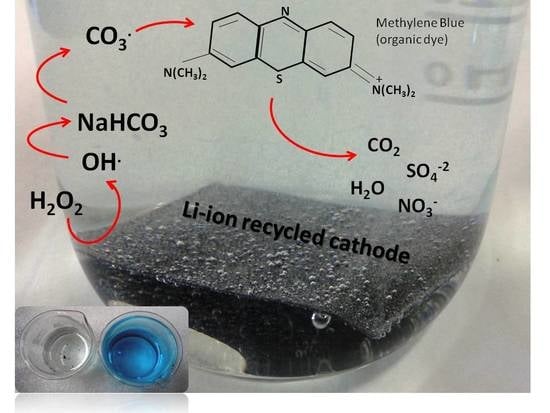
Fast Electrochemical Method for Organic Dye Decolorization Using Recycled Li-Ion Batteries
Abstract
:1. Introduction
2. Material and Methods
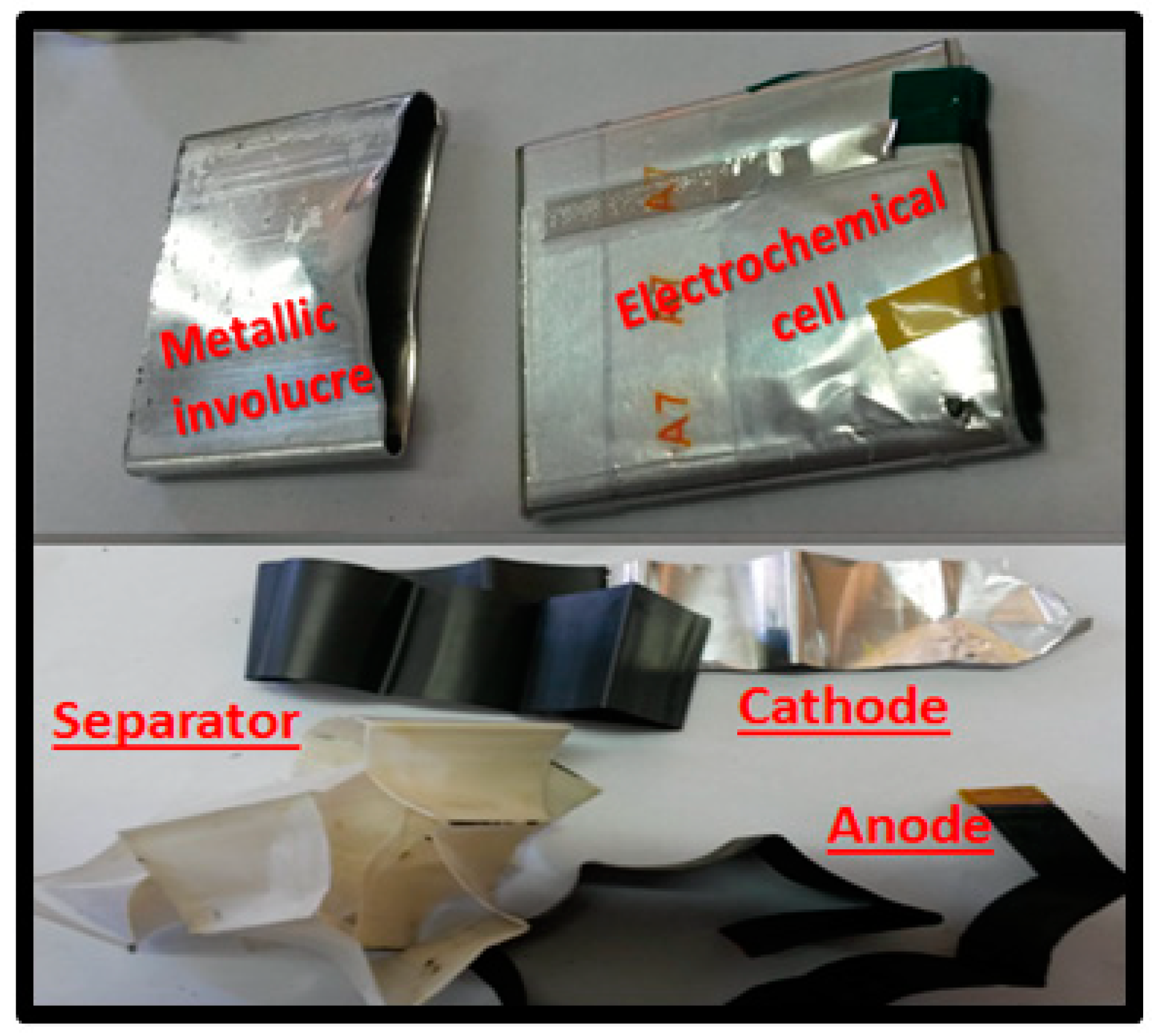
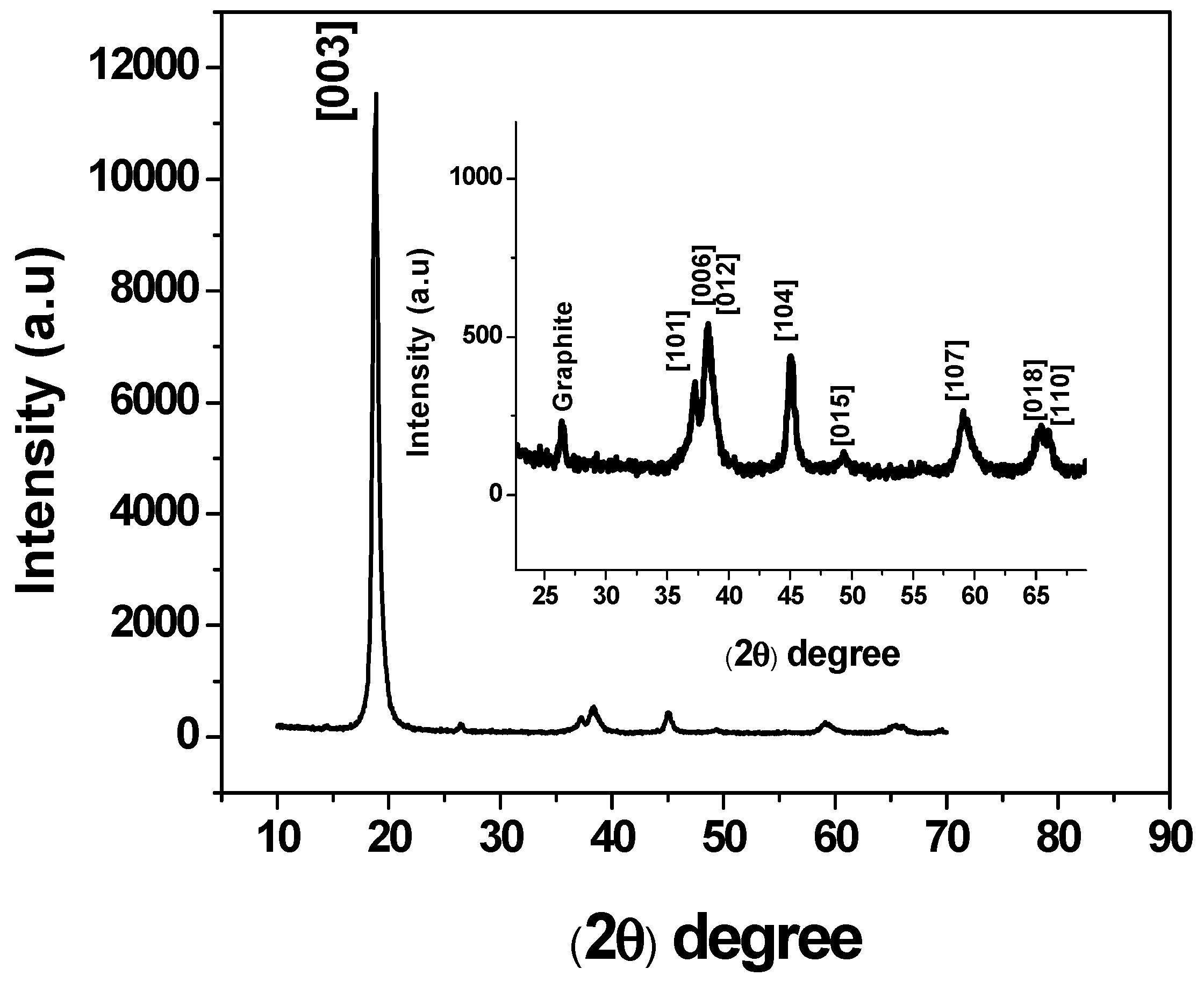
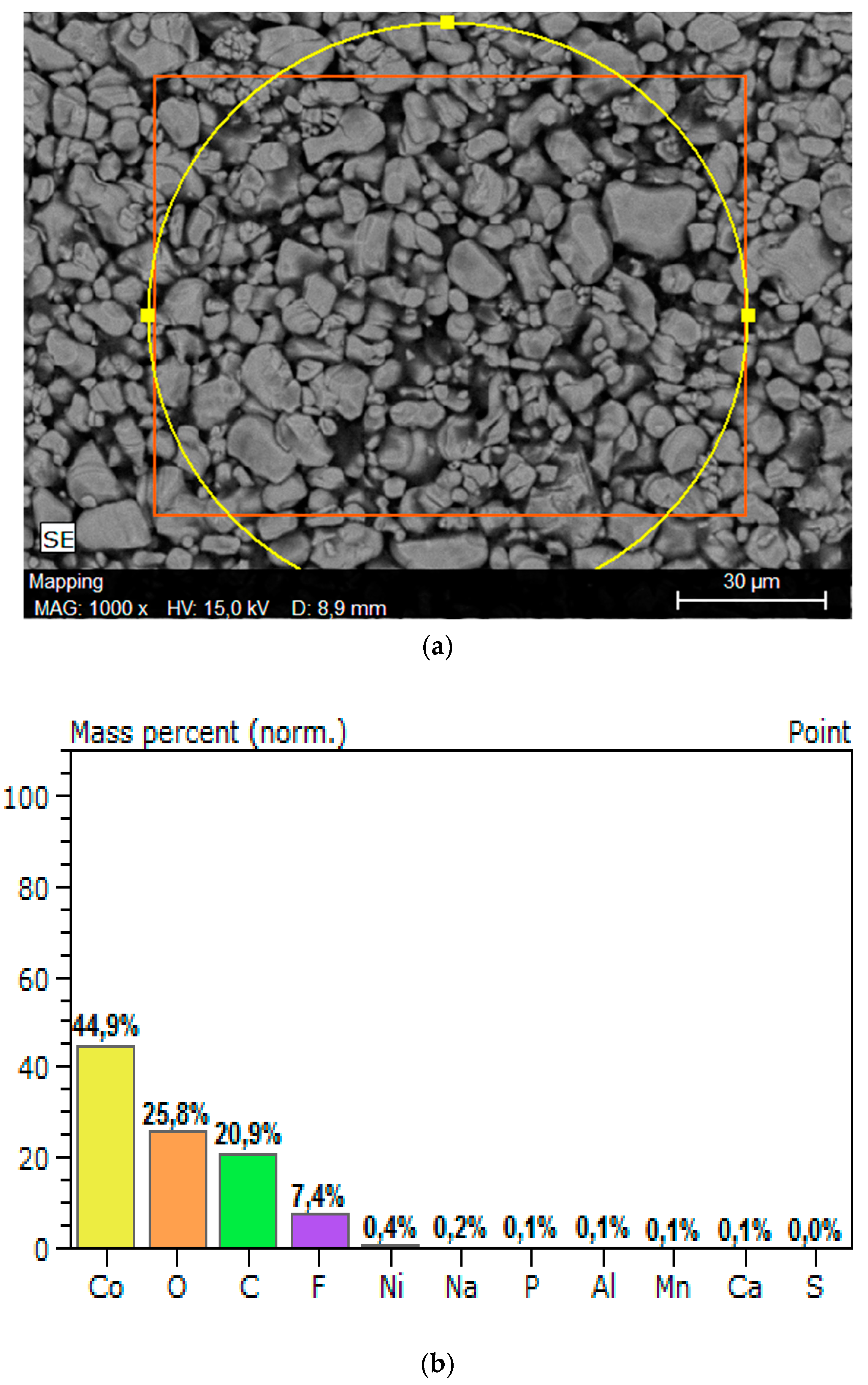
2.1. Obtaining and Characterization of Li-Ion Battery Spent Cathodes (LIB/SC)
2.2. Methylene Blue Decolorization Analysis
2.3. Linear Voltammetry Measurements
3. Results and Discussion
3.1. Characterization of Li-Ion Battery Spent Cathodes (LIB-SC)
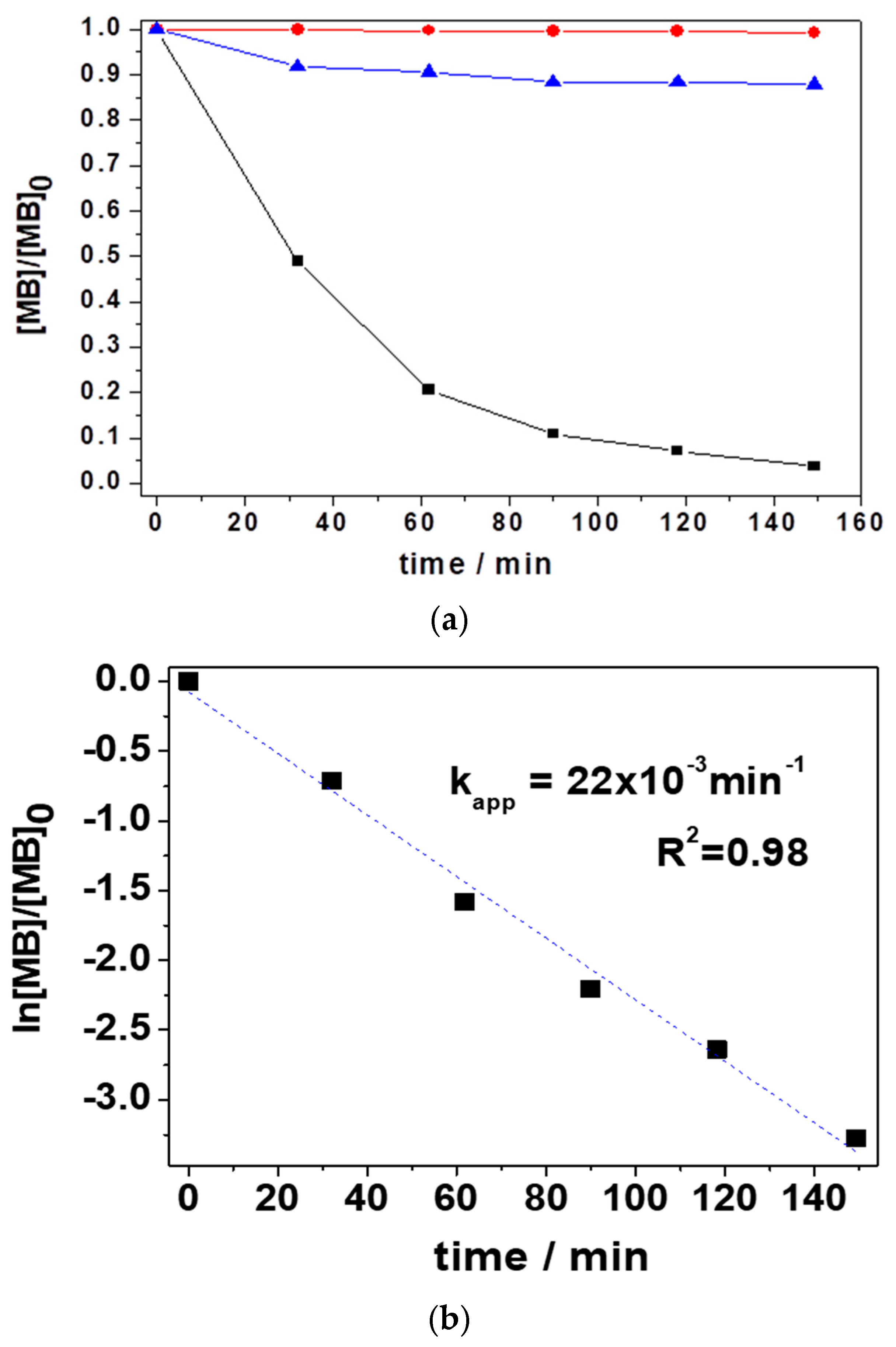
3.2. Decolorization of MB Solution Using LIB-SC+H2O2/NaHCO3 System
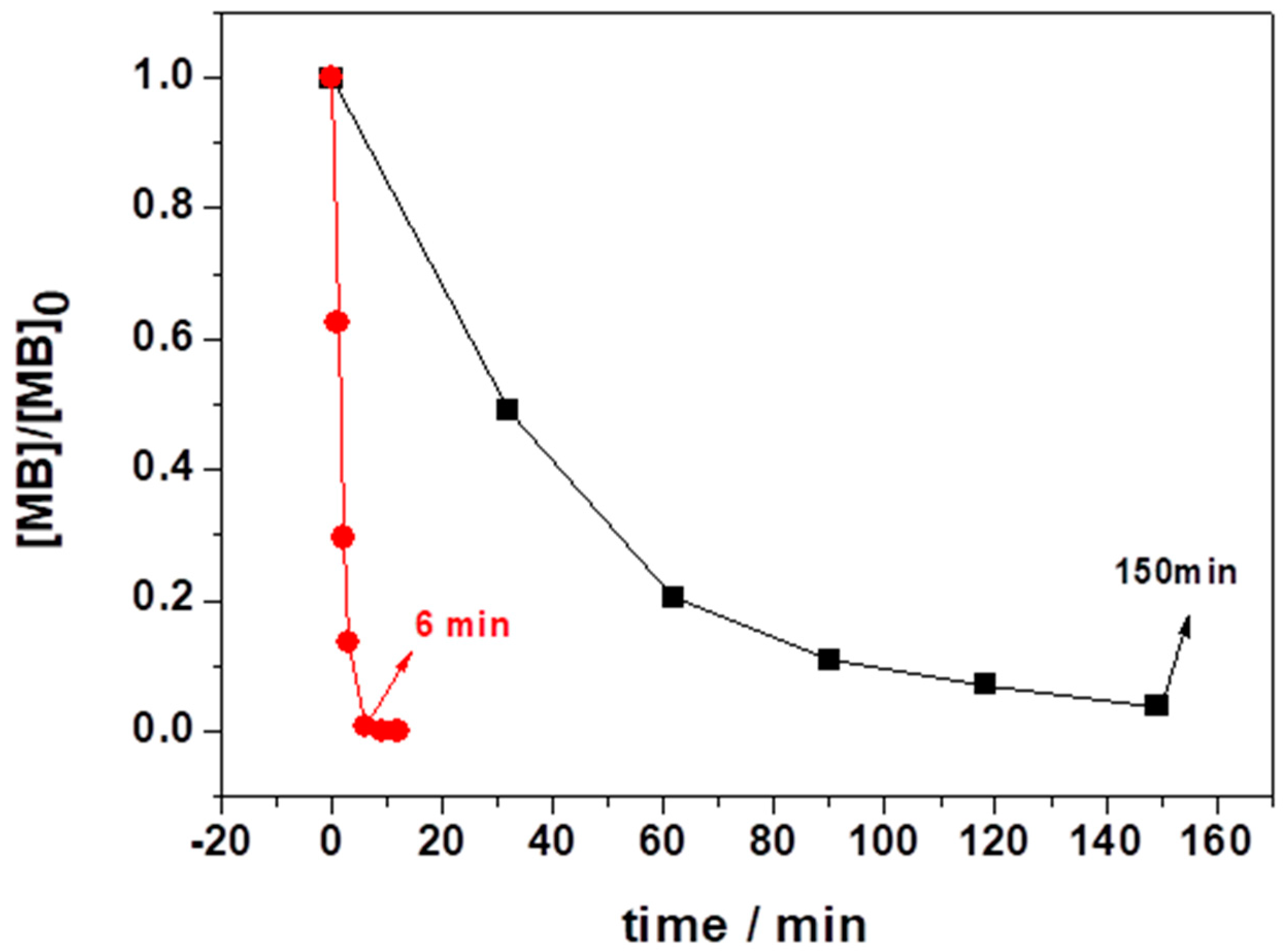
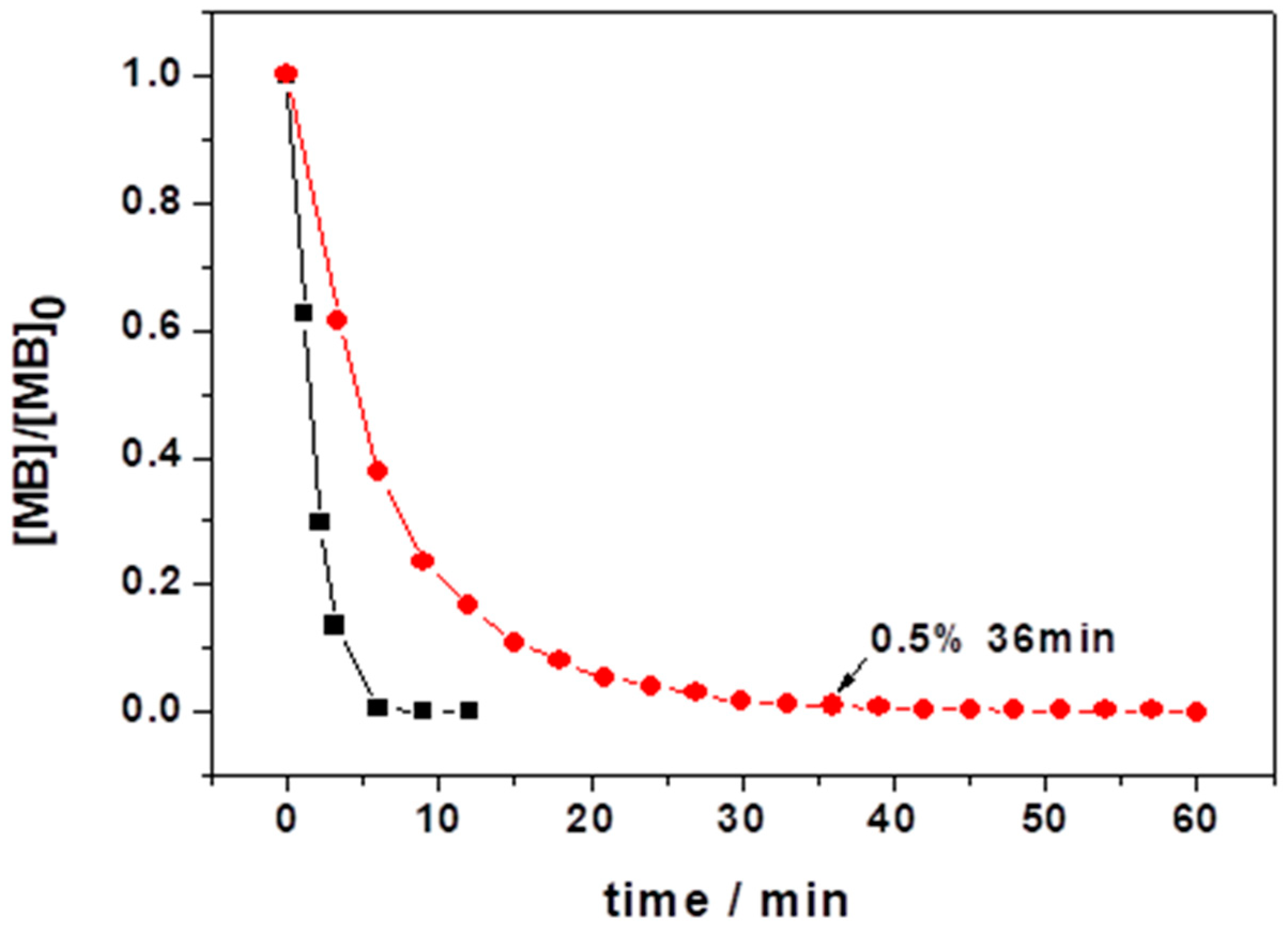
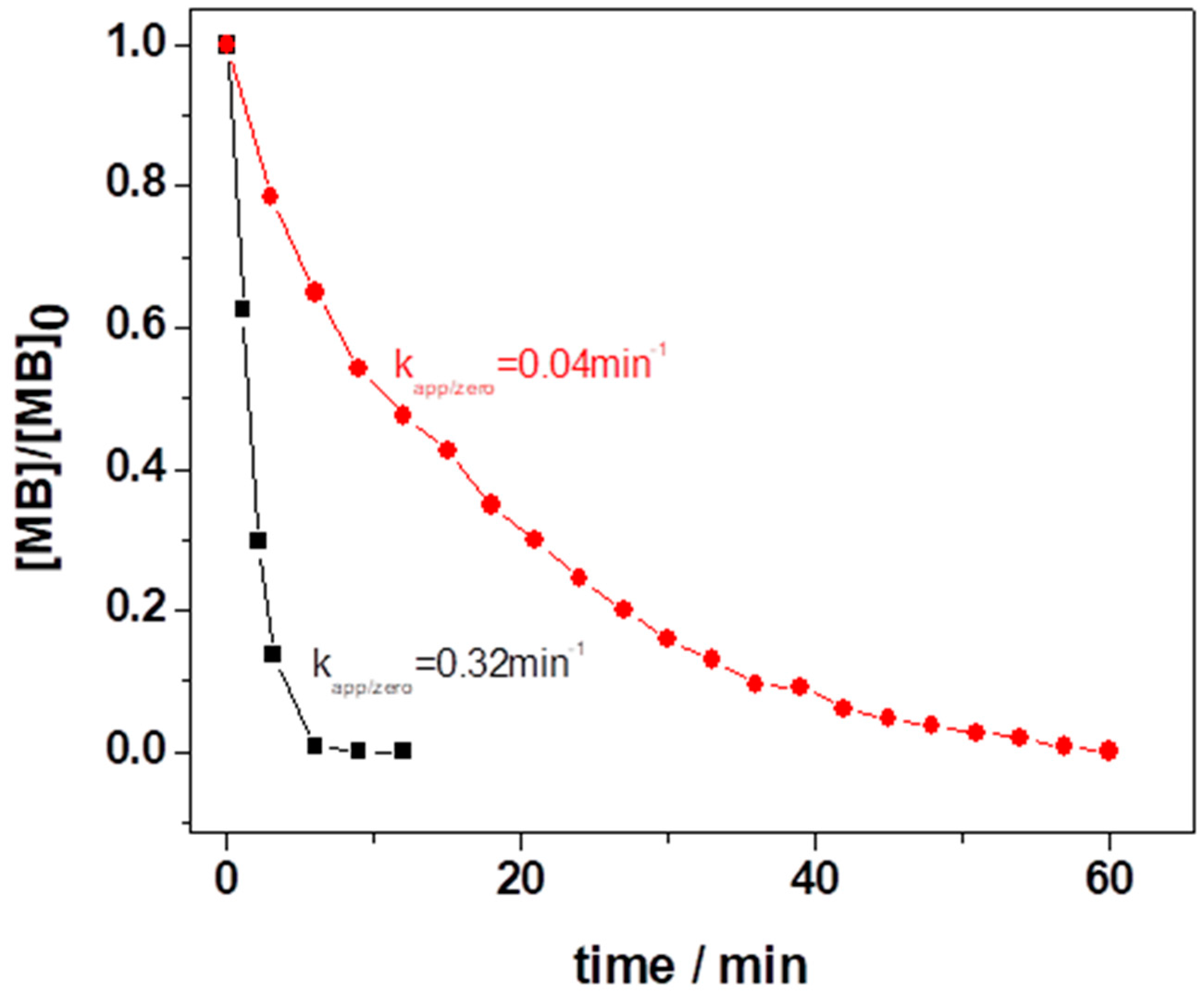
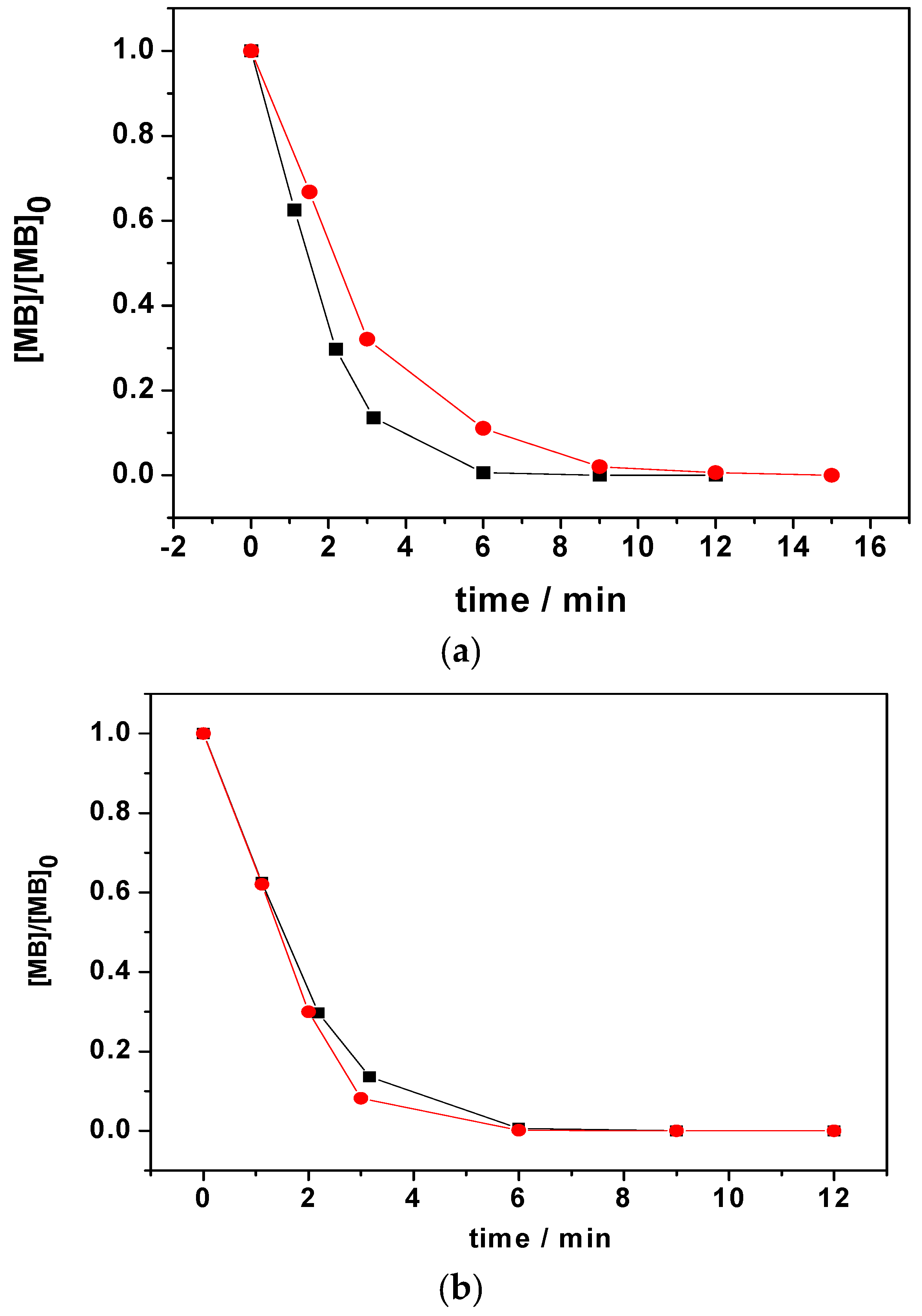
3.2.1. Variation of [MB]0 and LIB-SC Area in Decolorization of MB Using LIB-SC+H2O2/NaHCO3 System
3.2.2. Variation of [H2O2]0 and [NaHCO3]0 in Decolorization of MB Using LIB-SC+H2O2/NaHCO3 System
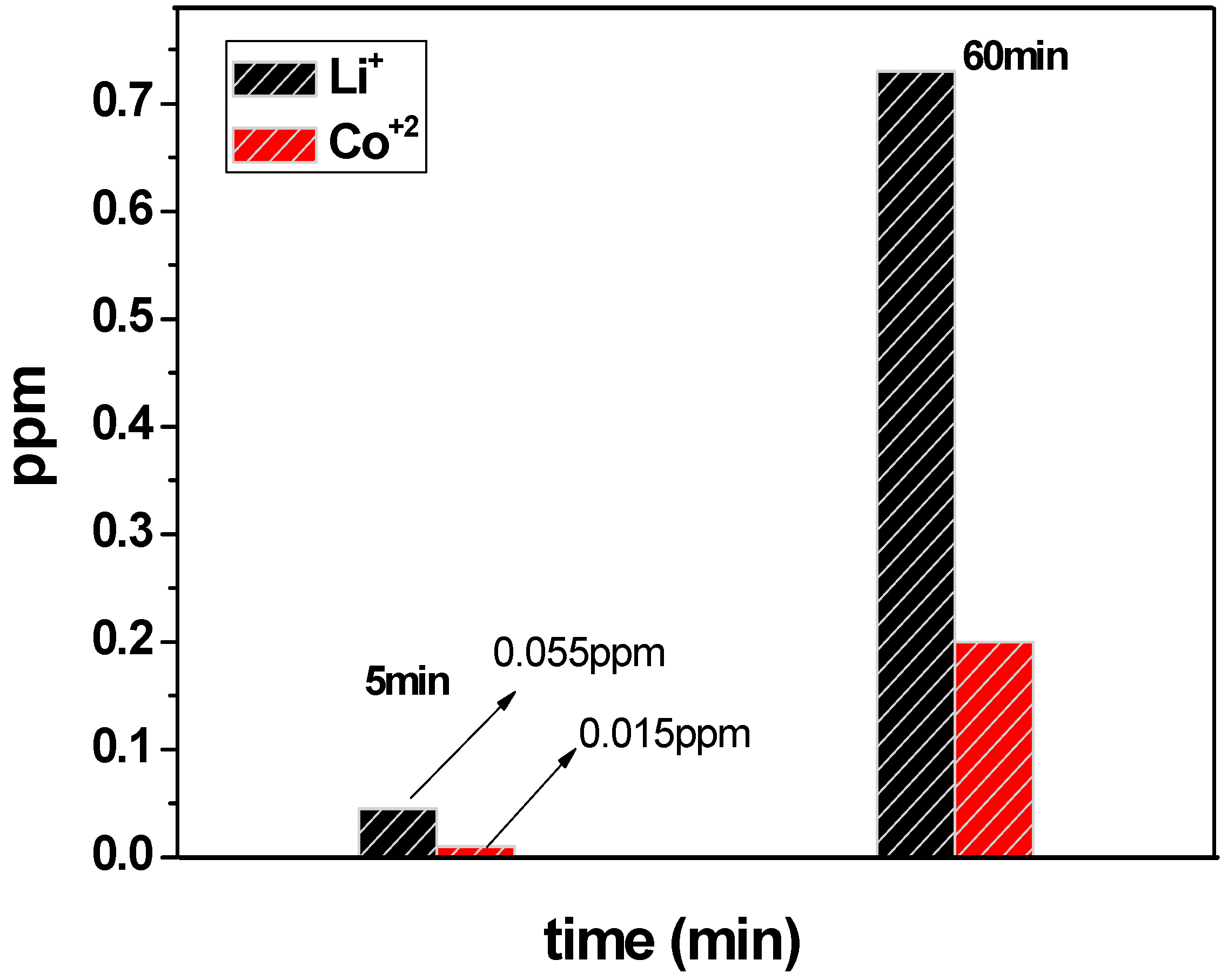
3.3. Linear Polarization Analyses and Co and Li Lixiviation
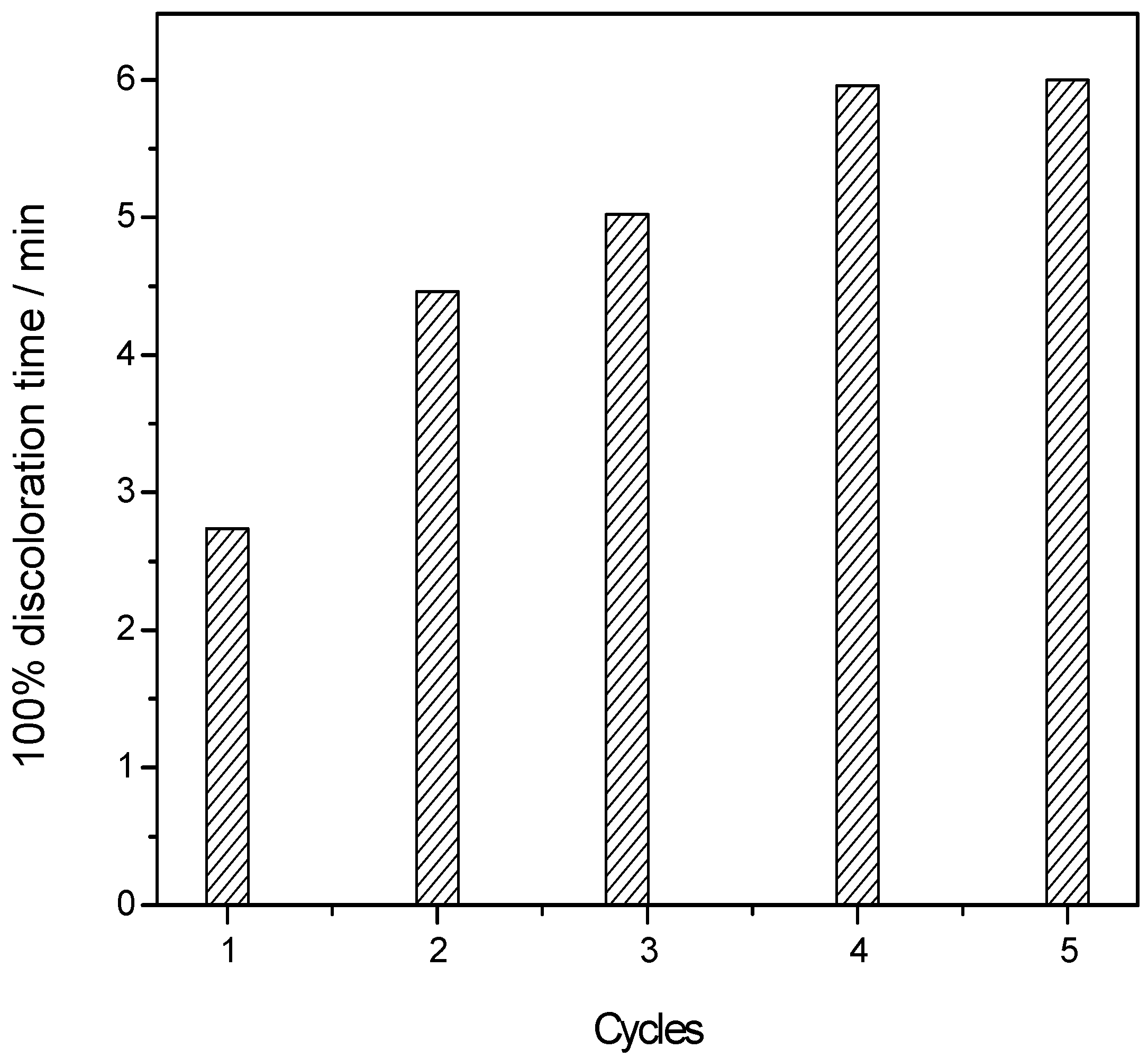
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brousselya, M.; Biensan, P.; Simon, B. Lithium insertion into host materials: The key to success for Li ion batteries. Electrochim. Acta 1999, 45, 3–22. [Google Scholar] [CrossRef]
- Garcia, E.M.; Taroco, H.A.; Matencio, T.; Domingues, R.Z.; Santos, J.A.F.; Freitas, M.B.J.G. Electrochemical recycling of cobalt from spent cathodes of lithium-ion batteries: Its application as coating on SOFC interconnects. J. Appl. Electrochem. 2011, 41, 1373–1379. [Google Scholar] [CrossRef]
- Georgi, M.T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Alessandro, P.; Raana, K.S.; Sajad, Y.; Michael, P.; William, E.M. High performance bi-metallic manganese cobalt oxide/carbon nanotube li-ion battery anodes. Electrochim. Acta 2016, 213, 620–625. [Google Scholar]
- Repp, S.; Harputlu, E.; Gurgen, S.; Castellano, M.; Kremer, N.; Pompe, N.; Worner, J.; Hoffmann, A.; Thomann, R.; Emen, F.M.; et al. Synergetic effects of Fe3+ doped spinel Li4Ti5O12 nanoparticles on reduced graphene oxide for high surface electrode hybrid supercapacitors. Nanoscale 2018, 10, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.G.; Ocakoglu, K.; Emre, E. High-Capacitance Hybrid Supercapacitor Based on Multi-Colored Fluorescent Carbon-Dots. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Quan, S.H.; Xie, C.J. A new supercapacitor and li-ion battery hybrid system for electric vehicle in ADVISOR. J. Phys.Conf. Ser. 2017. [Google Scholar] [CrossRef]
- Jelena, S.; Danica, B.B.; Divna, M.; Elena, T.; Jelena, P.; Milica, V. The synthesis of Li(Co-Mn-Ni)O2 cathode material from spent-Li ion batteries and the proof of its functionality in aqueous lithium and sodium electrolytic solutions. J. Power Sources 2017, 342, 690–703. [Google Scholar]
- Sami, V.M.F.F.; Antero, L.T.S. Solvent extraction fractionation of Li-ion battery leachate containing Li, Ni, and Co. Sep. Purif. Technol. 2017, 179, 274–282. [Google Scholar]
- Eric, M.G.; Hosane, A.T.; Ana, P.C.M.; Amauri, G.S.; Rafael, R.A.S.; Júlio, O.F.M.; Cristiane, G.T.; Quele, C.P.T. Application of spent Li-ion batteries cathode in methylene blue dye discoloration. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Kyle, S.H.; Timothy, W.M.; Ireneusz, J.; Kotchaphan, K.; Christopher, M.S.; David, M.B. Carbonate radical formation in radiolysis of sodium carbonate and bicarbonate solutions up to 250 °C and the mechanism of its second order decay. J. Phys. Chem. A 2010, 114, 2142–2150. [Google Scholar]
- Liu, W.; Qian, J.; Wang, K.; Xu, H.; Jiang, D.; Liu, Q.; Yang, X.W.; Li, H.; Huaming, L. Magnetically Separable Fe3O4 Nanoparticles-Decorated Reduced Graphene Oxide Nanocomposite for Catalytic Wet Hydrogen Peroxide Oxidation. J. Inorg. Organomet. Polym. 2013, 23, 907–916. [Google Scholar] [CrossRef]
- Keisuke, I.; Mohamed, G.E.-D. Aqueous pesticide degradation by hydrogen peroxide/ultraviolet irradiation and Fenton-type advanced oxidation processes: A review. J. Environ. Eng. Sci. 2006, 5, 81–135. [Google Scholar]
- Riccardo, R.; Fabio, L.M.; Colin, W.; Robert, A.H.; Yi, C. Electrochemical characterization of LiCoO2 as rechargeable electrode in aqueous LiNO3 electrolyte. Solid State Ion. 2011, 192, 289–292. [Google Scholar]
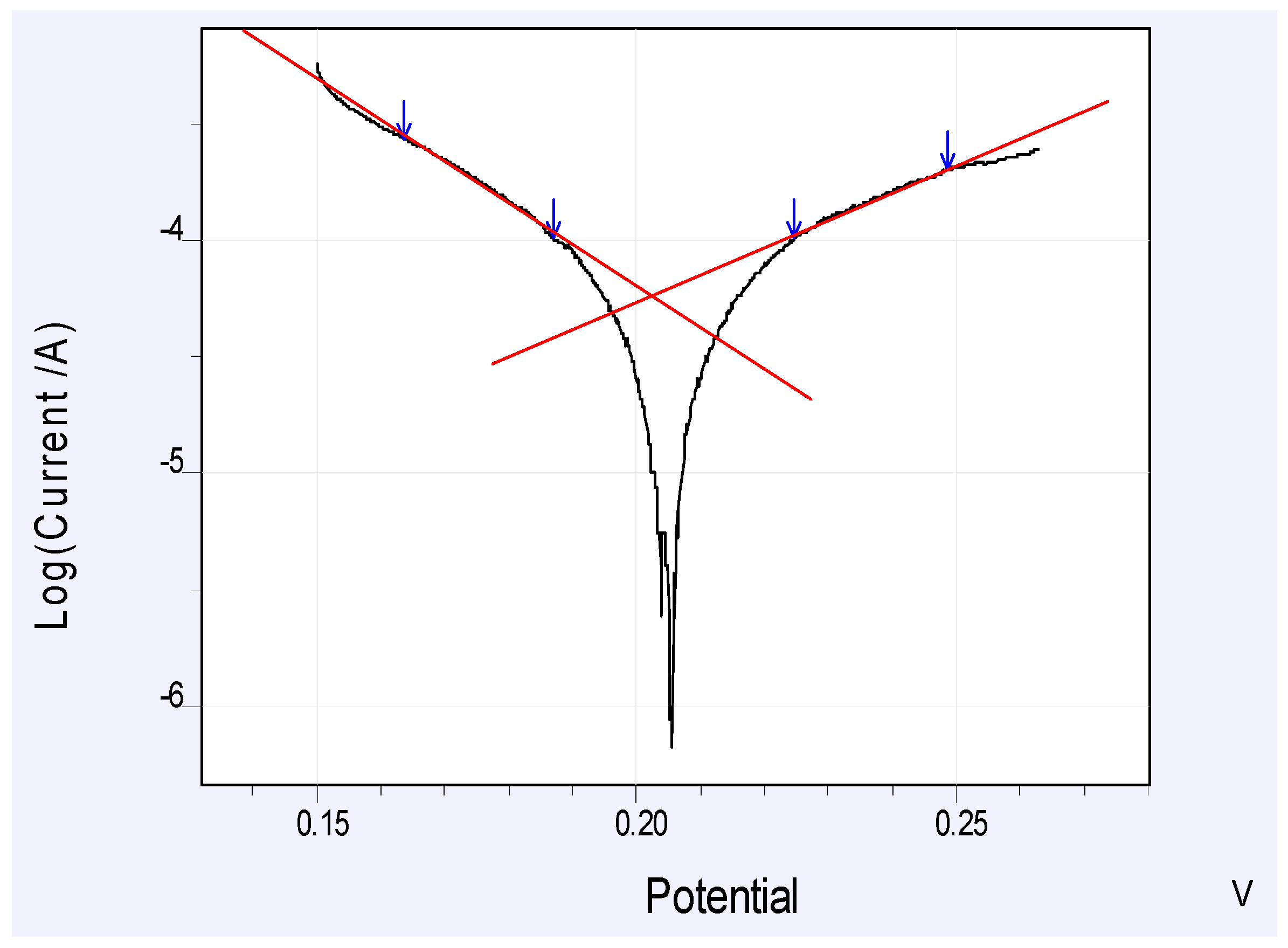
| Parameter | Value |
|---|---|
| E | 202.4 mV |
| i | 56.46 μAcm−2 |
| ba Vdec−1 | 0.152 |
| bc Vdec−1 | 0.096 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, E.M.; Taroco, H.A.; Teixeira, R.G. Fast Electrochemical Method for Organic Dye Decolorization Using Recycled Li-Ion Batteries. Recycling 2018, 3, 35. https://doi.org/10.3390/recycling3030035
Garcia EM, Taroco HA, Teixeira RG. Fast Electrochemical Method for Organic Dye Decolorization Using Recycled Li-Ion Batteries. Recycling. 2018; 3(3):35. https://doi.org/10.3390/recycling3030035
Chicago/Turabian StyleGarcia, Eric M., Hosane A. Taroco, and Rodrigo G. Teixeira. 2018. "Fast Electrochemical Method for Organic Dye Decolorization Using Recycled Li-Ion Batteries" Recycling 3, no. 3: 35. https://doi.org/10.3390/recycling3030035
APA StyleGarcia, E. M., Taroco, H. A., & Teixeira, R. G. (2018). Fast Electrochemical Method for Organic Dye Decolorization Using Recycled Li-Ion Batteries. Recycling, 3(3), 35. https://doi.org/10.3390/recycling3030035