, 365, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Straub, T.M.; Pepper, I.L.; Gerba, C.P. Hazards from Pathogenic Microorganisms in Land-Disposed Sewage Sludge. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 1993; Volume 132, pp. 55–91. ISBN 978-1-4684-7067-3. [Google Scholar] [CrossRef]
- Dumontet, S.; Scopa, A.; Kerje, S.; Krovacek, K. The Importance of Pathogenic Organisms in Sewage and Sewage Sludge. J. Air Waste Manag. Assoc. 2001, 51, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Arthurson, V. Proper Sanitization of Sewage Sludge: A Critical Issue for a Sustainable Society. Appl. Environ. Microbiol. 2008, 74, 5267–5275. [Google Scholar] [CrossRef] [PubMed]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Mei, X.; Tang, J.; Zhang, Y. Sludge stabilization: Characteristics of the end-products and an alternative evaluative methodology. Waste Manag. 2020, 105, 355–363. [Google Scholar] [CrossRef]
- Gadaleta, G.; Todaro, F.; Giuliano, A.; De Gisi, S.; Notarnicola, M. Co-Treatment of Food Waste and Municipal Sewage Sludge: Technical and Environmental Review of Biological and Thermal Technologies. Clean Technol. 2024, 6, 852–885. [Google Scholar] [CrossRef]
- Hoang, S.A.; Bolan, N.; Madhubashani, A.M.P.; Vithanage, M.; Perera, V.; Wijesekara, H.; Wang, H.; Srivastava, P.; Kirkham, M.B.; Mickan, B.S.; et al. Treatment processes to eliminate potential environmental hazards and restore agronomic value of sewage sludge: A review. Environ. Pollut. 2022, 293, 118564. [Google Scholar] [CrossRef]
- Jin, N.; Jin, B.; Zhu, N.; Yuan, H.; Ruan, J. Disinhibition of excessive volatile fatty acids to improve the efficiency of autothermal thermophilic aerobic sludge digestion by chemical approach. Bioresour. Technol. 2015, 175, 120–127. [Google Scholar] [CrossRef]
- González, D.; Colón, J.; Gabriel, D.; Sánchez, A. The effect of the composting time on the gaseous emissions and the compost stability in a full-scale sewage sludge composting plant. Sci. Total Environ. 2019, 654, 311–323. [Google Scholar] [CrossRef]
- Akrivos, J.; Mamais, D.; Katsara, K.; Andreadakis, A. Agricultural utilisation of lime treated sewage sludge. Water Sci. Technol. 2000, 42, 203–210. [Google Scholar] [CrossRef]
- Sabbas, T.; Polettini, A.; Pomi, R.; Astrup, T.; Hjelmar, O.; Mostbauer, P.; Cappai, G.; Magel, G.; Salhofer, S.; Speiser, C.; et al. Management of municipal solid waste incineration residues. Waste Manag. 2003, 23, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, A.; Hajinajaf, N.; Tavakoli, O.; Sarrafzadeh, M.-H. Cultivation of Mixed Microalgae Using Municipal Wastewater: Biomass Productivity, Nutrient Removal, and Biochemical Content. Iran. J. Biotechnol. 2020, 18, e2586. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Shen, Q.; Chen, X.; Li, F.; Wang, J.; Zhang, Z.; Lei, Z.; Yuan, T.; Shimizu, K. Energy saving and rapid establishment of granular microalgae system from tiny microalgae cells: Effect of decrease in upflow air velocity under intermittent aeration condition. Bioresour. Technol. 2022, 363, 127860. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yuan, Q. Nitrogen removal in photo sequence batch reactor using algae-bacteria consortium. J. Water Process Eng. 2018, 26, 108–115. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- González-González, L.M.; de-Bashan, L.E. Toward the Enhancement of Microalgal Metabolite Production through Microalgae–Bacteria Consortia. Biology 2021, 10, 282. [Google Scholar] [CrossRef]
- Amaro, H.M.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.M.; Esteves, A.F. Microalgae systems—Environmental agents for wastewater treatment and further potential biomass valorisation. J. Environ. Manag. 2023, 337, 117678. [Google Scholar] [CrossRef]
- Slompo, N.D.M.; Quartaroli, L.; Fernandes, T.V.; da Silva, G.H.R.; Daniel, L.A. Nutrient and pathogen removal from anaerobically treated black water by microalgae. J. Environ. Manag. 2020, 268, 110693. [Google Scholar] [CrossRef]
- Ruas, G.; Serejo, M.L.; Farias, S.L.; Scarcelli, P.; Boncz, M.Á. Removal of pathogens from domestic wastewater by microalgal-bacterial systems under different cultivation conditions. Int. J. Environ. Sci. Technol. 2022, 19, 10177–10188. [Google Scholar] [CrossRef]
- Mezzari, M.P.; Prandini, J.M.; Deon Kich, J.; Busi Da Silva, M.L. Elimination of Antibiotic Multi-Resistant Salmonella Typhimurium from Swine Wastewater by Microalgae-Induced Antibacterial Mechanisms. J. Bioremediat. Biodegrad. 2017, 8, 379. [Google Scholar] [CrossRef]
- Lee, C.S.; Lee, S.-A.; Ko, S.-R.; Oh, H.-M.; Ahn, C.-Y. Effects of photoperiod on nutrient removal, biomass production, and algal-bacterial population dynamics in lab-scale photobioreactors treating municipal wastewater. Water Res. 2015, 68, 680–691. [Google Scholar] [CrossRef]
- CEN/TR 15214-1:2006; Characterization of Sludges—Detection and Enumeration of Escherichia coli in Sludges, Soils, Soil Improvers, Growing Media and Biowastes—Part 1: Membrane Filtration Method for Quantification. 2006. Available online: https://standards.iteh.ai/catalog/standards/cen/2d5b6aa1-d372-4501-9301-8879a42b4882/cen-tr-15214-1-2006?srsltid=AfmBOopY7NQPusgol-BAQeeFqqU9MKA8uKGzVQeNuMAM9PgzyACxIYgO (accessed on 7 October 2024).
- ISO 6579-1:2017; Microbiologie de la Chaîne Alimentaire—Méthode Horizontale Pour la Recherche, le Dénombrement et le Sérotypage des Salmonella—Partie 1: Recherche des Salmonella spp. 2017. Available online: https://www.iso.org/standard/56712.html (accessed on 7 October 2024).
- Faye, M.C.A.S.; Zhang, K.K.; Peng, S.; Zhang, Y. Sludge dewaterability: The variation of extracellular polymeric substances during sludge conditioning with two natural organic conditioners. J. Environ. Manag. 2019, 251, 109559. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. APHA Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2017. [Google Scholar]
- Guo, Z.; Tong, Y.W. The interactions between Chlorella vulgaris and algal symbiotic bacteria under photoautotrophic and photoheterotrophic conditions. J. Appl. Phycol. 2014, 26, 1483–1492. [Google Scholar] [CrossRef]
- Zhu, S.; Feng, S.; Xu, Z.; Qin, L.; Shang, C.; Feng, P.; Wang, Z.; Yuan, Z. Cultivation of Chlorella vulgaris on unsterilized dairy-derived liquid digestate for simultaneous biofuels feedstock production and pollutant removal. Bioresour. Technol. 2019, 285, 121353. [Google Scholar] [CrossRef]
- He, Q.; Chen, L.; Zhang, S.; Chen, R.; Wang, H.; Zhang, W.; Song, J. Natural sunlight induced rapid formation of water-born algal-bacterial granules in an aerobic bacterial granular photo-sequencing batch reactor. J. Hazard. Mater. 2018, 359, 222–230. [Google Scholar] [CrossRef]
- Zhu, S.; Qin, L.; Feng, P.; Shang, C.; Wang, Z.; Yuan, Z. Treatment of low C/N ratio wastewater and biomass production using co-culture of Chlorella vulgaris and activated sludge in a batch photobioreactor. Bioresour. Technol. 2019, 274, 313–320. [Google Scholar] [CrossRef]
- Morales, M.; Sánchez, L.; Revah, S. The impact of environmental factors on carbon dioxide fixation by microalgae. FEMS Microbiol. Lett. 2018, 365, fnx262. [Google Scholar] [CrossRef]
- Shao, L.; Tianfeng, W.; Tianshui, L.; Fan, L.; Pinjing, H. Comparison of Sludge Digestion under Aerobic and Anaerobic Conditions with a Focus on the Degradation of Proteins at Mesophilic Temperature. Bioresour. Technol. 2013, 140, 131–137. [Google Scholar] [CrossRef]
- Cárdenas-Talero, J.L.; Silva-Leal, J.A.; Pérez-Vidal, A.; Torres-Lozada, P. The Influence of Municipal Wastewater Treatment Technologies on the Biological Stabilization of Sewage Sludge: A Systematic Review. Sustainability 2022, 14, 5910. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, W.; Liu, S.; Yan, M.; Song, K.; Mai, J.; Sun, J.; Ni, B.-J.; Gong, Y. Free Ammonia Pretreatment Improves Degradation of Secondary Sludge during Aerobic Digestion. ACS Sustain. Chem. Eng. 2018, 6, 1105–1111. [Google Scholar] [CrossRef]
- Duan, H.; Ye, L.; Lu, X.; Batstone, D.J.; Yuan, Z. Self-Sustained Nitrite Accumulation at Low pH Greatly Enhances Volatile Solids Destruction and Nitrogen Removal in Aerobic Sludge Digestion. Environ. Sci. Technol. 2019, 53, 1225–1234. [Google Scholar] [CrossRef]
- Azizi, S.; Kamika, I.; Tekere, M. Evaluation of the Digestibility of Attached and Suspended Growth Sludge in an Aerobic Digester for a Small Community. Water 2018, 10, 161. [Google Scholar] [CrossRef]
- Bernard, S. Aerobic digestion of pharmaceutical and domestic wastewater sludges at ambient temperature. Water Res. 2000, 34, 725–734. [Google Scholar] [CrossRef]
- d’Antonio, G. Aerobic digestion of thickened activated sludge. Water Res. 1983, 17, 1525–1531. [Google Scholar] [CrossRef]
- Murthy, S.N.; Novak, J.T. Factors Affecting Floc Properties during Aerobic Digestion: Implications for Dewatering. Water Environ. Res. 1999, 71, 197–202. [Google Scholar] [CrossRef]
- Novak, J.T.; Sadler, M.E.; Murthy, S.N. Mechanisms of floc destruction during anaerobic and aerobic digestion and the effect on conditioning and dewatering of biosolids. Water Res. 2003, 37, 3136–3144. [Google Scholar] [CrossRef]
- Bellinger, E.G.; Sigee, D.C. Freshwater Algae: Identification, Enumeration and Use as Bioindicators, 2nd ed.; John Wiley & Sons Inc.: Chichester, UK, 2015; ISBN 978-1-118-91713-8. [Google Scholar]
- Cui, H.; Yang, S.-S.; Pang, J.-W.; Mi, H.-R.; Nuer, C.-C.; Ding, J. An improved ASM-GDA approach to evaluate the production kinetics of loosely bound and tightly bound extracellular polymeric substances in biological phosphorus removal process. RSC Adv. 2020, 10, 2495–2506. [Google Scholar] [CrossRef]
- Yuan, D.Q.; Wang, Y.L.; Feng, J. Contribution of stratified extracellular polymeric substances to the gel-like and fractal structures of activated sludge. Water Res. 2014, 56, 56–65. [Google Scholar] [CrossRef]
- Huang, L.; Jin, Y.; Zhou, D.; Liu, L.; Huang, S.; Zhao, Y.; Chen, Y. A Review of the Role of Extracellular Polymeric Substances (EPS) in Wastewater Treatment Systems. Int. J. Environ. Res. Public Health 2022, 19, 12191. [Google Scholar] [CrossRef]
- Deng, S.; Wang, L.; Su, H. Role and influence of extracellular polymeric substances on the preparation of aerobic granular sludge. J. Environ. Manag. 2016, 173, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Ye, Y.; Li, Y. Effect of C/N ratio on extracellular polymeric substances (EPS) and physicochemical properties of activated sludge flocs. J. Hazard. Mater. 2011, 188, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Komolafe, O.; Velasquez Orta, S.B.; Monje-Ramirez, I.; Noguez, I.Y.; Harvey, A.P.; Orta Ledesma, M.T. Biodiesel production from indigenous microalgae grown in wastewater. Bioresour. Technol. 2014, 154, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Marchello, A.E.; Lombardi, A.T.; Dellamano-Oliveira, M.J.; Souza, C.W.O.D. Microalgae population dynamics in photobioreactors with secondary sewage effluent as culture medium. Braz. J. Microbiol. 2015, 46, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef]
- Ansa, E.D.O.; Lubberding, H.J.; Gijzen, H.J. The effect of algal biomass on the removal of faecal coliform from domestic wastewater. Appl. Water Sci. 2012, 2, 87–94. [Google Scholar] [CrossRef]
- Falaise, C.; François, C.; Travers, M.-A.; Morga, B.; Haure, J.; Tremblay, R.; Turcotte, F.; Pasetto, P.; Gastineau, R.; Hardivillier, Y.; et al. Antimicrobial Compounds from Eukaryotic Microalgae against Human Pathogens and Diseases in Aquaculture. Mar. Drugs 2016, 14, 159. [Google Scholar] [CrossRef]
- Inuwa, A.B.; Pervez, A.; Nazir, R. Microalgae-based wastewater treatment system: Current state, antibiotic resistant bacteria and antibiotic resistance genes reduction potentials. Int. J. Environ. Sci. Technol. 2023, 20, 14053–14072. [Google Scholar] [CrossRef]
- Rani, S.; Chowdhury, R.; Tao, W.; Nedbalová, L. Microalga-Mediated Tertiary Treatment of Municipal Wastewater: Removal of Nutrients and Pathogens. Sustainability 2021, 13, 9554. [Google Scholar] [CrossRef]


| Parameter | Raw Sludge | Treated Wastewater |
|---|---|---|
| pH | 7.05 ± 0.03 | 7.7 ± 0.70 |
| Conductivity (µs/cm) | 281.33 ± 3.78 | - |
| TS (g/L) | 6.16 ± 0.55 | - |
| VS (g/L) | 3.93 ± 0.58 | - |
| TSS (g/L) | 5.54 ± 0.14 | 0.004 ± 0.002 |
| VSS (g/L) | 3.59 ± 0.05 | - |
| DCO (mgO2/L) | - | 17.1 ± 4.10 |
| Fecal coliforms (CFU/gTS) | 8.67 × 107 ± 4.01 × 106 | - |
| E. coli (CFU/gTS) | 7.49 × 106 ± 3.83 × 105 | - |
| Salmonella spp. (in 25 gTS) | Present | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Hamed, H.; Debuigne, A.; Kleinjan, H.; Toye, D.; Léonard, A. Enhancing Sewage Sludge Stabilization, Pathogen Removal, and Biomass Production through Indigenous Microalgae Promoting Growth: A Sustainable Approach for Sewage Sludge Treatment. Recycling 2024, 9, 97. https://doi.org/10.3390/recycling9050097
Ben Hamed H, Debuigne A, Kleinjan H, Toye D, Léonard A. Enhancing Sewage Sludge Stabilization, Pathogen Removal, and Biomass Production through Indigenous Microalgae Promoting Growth: A Sustainable Approach for Sewage Sludge Treatment. Recycling. 2024; 9(5):97. https://doi.org/10.3390/recycling9050097
Chicago/Turabian StyleBen Hamed, Hajer, Antoine Debuigne, Hetty Kleinjan, Dominique Toye, and Angélique Léonard. 2024. "Enhancing Sewage Sludge Stabilization, Pathogen Removal, and Biomass Production through Indigenous Microalgae Promoting Growth: A Sustainable Approach for Sewage Sludge Treatment" Recycling 9, no. 5: 97. https://doi.org/10.3390/recycling9050097
APA StyleBen Hamed, H., Debuigne, A., Kleinjan, H., Toye, D., & Léonard, A. (2024). Enhancing Sewage Sludge Stabilization, Pathogen Removal, and Biomass Production through Indigenous Microalgae Promoting Growth: A Sustainable Approach for Sewage Sludge Treatment. Recycling, 9(5), 97. https://doi.org/10.3390/recycling9050097









