Acerola (Malpighia spp.) Waste: A Sustainable Approach to Nutraceutical, Pharmaceutical, and Energy Applications
Abstract
1. Introduction
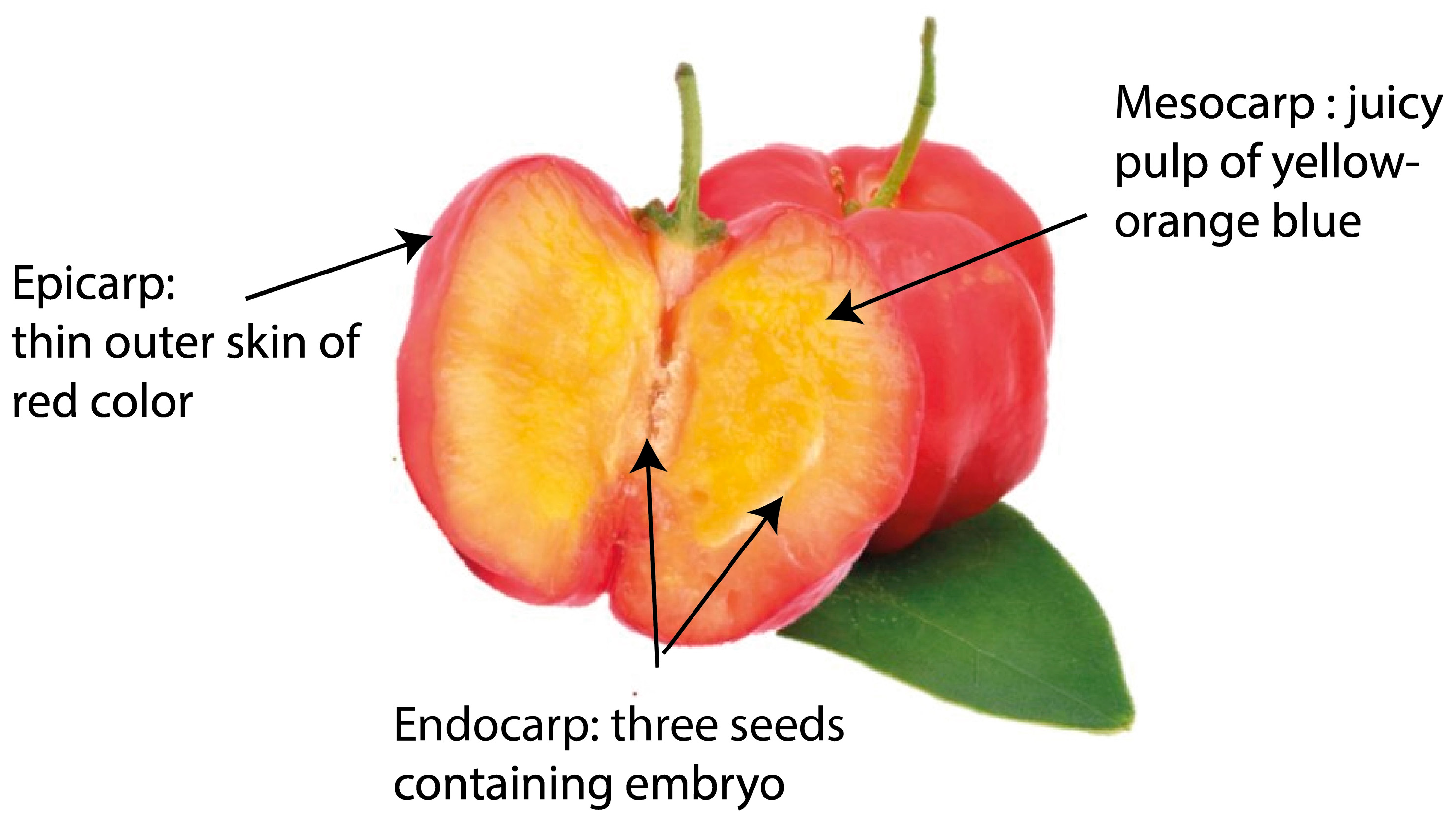
Acerola Plant and Fruit
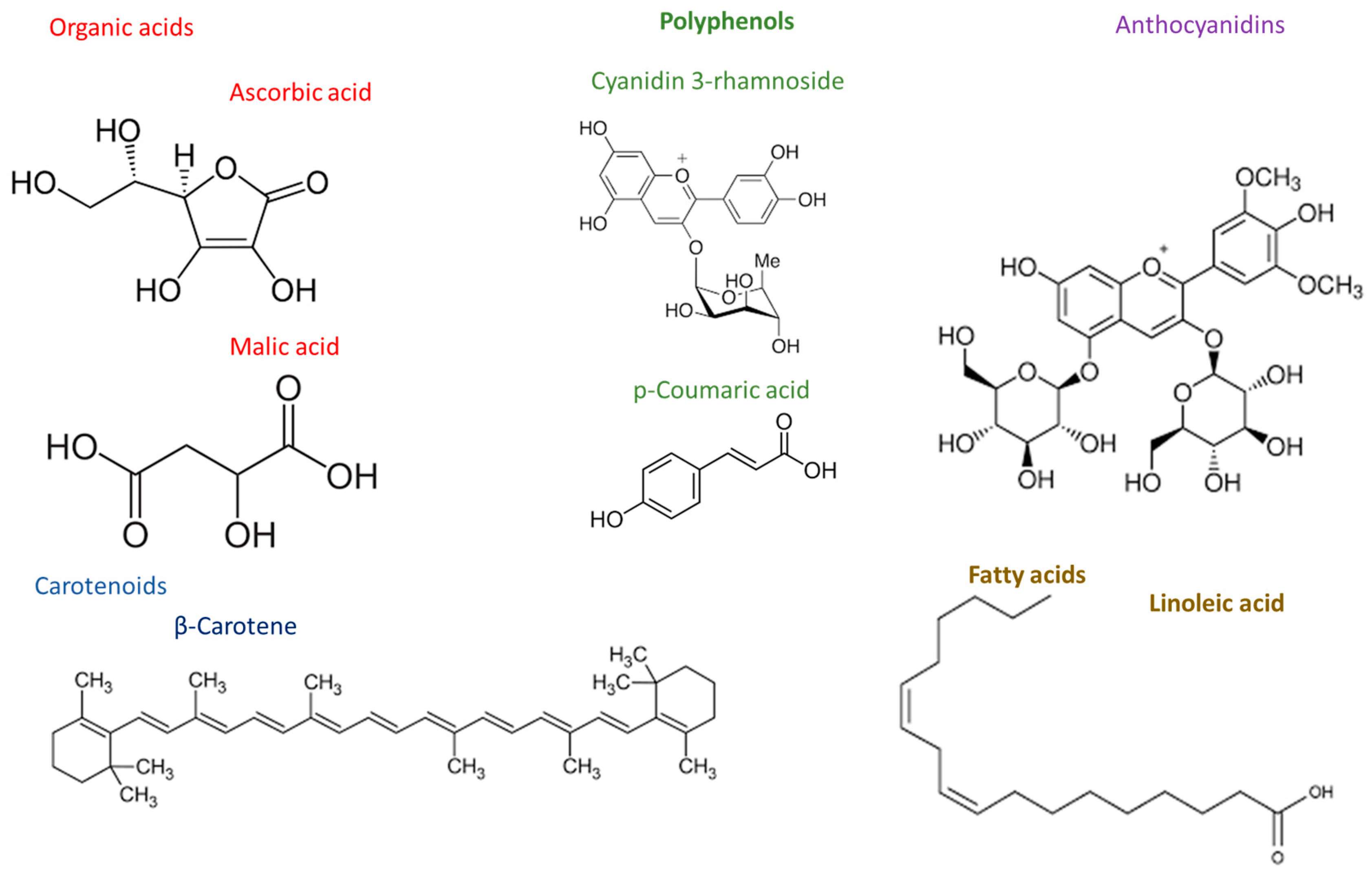
2. Chemical Composition and Characterization of Acerola Wastes
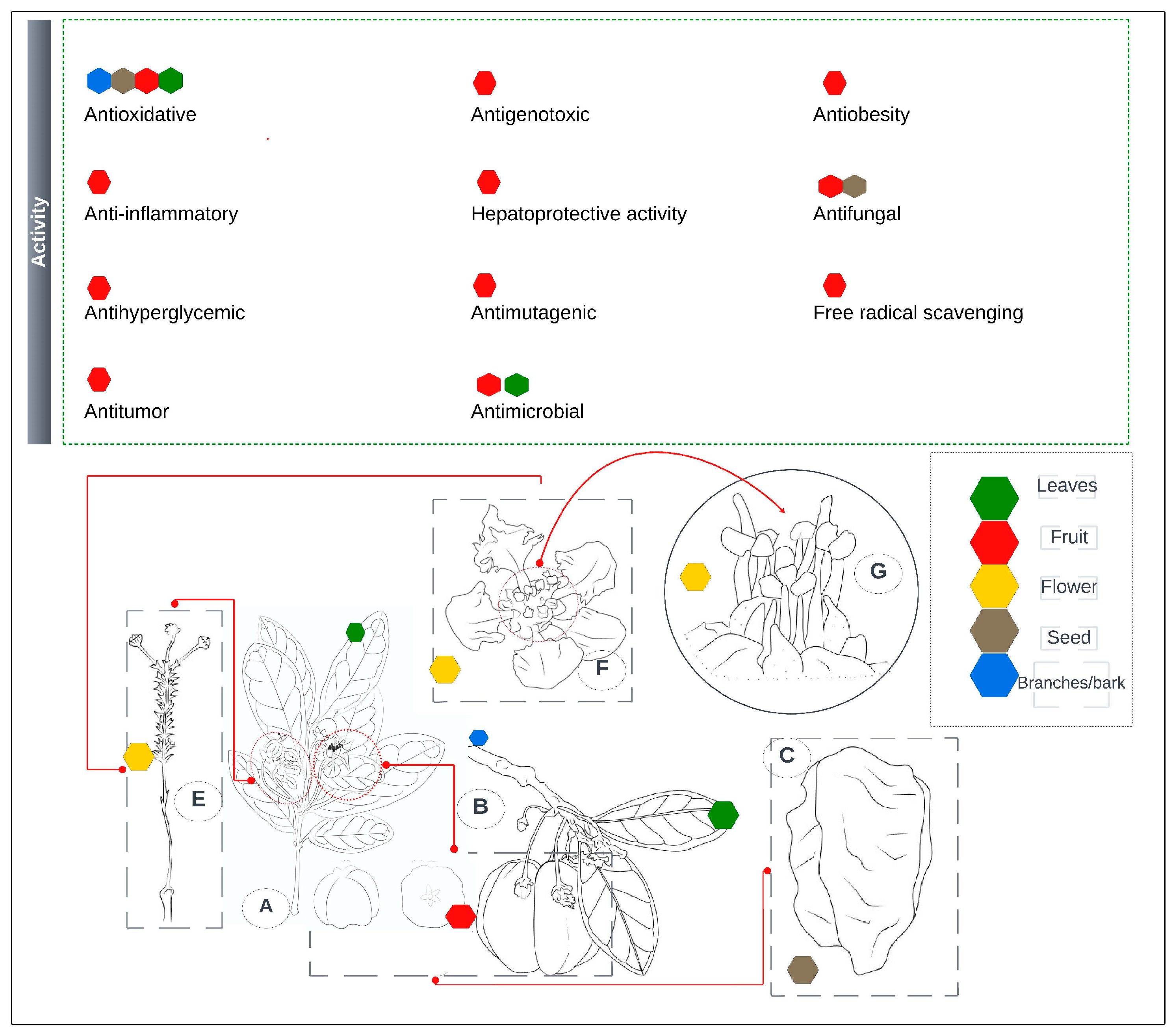
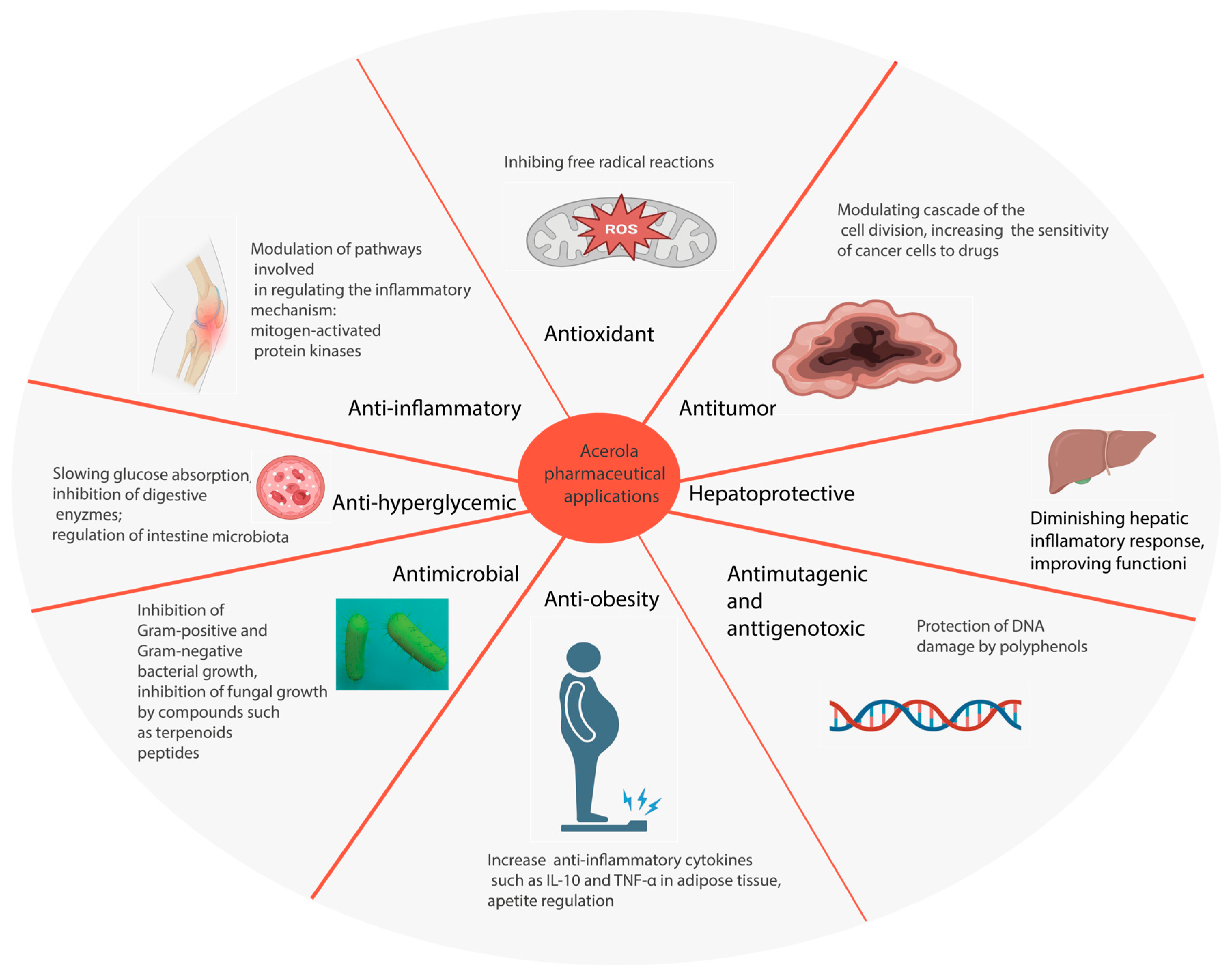
3. Pharmaceutical and Nutraceutical Applications
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Activity
3.3. Antihyperglycemic Activity
3.4. Antitumor Activity
3.5. Antigenotoxic Activity
3.6. Hepatoprotective Activity
3.7. Antimutagenic Effect
3.8. Antibacterial Activity
3.9. Antiobesity
3.10. Antifungal Activity
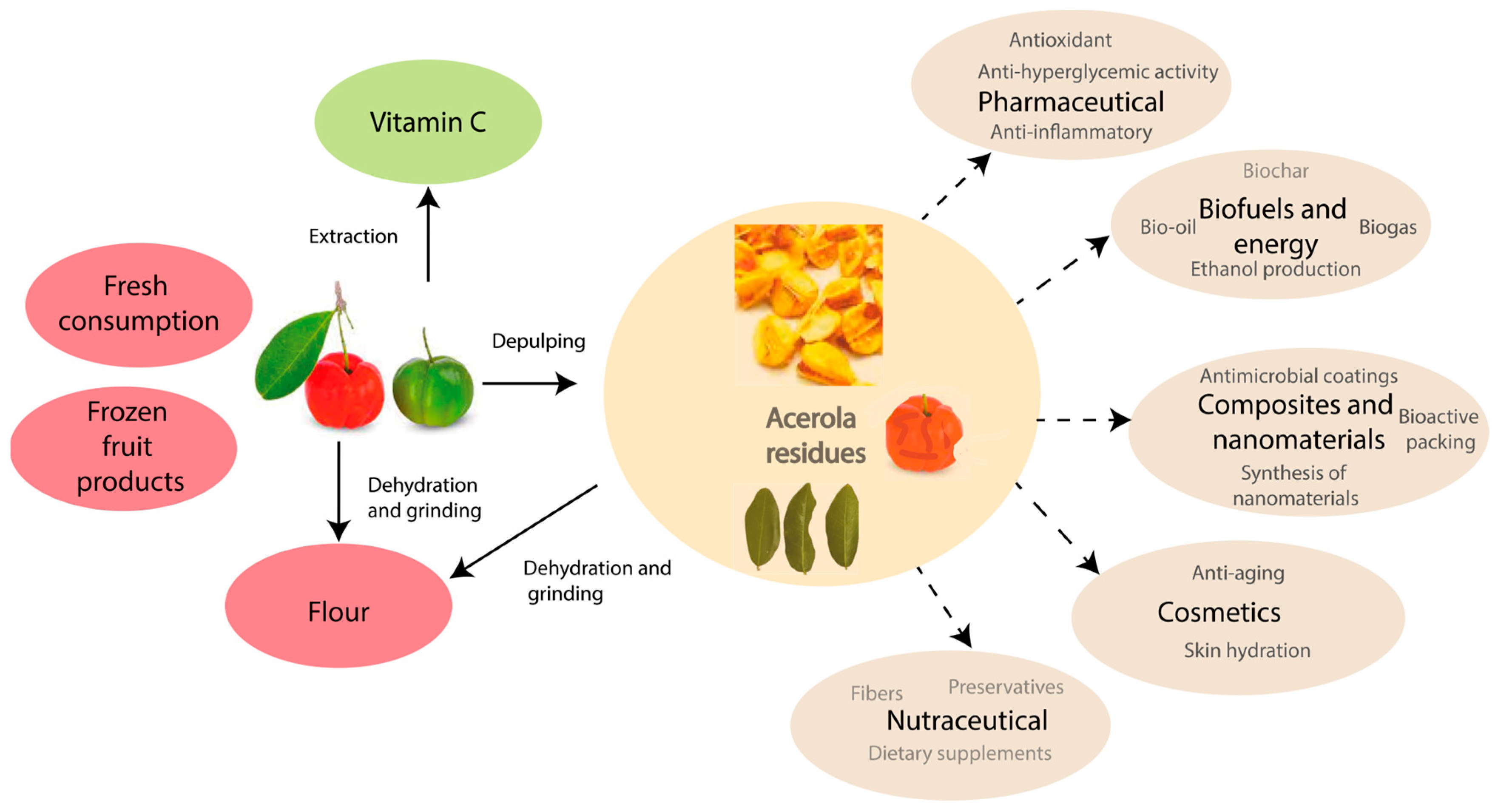
4. Nutraceutical
5. Cosmetics
6. Biofuels and Energy Applications
7. Composites and Nanomaterials
8. Waste Processing
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teixeira, B.F.; Aranha, J.B.; De Souza Vieira, T.M.F. Replacing synthetic antioxidants in food emulsions with microparticles from green acerola. Future Foods 2022, 5, 100130. [Google Scholar] [CrossRef]
- Yahoo Finance. Available online: https://finance.yahoo.com/news/acerola-extract-market-exceed-us-013000299.html (accessed on 8 November 2023).
- Ramadan, L.; Duarte, C.R.; Barrozo, M. New Hybrid System for Reuse of Agro-industrial Wastes of Acerola: Dehydration and Fluid Dynamic Analysis. Waste Biomass Valorization 2018, 10, 2273–2283. [Google Scholar] [CrossRef]
- Leonarski, E.; Guimarães, A.C.; Cesca, K.; Poletto, P. Production process and characteristics of kombucha fermented from alternative raw materials. Food Biosci. 2022, 49, 101841. [Google Scholar] [CrossRef]
- IBGE. 2017—Produção de Acerola. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/acerola/br (accessed on 14 August 2023).
- Carneiro, I.; Pereira, V.; Claudio, J.; de França, F.; Tonetto, S.; dos Santos, M. Brazilian varieties of acerola (Malpighia emarginata DC) produced under tropical semi-arid conditions: Bioactive phenolic compounds, sugars, organic acids, and antioxidant capacity. J. Food Biochem. 2021, 45, e13829. [Google Scholar]
- Prakash, A.; Baskaran, R. Acerola, an untapped functional superfruit: A review on latest frontiers. J. Food Sci. Technol. 2018, 55, 3373–3384. [Google Scholar] [CrossRef]
- Silva, P.B.; Nogueira, G.D.; Duarte, C.R.; Barrozo, M.A. A New Rotary Dryer Assisted by Infrared Radiation for Drying of Acerola Residues. Waste Biomass Valorization 2021, 12, 3395–3406. [Google Scholar] [CrossRef]
- Duzzioni, A.G.; Lenton, V.M.; Silva, D.I.S.; Barrozo, M.A.S. Effect of drying kinetics on main bioactive compounds and antioxidant activity of acerola (Malpighia emarginata D.C.) residue. Int. J. Food Sci. Technol. 2013, 48, 1041–1047. [Google Scholar] [CrossRef]
- Ellong, E.; Billard, C.; Adenet, S.; Rochefort, K. Polyphenols, Carotenoids, Vitamin C Content in Tropical Fruits and Vegetables and Impact of Processing Methods. Food Nutr. Sci. 2015, 6, 299–313. [Google Scholar] [CrossRef]
- Silva, V.; Da Silva, M.; Da Silva, R.; Ritcher, M.; Falcao, A.; Da Silva, J. A influência da origem geografica de amostras de acerola (Malpighia glabra L.) em relação ao seu potencial genotóxico e antigenotóxico. Rev. Inic. Cient. ULBRA 2008, 7, 37–45. [Google Scholar]
- Bortolotti, C.T.; Santos, K.G.; Francisquetti, M.C.; Duarte, C.R.; Barrozo, M.A. Hydrodynamic study of a mixture of West Indian Cherry Residue and Soybean Grains in a spouted bed. Can. J. Chem. Eng. 2013, 91, 1871–1880. [Google Scholar] [CrossRef]
- Souza, R.; do Nascimento, L.; Colussi, R. Bioactive compounds from acerola pomace: A review. Food Chem. 2023, 404, 134613. [Google Scholar]
- Mezadri, T.; Villaño, D.; Fernández, M.; García, M.; Troncoso, A. Antioxidant compounds and antioxidant activity in acerola (Malpighia emarginata DC.) fruits and derivatives. J. Food Compos. Anal. 2008, 21, 282–290. [Google Scholar] [CrossRef]
- Franco, M.; Belorio, M.; Gómez, M. Assessing Acerola Powder as Substitute for Ascorbic Acid as a Bread Improver. Foods 2022, 11, 1366. [Google Scholar] [CrossRef]
- Belwal, T.; Prasad, H.; Hassan, H.; Ahluwalia, S.; Fawzy, M.; Mocan, A.; Atanasov, A. Phytopharmacology of acerola (Malpighia spp.) and its potential as functional food. Trends Food Sci. Technol. 2018, 74, 99–106. [Google Scholar] [CrossRef]
- De Assis, S.; Fernandes, P.; Martins, A.; Faria, O. Acerola: Importance, culture conditions, production, and biochemical aspects. Fruits 2008, 63, 93–101. [Google Scholar] [CrossRef][Green Version]
- Asenjo, C.F.; Freire De Guzmán, A.R. The high ascorbic acid content of the West Indian cherry. Science 1946, 103, 219. [Google Scholar] [CrossRef]
- Farinelli, D.; Portarena, S.; Da Silva, D.F.; Traini, C.; Da Silva, G.M.; Da Silva, E.C.; Da Veiga, J.F.; Pollegioni, P.; Villa, F. Variability of Fruit Quality among 103 Acerola (Malpighia emarginata D.C.) Phenotypes from the Subtropical Region of Brazil. Agriculture 2021, 11, 1078. [Google Scholar] [CrossRef]
- Woodson, R.E.; Schery, R.W.; Cuatrecasas, J.; Croat, T.; Vivaldi, J. Flora of Panama. Part VI. Family 93. Malpighiaceae. Ann. Mo. Bot. Gard. 1980, 67, 851–945. [Google Scholar] [CrossRef]
- Brücher, H. Useful Plants of Neotropical Origin: And Their Wild Relatives; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar] [CrossRef]
- Barros, B.; Barboza, B.; Ramos, B.; de Moura, M.; Coelho, L.; Napoleao, T.; Correia, T.; Paiva, P.; da Cruz, I.; Da Silva, T.; et al. Saline Extract from Malpighia emarginata DC Leaves Showed Higher Polyphenol Presence, Antioxidant and Antifungal Activity and Promoted Cell Proliferation in Mice Splenocytes. An. Acad. Bras. Ciências 2019, 91, 1–15. [Google Scholar] [CrossRef]
- Guedes, T.; Rajan, M.; Barbosa, P.; Silva, E.; Machado, T.; Narain, N. Phytochemical Composition and Antioxidant Potential of Different Varieties viz. Flor Branca, Costa Rica and Junco of Green Unripe Acerola (Malphigia emarginata D.C.) Fruits. Food Sci. Technol. 2022, 42, e46320. [Google Scholar] [CrossRef]
- El-Hawary, S.; Mousa, O.; Ahmed, R.; El Gedaily, R. Cytotoxic, Antimicrobial Activities, and Phytochemical Investigation of Three Peach Cultivars and Acerola Leaves. J. Rep. Pharm. Sci. 2022, 9, 221–234. [Google Scholar]
- Da Silva, R.; Silva, V.; Da Silva, M.; Ritcher, M.; Abin, J.; Martínez, M.; Falcão, A.; Da Silva, J. Genotoxic and Antigenotoxic Activity of Acerola (Malpighia glabra L.) Extract in Relation to the Geographic Origin. Phytother. Res. 2013, 27, 1495–1501. [Google Scholar] [CrossRef]
- Sato, Y.; Uchida, E.; Aoki, H.; Hanamura, T.; Nagamine, K.; Kato, H.; Koizumi, T.; Ishigami, A. Acerola (Malpighia emarginata DC.) Juice Intake Suppresses UVB-Induced Skin Pigmentation in SMP30/GNL Knockout Hairless Mice. PLoS ONE 2017, 12, e0170438. [Google Scholar] [CrossRef]
- Gomes, N.; Mota, E.; Sousa, D.; Freitas, C.; de Oliveira, M.; Marinho, A.; Alcântara, M.; Fernandes, D. Effect of the Pretreatment with Acerola (Malpighia emarginata DC.) Juice on Ethanol-Induced Oxidative Stress in Mice e Hepato-protective Potential of Acerola Juice. Free Radic. Antioxid. 2013, 3, 16–21. [Google Scholar]
- Stafussa, A.P.; Maciel, G.M.; Rampazzo, V.; Bona, E.; Makara, C.N.; Junior, B.D.; Haminiuk, C.W.I. Bioactive compounds of 44 traditional and exotic Brazilian fruit pulps: Phenolic compounds and antioxidant activity. Int. J. Food Prop. 2018, 21, 106–118. [Google Scholar] [CrossRef]
- Pedro, R.E.R.; Dos Santos Ricardo, C.; De Melo Filho Antonio, A.; Ricardo, S.A.; Jacqueline, A.T.; Selvin, A.S.M.; Edvan, A.C.; Ismael, M.F.; Jhunior, A.M.F.; Pollyana, C.C.; et al. Antimicrobial activity and acetilcolinesterase inhibition of oils and Amazon fruit extracts. J. Med. Plants Res. 2020, 14, 88–97. [Google Scholar] [CrossRef]
- Cruz, R.G.D.; Beney, L.; Gervais, P.; Lira, S.P.D.; Vieira, T.M.F.D.S.; Dupont, S. Comparison of the antioxidant property of acerola extracts with synthetic antioxidants using an in vivo method with yeasts. Food Chem. 2019, 277, 698–705. [Google Scholar] [CrossRef]
- Hoang, Q.B.; Pham, N.T.; Le, T.T.; Duong, T.N.D. Bioactive compounds and strategy processing for acerola: A review. Can Tho Univ. J. Sci. 2022, 14, 46–60. [Google Scholar] [CrossRef]
- Oliveira, C.; Andrade, J.; Rajan, M.; Narain, N. Influence of the Phytochemical Profile on the Peel, Seed and Pulp of Margarida, Breda and Geada Varieties of Avocado (Persea americana Mill) Associated with Their Antioxidant Potential. Food Sci. Technol. 2022, 42, e25822. [Google Scholar] [CrossRef]
- Vasavilbazo, A.; Almaraz, N.; González, H.; Ávila, J.; González, A.; Delgado, E.; Torres, R. Caracterización Fitoquímica y Propiedades Antioxidantes de la Acerola Silvestre Malpighia umbellata Rose. CyTA J. Food 2018, 16, 698–706. [Google Scholar] [CrossRef]
- Delva, L.; Goodrich, R. Antioxidant Activity and Antimicrobial Properties of Phenolic Extracts from Acerola (Malpighia emarginata DC) Fruit. Int. J. Food Sci. Technol. 2013, 48, 1048–1056. [Google Scholar] [CrossRef]
- Garcia, V.; Borges, J.; Chagas, E.; Yoshida, C.; Vanin, F.; Laurindo, J.; Carvalho, R. Production and Characterization of Dehydrated Acerola Pulp: A Comparative Study of Freeze and Refractance Window Drying. Food Sci. Eng. 2022, 4, 20–29. [Google Scholar] [CrossRef]
- Delva, L.; Goodrich, R. Acerola (Malpighia emarginata DC): Production, Postharvest Handling, Nutrition, and Biological Activity. Food Rev. Int. 2013, 29, 107–126. [Google Scholar] [CrossRef]
- Chang, S.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, Antioxidant Efficacies, and Health Effects—A Com-prehensive Review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1580–1604. [Google Scholar] [CrossRef]
- Poletto, P.; Álvarez-Rivera, G.; López, G.-D.; Borges, O.M.A.; Mendiola, J.A.; Ibáñez, E.; Cifuentes, A. Recovery of ascorbic acid, phenolic compounds and carotenoids from acerola by-products: An opportunity for their valorization. LWT 2021, 146, 111654. [Google Scholar] [CrossRef]
- Gualberto, N.C.; Oliveira, C.S.; Nogueira, J.P.; Jesus, M.S.; Araujo, H.C.S.; Rajan, M.; Neta, M.T.S.L.; Narain, N. Bioactive Compounds and Antioxidant Activities in the Agro-Industrial Residues of Acerola (Malpighia emarginata L.), Guava (Psidium guajava L.), Genipap (Genipa americana L.) and Umbu (Spondias tuberosa L.) Fruits Assisted by Ultrasonic or Shaker Extraction. Food Res. Int. 2021, 147, 110538. [Google Scholar] [CrossRef]
- Van Le, H.; Viet, V. Comparison of enzyme-assisted and ultrasound-assisted extraction of vitamin C and phenolic compounds from acerola (Malpighia emarginata DC.) fruit. Int. J. Food Sci. Technol. 2012, 47, 1206–1214. [Google Scholar] [CrossRef]
- Righetto, A.M.; Netto, F.M.; Carraro, F. Chemical Composition and Antioxidant Activity of Juices from Mature and Immature Acerola (Malpighia emarginata DC). Food Sci. Technol. Int. 2005, 11, 315–321. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef]
- Hrelia, S.; Angeloni, C.; Barbalace, M.C. Agri-Food Wastes as Natural Source of Bioactive Antioxidants. Antioxidants 2023, 12, 351. [Google Scholar] [CrossRef]
- Teshome, E.; Forsido, S.F.; Vasantha Rupasinghe, H.P.; Keyata, E.O. Potentials of Natural Preservatives to Enhance Food Safety and Shelf Life: A Review. Sci. World J. 2021, 2022, 1–11. [Google Scholar] [CrossRef]
- Corrêa, C.V.; Gouveia, A.M.S.; Martins, B.N.M.; Jorge, L.G.; Lanna, N.B.L.; Tavares, A.E.B.; Mendonça, V.Z.; Evangelista, R.M. Influence of ripening stages on physicochemical characteristics of acerola fruits. Rev. De Ciências Agrárias 2017, 40, 808–813. [Google Scholar] [CrossRef]
- Vendramini, A.; Trugo, L. Chemical composition of acerola fruit (Malpighia punicifolia L.) at three stages of maturity. Food Chem. 2000, 71, 195–198. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- Câmara, J.S.; Locatelli, M.; Pereira, J.A.M.; Oliveira, H.; Arlorio, M.; Fernandes, I.; Perestrelo, R.; Freitas, V.; Bordiga, M. Behind the Scenes of Anthocyanins—From the Health Benefits to Potential Applications in Food, Pharmaceutical and Cosmetic Fields. Nutrients 2022, 14, 5133. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavaila-bility and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A Comprehensive Review on Carotenoids in Foods and Feeds: Status Quo, Applications, Patents, and Research Needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Batista, K.S.; Soares, N.L.; Dorand, V.A.M.; Alves, A.F.; Dos Santos Lima, M.; de Alencar Pereira, R.; Leite de Souza, E.; Magnani, M.; Persuhn, D.C.; de Souza Aquino, J. Acerola Fruit By-Product Alleviates Lipid, Glucose, and Inflammatory Changes in the Enterohepatic Axis of Rats Fed a High-Fat Diet. Food Chem. 2023, 403, 134322. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Gullón, B.; Gómez, B.; Martínez-Sabajanes, M.; Yáñez, R.; Parajó, J.; Alonso, J. Pectic oligosaccharides: Manufacture and functional properties. Trends Food Sci. Technol. 2013, 30, 153–161. [Google Scholar] [CrossRef]
- Sun, R.; Niu, Y.; Li, M.; Liu, Y.; Wang, K.; Gao, Z.; Wang, Z.; Yue, T.; Yuan, Y. Emerging trends in functional pectin pro-cessing and its fortification for synbiotics: A review. Trends Food Sci. Technol. 2023, 134, 80–97. [Google Scholar] [CrossRef]
- Porcu, O.M.; Rodriguez-Amaya, D.B. Variation in the carotenoid composition of acerola and its processed products. J. Sci. Food Agric. 2006, 86, 1916–1920. [Google Scholar] [CrossRef]
- Hanamura, T.; Hagiwara, T.; Kawagishi, H. Structural and functional characterization of polyphenols isolated from acerola (Malpighia emarginata DC.) fruit. Biosci. Biotechnol. Biochem. 2005, 69, 280–286. [Google Scholar] [CrossRef]
- Vendramini, A.; Trugo, L. Phenolic Compounds in Acerola Fruit (Malpighia punicifolia, L.). J. Braz. Chem. Soc. 2004, 15, 664–668. [Google Scholar] [CrossRef]
- Carmo, J.; Nazareno, L.; Rufino, M. Characterization of the Acerola Industrial Residues and Prospection of Their Potential Application as Antioxidant Dietary Fiber Source. Food Sci. Technol. 2018, 38, 236–241. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R. Volatile Flavor Constituents of Acerola (Malpighia emarginata DC.) Fruit. J. Agric. Food Chem. 2001, 49, 5880–5882. [Google Scholar] [CrossRef]
- Monteiro, S.; Barbosa, M.; Silva, F.; Bezerra, R.; Maia, K. Preparation, phytochemical and bromatological evaluation of flour obtained from the acerola (Malpighia punicifolia) agroindustrial residue with potential use as fiber source. LWT-Food Sci. Technol. 2020, 134, 110142. [Google Scholar] [CrossRef]
- Da Silva, B.; Dias, D.; Cavalcante, D.; da Costa, F.; Nunes, A.; da Cruz, I.; de Oliveira, M.; Lagos, C. Phytochemical Analysis, Nutritional Profile and Immunostimulatory Activity of Aqueous Extract from Malpighia emarginata DC Leaves. Biocatal. Agric. Biotechnol. 2020, 23, 101442. [Google Scholar] [CrossRef]
- Fekry, A.; Elsabbagh, W.; Abu, B.M.; El-Ghazaly, M.; Mohamed, A.E.S. Antioxidant activity of Malpighia glabra L., leaves extract. Azhar Int. J. Pharm. Med. Sci. 2021, 1, 88–93. [Google Scholar] [CrossRef]
- Shreelakshmi, S.V.; Chaitrashree, N.; Nazareth, M.S.; Kumar, S.S.; Shetty, N.P.; Giridhar, P. Bioactive compounds and antioxidant activity during ripening of Malpighia glabra fruits. J. Food Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Mezadri, T.; Pérez, A.; Hornero, D. Carotenoid Pigments in Acerola Fruits (Malpighia emarginata DC.) and Derived Products. Eur. Food Res. Technol. 2005, 220, 63–69. [Google Scholar] [CrossRef]
- Mesquita, C.; Brito, K.; Nayara, F.; da Cruz, E.; dos Santos, M.; Bordin, V.; Fernandes, E.; Gómez, A.; Leite, E.; Gomes, M. Protective Effects of Tropical Fruit Processing Coproducts on Probiotic Lactobacillus Strains During Freeze-Drying and Storage. Microorganisms 2020, 8, 96. [Google Scholar]
- Marques, T.; Caetano, A.; Simão, A.; Castro, F.; de Oliveira, V.; Corrêa, A. Methanolic extract of Malpighia emarginata ba-gasse: Phenolic compounds and inhibitory potential on digestive enzymes. Rev. Bras. Farmacogn. 2016, 26, 191–196. [Google Scholar] [CrossRef]
- Duarte, S.; Mesquita, C.; Da Silva, G.; dos Santos, M.; Bordin, V.; Fernandes, E.; Leite, E.; Gomes, M. Improvement in Physicochemical Characteristics, Bioactive Compounds and Antioxidant Activity of Acerola (Malpighia emarginata D.C.) and Guava (Psidium guajava L.) Fruit By-products Fermented with Potentially Probiotic Lactobacilli. Food Sci. Technol. 2020, 134, 110200. [Google Scholar]
- Caetano, A.C.D.S.; Araújo, C.R.D.; Lima, V.L.A.G.D.; Maciel, M.I.S.; Melo, E.D.A. Evaluation of antioxidant activity of agroindustrial waste of acerola (Malpighia emarginata D.C.) fruit extracts. Ciência E Tecnol. De Aliment. 2011, 31, 769–775. [Google Scholar] [CrossRef]
- Hanada, T.; Yoshimura, A. Regulation of Cytokine Signaling and Inflammation. Cytokine Growth Factor Rev. 2002, 13, 413–421. [Google Scholar] [CrossRef]
- Bellik, Y.; Boukraâ, L.; Alzahari, H.; Bakhotmah, B.; Abdellah, F.; Hammoudi, S.; Iguer, M. Molecular Mechanism Underlying Anti-Inflammatory and Anti-Allergic Activities of Phytochemicals: An Update. Molecules 2012, 18, 322–353. [Google Scholar] [CrossRef]
- Cabral, N.; de Oliveira, E.; Bezerra, I.; Lisboa, H.; Paiva, E.; Rangel, E.; Pens, D.; Fonseca, J.; Siqueira, R.; de Bittencourt, M.A. Anti-Inflammatory and Antioxidant Properties of Blend Formulated with Compounds of Malpighia emarginata D.C (Ac-erola) and Camellia sinensis L. (Green Tea) in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Biomed. Pharmacother. 2020, 128, 110277. [Google Scholar]
- Albuquerque, M.; Levit, R.; Beres, C.; Bedani, R.; de Moreno, A.; Isay, S.; Leblanc, J. Tropical Fruit By-Products Water Extracts of Tropical Fruit By-Products as Sources of Soluble Fibers and Phenolic Compounds with Potential Antioxidant, Anti-Inflammatory, and Functional Properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Pereira, F.; Xiong, J.; Borges, K.; Targino, R.; Esposito, D. Phytochemical Content, Antioxidant, Anti-Inflammatory Ac-tivities, and Wound Healing Properties of Freeze-Dried Fruits. Acta Sci. Nutr. Health 2020, 4, 63–71. [Google Scholar] [CrossRef]
- Milanez, F.; Dimer, D.; Daumann, F.; de Oliveira, S.; Luciano, T.; Correa, J.; Alves, A.; Neves, R.; Rosa, J.; Missae, L.; et al. Acerola (Malpighia emarginata DC.) Juice Intake Protects Against Alter-ations to Proteins Involved in Inflammatory and Lipolysis Pathways in the Adipose Tissue of Obese Mice Fed a Cafeteria Diet. Lipids Health Dis. 2014, 13, 13–24. [Google Scholar]
- Asgar, A. The Anti-Diabetic Potential of Phenolic Compounds: A Review. Int. J. Food Prop. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Capanoglu, E.; Paoli, P.; Simal, J.; Mohanram, K.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary Polyphenols as Anti-diabetic Agents: Advances and Opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Barbalho, S.; Damasceno, D.; Machado, A.; Palhares, M.; Aparecida, K.; Oshiiwa, M.; Sazaki, V.; Sellis, V. Evaluation of Glycemic and Lipid Profile of Offspring of Diabetic Wistar Rats Treated with Malpighia emarginata Juice. Exp. Diabetes Res. 2011, 10, 1–6. [Google Scholar] [CrossRef]
- Hanamura, T.; Mayama, C.; Aoki, H.; Hirayama, Y.; Shimizu, M. Antihyperglycemic Effect of Polyphenols from Acerola (Malpighia emarginata DC.) Fruit. Biosci. Biotechnol. Biochem. 2006, 70, 1813–1820. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Zhao, J.; Liu, M.; Shu, X.; Li, Q.; Wang, Y.; Zhou, Y. Polyphenol Mechanisms Against Gastric Cancer and Their Interactions with Gut Microbiota: A Review. Curr. Oncol. 2022, 29, 5247–5261. [Google Scholar] [CrossRef]
- Mitra, T.; Bhattacharya, R. Phytochemicals modulate cancer aggressiveness: A review depicting the anticancer efficacy of dietary polyphenols and their combinations. J. Cell. Physio. 2020, 235, 7696–7708. [Google Scholar] [CrossRef]
- Motohashi, N.; Wakabayashi, H.; Kurihara, T.; Fukushima, H.; Yamada, T.; Kawase, M.; Sohara, Y.; Tani, S.; Shirataki, Y.; Sakagami, H.; et al. Biological Activity of Barbados Cherry (Acerola Fruits, Fruit of Malpighia emarginata DC) Extracts and Fractions. Phytother. Res. 2004, 18, 212–223. [Google Scholar] [CrossRef]
- Nagamine, I.; Akiyama, T.; Kainuma, M.; Kumagai, H.; Satoh, H.; Yamada, K.; Yano, T.; Sakurai, H. Effect of Acerola Cherry Extract on Cell Proliferation and Activation of Ras Signal Pathway at the Promotion Stage of Lung Tumorigenesis in Mice. J. Nutr. Sci. Vitaminol. 2002, 48, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.; Franco, M.; Galhardo, R.; Pessanha, M.; Henrique, A.; Barbisan, L. Protective Effects of Spray-Dried Açaí (Euterpe oleracea Mart) Fruit Pulp against Initiation Step of Colon Carcinogenesis. Food Res. Int. 2015, 77, 432–440. [Google Scholar]
- Nunes, R.; Silva, V.; da Silva, M.; Silva, M.; Maglione, C.; Moraes, V.; Falcão, A.; Da Silva, J. Protective Effects of Acerola Juice on Genotoxicity Induced by Iron In Vivo. Genet. Mol. Biol. 2016, 39, 122–128. [Google Scholar]
- Silva, R.; Silva, V.; Da Silva, M.; Richter, M.; Costa, L.; Rocha, A.; Abin, J.; Martínez, M.; Ferronato, S.; Falcão, A.; et al. Antigenotoxicity and Antioxidant Activity of Acerola Fruit (Malpighia glabra L.) at Two Stages of Ripeness. Plant Foods Hum. Nutr. 2011, 66, 129–135. [Google Scholar]
- Dimer, D.; Da Silva, J.; Daumann, F.; Formentin, A.; Longaretti, L.; Paganini, A.; de Lira, F.; Campos, F.; Falcão, A.; Silva, D.; et al. Corrective Effects of Acerola (Malpighia emarginata DC.) Juice Intake on Biochemical and Genotoxic Parameters in Mice Fed on a High-Fat Diet. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2013, 770, 144–152. [Google Scholar]
- Macvanin, M.; Gluvic, Z.; Yafirovic, S.; Gao, X.; Essack, M.; Isenovic, E. The protective role of nutritional antioxidants against oxidative stress in thyroid disorders. Front. Endocrinol. 2023, 13, 1092837. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, I.; Fujita, M.; Hongo, I.; Nguyen, H.; Miyahara, M.; Parkányiová, J.; Pokorný, J.; Dostálová, J.; Sakurai, H. Hepatoprotective Effects of Acerola Cherry Extract Powder against D-Galactosamine-Induced Liver Injury in Rats and its Bioactive Compounds. Czech J. Food Sci. 2004, 22, 159–162. [Google Scholar] [CrossRef]
- Marques, T.R.; Caetano, A.A.; Henrique, S.; Cesar, P.; Braga, M.A.; Henrique, A.; Machado, G.; Sousa, R.V.; Corrêa, A.D. Antioxidant activity and hepatoprotective potential of lyophilized extract of acerola bagasse against CCl 4-induced hepatotoxicity in Wistar rats. J. Food Biochem. 2018, 42, e12670. [Google Scholar] [CrossRef]
- Düsman, E.; Almeida, L.; Tnin, L.; Vicentini, V. In vivo antimutagenic effects of the Barbados cherry fruit (Malpighia glabra Linnaeus) in a chromosomal aberration assay. Genet. Mol. Res. 2016, 15, 1–9. [Google Scholar] [CrossRef]
- Almeida, I.; Düsman, E.; Heck, M.; Pamphile, J.; Lopes, N.; Tonin, L.; Vicentini, V. Cytotoxic and mutagenic effects of iodine-131 and radioprotection of acerola (Malpighia glabra L.) and beta-carotene in vitro. Genet. Mol. Res. 2013, 12, 6402–6413. [Google Scholar] [CrossRef]
- Spada, P.; Nunes, G.; Bortoloni, G.; Henriques, J.; Salvador, M. Antioxidant, Mutagenic, and Antimutagenic Activity of Frozen Fruits. J. Med. Food 2008, 11, 144–151. [Google Scholar] [CrossRef]
- Carvalho, L.; Dionísio, A.; da Silva, A.; Wurlitzer, N.; Sousa, E.; Anceski, G.; Montenegro, I.; Nogueira, M.; Hai, R. Antiproliferative, antimutagenic and antioxidant activities of a Brazilian tropical fruit juice. LWT-Food Sci. Technol. 2014, 59, 1319–1324. [Google Scholar] [CrossRef]
- Montero, I.; Alves, E.; Saravia, S.; Aparecida, J.; Santos, R.; de Malo, A.; Carvalho, R.; Estevam, P.; Marcia, J.; Cardoso, P.; et al. Antimicrobial activity and acetylcholinesterase inhibition of oils and Amazon fruit extracts. J. Med. Plants Res. 2020, 14, 88–97. [Google Scholar]
- Schmourlo, G.; de Morais, Z.; de Oliveira, D.; Costa, S.; Mendonça, R.; Alviano, C.; Miranda, A. Antioxidant and Anti-microbial Activity of Edible Plants and Their Potential Use as Nutraceuticals. Acta Hort. 2007, 756, 355–368. [Google Scholar] [CrossRef]
- Rezende, T.; Caetano, A.; Avelar, L.; Simão, A.; Andrade, G.; Duarte, A. Characterization of phenolic compounds, antioxidant, and antibacterial potential the extract of acerola bagasse flour. Acta Sci. Technol. 2017, 39, 143–148. [Google Scholar]
- Tremonte, P.; Sorrentino, E.; Succi, M.; Tipaldi, L.; Panella, G.; Ibañez, E.; Mendiola, J.; Di Renzo, T.; reale, A.; Coppola, R. Antimicrobial Effect of Malpighia punicifolia and Extension of Water Buffalo Steak Shelf-Life. J. Food Sci. 2015, 81, M97–M105. [Google Scholar] [CrossRef]
- Paz, M.; Gúllon, P.; Fátima, M.; Carvalho, A.; Domingues, V.; Gomes, A.; Becker, H.; Longhinotti, E.; Deleure, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef]
- Pinheiro, A.; Linhares, A.; Silva, A.; Moraes, L.; Machado, P.; Wilane, R. Bioactive potential of nanoparticles of acerola byproduct (Malpighia spp.): Bioaccessibility in nectar. Res. Soc. Dev. 2020, 9, e159996691. [Google Scholar]
- Vital, A.; Alencar, M.; Alves, K.; de Freitas, P.; Silveira, N.; Alves, L.; Chaves, S.; Gonçalves, S.; de Souza, J.; Leite, E.; et al. Effects of consumption of acerola, cashew and guava by-products on adiposity and redox homeostasis of adipose tissue in obese rats. Clin. Nutr. ESPEN 2021, 43, 283–289. [Google Scholar]
- Dimer, D.; Tezza, G.; Daumann, F.; Longaretti, L.; Dajori, A.; Mezari, L.; Carvalho, M.; Streck, E.; Moraes, V. Effects of acerola (Malpighia emarginata DC.) Juice Intake on Brain Energy Metabolism of Mice Fed a Cafeteria Diet. Mol. Neurobiol. 2017, 54, 954–963. [Google Scholar]
- Sousa, M.S.B.; Vieira, L.M.; Silva, M.J.M.; Lima, A. Caracterização nutricional e compostos antioxidantes em resíduos de polpas de frutas tropicais. Ciência E Agrotecnologia 2011, 35, 554–559. [Google Scholar] [CrossRef]
- Magalhães, M.P.D.; Gandra, K.M.B.; Cunha, L.R.D.; Lima, E.M.F. Obtaining flour from acerola processing residue and evaluating bioactive and nutritive compounds. Res. Soc. Dev. 2021, 10, e188101420714. [Google Scholar] [CrossRef]
- Soares, E.; Oliveira, G.S.F.; Maia, G.A.; Monteiro, J.C.S.; Silva, J.A.; Filho, M.S.S. Desidratação da polpa de acerola (Malpighia emarginata d.c.) pelo processo “foam-mat”. Ciência E Tecnol. De Aliment. 2021, 21, 164–170. [Google Scholar] [CrossRef]
- Abud, A.K.S.; Narain, N. Characterization and alternatives to use acerola residue. Acta Hortic. 2018, 1198, 145–154. [Google Scholar] [CrossRef]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Di Francesco, S.; Capillo, M.C.; Rauso, R.; Herrera, M.; Bencini, P.L.; Guida, S.; Mocchi, R. The Anti-Ageing and Whitening Potential of a Cosmetic Serum Containing 3-O-ethyl-l-ascorbic Acid. Life 2021, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Hanamura, T.; Uchida, E.; Aoki, H. Skin-lightening effect of a polyphenol extract from Acerola (Malpighia emarginata DC.) fruit on UV-induced pigmentation. Biosci. Biotechnol. Biochem. 2008, 72, 3211–3218. [Google Scholar] [CrossRef]
- Dornoff Jeffrey, M.; O’toole Deborah, A.; Davies Michael, B. Skin Whitening Agent Containing Acerola Cherry Fermentation Material. 1997. Available online: https://patents.google.com/patent/KR100315502B1/en (accessed on 11 November 2023).
- Zimmerman, A.C.; Beio, D.V. Method of Increasing Skin Cell Renewal Rate Using Acerola Cherry Fermentate. 2000. Available online: https://patents.google.com/patent/US6074647A/en (accessed on 11 November 2023).
- Nogueira, G.; Duarte, C.; Barrozo, M. Hydrothermal carbonization of acerola (Malphigia emarginata D.C.) wastes and its application as an adsorbent. Waste Manag. 2019, 95, 466–475. [Google Scholar] [CrossRef]
- Oliveira, J.A.R.; Conceição, A.C.; Silva, L.H.M.; Moreira, D.K.T.; Passos, M.F.; Komesu, A. Evaluation of the technological potential of four wastes from Amazon fruit industry in glucose and ethanol production. J. Food Process Eng. 2020, 44, e13610. [Google Scholar] [CrossRef]
- André, A.C.L.; Barros, A.E.S.C.; Silva, P.T.d.S.E.; Lourençoni, D.; Amorim, M.C.C.D. Anaerobic co-digestion of acerola (Malphigia emarginata) agro-industry effluent with domestic sewage at mesophilic and thermophilic conditions. Semin. Ciências Exatas E Tecnológicas 2021, 42, 85–96. [Google Scholar] [CrossRef]
- Magama, P.; Chiyanzu, I.; Mulopo, J. A systematic review of sustainable fruit and vegetable waste recycling alternatives and possibilities for anaerobic biorefinery. Bioresour. Technol. Rep. 2022, 18, 101031. [Google Scholar] [CrossRef]
- León Gutiérrez, G. Nanoparticulate Titanium Dioxide Nanomaterial Modified with Functional Groups and with Citric Extracts Adsorbed on the Surface, for the Removal of a Wide Range of Microorganisms. U.S. Patent No. 10,342,840, 9 July 2019. [Google Scholar]
- Reinaldo, J.S.; Milfont, C.H.R.; Gomes, F.P.C.; Mattos, A.L.A.; Medeiros, F.G.M.; Lopes, P.F.N.; Ito, E.N. Influence of grape and acerola residues on the antioxidant, physicochemical and mechanical properties of cassava starch biocomposites. Polym. Test. 2021, 93, 107015. [Google Scholar] [CrossRef]
- Nóbrega, E.; Oliveira, E.; Genovese, M.; Correia, R. The impact of hot air drying on the physi-cal-chemical characteristics, bioactive compounds and antioxidant activity of acerola (Malphigia emarginata) residue. J. Food Process. Preserv. 2015, 39, 131–141. [Google Scholar] [CrossRef]
- Xavier, F.; Maia, S.; Oliveira, T.; Mendonça, E. Biomassa microbiana e matéria orgânica leve em solos sob sistemas agrícolas orgânico e convencional na Chapada da Ibiapaba—CE. Rev. Bras. De Ciência Do Solo 2006, 30, 247–258. [Google Scholar] [CrossRef]
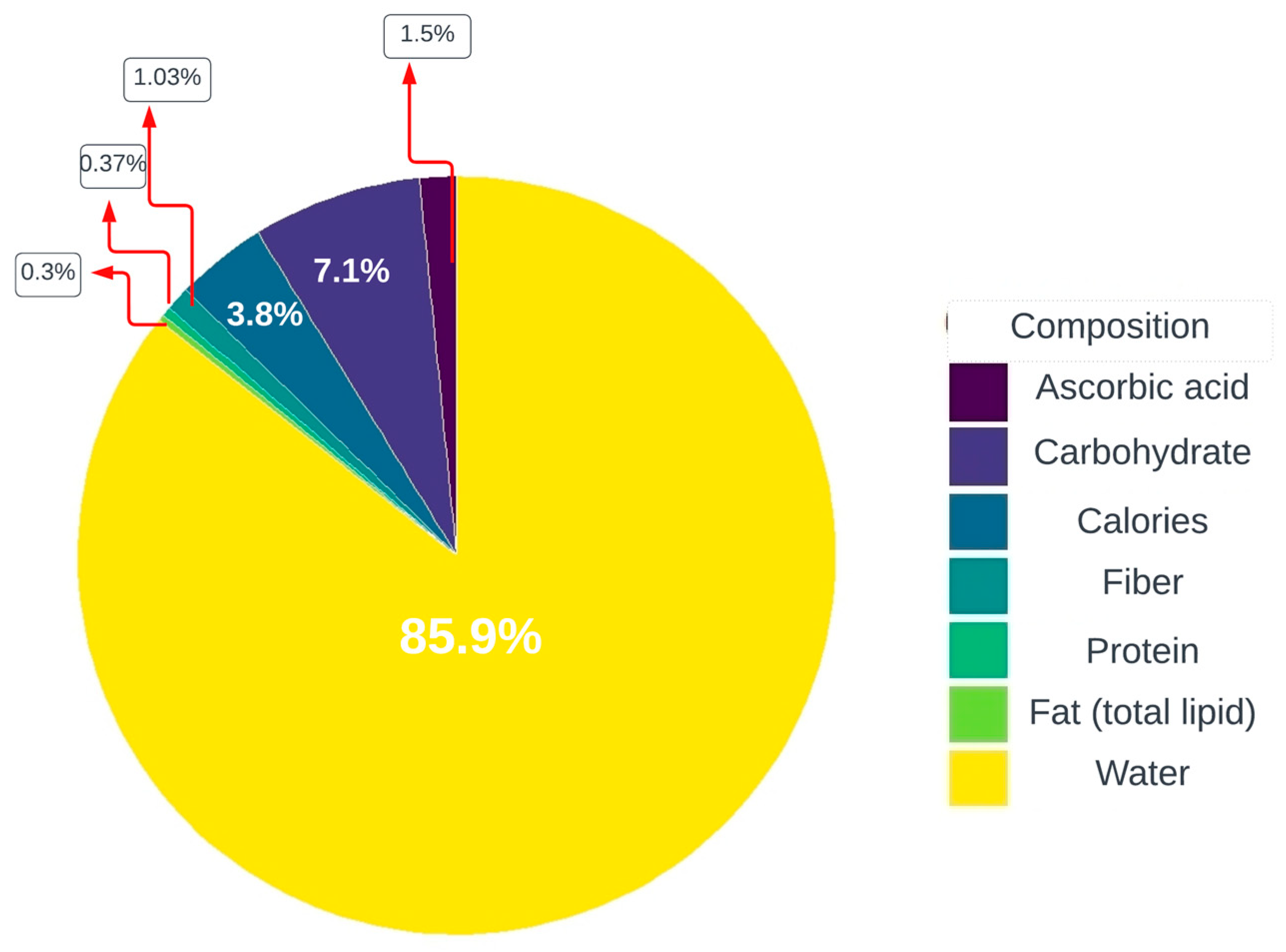
| Source | Bioactive Compound | Concentration |
|---|---|---|
| Bagasse | (9Z)-lutein | 3.40 ± 0 [38] a |
| (All-E)-B-carotene | <0.05 ± 0 [38] a | |
| B-carotene | 5.84 ± 0.01 [39] b | |
| Carotenoids | 5.84 ± 0.01 [39] b | |
| Catechin | 22.46 ± 0.08 [39] b | |
| Epicatechin | 21.73 ± 0.35 [39] b | |
| Ferulic acid | 51.30 ± 3.73 [39] b | |
| Flavonoids total | 571.98 ± 40.29 [39] b | |
| Kaempferol | 27.59 ± 0.32 [39] b | |
| Naringenin | 810.40 ± 76.76 [39] b | |
| Organic acids | 1235.00 ± 25.00 [39] c | |
| P-coumaric acid | 59.48 ± 0.82 [39] b | |
| Rutin | 53.25 ± 2.81 [39] b | |
| Succinic acid | 119.00 ± 23.00 [39] c | |
| Tartaric acid | 1116.00 ± 2.00 [39] c | |
| Juice powder | L-ascorbic acid | 314.50 ± 5.80 [38] a |
| Nonpomace | (9Z)-lutein | 11.10 ± 0 [38] a |
| (All-E)-B-carotene | 5.80 ± 0 [38] a | |
| L-ascorbic acid | 158.00 ± 0.40 [38] a |
| Property or Activity | Value |
|---|---|
| pH | 3.38 |
| Total sugars (% glucose) | 1.93 |
| Total anthocyanins (mg/100 g) | 19.43 |
| Yellow flavonoids (mg/100 g) | 36.56 |
| Total polyphenols (mg gallic acid/100 g) | 545.98 |
| Total antioxidant activity (μM Trolox/g) | 17.70 |
| Organic Sample | Extract Condition | DPPH | ABTS− | ORAC | FRAP | Source |
|---|---|---|---|---|---|---|
| Leaves | Methanolic | 10.88 + 0.38 a | 12.62 + 1.08 a | [33] | ||
| Methanolic | 4.98 a | [63] | ||||
| Saline | 38.59 + 1.20 e | 416.11 + 0.46 e | [22] | |||
| Ethanolic | 1.75 + 1.06 i | 0.41 + 0.16 i | 3.875 + 0.18 i | [64] | ||
| Hydroethanolic | 68.8 + 0.18 a | 38.1 + 0.03 | [20] | |||
| Methanolic | 21.69 + 1.62 a | 18.78 + 10.72 | [33] | |||
| Methanolic | 1.625 + 0.9 i | 0.31 + 0.01 i | 1.63 + 0.9 i | [64] | ||
| Ripe fruit | Water and methanol | 125.66 + 8.37 b1 | 91.76 + 6.24 b1 | 76.71 + 1.34 b2 | [14] | |
| Water and methanol | 82.22 + 2.9 | 64.57 + 2.47 | 57.77 + 1.61 | [65] | ||
| Water and methanol | 0.75 + 0.35 i | 0.305 + 0.02 i | 7.0 + 4.24 i | [64] | ||
| Unripe fruit | Ethanolic | 1910 c | 8613.54 c | 2454.42 c | 1166.09 c | [23] |
| Methanolic | 21.16 + 0.91 | 16.355 + 1.34 | [33] | |||
| Ethanolic | 0.3 + 0.2 i | 0.28 + 0.01 i | 5.0 + 0.0 i | [64] | ||
| Methanolic | 0.375 + 0.18 i | 0.195 + 0.01 i | 3.5 + 2.1 i | [64] | ||
| Water | 0.375 + 0.18 i | 0.17 + 0.03 i | [64] | |||
| Juice | Seeds in water Pulp in water | 0.18 + 0.013 d 3.44 + 0.12 d | [66] | |||
| Pulp | Hydroethanolic | 7433 + 26.2 e | 8512 + 61.4 e | [27] | ||
| Hydromethanolic | 19.65 + 1.92 e | 17.1 + 0.30 e | 11.815 + 0.37 | [14] | ||
| Hydroethanolic | ||||||
| Bark | Methanolic | 15.36 + 0.18 a | 10.91 + 1.15 a | [33] | ||
| Bagasse | 16.14 + 0.01 f | 0.92 + 0.01 f | [28] | |||
| Hydromethanolic | 405.11 + 1.83 | [67] | ||||
| Seeds/peels/barks mixed. | Methanolic | 790 + 14.00 g | 2348.65 + 11.21 g | [68] | ||
| Hydroacetone | 0.33 + 0.02 h2 | 291.71 + 20.90 h1 | [69] | |||
| Hydroethanolic extract | 0.22 + 0.01 h2 | 1445.10 + 73.07 h1 | [69] | |||
| Hydromethanolic extract | 0.23 + 0.01 h2 | 1145.50 + 45.81 h1 | [69] | |||
| Hydroacetone | 291.71 ± 20.90 h1 | [69] | ||||
| Hydroethanolic | 1445.10 ± 73.07 h1 | [69] | ||||
| Hydromethanolic | 1145.50 ± 45.81 h1 | [69] | ||||
| Hydroacetone | 2228.51 + 6.29 i | 5180.85 + 184.785 i | 45,325.64 + 2245.64 i | [39] | ||
| Hydroethanolic extract | 2265.755 + 33.64 i | 1460.21 + 22.51 i | 54,917.62 + 2007.535 i | [39] | ||
| Hydromethanolic extract | 2305.83 + 37.355 i | 3349.055 + 90.69 i | 41,023.735 + 3350.54 i | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Baudrit, J.R.; Camacho, M.; Batista-Menezes, D.; Corrales-Ureña, Y.; Zúñiga, J.M.; Chacón, A.M.; Lecot, N.; Henríquez, L.C.; Lopretti, M. Acerola (Malpighia spp.) Waste: A Sustainable Approach to Nutraceutical, Pharmaceutical, and Energy Applications. Recycling 2023, 8, 96. https://doi.org/10.3390/recycling8060096
Vega-Baudrit JR, Camacho M, Batista-Menezes D, Corrales-Ureña Y, Zúñiga JM, Chacón AM, Lecot N, Henríquez LC, Lopretti M. Acerola (Malpighia spp.) Waste: A Sustainable Approach to Nutraceutical, Pharmaceutical, and Energy Applications. Recycling. 2023; 8(6):96. https://doi.org/10.3390/recycling8060096
Chicago/Turabian StyleVega-Baudrit, José Roberto, Melissa Camacho, Diego Batista-Menezes, Yendry Corrales-Ureña, Juan Miguel Zúñiga, Arturo Mora Chacón, Nicole Lecot, Luis Castillo Henríquez, and Mary Lopretti. 2023. "Acerola (Malpighia spp.) Waste: A Sustainable Approach to Nutraceutical, Pharmaceutical, and Energy Applications" Recycling 8, no. 6: 96. https://doi.org/10.3390/recycling8060096
APA StyleVega-Baudrit, J. R., Camacho, M., Batista-Menezes, D., Corrales-Ureña, Y., Zúñiga, J. M., Chacón, A. M., Lecot, N., Henríquez, L. C., & Lopretti, M. (2023). Acerola (Malpighia spp.) Waste: A Sustainable Approach to Nutraceutical, Pharmaceutical, and Energy Applications. Recycling, 8(6), 96. https://doi.org/10.3390/recycling8060096







