Design of an Energy-Efficient Pilot-Scale Pyrolysis Reactor Using Low-Cost Insulating Materials
Abstract
1. Introduction
2. Results
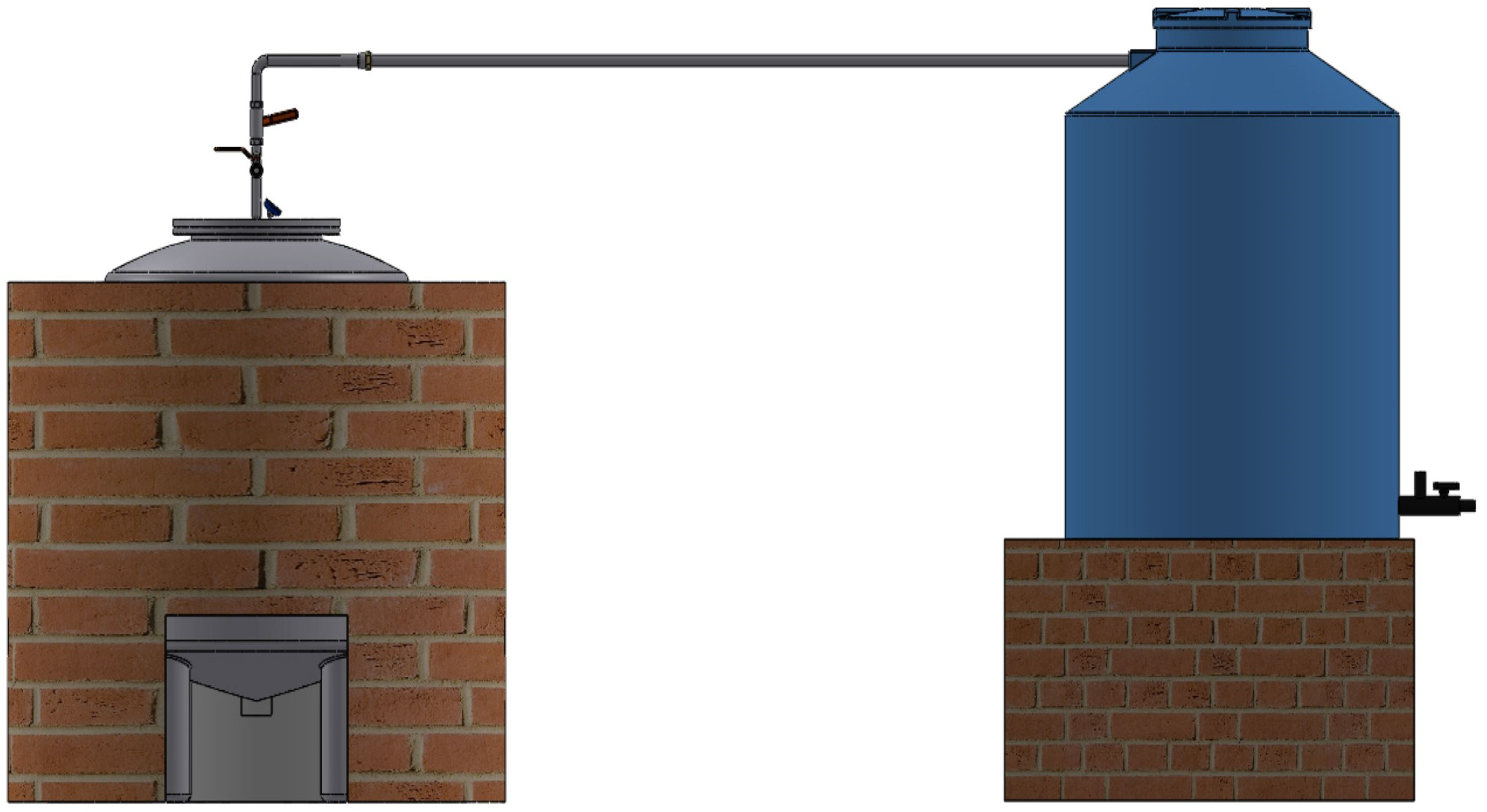
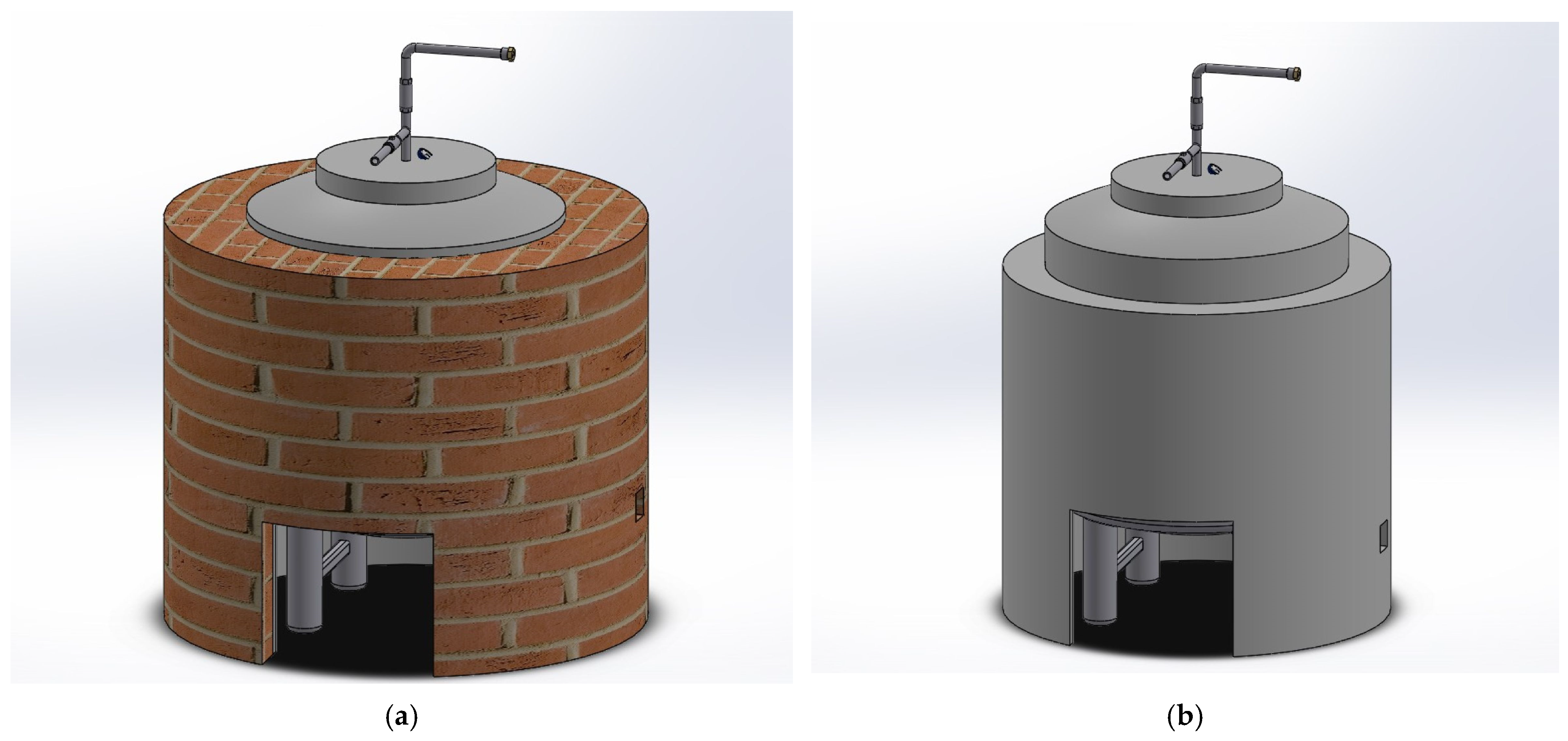
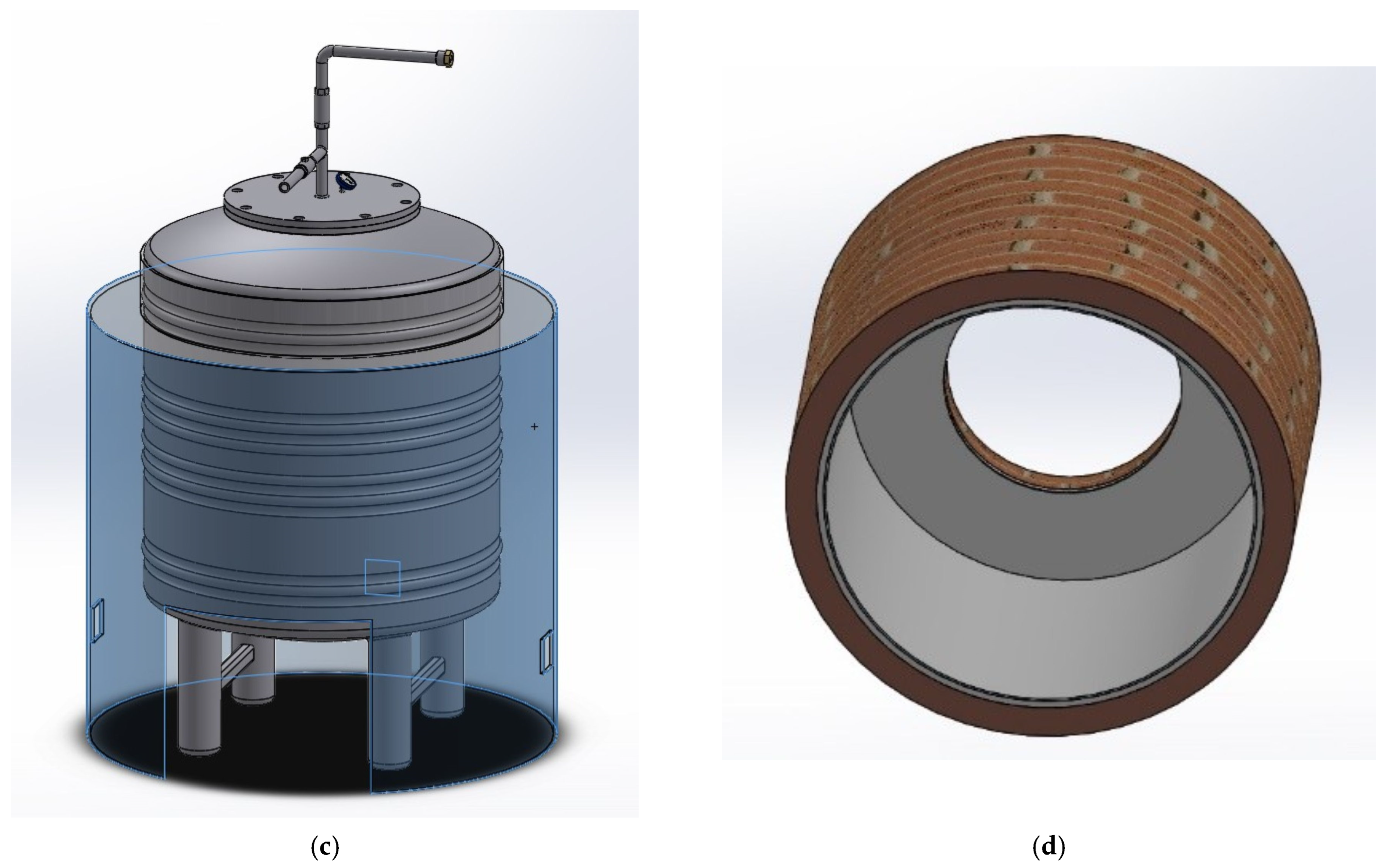
2.1. Prototype Description
2.2. Thermal Properties of Materials
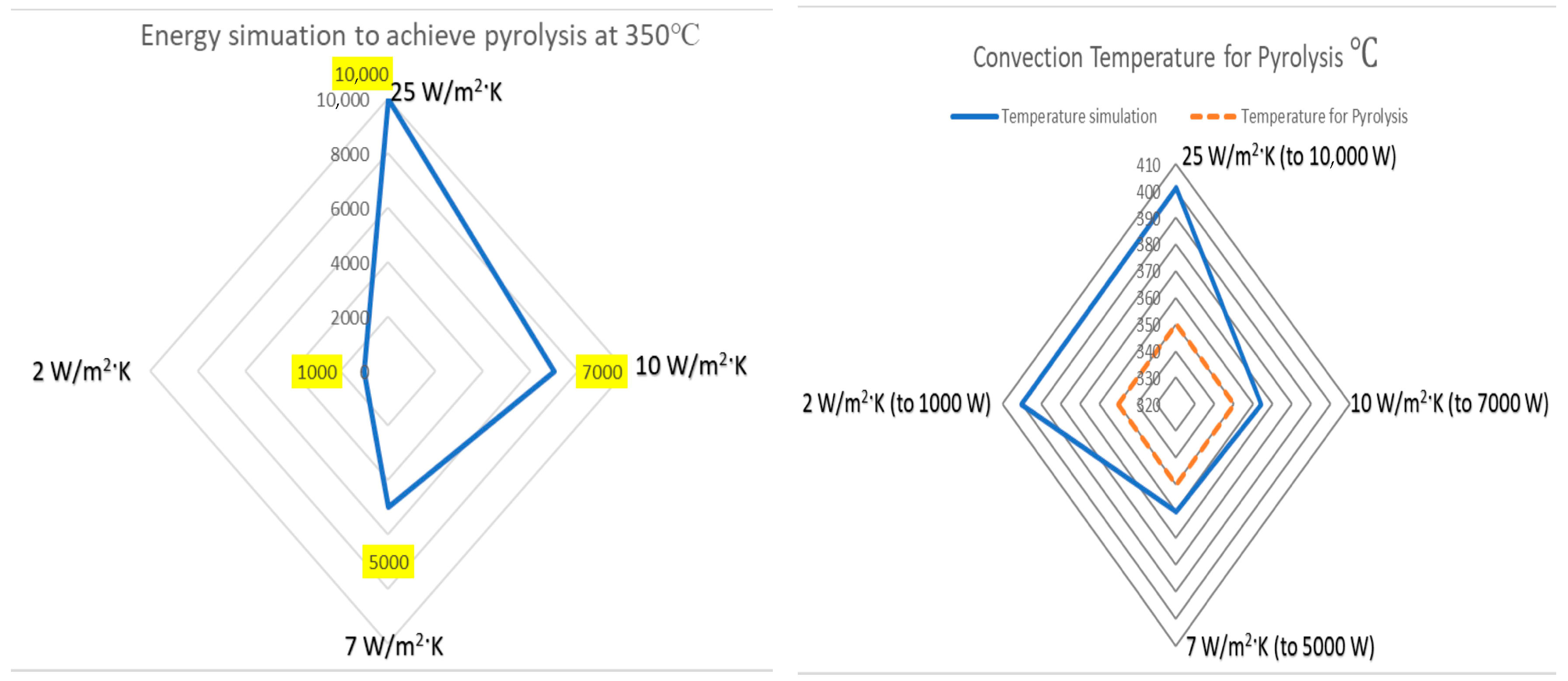
2.3. Heat Transfer and Thermal Efficiency
3. Discussion
4. Materials and Methods
4.1. Reactor Tank Material
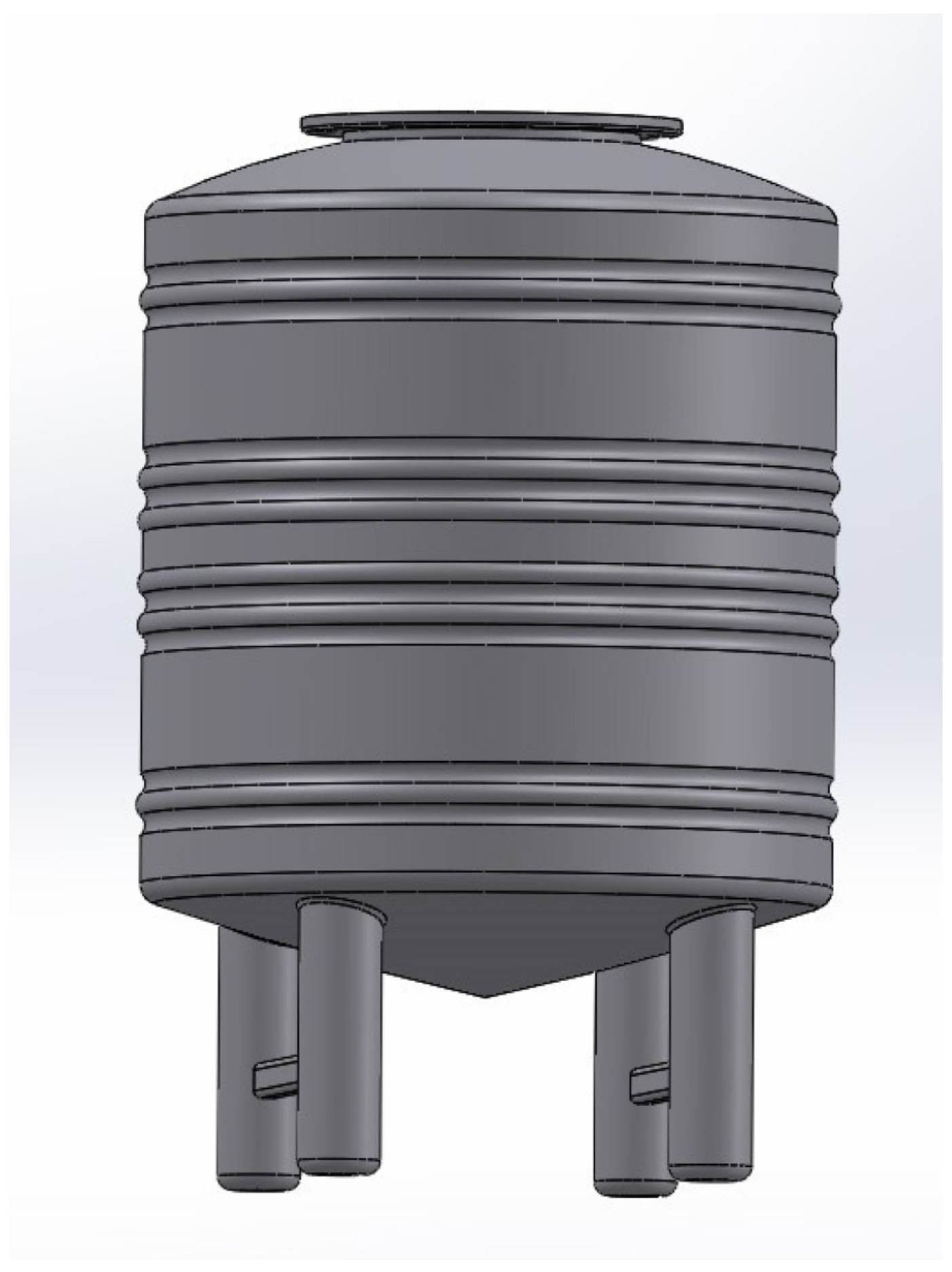
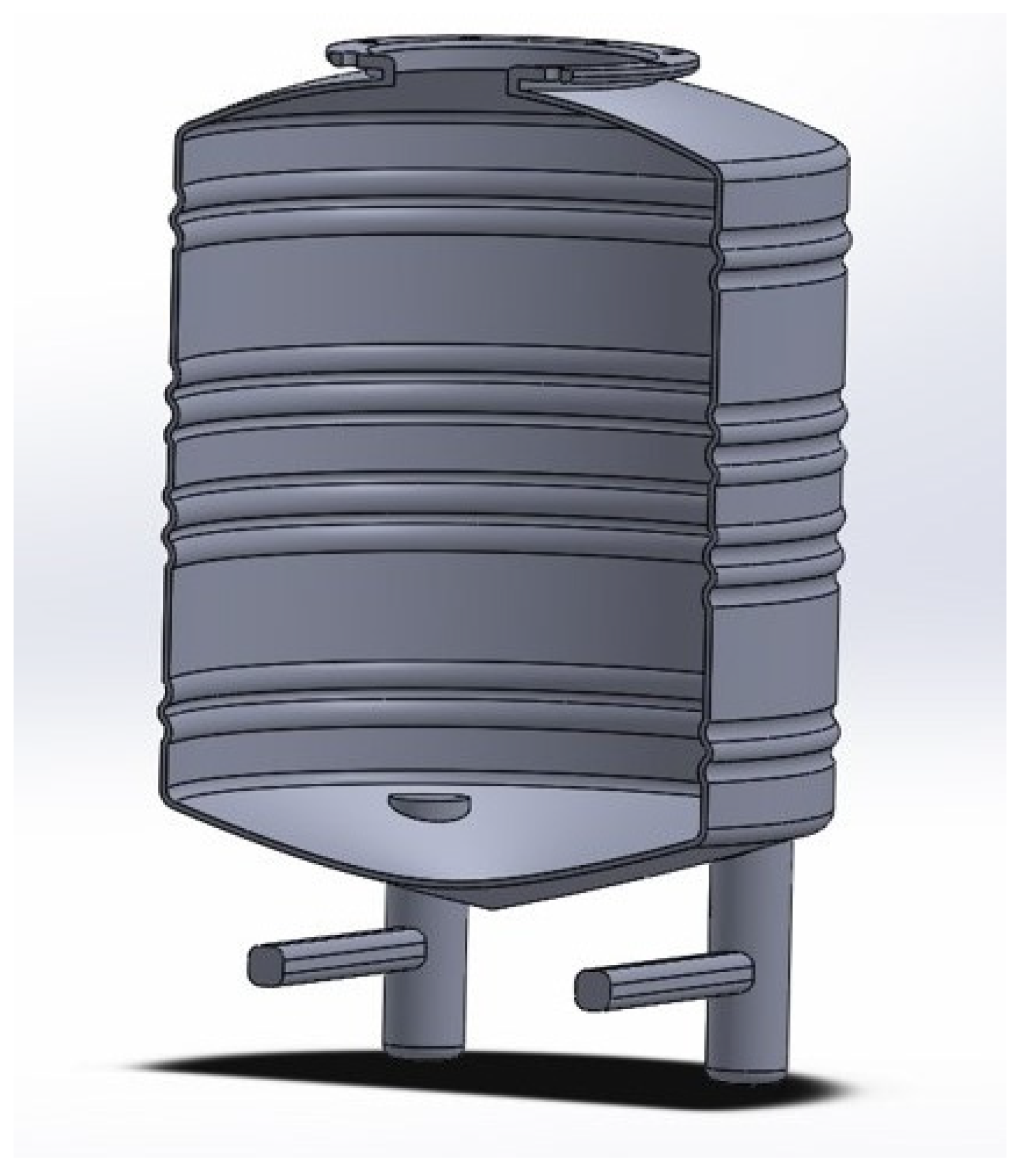
4.2. Reactor Tank Design
4.3. Reactor Jacket
4.4. Simulation Parameters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halkos, G.E.; Gkampoura, E.-C. Reviewing usage, potentials, and limitations of renewable energy sources. Energies 2020, 13, 2906. [Google Scholar] [CrossRef]
- Harjanne, A.; Korhonen, J.M. Abandoning the concept of renewable energy. Energy Policy 2019, 127, 330–340. [Google Scholar] [CrossRef]
- Taghizad-Tavana, K.; Alizadeh, A.A.; Ghanbari-Ghalehjoughi, M.; Nojavan, S. A comprehensive review of electric vehicles in energy systems: Integration with renewable energy sources, charging levels, different types, and standards. Energies 2023, 16, 630. [Google Scholar] [CrossRef]
- Dinh, H.T.; Yun, J.; Kim, D.M.; Lee, K.-H.; Kim, D. A home energy management system with renewable energy and energy storage utilizing main grid and electricity selling. IEEE Access 2020, 8, 49436–49450. [Google Scholar] [CrossRef]
- Piotr, W.; Mariusz, N. Assessment of the possibility of using various types of renewable energy sources installations in single-family buildings as part of saving final energy consumption in Polish conditions. Energies 2022, 15, 1329. [Google Scholar] [CrossRef]
- Dzwigol, H.; Kwilinski, A.; Lyulyov, O.; Pimonenko, T. The role of environmental regulations, renewable energy, and energy efficiency in finding the path to green economic growth. Energies 2023, 16, 3090. [Google Scholar] [CrossRef]
- Sebestyén, V. Renewable and Sustainable Energy Reviews: Environmental impact networks of renewable energy power plants. Renew. Sustain. Energy Rev. 2021, 151, 111626. [Google Scholar] [CrossRef]
- Qazi, A.; Hussain, F.; Rahim, N.A.; Hardaker, G.; Alghazzawi, D.; Shaban, K.; Haruna, K. Towards sustainable energy: A systematic review of renewable energy sources, technologies, and public opinions. IEEE Access 2019, 7, 63837–63851. [Google Scholar] [CrossRef]
- Harusasni, M.M.; Sapuan, S.M.; Khalina, A.; Ilyas, R.A.; Hazrol, M.D. Review on green technology pyrolysis for plastic waste. In Proceedings of the 7th Postgraduate Seminar on Natural Fibre Reinforced Polymer Composites, Serdang, Selangor, Malaysia, 17 November 2020; pp. 50–53. [Google Scholar]
- Papari, S.; Hawboldt, K.; Fransham, P. Study of selective condensation for woody biomass pyrolysis oil vapours. Fuel 2019, 245, 233–239. [Google Scholar] [CrossRef]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Review and Design Overview of Plastic Waste-to-Pyrolysis Oil Conversion with Implications on the Energy Transition. J. Energy 2023, 2023, 1821129. [Google Scholar] [CrossRef]
- Piotr, M.P. Experimental and theoretical study on the internal convective and radiative heat transfer coefficients for a vertical wall in a residential building. Energies 2021, 14, 5953. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Zhang, P.; Su, C.; Zhang, P.; Chi, B.; Xu, K.; Ma, F. Wear properties of self-lubricating Cr x S y/Ni coatings on 304 stainless steel by pre-placed laser cladding. Surf. Eng. 2021, 37, 1541–1549. [Google Scholar] [CrossRef]
- Panmongkol, P.; Phung-On, I. Effect of backing gas mixtures on corrosion properties of stainless steel grade 304 weld metal by autogenous GTAW. J. Mater. Res. Technol. 2021, 11, 2238–7854. [Google Scholar] [CrossRef]
- American Society of Mechanical Engineers. VIII, Division 1, Rules for Construction of Pressure Vessels; American Society of Mechanical Engineers: New York, NY, USA, 2013. [Google Scholar]
- PEMEX. Diseño y Construccion de Recipientes a Precion; NRF-028.PEMEX-2010; PEMEX: Mexico City, Mexico, 2010. [Google Scholar]
- Damián Valerio, R.S. Estudio del Estado Tensional en Uniones Atornilladas Mediante Solidworks. Bachelor’s Thesis, Universidad Politecnica de Cartagena, Cartagena, Spain, March 2018. [Google Scholar]
- Le Duong, H.A.; Zolt, P. An overview of factors influencing thermal conductivity of building insulation materials. J. Build. Eng. 2001, 1, 102604. [Google Scholar]
- Basim, A.-J.; Mourad, A.-H.; Hittini, W.; Hassan, M.; Hameedi, S. Traditional, state-of-the-art and renewable thermal building insulation materials: An overview. Constr. Build. Mater. 2019, 214, 709–735. [Google Scholar]
- Michalak, P. xperimental Study on Heat Transfer Coefficients in an Office Room with a Radiant Ceiling During Low Heating Loads. Energies 2025, 18, 1591. [Google Scholar] [CrossRef]
- Moriarty, P.; Honnery, D. Energy accounting for a renewable energy future. Energies 2019, 12, 4280. [Google Scholar] [CrossRef]
- Reid, W.V.; Ali, M.K.; Field, C.B. The future of bioenergy. Glob. Chang Biol. 2020, 26, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Mandley, S.; Wicke, B.; Junginger, H.; van Vuuren, D.; Daioglou, V. Integrated assessment of the role of bioenergy within the EU energy transition targets to 2050. GCB Bioenergy 2022, 14, 157–172. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. A technical review of bioenergy and resource recovery from municipal solid waste. J. Hazard. Mater. 2021, 402, 123970. [Google Scholar] [CrossRef]
- Jonghun, L.; Yuchan, A.; Junghwan, K. Optimal sorting and recycling of plastic waste as a renewable energy resource considering economic feasibility and environmental pollution. Process Saf. Environ. Prot. 2023, 169, 695–696. [Google Scholar]
- Armenise, S.; SyieLuing, W.; Ramírez-Velásquez, J.M.; Launay, F.; Wuebben, D.; Ngadi, N.; Rams, J.; Muñoz, M. Plastic waste recycling via pyrolysis: A bibliometric survey and literature review. J. Anal. Appl. Pyrolysis 2021, 158, 105–265. [Google Scholar] [CrossRef]
- Vijayakurmar, A.; Sebastian, J. Pyrolysis process to produce fuel from diffent types of plastic a review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 396, 012062. [Google Scholar] [CrossRef]
- Zhou, N.; Dai, L.; Lv, Y.; Li, H.; Deng, W.; Guo, F.; Ruan, R. Catalytic pyrolysis of plastic wastws in a continuous microwave assisted pyrolysis sistem for fuel production. Chem. Eng. J. 2021, 418, 129412. [Google Scholar] [CrossRef]
- Jha, K.K.; Kannan, T. Recicling of plastic waste into fuel by pyrolysis a review. Mater. Today Proc. 2021, 37, 3718–3720. [Google Scholar]
- Maqsood, T.; Dai, J.; Zhang, Y.; Guang, M.; Li, B. Pyrolysis of plastic species: A review of resorces and products. J. Anal. Appl. Pyrolysis 2021, 159, 105295. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Sharuddin, S.; Abnisa, F.; Daud, W.; Auroua, M. Pyrolysis of plastic waste for liquid fuel production as prospective energy resource. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012001. [Google Scholar] [CrossRef]
- Abdy, C.; Zhang, Y.; Wang, J.; Yang, Y.; Artamendi, I.; Allen, B. Pyrolysis of polyolefin plastic waste and potential applications in asphalt road construction. A technical review. Resour. Conserv. Recycl. 2022, 180, 106213. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Tovar, J.A.T.; Campuzano, H.S.; Avilés, M.G.; Sobral, H.; Ruiz, F.J.S. Degradation of Plastic Materials through Small-Scale Pyrolysis Characterization of the Obtained Hydrocarbons and Life. Recycling 2024, 9, 5. [Google Scholar] [CrossRef]
- Bagri, R.; Williams, P.T. Catalytic pyrolysis of polyethylene. J. Anal. Appl. Pyrolysis 2002, 63, 29–41. [Google Scholar] [CrossRef]
- Wong, S.L.; Armenise, S.; Nyakuma, B.B.; Bogush, A.; Towers, S.; Lee, C.H.; Wong, K.Y.; Lee, T.H.; Rebrov, E.; Muñoz, M. Plastic pyrolysis over HZSM-5 zeolite and fluid catalytic cracking catalyst under ultra-fast heating. J. Anal. Appl. Pyrolysis 2023, 169, 105793. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M.; Navarro, R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86. [Google Scholar] [CrossRef]
- Susastriawan, A.; Purnomo; Sandria, A. Experimental study the influence of zeolite size on low-temperature pyrolysis of low-density polyethylene plastic waste. Therm. Sci. Eng. Prog. 2020, 17, 100497. [Google Scholar] [CrossRef]
- Miandad, R.; Nizami, A.; Rehan, M.; Barakat, M.; Khan, M.; Mustafa, A.; Ismail, I.; Murphy, J. Influence of temperature and reaction time on the conversion of polystyrene waste to pyrolysis liquid oil. Waste Manag. 2016, 58, 250–259. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Verma, V.; Thangavel, S.; Dutt, N.; Kumar, A.; Weerasinghe, R. (Eds.) Highly Efficient Thermal Renewable Energy Systems: Design, Optimization and Applications; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Sair, S.; Oushabi, A.; Kammouni, A.; Tanane, O.; Abboud, Y.; El Bouari, A. Mechanical and thermal conductivity properties of hemp fiber reinforced polyurethane composites. Case Stud. Constr. Mater. 2018, 8, 203–212. [Google Scholar] [CrossRef]
- Duque, J.F. A Novel Pathway to Recover Hydrocarbons from Polyethylene Residues Through the Combustion-Driven Pyrolysis Process. Ph.D. Dissertation, Institut National Polytechnique de Toulouse—INPT, Vitória, Brasil, 2021. [Google Scholar]
- Ashby, M.; Shercliff, H.; Cebon, D. (Eds.) Chapter 12—Materials and heat. In Materials, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 303–333. ISBN 9780081023761. [Google Scholar]
- Yan, H.; Guo, H. Efficiency and Its Bounds for Thermal Engines at Maximum Power Using Newton’s Law of Cooling. Phys. Rev. 2012, 85, 011146. [Google Scholar] [CrossRef] [PubMed]
- Kaviany, M. Principles of Heat Transfer in Porous Media; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Chisti, Y. Airlift Bioreactors; Elsevier Applied Science: New York, NY, USA, 1989. [Google Scholar]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An overview of recent developments in biomass pyrolysis technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, A.; Singh, E.; Kumar, S. Recent Research Advancements in Catalytic Pyrolysis of Plastic Waste. ACS Sustain. Chem. Eng. 2023, 11, 2033–2049. [Google Scholar] [CrossRef]
- Oni, T.O.; Ayodeji, S.O. Design; construction, and performance testing of a fixed-bed pyrolysis reactor for production of pyrolytic fuel. Eng. Res. Express 2020, 2, 035027. [Google Scholar] [CrossRef]
- Fang, S.; Jiang, L.; Li, P.; Bai, J.; Chang, C. Study on pyrolysis products characteristics of medical waste and fractional condensation of the pyrolysis oil. Energy 2020, 195, 116969. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A review on condensing system for biomass pyrolysis process. Fuel Process. Technol. 2018, 180, 1–13. [Google Scholar] [CrossRef]
- ASTM D792-13; Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement. ASTM International: West Conshohocken, PA, USA, 2013.
- Bojanovský, J.; Máša, V.; Hudák, I.; Skryja, P.; Hopjan, J. Rotary kiln, a unit on the Border of the process and Energy In-dustry—Current State and perspectives. Sustainability 2022, 14, 13903. [Google Scholar] [CrossRef]
- Papuga, S.; Savković, J.; Djurdjevic, M.; Ciprioti, S.V. Effect of feed mass, reactor temperature, and time on the yield of waste polypropylene pyrolysis oil produced via a fixed-bed reactor. Polymers 2024, 16, 1302. [Google Scholar] [CrossRef] [PubMed]
- Mkhize, N.M.; Danon, B.; Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M.; van der Gryp, P. Influence of reactor and condensation system design on tyre pyrolysis products yields. J. Anal. Appl. Pyrolysis 2019, 143, 104683. [Google Scholar] [CrossRef]
- Systemes, D. SOLIDWORKS 2011 SP05. Dassaults Systemes, 01 ENERO 2011. Available online: https://help.solidworks.com/2011/spanish/SolidWorks/cworks/LegacyHelp/Simulation/AnalysisBackground/ThermalAnalysis/Convection_Topics/Convection_Heat_Coefficient.htm?utm (accessed on 16 June 2025).



| Material | Thermal Conductivity | Thickness m |
|---|---|---|
| Ceramics | 1.4 | 0.025 |
| Refractory Cement | 1.2 | 0.01 |
| Red clay brick | 0.9 | 0.12 |
| Steel 304-2B | 16.2 | 0.0016 |
| Material | Value of R (m2·K/W) |
|---|---|
| Ceramics | 0.018 |
| Refractory cement | 0.0083 |
| Red clay brick | 0.133 |
| Stainless steel 304-2B | 0.000098 |
| Radiation Steel Emissivity | Heat Flux W/m2 | Convection W/m2·K | Heat Power W | Temperature Max. °C | Temperature Min. °C | Surface Temperature °C |
|---|---|---|---|---|---|---|
| 0.3 | 656.4 | 25 | 3000 | 268 | 49 | 151 |
| 0.3 | 1094.0 | 25 | 5000 | 411 | 66 | 238 |
| 0.3 | 2188.0 | 25 | 10,000 | 697 | 106 | 401 |
| 0.3 | 4376.0 | 25 | 20,000 | 1000 | 215 | 659 |
| 0.3 | 656.45 | 10 | 3000 | 507 | 161 | 265 |
| 0.3 | 1094.09 | 10 | 5000 | 645 | 199 | 310 |
| 0.3 | 1531.17 | 10 | 7000 | 757 | 234 | 364 |
| 0.3 | 1750.54 | 10 | 8000 | 806 | 251 | 375 |
| 0.3 | 2188.18 | 10 | 10,000 | 895 | 285 | 420 |
| 0.3 | 656.45 | 7 | 3000 | 551 | 214 | 340 |
| 0.3 | 875.27 | 7 | 4000 | 622 | 234 | 345 |
| 0.3 | 1094.09 | 7 | 5000 | 685 | 255 | 360 |
| 0.3 | 1312.91 | 7 | 6000 | 742 | 276 | 380 |
| 0.3 | 1750.54 | 7 | 8000 | 841 | 316 | 450 |
| 0.3 | 218.0 | 2 | 1000 | 467 | 322 | 400 |
| 0.3 | 328.0 | 2 | 1500 | 513 | 339 | 444 |
| 0.3 | 437.0 | 2 | 2000 | 556 | 355 | 456 |
| 0.3 | 656.0 | 2 | 3000 | 631 | 387 | 533 |
| 0.3 | 1094.0 | 2 | 5000 | 755 | 419 | 587 |
| Thickness (mm) | C | Mn | Si | Cr | Ni | Mo |
|---|---|---|---|---|---|---|
| 1.5 | 0.09 | 1.04 | 0.44 | 17.75 | 7.69 | 0.13 |
| 2.0 | 0.05 | 0.99 | 0.56 | 17.60 | 8.00 | 0.14 |
| 3.0 | 0.09 | 1.02 | 0.40 | 17.97 | 8.06 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres Tovar, J.A.; Servín-Campuzano, H.; González-Avilés, M.; Sobral, H.; Sánchez-Ruiz, F.J.; Trujillo, S.L.H. Design of an Energy-Efficient Pilot-Scale Pyrolysis Reactor Using Low-Cost Insulating Materials. Recycling 2025, 10, 199. https://doi.org/10.3390/recycling10060199
Torres Tovar JA, Servín-Campuzano H, González-Avilés M, Sobral H, Sánchez-Ruiz FJ, Trujillo SLH. Design of an Energy-Efficient Pilot-Scale Pyrolysis Reactor Using Low-Cost Insulating Materials. Recycling. 2025; 10(6):199. https://doi.org/10.3390/recycling10060199
Chicago/Turabian StyleTorres Tovar, José Alfredo, Hermelinda Servín-Campuzano, Mauricio González-Avilés, Hugo Sobral, Francisco Javier Sánchez-Ruiz, and Saúl Leonardo Hernández Trujillo. 2025. "Design of an Energy-Efficient Pilot-Scale Pyrolysis Reactor Using Low-Cost Insulating Materials" Recycling 10, no. 6: 199. https://doi.org/10.3390/recycling10060199
APA StyleTorres Tovar, J. A., Servín-Campuzano, H., González-Avilés, M., Sobral, H., Sánchez-Ruiz, F. J., & Trujillo, S. L. H. (2025). Design of an Energy-Efficient Pilot-Scale Pyrolysis Reactor Using Low-Cost Insulating Materials. Recycling, 10(6), 199. https://doi.org/10.3390/recycling10060199







