Biodismantling, a Novel Application of Bioleaching in Recycling of Electronic Wastes
Abstract
1. Introduction
2. Results
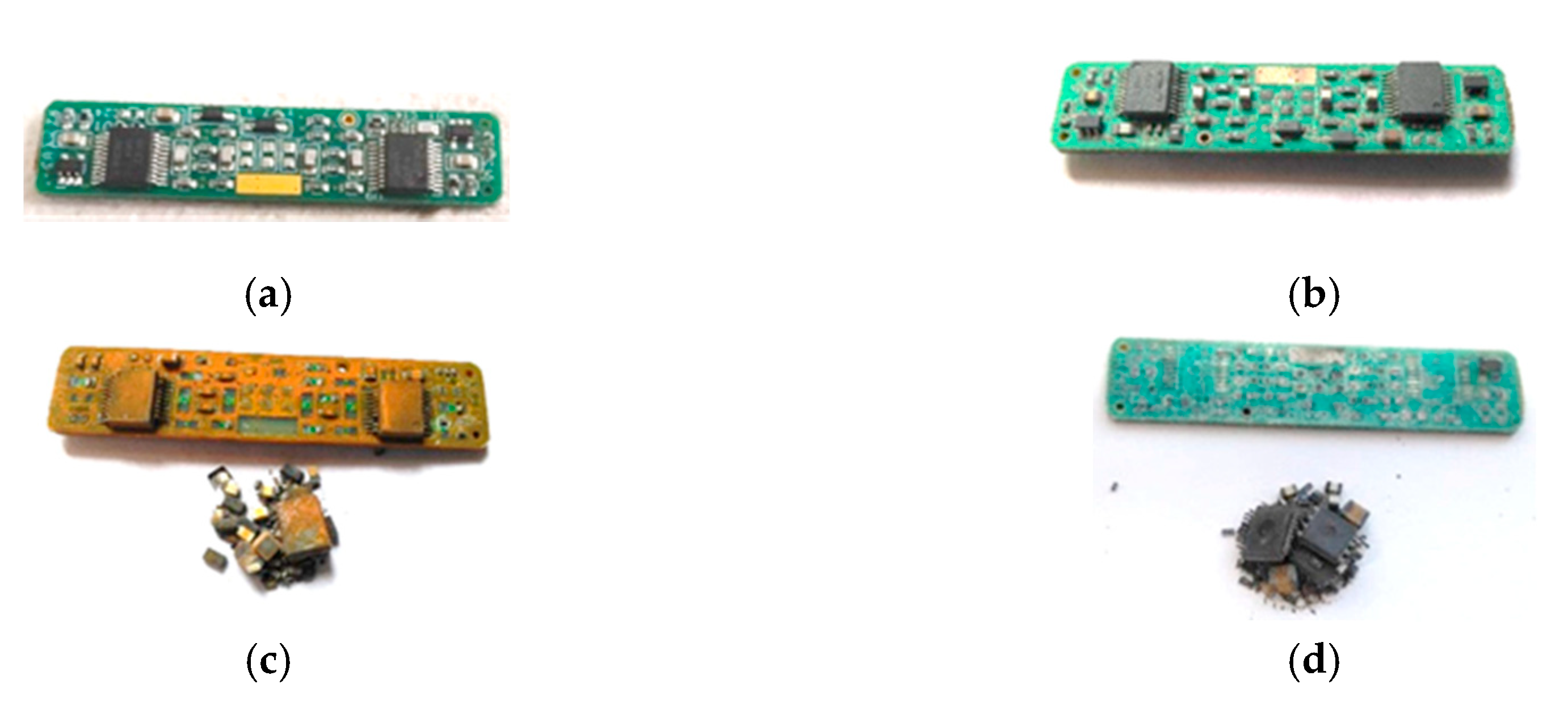
2.1. Dismantling
2.2. Characterization of Component Fractions
3. Discussion
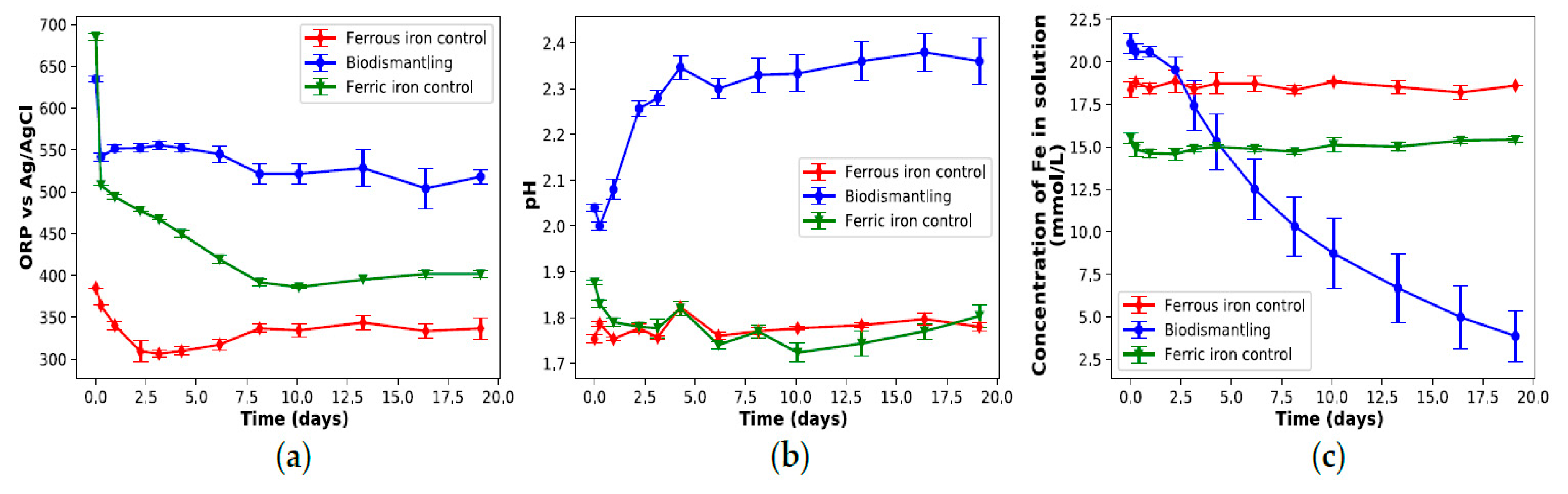
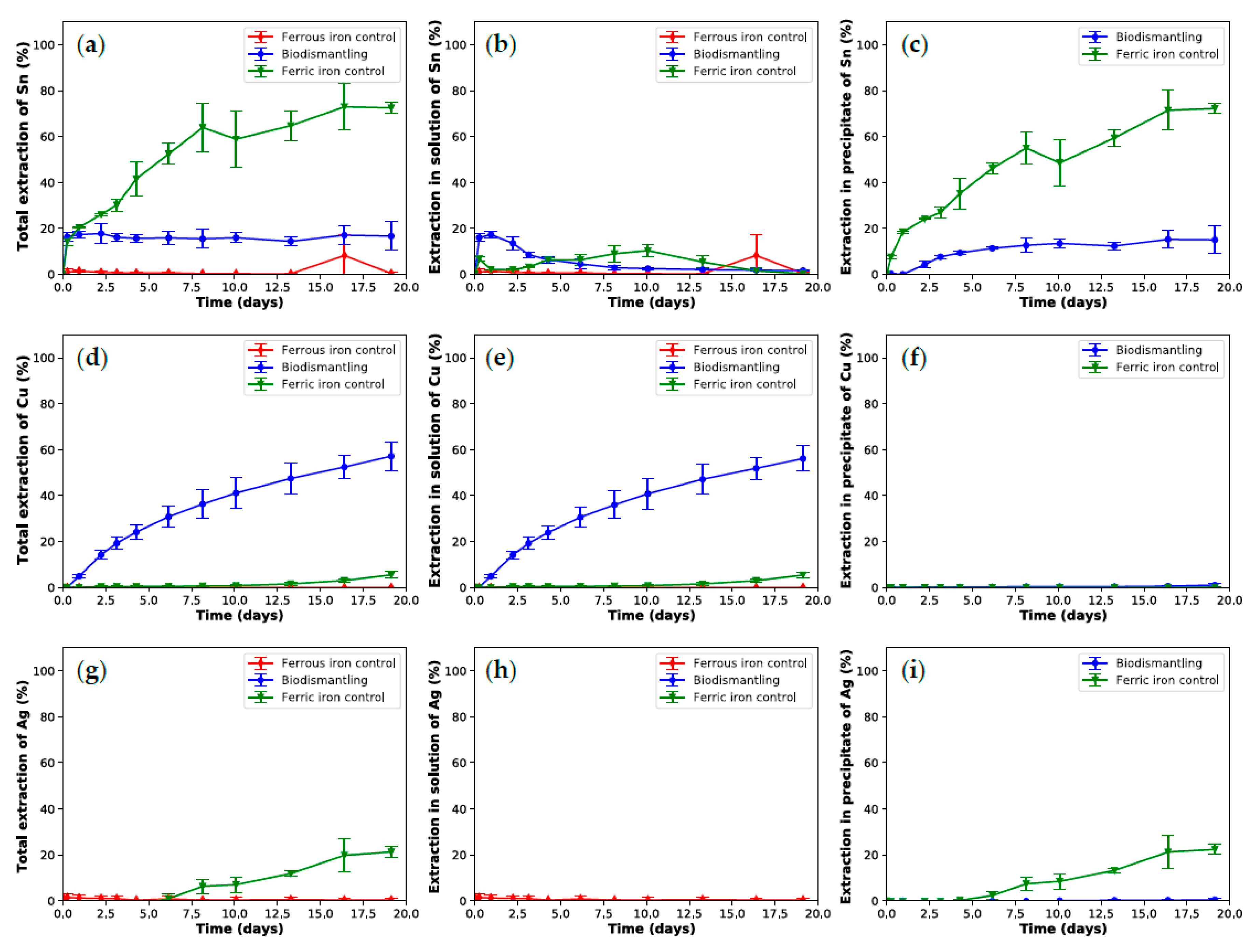
3.1. Biodismantling Concept
3.2. Recovery of Metal Released during Biodismantling
4. Materials and Methods
4.1. Printed Circuit Boards (PCBs)
4.2. Characterization of the Circuit Boards and Components
4.3. Strains and Growth Conditions
4.4. Dismantling Experiment
4.5. Chemical Measurements
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Element | Diode-Like | Big Integrated Circuits | Small Integrated Circuits | White-Capacitors-Like | Resistors-Like | Very Small EC | Brown-Capacitors-Like | Depopulated PCB | Total Waste |
|---|---|---|---|---|---|---|---|---|---|
| (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | |
| S | <LD 1 | <LD | <LD | <LD | <LD | <LD | <LD | <LD | <LD |
| Fe | 15.10 | 0.19 | 0.86 | <LD | <LD | <LD | <LD | <LD | 0.17 |
| Ni | 10.32 | <LD | 0.55 | 7.76 | 3.91 | 8.72 | 2.99 | 0.55 | 1.07 |
| Cu | 0.81 | 8.62 | 35.96 | 6.00 | 1.61 | 6.43 | 4.07 | 15.26 | 12.88 |
| Zn | 1.51 | 0.03 | 0.31 | 0.15 | 0.32 | 0.50 | 0.11 | <LD | 0.04 |
| Sn | 3.25 | 1.03 | 1.50 | 2.01 | 6.39 | 8.64 | 0.57 | 2.79 | 2.36 |
| Ba | <LD | <LD | <LD | 0.12 | 0.01 | 35.89 | 11.14 | 0.03 | 1.16 |
| (µg/g) | (µg/g) | (µg/g) | (µg/g) | (µg/g) | (µg/g) | (µg/g) | (µg/g) | (µg/g) | |
| Mn | 1363 | <LD | <LD | 2450 | 192 | 586 | 672 | <LD | 149 |
| Co | 799 | 1 | 80 | 37 | 37 | 57 | 8 | <LD | 11 |
| Ga | 19 | <LD | <LD | <LD | <LD | <LD | <LD | <LD | <1 |
| As | <LD | <LD | <LD | <LD | <LD | <LD | <LD | <LD | <LD |
| Br | <LD | <LD | <LD | <LD | <LD | <LD | <LD | <LD | <LD |
| Y | <LD | <LD | <LD | 13 | <1 | 24 | 714 | <LD | 57 |
| Ag | 365 | 35 | 211 | 380 | 228 | 729 | 41 | 1772 | 1226 |
| Cd | <LD | <LD | <LD | 7 | <LD | <LD | <LD | <LD | 0 |
| In | 107 | 32 | 48 | 65 | 206 | 278 | 18 | 86 | 73 |
| Sb | 15 | 2 | 7 | 4 | 10 | <LD | <LD | <LD | 1 |
| Ce | <LD | <LD | <LD | 3 | <LD | <LD | 2 | <LD | 5 |
| Pr | <LD | <LD | <LD | <1 | <LD | <LD | <1 | <LD | <1 |
| Nd | <LD | <LD | <LD | 1 | <LD | <LD | <1 | <LD | <1 |
| Eu | <1 | <LD | <LD | <1 | <LD | 43 | 14 | <LD | 1 |
| Gd | <LD | <LD | <LD | <1 | <LD | 2 | 39 | <LD | 3 |
| Dy | <LD | <LD | <LD | 1 | <LD | 9173 | 50 | <LD | 72 |
| Pb | <LD | 9 | <LD | <LD | 582 | <LD | <LD | <LD | 10 |
| Ru | <LD | <LD | <LD | <LD | 44 | <LD | <LD | NA 2 | 1 |
| Pd | 1 | 1 | 339 | 21 | 197 | 20 | 30 | NA | 12 |
| Os | 1 | <1 | <LD | <LD | <LD | <LD | <LD | NA | 0 |
| Ir | <LD | <LD | <LD | 1 | <LD | <LD | <LD | NA | 0 |
| Pt | <LD | <LD | <LD | 4 | <LD | <LD | <1 | NA | 0 |
Appendix B

Appendix C

References
- Eurostat. Waste Electrical and Electronic Equipment (WEEE) by Waste Management Operations. Available online: https://ec.europa.eu/eurostat/web/products-datasets/-/env_waselee (accessed on 27 June 2020).
- European Commission. Study on the review of the list of critical raw materials: Final report. Publ. Off. Eur. Union 2017. [Google Scholar] [CrossRef]
- Ueberschaar, M. Assessing Recycling Strategies for Critical Raw Materials in Waste Electrical and Electronic Equipment. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2017. [Google Scholar]
- Cucchiella, F.; D’Adamo, I.; Lenny Koh, S.C.; Rosa, P. Recycling of WEEEs: An economic assessment of present and future e-waste streams. Renew. Sustain. Energy Rev. 2015, 51, 263–272. [Google Scholar] [CrossRef]
- Umicore. Lanthanum. Available online: https://www.umicore.com/en/about/elements/lanthanum/ (accessed on 6 May 2020).
- White, N. Thick films. In Springer Handbook of Electronic and Photonic Materials, 2nd ed.; Kasap, S., Capper, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 707–721. ISBN 978-3-319-48931-5. [Google Scholar]
- John, G. Basic Tantalum Capacitor Technology. Available online: http://www.avx.com/docs/techinfo/3_Basic.pdf (accessed on 27 June 2020).
- Kaya, M. WPCB Recycling chain and treatment options. In Electronic Waste and Printed Circuit Board Recycling Technologies; Kaya, M., Ed.; Springer: Cham, Switzerland, 2019; pp. 59–68. ISBN 978-3-030-26593-9. [Google Scholar]
- Kaya, M. Printed circuit boards (PCBs). In Electronic Waste and Printed Circuit Board Recycling Technologies; Kaya, M., Ed.; Springer: Cham, Switzerland, 2019; pp. 33–57. ISBN 978-3-030-26593-9. [Google Scholar]
- Lead-free Soldering; Bath, J., Ed.; Springer: New York, NY, USA, 2007; ISBN 978-0-387-32466-1. [Google Scholar]
- Ma, H.; Suhling, J.C.; Lall, P.; Bozack, M.J. Reliability of the aging lead free solder joint. In Proceedings of the 56th Electronic Components and Technology Conference 2006, San Diego, CA, USA, 30 May–2 June 2006; pp. 849–864, ISBN 1-4244-0152-6. [Google Scholar]
- Abdelbasir, S.M.; El-Sheltawy, C.T.; Abdo, D.M. Green processes for electronic waste recycling: A review. J. Sustain. Metall. 2018, 4, 295–311. [Google Scholar] [CrossRef]
- Schippers, A.; Hedrich, S.; Vasters, J.; Drobe, M.; Sand, W.; Willscher, S. Biomining: Metal recovery from ores with microorganisms. Adv. Biochem. Eng. Biotechnol. 2014, 141, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D. The evolution, current status, and future prospects of using biotechnologies in the mineral extraction and metal recovery sectors. Minerals 2018, 8, 343. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019. [Google Scholar] [CrossRef]
- Hubau, A.; Minier, M.; Chagnes, A.; Joulian, C.; Silvente, C.; Guezennec, A.-G. Recovery of metals in a double-stage continuous bioreactor for acidic bioleaching of printed circuit boards (PCBs). Sep. Purif. Technol. 2020, 238, 116481. [Google Scholar] [CrossRef]
- Adhapure, N.N.; Dhakephalkar, P.K.; Dhakephalkar, A.P.; Tembhurkar, V.R.; Rajgure, A.V.; Deshmukh, A.M. Use of large pieces of printed circuit boards for bioleaching to avoid ‘precipitate contamination problem’ and to simplify overall metal recovery. MethodsX 2014, 1, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hocheng, H.; Hong, T.; Jadhav, U. Microbial leaching of waste solder for recovery of metal. Appl. Biochem. Biotechnol. 2014, 173, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Pourbaix, M. Atlas of Electrochemical Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Pu, Y.; Chen, W.; Chen, S.; Langhammer, H.T. Microstructure and dielectric properties of dysprosium-doped barium titanate ceramics. Cerâmica 2005, 51, 214–218. [Google Scholar] [CrossRef]
- Wang, M.-J.; Yang, H.; Zhang, Q.-L.; Lin, Z.-S.; Zhang, Z.-S.; Yu, D.; Hu, L. Microstructure and dielectric properties of BaTiO3 ceramic doped with yttrium, magnesium, gallium and silicon for AC capacitor application. Mater. Res. Bull. 2014, 60, 485–491. [Google Scholar] [CrossRef]
- Zapp, P.; Marx, J.; Schreiber, A.; Friedrich, B.; Voßenkaul, D. Comparison of dysprosium production from different resources by life cycle assessment. Resour. Conserv. Recycl. 2018, 130, 248–259. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Comprehensive characterization of printed circuit boards of various end-of-life electrical and electronic equipment for beneficiation investigation. Waste Manag. 2018, 75, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Chi-lung, Y. Abundance of chemical elements in the earth’s crust and its major tectonic units. Int. Geol. Rev. 1970, 12, 778–786. [Google Scholar] [CrossRef]
- Ho, J.; Jow, T.R.; Boggs, S. Historical introduction to capacitor technology. IEEE Electr. Insul. Mag. 2010, 26, 20–25. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S. A Review of current flame retardant systems for epoxy resins. J. Fire Sci. 2016, 22, 25–40. [Google Scholar] [CrossRef]
- Guo, J.; Guo, J.; Xu, Z. Recycling of non-metallic fractions from waste printed circuit boards: A review. J. Hazard. Mater. 2009, 168, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Jones, F.S.; Bigham, J.M.; Tuovinen, O.H. Synthesis of argentojarosite with simulated bioleaching solutions produced by Acidithiobacillus ferrooxidans. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Giebner, F.; Kaden, L.; Wiche, O.; Tischler, J.; Schopf, S.; Schlömann, M. Bioleaching of cobalt from an arsenidic ore. Miner. Eng. 2019, 131, 73–78. [Google Scholar] [CrossRef]
| Sample | Time (day) | Mass Share (%) of Metal in Precipitate of | Sum (mg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | Ni | Cu | Zn | Ag | In | Sn | |||
| Biodismantling | 19 | 89.3 | 0.1 | 2.8 | 0.4 | <0.1 | <0.1 | 7.4 | 181 |
| Ferric control | 19 | 8.4 | 0.1 | 0.5 | 0.9 | 1.4 | 0.3 | 88.4 | 72.5 |
| Element | Diode Like | Big Integrated Circuits | Small Integrated Circuits | White Capacitors-Like | Resistors-Like | Very Small EC | Brown Capapacitors-like | Depopulated PCB | Total Waste |
|---|---|---|---|---|---|---|---|---|---|
| (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | |
| Fe | 15.1 | 0.19 | 0.86 | <LD 1 | <LD | <LD | <LD | <LD | 0.17 |
| Ni | 10.3 | <LD | 0.55 | 7.76 | 3.91 | 8.72 | 2.99 | 0.55 | 1.07 |
| Cu | 0.81 | 8.62 | 36.0 | 6.00 | 1.61 | 6.43 | 4.07 | 15.3 | 12.9 |
| Zn | 1.51 | 0.03 | 0.31 | 0.15 | 0.32 | 0.50 | 0.11 | <LD | 0.04 |
| Sn | 3.25 | 1.03 | 1.50 | 2.01 | 6.39 | 8.64 | 0.57 | 2.79 | 2.36 |
| Ba | <LD | <LD | <LD | 0.12 | 0.01 | 35.9 | 11.1 | 0.03 | 1.16 |
| (µg/g) | (µg/g) | (µg/g) | (µg/g) | (µg/g) | (µg/g) | (µg/g) | (µg/g) | (µg/g) | |
| Mn | 1363 | <LD | <LD | 2450 | 192 | 586 | 672 | <LD | 149 |
| Co | 799 | 1 | 80 | 37 | 37 | 57 | 8 | <LD | 11 |
| Ga | 19 | <LD | <LD | <LD | <LD | <LD | <LD | <LD | <1 |
| As | <LD | <LD | <LD | <LD | <LD | <LD | <LD | <LD | <LD |
| Y | <LD | <LD | <LD | 13 | <1 | 24 | 714 | <LD | 57 |
| Ag | 365 | 35 | 211 | 380 | 228 | 729 | 41 | 1772 | 1226 |
| In | 107 | 32 | 48 | 65 | 206 | 278 | 18 | 86 | 73 |
| Sb | 15 | 2 | 7 | 4 | 10 | <LD | <LD | <LD | 1 |
| Dy | <LD | <LD | <LD | 1 | <LD | 9173 | 50 | <LD | 72 |
| Pb | <LD | 9 | <LD | <LD | 582 | <LD | <LD | <LD | 10 |
| Ru | <LD | <LD | <LD | <LD | 44 | <LD | <LD | NA 2 | 1 |
| Pd | 1 | 1 | 339 | 21 | 197 | 20 | 30 | NA | 12 |
| Fraction Name | Mass of Fraction. Mean and Standard Deviation (mg) | Part of the Total Waste (wt.%) |
|---|---|---|
| Depopulated PCB | 2511 ± 5 | 67.3 |
| Diode-like | 30 ± 0 | 0.8 |
| Big integrated circuits | 638 ± 2 | 17.1 |
| Small integrated circuits | 61 ± 1 | 1.6 |
| White capacitor-like | 119 ± 5 | 3.2 |
| Brown capacitor-like | 295 ± 2 | 7.9 |
| Resistor-like | 61 ± 3 | 1.6 |
| Very small ECs 1 | 27 ± 2 | 0.7 |
| Total | 3733 ± 14 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monneron-Enaud, B.; Wiche, O.; Schlömann, M. Biodismantling, a Novel Application of Bioleaching in Recycling of Electronic Wastes. Recycling 2020, 5, 22. https://doi.org/10.3390/recycling5030022
Monneron-Enaud B, Wiche O, Schlömann M. Biodismantling, a Novel Application of Bioleaching in Recycling of Electronic Wastes. Recycling. 2020; 5(3):22. https://doi.org/10.3390/recycling5030022
Chicago/Turabian StyleMonneron-Enaud, Benjamin, Oliver Wiche, and Michael Schlömann. 2020. "Biodismantling, a Novel Application of Bioleaching in Recycling of Electronic Wastes" Recycling 5, no. 3: 22. https://doi.org/10.3390/recycling5030022
APA StyleMonneron-Enaud, B., Wiche, O., & Schlömann, M. (2020). Biodismantling, a Novel Application of Bioleaching in Recycling of Electronic Wastes. Recycling, 5(3), 22. https://doi.org/10.3390/recycling5030022






