Harnessing Wheat Bran as a Phytochemical Bioresource: Release of Ferulic Acid Using Organosolv Treatment with Acidic/Alkaline Deep Eutectic Solvents
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Treatment Severity
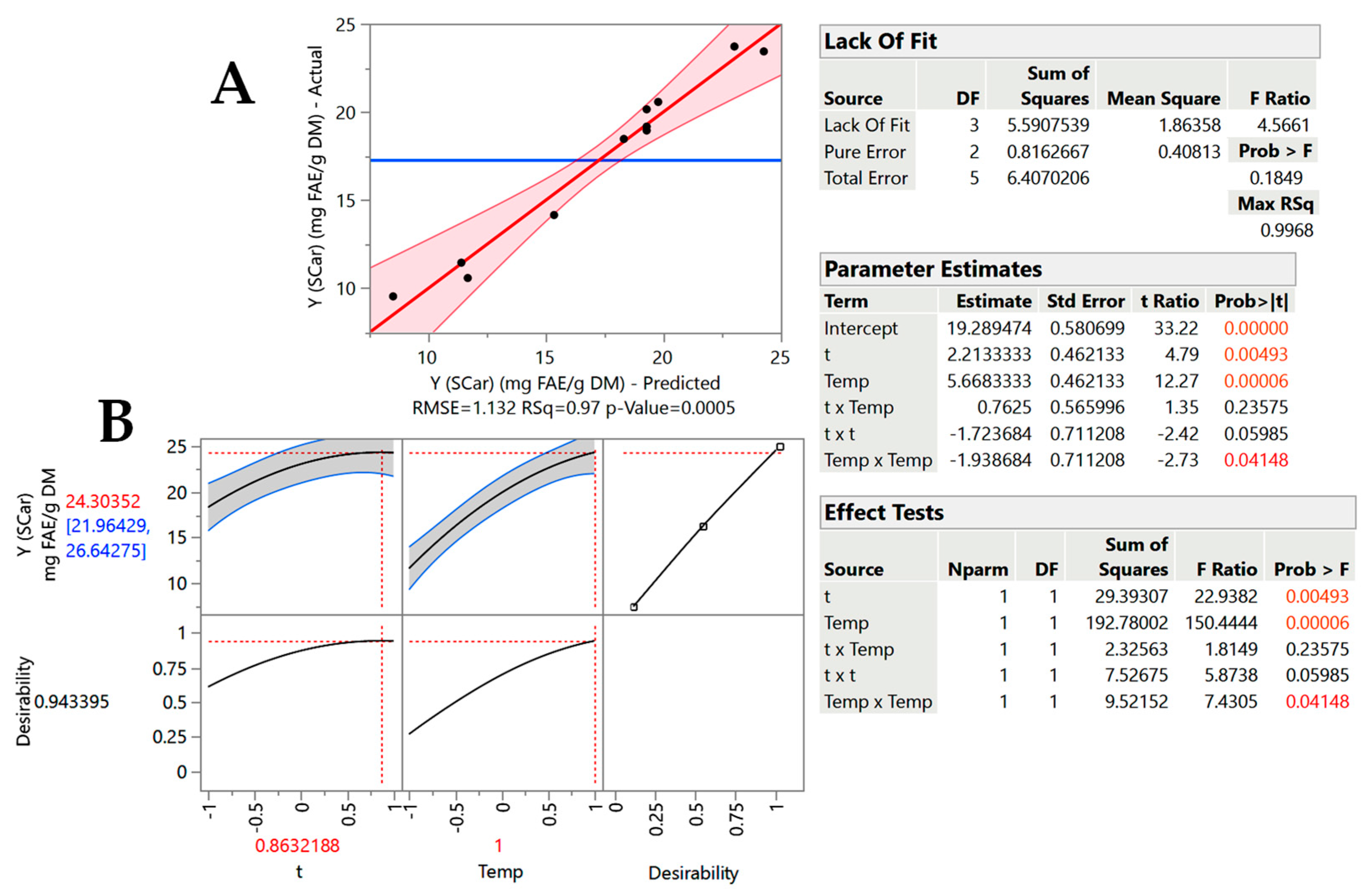
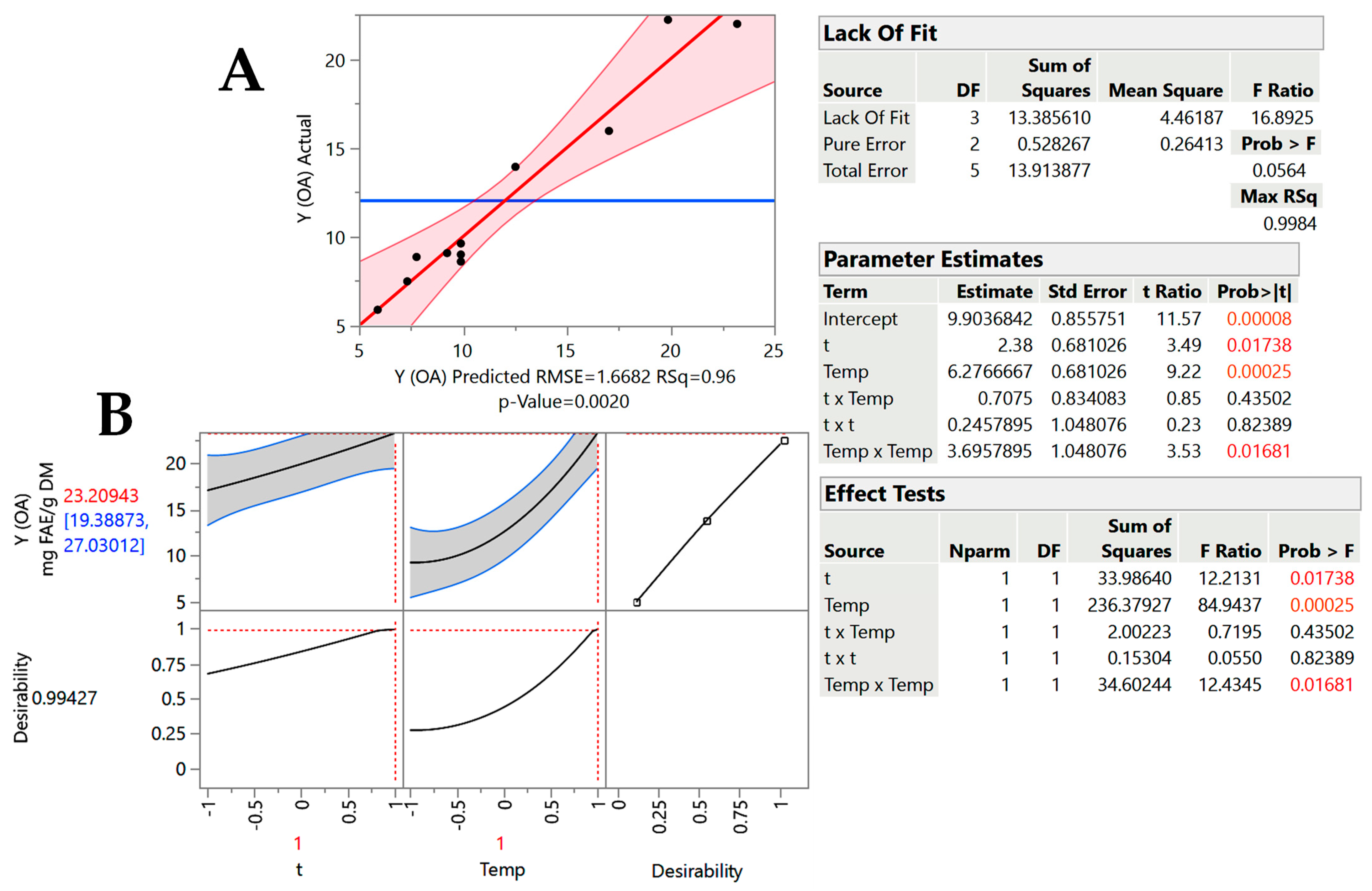
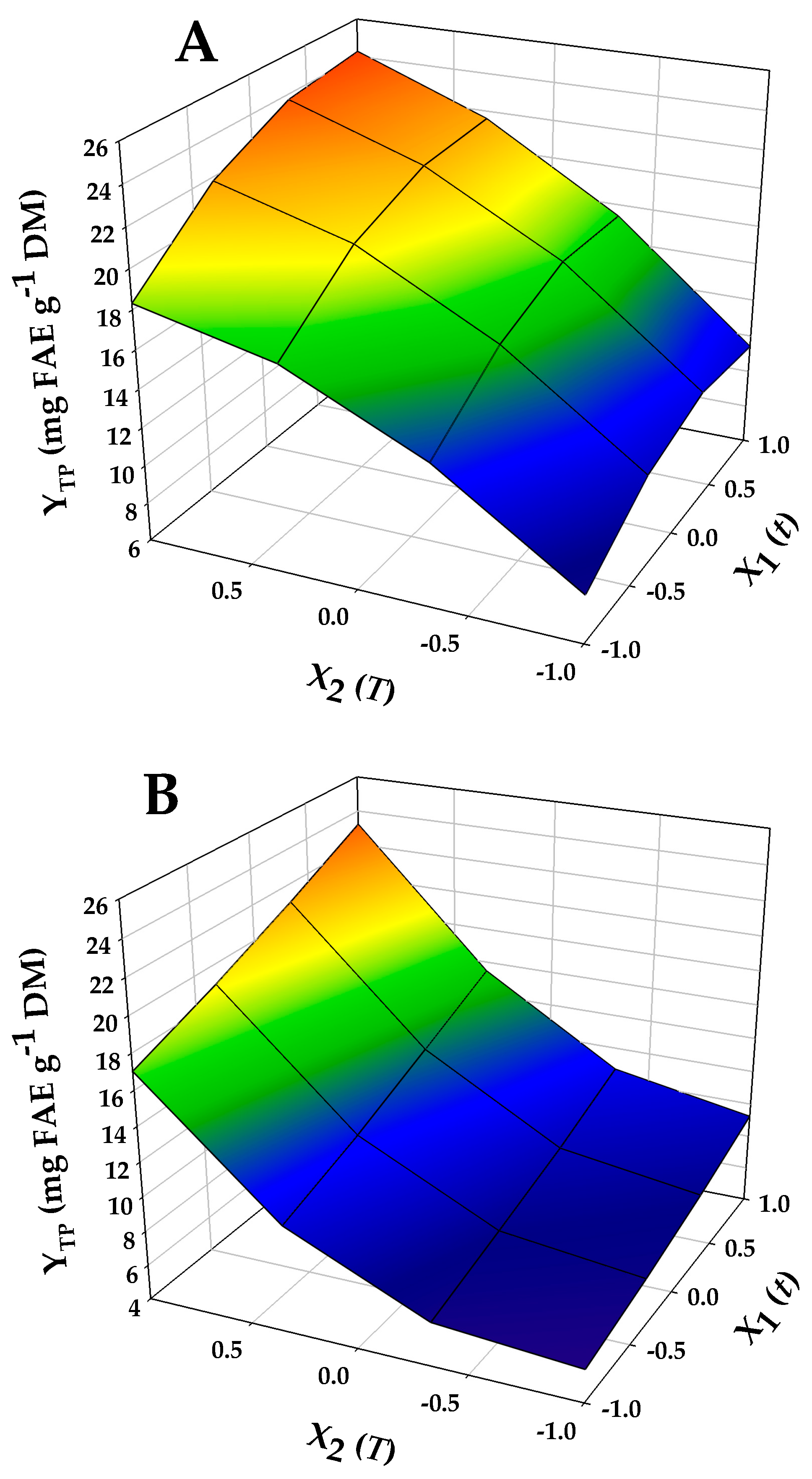
2.2. Treatment Optimization
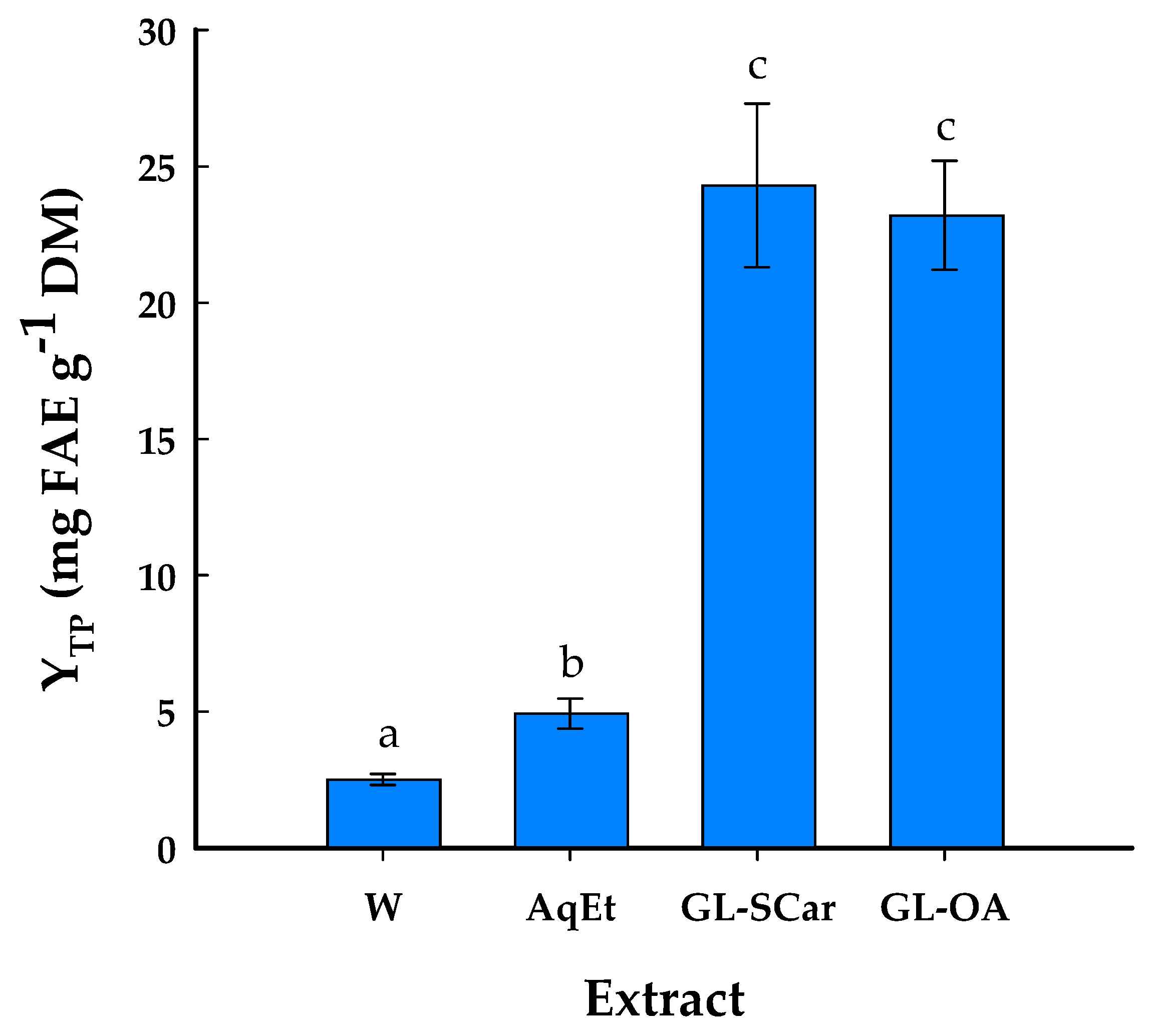
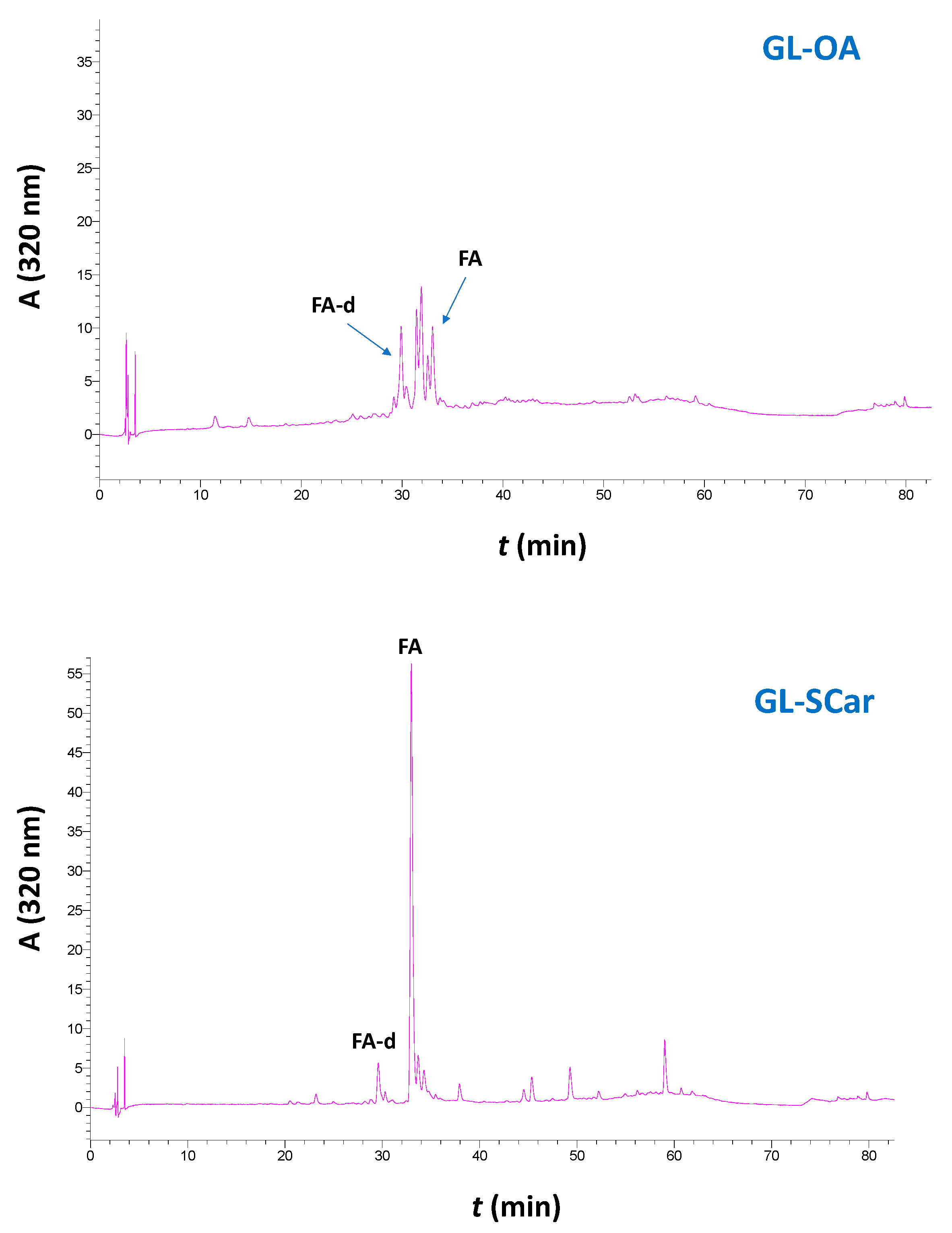
2.3. Polyphenolic Profile and Antioxidant Effects
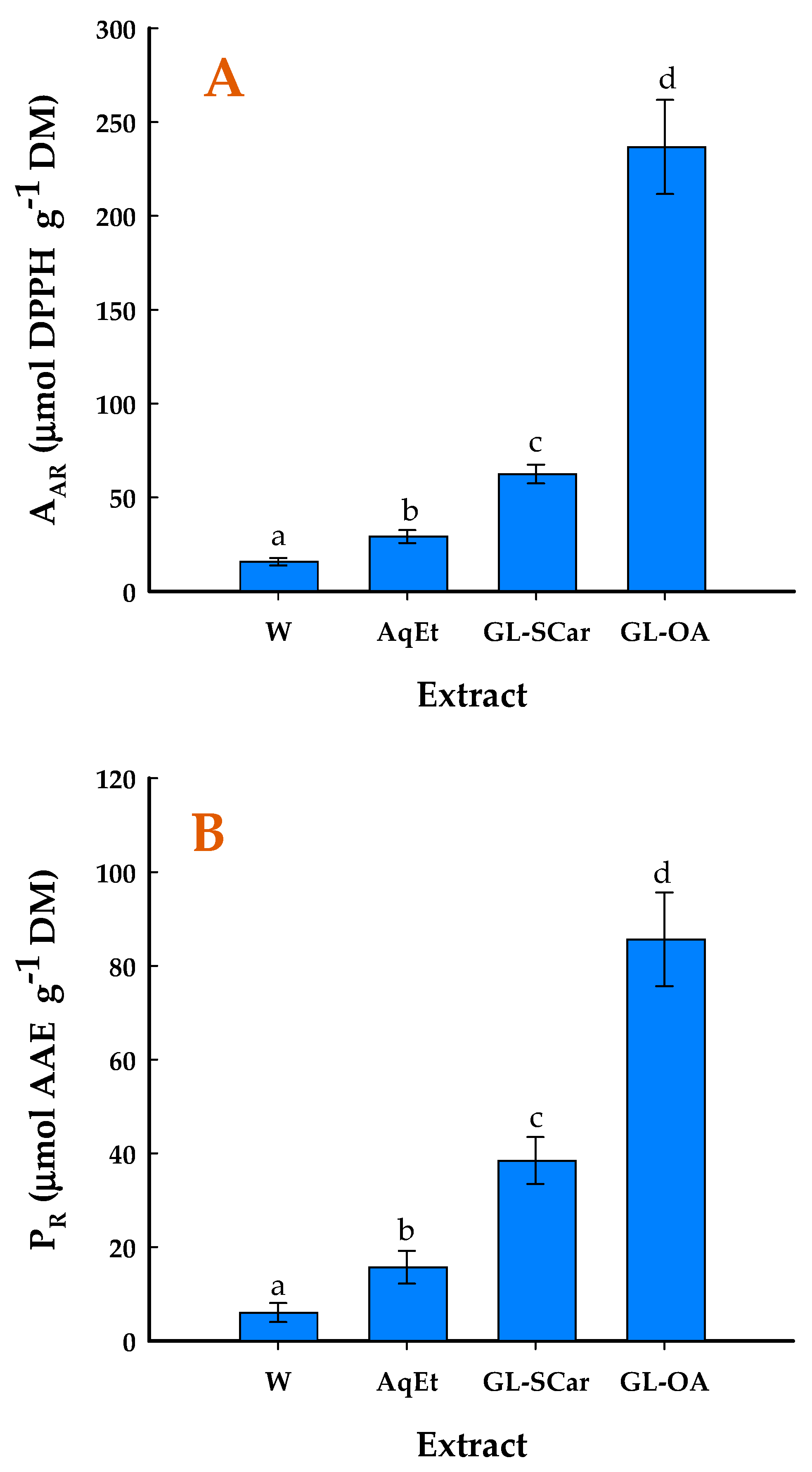
2.4. Antioxidant Effects
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Deep Eutectic Solvent Synthesis
3.3. Wheat Bran Procurement
3.4. DES Organosolv Treatments
3.5. Treatment Severity Assessment
3.6. Response Surface Treatment Optimization
3.7. Reference Alkaline Hydrolysis
3.8. Polyphenol Yield and Antioxidant Activity Determinations
3.9. Chromatographic Analyses
3.10. Data Processing—Statistics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Ouro-Salim, O.; Guarnieri, P. Circular economy of food waste: A literature review. Environ. Qual. Manag. 2022, 32, 225–242. [Google Scholar] [CrossRef]
- Jin, Q.; Yang, L.; Poe, N.; Huang, H. Integrated processing of plant-derived waste to produce value-added products based on the biorefinery concept. Trends Food Sci. Technol. 2018, 74, 119–131. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- El Mekawy, A.; Diels, L.; De Wever, H.; Pant, D. Valorization of cereal based biorefinery byproducts: Reality and expectations. Environ. Sci. Technol. 2013, 47, 9014–9027. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Sustainable applications for the valorization of cereal processing by-products. Foods 2022, 11, 241. [Google Scholar] [CrossRef]
- Rudjito, R.C.; Matute, A.C.; Jiménez-Quero, A.; Olsson, L.; Stringer, M.A.; Krogh, K.B.R.M.; Eklöf, J.; Vilaplana, F. Integration of subcritical water extraction and treatment with xylanases and feruloyl esterases maximises release of feruloylated arabinoxylans from wheat bran. Bioresour. Technol. 2024, 395, 130387. [Google Scholar] [CrossRef]
- Apprich, S.; Tirpanalan, Ö.; Hell, J.; Reisinger, M.; Böhmdorfer, S.; Siebenhandl-Ehn, S.; Novalin, S.; Kneifel, W. Wheat bran-based biorefinery 2: Valorization of products. LWT Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- Katileviciute, A.; Plakys, G.; Budreviciute, A.; Onder, K.; Damiati, S.; Kodzius, R. A sight to wheat bran: High value-added products. Biomolecules 2019, 9, 887. [Google Scholar] [CrossRef]
- Silva, E.d.O.; Batista, R. Ferulic acid and naturally occurring compounds bearing a feruloyl moiety: A review on their structures, occurrence, and potential health benefits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef]
- Fărcaș, A.; Drețcanu, G.; Pop, T.D.; Enaru, B.; Socaci, S.; Diaconeasa, Z. Cereal processing by-products as rich sources of phenolic compounds and their potential bioactivities. Nutrients 2021, 13, 3934. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.-X.; Guo, S.-D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E. Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Razavi, S.H. The role of bioconversion processes to enhance bioaccessibility of polyphenols in rice. Food Biosci. 2020, 35, 100605. [Google Scholar] [CrossRef]
- Barberousse, H.; Roiseux, O.; Robert, C.; Paquot, M.; Deroanne, C.; Blecker, C. Analytical methodologies for quantification of ferulic acid and its oligomers. J. Sci. Food Agric. 2008, 88, 1494–1511. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Patto, M.C.V.; Bronze, M.d.R. Relevance, structure and analysis of ferulic acid in maize cell walls. Food Chem. 2018, 246, 360–378. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Database of deep eutectic solvents and their physical properties: A review. J. Mol. Liq. 2023, 384, 121899. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Row, K.H. Development of deep eutectic solvents for sustainable chemistry. J. Mol. Liq. 2022, 362, 119654. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Yang, Z. Natural deep eutectic solvents and their applications in biotechnology. Appl. Ion. Liq. Biotechnol. 2019, 31–59. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Tripathi, M.; Lukk, T.; Karpichev, Y.; Gathergood, N.; Singh, B.N.; Thakur, V.K.; Tabatabaei, M.; Gupta, V.K. Biobased natural deep eutectic system as versatile solvents: Structure, interaction and advanced applications. Sci. Total Environ. 2023, 881, 163002. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural deep eutectic solvents (NADES): Phytochemical extraction performance enhancer for pharmaceutical and nutraceutical product development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep eutectic solvents for the extraction of bioactive compounds from natural sources and agricultural by-products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Bozinou, E.; Palaiogiannis, D.; Mourtzinos, I.; Chatzilazarou, A.; Gkatzionis, C.; Makris, D.P. The use of tailor-made acidic deep eutectic solvents for preparation of quercetin-enriched extracts from onion solid wastes. Environ. Qual. Manag. 2023, 33, 147–155. [Google Scholar] [CrossRef]
- Papadaki, E.; Grigorakis, S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P. Hydrothermal treatment of wheat bran under mild acidic or alkaline conditions for enhanced polyphenol recovery and antioxidant activity. Molecules 2024, 29, 1193. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, E.S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P.; Makris, D.P. Polyphenol release from wheat bran using ethanol-based organosolv treatment and acid/alkaline catalysis: Process modeling based on severity and response surface optimization. Antioxidants 2022, 11, 2457. [Google Scholar] [CrossRef]
- Casasni, S.; Guenaoui, A.; Grigorakis, S.; Makris, D.P. Acid-catalyzed organosolv treatment of potato peels to boost release of polyphenolic compounds using 1-and 2-propanol. Appl. Sci. 2023, 13, 9484. [Google Scholar] [CrossRef]
- Guenaoui, A.; Casasni, S.; Grigorakis, S.; Makris, D.P. Alkali-catalyzed organosolv treatment of oat bran for enhanced release of hydroxycinnamate antioxidants: Comparison of 1-and 2-propanol. Environments 2023, 10, 118. [Google Scholar] [CrossRef]
- Pazo-Cepeda, V.; Benito-Román, Ó.; Navarrete, A.; Alonso, E. Valorization of wheat bran: Ferulic acid recovery using pressurized aqueous ethanol solutions. Waste Biomass Valor. 2020, 11, 4701–4710. [Google Scholar] [CrossRef]
- Papadimitriou, G.; Zarnavalou, V.; Chatzimitakos, T.; Palaiogiannis, D.; Athanasiadis, V.; Lalas, S.I.; Makris, D.P. Comparison of sodium hydroxide and sodium carbonate as alkali catalysts in ethanol organosolv treatment of cotton stalks for the release of hydroxycinnamates. Recycling 2024, 9, 21. [Google Scholar] [CrossRef]
- Kottaras, P.; Koulianos, M.; Makris, D.P. Low-transition temperature mixtures (LTTMs) made of bioorganic molecules: Enhanced extraction of antioxidant phenolics from industrial cereal solid wastes. Recycling 2017, 2, 3. [Google Scholar] [CrossRef]
- Abozed, S.S.; El-Kalyoubi, M.; Abdelrashid, A.; Salama, M.F. Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Ann. Agric. Sci. 2014, 59, 63–67. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, R.; Liu, C.; Zheng, X.; Liu, B. Enhancing antioxidant activity and antiproliferation of wheat bran through steam flash explosion. J. Food Sci. Technol. 2016, 53, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
- Martín-Diana, A.B.; Tomé-Sánchez, I.; García-Casas, M.J.; Martínez-Villaluenga, C.; Frías, J.; Rico, D. A novel strategy to produce a soluble and bioactive wheat bran ingredient rich in ferulic acid. Antioxidants 2021, 10, 969. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT Food Sci. Technol. 2004, 37, 717–721. [Google Scholar] [CrossRef]
- Guerrini, A.; Burlini, I.; Lorenzo, B.H.; Grandini, A.; Vertuani, S.; Tacchini, M.; Sacchetti, G. Antioxidant and antimicrobial extracts obtained from agricultural by-products: Strategies for a sustainable recovery and future perspectives. Food Bioprod. Process. 2020, 124, 397–407. [Google Scholar] [CrossRef]
- Verma, B.; Hucl, P.; Chibbar, R. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chem. 2009, 116, 947–954. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Arranz, S.; Calixto, F.S. Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J. Cereal Sci. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Chen, X.; Sun, K.; Zhuang, K.; Ding, W. Comparison and Optimization of Different Extraction Methods of Bound Phenolics from Jizi439 Black Wheat Bran. Foods 2022, 11, 1478. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazza, G. Lignin in straw of herbaceous crops. Ind. Crops Prod. 2008, 28, 237–259. [Google Scholar] [CrossRef]
- Ng, M.H.; Hadi, N.A. Extraction of ferulic acid from oil palm pressed fiber by a choline chloride based deep eutectic solvent. J. Am. Oil Chem. Soc. 2022, 99, 443–453. [Google Scholar] [CrossRef]
- Chadni, M.; Haudrechy, A.; Couvreur, J.; Allais, F. Investigation of ferulic acid recovery from enzymatic hydrolysate of wheat bran using various solvents and liquid-liquid extraction assisted by membrane contactor. J. Mol. Liq. 2024, 400, 124538. [Google Scholar] [CrossRef]
- Makris, D.P.; Lalas, S. Glycerol and glycerol-based deep eutectic mixtures as emerging green solvents for polyphenol extraction: The evidence so far. Molecules 2020, 25, 5842. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Finger-Teixeira, A.; Mota, T.R.; Salvador, V.H.; Moreira-Vilar, F.C.; Molinari, H.B.C.; Mitchell, R.A.C.; Marchiosi, R.; Ferrarese-Filho, O.; Santos, W.D.D. Ferulic acid: A key component in grass lignocellulose recalcitrance to hydrolysis. Plant Biotechnol. J. 2015, 13, 1224–1232. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazza, G. Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chem. 2009, 115, 1542–1548. [Google Scholar] [CrossRef]
- Linh, T.N.; Fujita, H.; Sakoda, A. Release kinetics of esterified p-coumaric acid and ferulic acid from rice straw in mild alkaline solution. Bioresour. Technol. 2017, 232, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Scelsi, E.; Angelini, A.; Pastore, C. Deep eutectic solvents for the valorisation of lignocellulosic biomasses towards fine chemicals. Biomass 2021, 1, 29–59. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Valério, R.; Torres, C.A.; Brazinha, C.; da Silva, M.G.; Coelhoso, I.M.; Crespo, J.G. Purification of ferulic acid from corn fibre alkaline extracts for bio-vanillin production using an adsorption process. Separ. Purif. Technol. 2022, 298, 121570. [Google Scholar] [CrossRef]
- Pazo-Cepeda, M.V.; Aspromonte, S.G.; Alonso, E. Extraction of ferulic acid and feruloylated arabinoxylo-oligosaccharides from wheat bran using pressurized hot water. Food Biosci. 2021, 44, 101374. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, L.; Shen, F.; Hu, J.; Huang, M.; He, J.; Zhang, Y.; Deng, S.; Li, Q.; Tian, D. Insights into lignocellulosic waste fractionation for lignin nanospheres fabrication using acidic/alkaline deep eutectic solvents. Chemosphere 2022, 286, 131798. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; Vandenberghe, L.P.d.S.; Neto, D.P.d.C.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance–Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Xie, J.; Cheng, Z.; Zhu, S.; Xu, J. Lewis base enhanced neutral deep eutectic solvent pretreatment for enzymatic hydrolysis of corn straw and lignin characterization. Renew. Energy 2022, 188, 320–328. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Li, D.; Zhang, J.; Nawaz, H.; You, T.; Xu, F. Highly-efficient pretreatment using alkaline enhanced aqueous deep eutectic solvent to unlock poplar for high yield of fermentable sugars: Synergistic removal of lignin and mannan. Bioresour. Technol. 2022, 351, 126993. [Google Scholar] [CrossRef]
- Yue, X.; Suopajarvi, T.; Mankinen, O.; Mikola, M.; Mikkelson, A.; Ahola, J.; Hiltunen, S.; Komulainen, S.; Kantola, A.M.; Telkki, V.-V. Comparison of lignin fractions isolated from wheat straw using alkaline and acidic deep eutectic solvents. J. Agric. Food Chem. 2020, 68, 15074–15084. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; You, T.; Li, D.; Nawaz, H.; Zhang, X.; Xu, F. Insights into alkaline choline chloride-based deep eutectic solvents pretreatment for Populus deltoides: Lignin structural features and modification mechanism. Int. J. Biol. Macromol. 2021, 193, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-Y.; Xu, L.-H.; Sun, Q.; Sun, S.-N.; Cao, X.-F.; Wen, J.-L.; Yuan, T.-Q. Ultrafast alkaline deep eutectic solvent pretreatment for enhancing enzymatic saccharification and lignin fractionation from industrial xylose residue. Bioresour. Technol. 2022, 352, 127065. [Google Scholar] [CrossRef] [PubMed]
- Kylli, P.; Nousiainen, P.; Biely, P.; Sipilä, J.; Tenkanen, M.; Heinonen, M. Antioxidant potential of hydroxycinnamic acid glycoside esters. J. Agric. Food Chem. 2008, 56, 4797–4805. [Google Scholar] [CrossRef]
- Ohta, T.; Nakano, T.; Egashira, Y.; Sanada, H. Antioxidant activity of ferulic acid β-glucuronide in the LDL oxidation system. Biosci. Biotechnol. Biochem. 1997, 61, 1942–1943. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity–relating pH to biomatrix opening. New Biotechnol. 2010, 27, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Mota, I.; Pinto, P.C.R.; Novo, C.; Sousa, G.; Guerreiro, O.; Guerra, A.R.; Duarte, M.F.; Rodrigues, A.E. Extraction of polyphenolic compounds from Eucalyptus globulus bark: Process optimization and screening for biological activity. Ind. Eng. Chem. Res. 2012, 51, 6991–7000. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef]
- Lakka, A.; Karageorgou, I.; Kaltsa, O.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D. Polyphenol extraction from Humulus lupulus (hop) using a neoteric glycerol/L-alanine deep eutectic solvent: Optimisation, kinetics and the effect of ultrasound-assisted pretreatment. AgriEngineering 2019, 1, 403–417. [Google Scholar] [CrossRef]
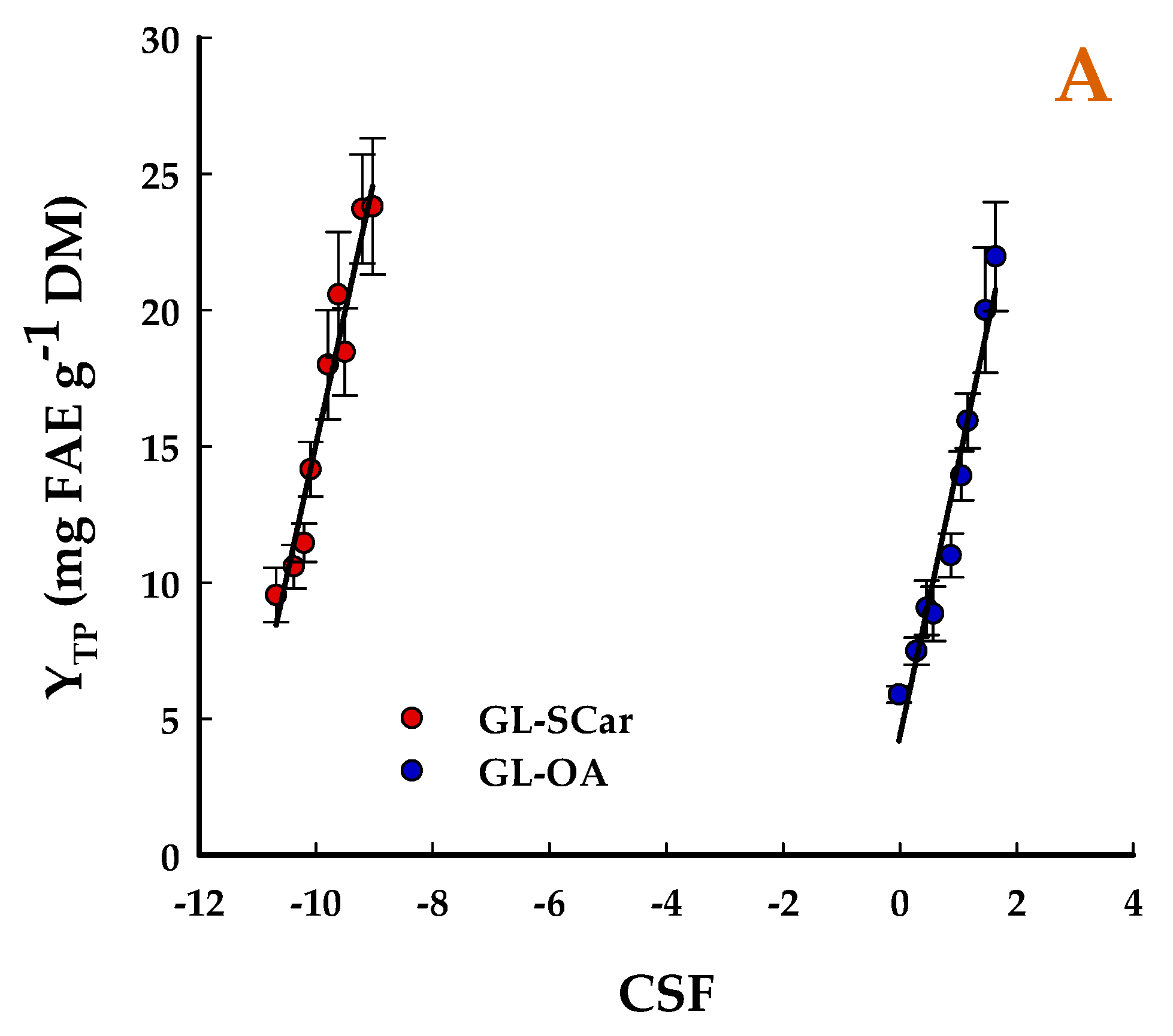
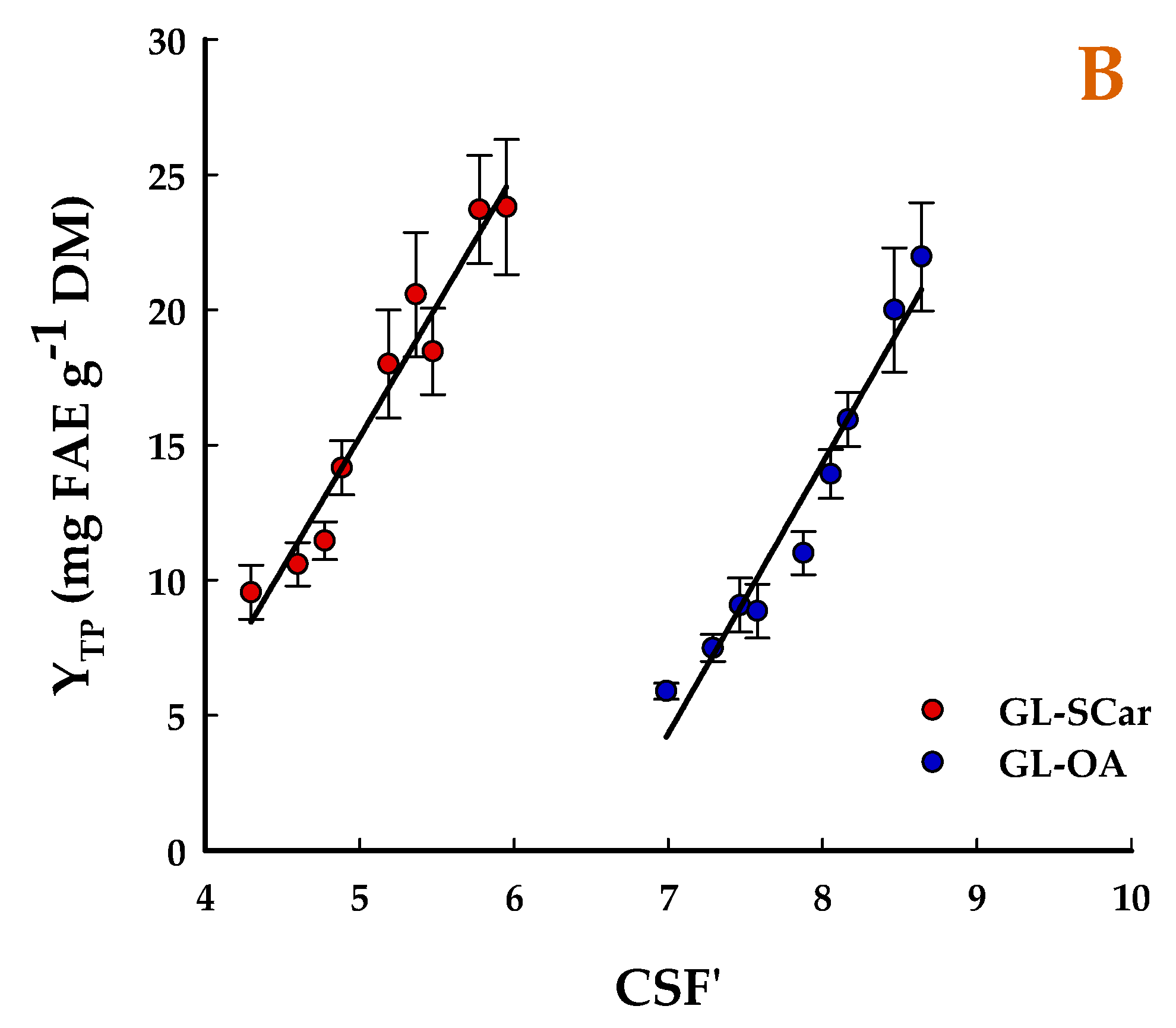
| t (min) | T (°C) | SF | CSF | CSF′ | YTP (mg FAE g−1 DM) | |||
|---|---|---|---|---|---|---|---|---|
| GL-SCar | GL-OA | GL-SCar | GL-OA | GL-SCar | GL-OA | |||
| 60 | 50 | 0.31 | −10.68 | −0.01 | 4.30 | 6.99 | 9.55 ± 0.13 a | 5.89 ± 0.08 a |
| 120 | 50 | 0.61 | −10.38 | 0.29 | 4.60 | 7.29 | 10.59 ± 0.70 b | 7.49 ± 0.10 b |
| 180 | 50 | 0.78 | −10.21 | 0.46 | 4.77 | 7.46 | 11.46 ± 0.71 b | 9.08 ± 0.16 c |
| 60 | 70 | 0.89 | −10.10 | 0.57 | 4.88 | 7.57 | 14.16 ± 0.28 c | 8.86 ± 0.14 c |
| 120 | 70 | 1.20 | −9.79 | 0.88 | 5.19 | 7.88 | 18.58 ± 0.29 d | 11.00 ± 0.93 d |
| 180 | 70 | 1.37 | −9.62 | 1.05 | 5.36 | 8.05 | 20.57 ± 0.24 e | 13.93 ± 0.22 e |
| 60 | 90 | 1.48 | −9.51 | 1.16 | 5.47 | 8.16 | 18.47 ± 0.34 d | 15.95 ± 0.40 f |
| 120 | 90 | 1.78 | −9.21 | 1.46 | 5.77 | 8.46 | 23.71 ± 0.30 f | 20.20 ± 0.28 g |
| 180 | 90 | 1.96 | −9.03 | 1.64 | 5.95 | 8.64 | 23.81 ± 0.27 f | 21.97 ± 0.21 h |
| Design Point | Independent Variables | Response (YTP, mg FAE g−1 DM) | ||||
|---|---|---|---|---|---|---|
| X1 (t, min) | X2 (T, °C) | GL-SCar | GL-OA | |||
| Measured | Predicted | Measured | Predicted | |||
| 1 | −1 (60) | −1 (50) | 9.55 | 8.51 | 5.89 | 5.90 |
| 2 | −1 (60) | 1 (90) | 18.47 | 18.32 | 15.95 | 17.03 |
| 3 | 1 (180) | −1 (50) | 11.46 | 11.41 | 9.08 | 9.24 |
| 4 | 1 (180) | 1 (90) | 23.81 | 24.27 | 21.97 | 23.21 |
| 5 | −1 (60) | 0 (70) | 14.16 | 15.35 | 8.86 | 7.77 |
| 6 | 1 (180) | 0 (70) | 20.57 | 19.78 | 13.93 | 12.53 |
| 7 | 0 (120) | −1 (50) | 10.59 | 11.68 | 7.49 | 7.32 |
| 8 | 0 (120) | 1 (90) | 23.71 | 23.02 | 20.20 | 19.88 |
| 9 | 0 (120) | 0 (70) | 20.15 | 19.29 | 11.62 | 9.90 |
| 10 | 0 (120) | 0 (70) | 19.17 | 19.29 | 11.00 | 9.90 |
| 11 | 0 (120) | 0 (70) | 18.58 | 19.29 | 10.60 | 9.90 |
| Extraction Medium | Extraction Yield (μg g−1 DM) | ||
|---|---|---|---|
| Ferulic Acid | Ferulate Derivative | Total | |
| Reference hydrolysis | 2232.30 ± 98.54 a | 208.21 ± 14.32 a | 2440.51 |
| Water | 33.25 ± 2.28 b | 17.44 ± 1.45 b | 50.69 |
| 60% EtOH | 40.51 ± 3.58 c | 20.69 ± 1.89 c | 61.20 |
| GL-OA | 102.82 ± 9.63 d | 123.93 ± 10.44 d | 226.75 |
| GL-SCar | 943.18 ± 78.12 e | 98.82 ± 7.89 e | 1042.00 |
| Treatment Variables | Codes | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| t (min) | X1 | 60 | 120 | 180 |
| T (°C) | X2 | 50 | 70 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorakis, S.; Makris, D.P. Harnessing Wheat Bran as a Phytochemical Bioresource: Release of Ferulic Acid Using Organosolv Treatment with Acidic/Alkaline Deep Eutectic Solvents. Recycling 2025, 10, 178. https://doi.org/10.3390/recycling10050178
Grigorakis S, Makris DP. Harnessing Wheat Bran as a Phytochemical Bioresource: Release of Ferulic Acid Using Organosolv Treatment with Acidic/Alkaline Deep Eutectic Solvents. Recycling. 2025; 10(5):178. https://doi.org/10.3390/recycling10050178
Chicago/Turabian StyleGrigorakis, Spyros, and Dimitris P. Makris. 2025. "Harnessing Wheat Bran as a Phytochemical Bioresource: Release of Ferulic Acid Using Organosolv Treatment with Acidic/Alkaline Deep Eutectic Solvents" Recycling 10, no. 5: 178. https://doi.org/10.3390/recycling10050178
APA StyleGrigorakis, S., & Makris, D. P. (2025). Harnessing Wheat Bran as a Phytochemical Bioresource: Release of Ferulic Acid Using Organosolv Treatment with Acidic/Alkaline Deep Eutectic Solvents. Recycling, 10(5), 178. https://doi.org/10.3390/recycling10050178








