Minimizing Organic Waste Generated by Pineapple Crown: A Simple Process to Obtain Cellulose for the Preparation of Recyclable Containers
Abstract
1. Introduction
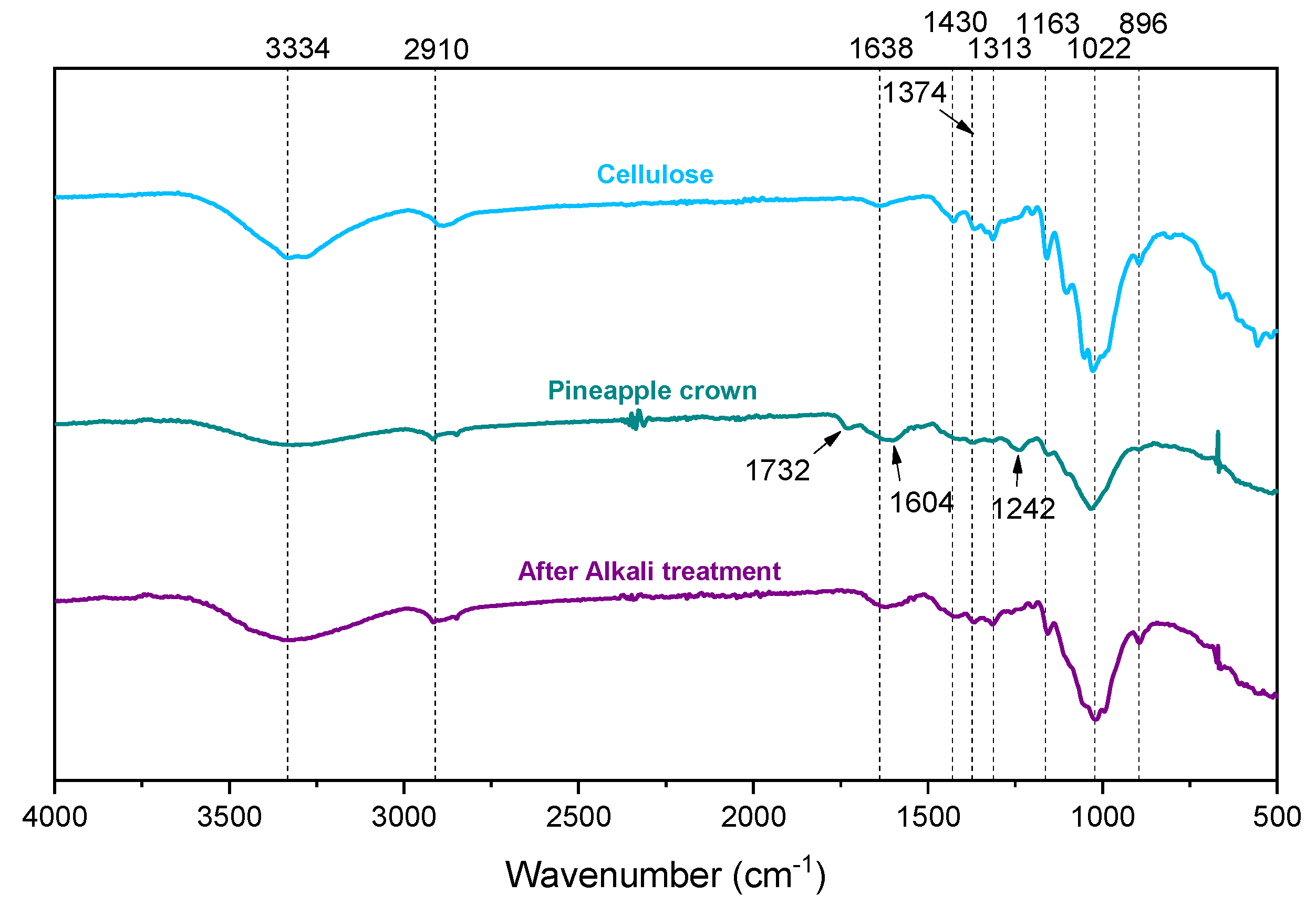
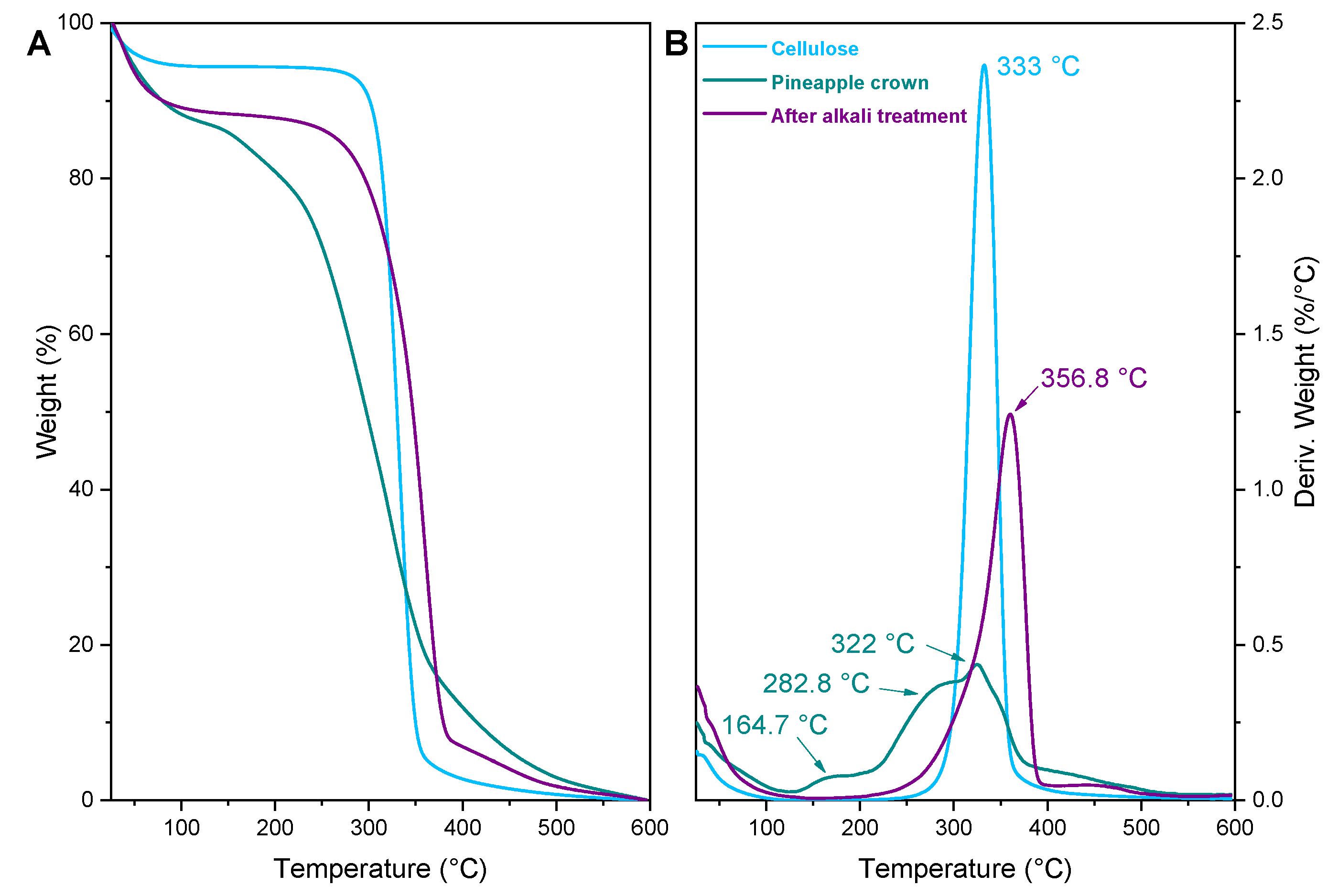
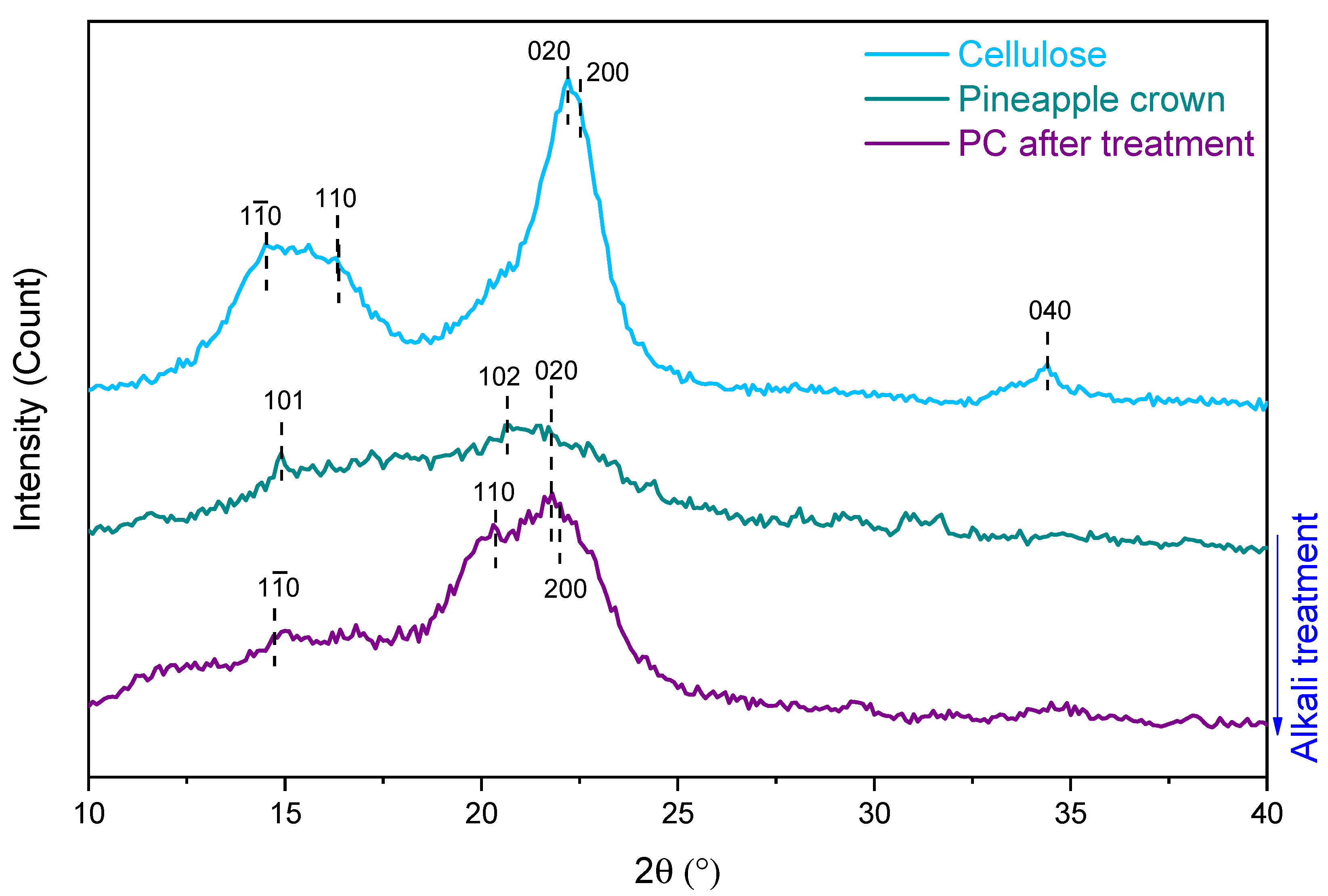
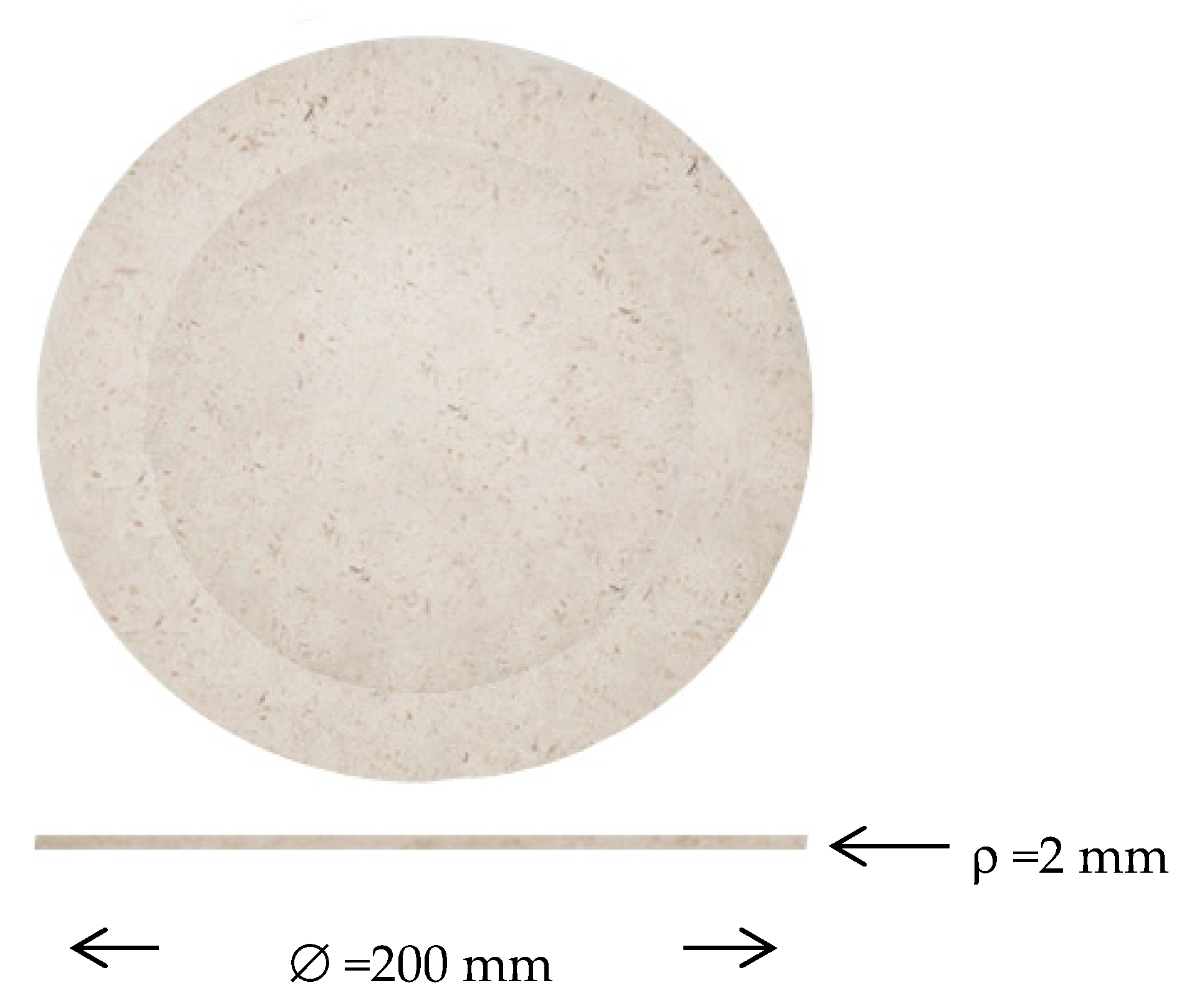
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Cellulose Source
3.3. Cellulose from Pineapple Crown
3.4. Composition of Pineapple Crown Ananas comosus
3.5. Fourier-Transform Infrared Spectroscopy Analyses (FTIR)
3.6. Thermogravimetric Analysis (TGA)
3.7. X-ray Diffraction Analysis (XRD)
3.8. Container Elaboration
3.9. Mechanical Proprieties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Upadhyay, A.; Lama, J.P.; Tawata, S. Utilization of Pineapple Waste: A Review. J. Food Sci. Technol. Nepal 2013, 6, 10–18. [Google Scholar] [CrossRef]
- Prado, K.S.; Spinacé, M.A.S. Isolation and characterization of cellulose nanocrystals from pineapple crown waste and their potential uses. Int. J. Biol. Macromol. 2019, 122, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, A. Chemical analysis and pulping study of pineapple crown leaves. Ind. Crops Prod. 2006, 24, 66–74. [Google Scholar] [CrossRef]
- Mukherjee, P.S.; Satyanarayana, K.G. Structure and properties of some vegetable fibres—Part 1 Sisal fibre. J. Mater. Sci. 1984, 19, 3925–3934. [Google Scholar] [CrossRef]
- Ghinea, C.; Leahu, A. Monitoring of Fruit and Vegetable Waste Composting Process: Relationship between Microorganisms and Physico-Chemical Parameters. Processes 2020, 8, 302. [Google Scholar] [CrossRef]
- Maraveas, C. Production of Sustainable and Biodegradable Polymers from Agricultural Waste. Polymers 2020, 12, 1127. [Google Scholar] [CrossRef]
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Asrofi, M. Production of Nanocellulose from Pineapple Leaf Fibers via High-Shear Homogenization and Ultrasonication. Fibers 2018, 6, 28. [Google Scholar] [CrossRef]
- Vignon, M.R.; Heux, L.; Malainine, M.E.; Mahrouz, M. Arabinan-cellulose composite in Opuntia ficus-indica prickly pear spines. Carbohydr. Res. 2004, 339, 123–131. [Google Scholar] [CrossRef]
- Rong, M.Z.l.; Zhang, M.Q.; Liu, Y.; Yang, G.C.; Zeng, H.M. The effect of fiber treatment on the mechanical properties of unidirectional sisal-reinforced epoxy composites. Compos. Sci. Technol. 2001, 61, 1437–1447. [Google Scholar] [CrossRef]
- Saha, S.C.; Das, B.K.; Ray, P.K.; Pandey, S.N.; Goswami, K. SEM Studies of the Surface and Fracture Morphology of Pineapple Leaf Fibers. Text Res. J. 1990, 60, 726–731. [Google Scholar] [CrossRef]
- Asim, M.; Khalina, A.; Jawaid, M.; Nasir, M.; Dashtizadeh, Z.; Ishak, M.R.; Hoque, M.E. A review on pineapple leaves fibre and its composites. Int. J. Polym. Sci. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 154–169. [Google Scholar] [CrossRef] [PubMed]
- De Godoy, M.R.C.; Kerr, K.R.; Fahey, J.G.C.; Fahey, G.C. Alternative dietary fiber sources in companion animal nutrition. Nutrients 2013, 94, 3099–3117. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.T.; Arslan, M.B. A study on physical and chemical properties of cellulose paper immersed in various solvent mixtures. Int. J. Mol. Sci. 2008, 9, 78–88. [Google Scholar] [CrossRef]
- Ma, Y.; Hummel, M.; Määttänen, M.; Särkilahti, A.; Harlin, A.; Sixta, H. Upcycling of waste paper and cardboard to textiles. Green Chem. 2016, 18, 858–866. [Google Scholar] [CrossRef]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- De Assis, A.C.L.; Alves, L.P.; Malheiro, J.P.T.; Barros, A.R.A.; Pinheiro-Santos, E.E.; De Azevedo, E.P.; Alves, H.D.S.; Junior, J.A.O.; Damasceno, B.P.G.L. Opuntia Ficus-Indica L. Miller (Palma Forrageira) as an Alternative Source of Cellulose for Production of Pharmaceutical Dosage Forms and Biomaterials: Extraction and Characterization. Polymers 2019, 11, 1124. [Google Scholar] [CrossRef]
- Dos Santos, R.M.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Cherian, B.M.; Leão, A.L.; De Souza, S.F.; Costa, L.M.M.; De Olyveira, G.M.; Kottaisamy, M.; Nagarajan, E.; Thomas, S. Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medical applications. Carbohydr. Polym. 2011, 86, 1790–1798. [Google Scholar] [CrossRef]
- Kengkhetkit, N.; Amornsakchai, T. Utilisation of pineapple leaf waste for plastic reinforcement: 1. A novel extraction method for short pineapple leaf fiber. Ind. Crops Prod. 2012, 40, 55–61. [Google Scholar] [CrossRef]
- Leão, A.L.; Souza, S.F.; Cherian, B.M.; Frollini, E.; Thomas, S.; Pothan, L.A.; Kottaisamy, M. Agro-Based Biocomposites for Industrial Applications. Mol. Cryst. Liq. Cryst. 2010, 522, 18–27. [Google Scholar] [CrossRef]
- Threepopnatkul, P.; Kaerkitcha, N.; Athipongarporn, N. Effect of surface treatment on performance of pineapple leaf fiber-polycarbonate composites. Compos. Part B Eng. 2009, 40, 628–632. [Google Scholar] [CrossRef]
- Resende, J.M.; Lima De Oliveira, F.; Mulinari, D.R. Avaliação de Compósitos Híbridos Para Aplicações em Engenharia. Cad. UniFOA 2009, 615, 11–17. Available online: http://moodleead.unifoa.edu.br/revistas/index.php/cadernos/article/view/1027 (accessed on 23 August 2020). (In Portuguese).
- Marcon, J.S.; Mulinari, D.R.; Odila, M.; Cioffi, H.; Voorwald, H.J.C. Estudo da Modificação da Fibra Proveniente da Coroa de Abacaxi Para a Formação de Compósitos Poliméricos. Ipen.br. Foz do Iguaçu, PR: Anais do 10o Congresso Brasileiro de Polímeros. 2009, p. 7. Available online: https://www.ipen.br/biblioteca/cd/cbpol/2009/PDF/924.pdf (accessed on 23 August 2020). (In Portuguese).
- Sipiao, B.; Paiva, R.; Goulart, S.; Mulinari, D. Effect of chemical modification on mechanical behaviour of polypropylene reinforced pineapple crown fibers composites. Procedia Eng. 2011, 10, 2028–2033. [Google Scholar] [CrossRef]
- Braga, R.M.; Queiroga, T.S.; Calixto, G.Q.; Almeida, H.N.; Melo, D.M.D.A.; Melo, M.A.F.; Freitas, J.C.; Curbelo, F.D.D.S. The energetic characterization of pineapple crown leaves. Environ. Sci. Pollut. Res. 2015, 22, 18987–18993. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Yuan, Z.; Fan, Q.; Dai, X.; Zhao, Y.; Wang, Z.; Qin, M. Characterisation of cellulose films regenerated from acetone/water coagulants. Carbohydr. Polym. 2014, 102, 438–444. [Google Scholar] [CrossRef]
- Cataldi, P.; Profaizer, M.; Bayer, I. Preventing Water-Induced Mechanical Deterioration of Cardboard by a Sequential Polymer Treatment. Ind. Eng. Chem. Res. 2019, 58, 6456–6465. [Google Scholar] [CrossRef]
- Tran, T.N.; Paul, U.; Heredia-Guerrero, J.A.; Liakos, I.L.; Marras, S.; Scarpellini, A.; Ayadi, F.; Athanassiou, A.; Bayer, I. Transparent and flexible amorphous cellulose-acrylic hybrids. Chem. Eng. J. 2016, 287, 196–204. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Júnior, H.L.O.; Coutinho, L.V.; Duchemin, B.; Cioffi, M.O.H. Obtaining cellulose nanocrystals from pineapple crown fibers by free-chlorite hydrolysis with sulfuric acid: Physical, chemical and structural characterization. Cellulose 2020, 27, 5745–5756. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Flury, M. Is Biodegradable Plastic Mulch the Solution to Agriculture’s Plastic Problem? Environ. Sci. Technol. 2017, 51, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Chavez Osorio, A.; Janet, I.; de Oca Lamas, M.; Zavaleta, R.; Victoria, C.; TorRes, V.; Velásquez García, E. Q’umir: Reutilización y transformación de botellas de vidrio a vasos [Internet]. Universidad Peruana de Ciencias Aplicadas, 2019. Available online: http://hdl.handle.net/10757/625532 (accessed on 23 August 2020). (In Spanish).
- Mishra, S.; Mohanty, A.K.; Drzal, L.T.; Misra, M.; Hinrichsen, G. A review on pineapple leaf fibers, sisal fibers and their biocomposites [Internet]. Macromol. Mater. Eng. 2004, 289, 955–974. [Google Scholar] [CrossRef]
- Song, K.; Zhu, X.; Zhu, W.; Li, X. Preparation and characterization of cellulose nanocrystal extracted from Calotropis procera biomass. Bioresour. Bioprocess. 2019, 6, 45. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Santos, E.B.C.; Moreno, C.G.; Barros, J.J.P.; De Moura, D.A.; Fim, F.C.; Ries, A.; Wellen, R.M.R.; Silva, L.B. Effect of alkaline and hot water treatments on the structure and morphology of piassava fibers. Mater. Res. 2018, 21, e20170365. [Google Scholar] [CrossRef]
- Khenblouche, A.; Bechki, D.; Gouamid, M.; Charradi, K.; Segni, L.; Hadjadj, M.; Boughali, S. Extraction and characterization of cellulose microfibers from Retama raetam stems. Polimeros 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Liu, C.; Ren, J.-L.; Xu, F.; Liu, J.-J.; Sun, J.-X.; Sun, R.-C. Isolation and characterization of cellulose obtained from ultrasonic irradiated sugarcane bagasse. J. Agric. Food Chem. 2006, 54, 5742–5748. [Google Scholar] [CrossRef]
- Gao, X.; Chen, K.-L.; Zhang, H.; Peng, L.; Liu, Q.-X. Isolation and characterization of cellulose obtained from bagasse pith by oxygen-containing agents. BioResources 2014, 9, 4094–4107. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, N.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Dhlamini, M.; Mothudi, B.M.; Kommula, V.P.; Zhang, J.; Zhang, J.; Rajulu, A.V. Extraction and characterization of cellulose single fibers from native african napier grass. Carbohydr. Polym. 2018, 188, 85–91. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, S.; Zhang, X.; Takabe, K.; Xu, F. Unraveling variations of crystalline cellulose induced by ionic liquid and their effects on enzymatic hydrolysis. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Maraveas, C. Environmental Sustainability of Greenhouse Covering Materials. Sustainability 2019, 11, 6129. [Google Scholar] [CrossRef]
- Cherian, B.M.; Leão, A.L.; De Souza, S.F.; Thomas, S.; Pothen, L.; Kottaisamy, M. Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr. Polym. 2010, 81, 720–725. [Google Scholar] [CrossRef]
- Prado, K.D.S.D.; Spinacé, M.A. Characterization of fibers from pineapple’s crown, rice husks and cotton textile residues. Mater. Res. 2015, 18, 530–537. [Google Scholar] [CrossRef]
- Dai, H.; Huang, H. Synthesis, characterization and properties of pineapple peel cellulose-g-acrylic acid hydrogel loaded with kaolin and sepia ink. Cellulose 2017, 24, 69–84. [Google Scholar] [CrossRef]
- Espino, E.; Cakir, M.; Domenek, S.; Roman-Gutierrez, A.D.; Belgacem, N.; Bras, J.; Belgacem, M.N. Isolation and characterization of cellulose nanocrystals from industrial by-products of Agave tequilana and barley. Ind. Crops Prod. 2014, 62, 552–559. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- French, A.D.; Cintrón, M.S. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.-J.; Kamdem, D.P. Chemical composition, crystallinity and crystallite cellulose size in populus hybrids and aspen. Cell. Chem. Technol. 2009, 43, 229–234. [Google Scholar]
- Wattanakornsiri, A.; Pachana, K.; Kaewpirom, S.; Sawangwong, P.; Migliaresi, C. Green composites of thermoplastic corn starch and recycled paper cellulose fibers. Songklanakarin J. Sci. Technol. 2011, 33, 461–467. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Treatment | Cellulose (%) | Lignin (%) | Hemicellulose (%) | Others (%) |
|---|---|---|---|---|
| PC | 56.0 ± 5.2 | 13.1 ± 3.2 | 16.8 ± 2.2 | 14.1 ± 2.0 |
| Alkali PC | 84.7 ± 6.0 * | 4.7 ± 1.0 * | 6.8 ± 0.8 * | 3.8 ± 0.4 * |
| Mechanical Proprieties | Dry Condition | Humidity Condition |
|---|---|---|
| Tensile stress (σ, MPa) | 49 ± 2 p | 11 ± 2 p * |
| 46 ± 3 t | 9.2 ± 1.1 t * | |
| Elongation (ε, %) | 3.0 ± 0.2 p | 2.1 ± 0.4 p * |
| 2.7 ± 0.3 t | 1.8 ± 0.3 t * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choquecahua Mamani, D.; Otero Nole, K.S.; Chaparro Montoya, E.E.; Mayta Huiza, D.A.; Pastrana Alta, R.Y.; Aguilar Vitorino, H. Minimizing Organic Waste Generated by Pineapple Crown: A Simple Process to Obtain Cellulose for the Preparation of Recyclable Containers. Recycling 2020, 5, 24. https://doi.org/10.3390/recycling5040024
Choquecahua Mamani D, Otero Nole KS, Chaparro Montoya EE, Mayta Huiza DA, Pastrana Alta RY, Aguilar Vitorino H. Minimizing Organic Waste Generated by Pineapple Crown: A Simple Process to Obtain Cellulose for the Preparation of Recyclable Containers. Recycling. 2020; 5(4):24. https://doi.org/10.3390/recycling5040024
Chicago/Turabian StyleChoquecahua Mamani, Diana, Kristy Stefany Otero Nole, Efrén Eugenio Chaparro Montoya, Dora Amalia Mayta Huiza, Roxana Yesenia Pastrana Alta, and Hector Aguilar Vitorino. 2020. "Minimizing Organic Waste Generated by Pineapple Crown: A Simple Process to Obtain Cellulose for the Preparation of Recyclable Containers" Recycling 5, no. 4: 24. https://doi.org/10.3390/recycling5040024
APA StyleChoquecahua Mamani, D., Otero Nole, K. S., Chaparro Montoya, E. E., Mayta Huiza, D. A., Pastrana Alta, R. Y., & Aguilar Vitorino, H. (2020). Minimizing Organic Waste Generated by Pineapple Crown: A Simple Process to Obtain Cellulose for the Preparation of Recyclable Containers. Recycling, 5(4), 24. https://doi.org/10.3390/recycling5040024






