Exploring Refuse-Derived Fuel Production from Seafood-Processing Sludge and Landfill-Mined Plastic Waste Co-Pelletization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pelletization
2.3. Chemical and Physical Analysis
2.4. Estimation of CO2 Emission Factors of Solid Fuels
2.5. Estimation of GHG Emissions from Treatment and Disposal Scenarios
2.5.1. Dewatered Sludge Landfilling
2.5.2. RDF Production and Combustion
2.5.3. Pellet Production and Combustion
(Emissionssludge prep × WFsludge) + Emissionspelletization + Emissionscombustion
2.5.4. Coal Mining and Combustion
3. Results and Discussion
3.1. Characteristics of the Sludge and Plastic Waste
3.2. Characteristics of Pellets
3.2.1. Compressive Strength
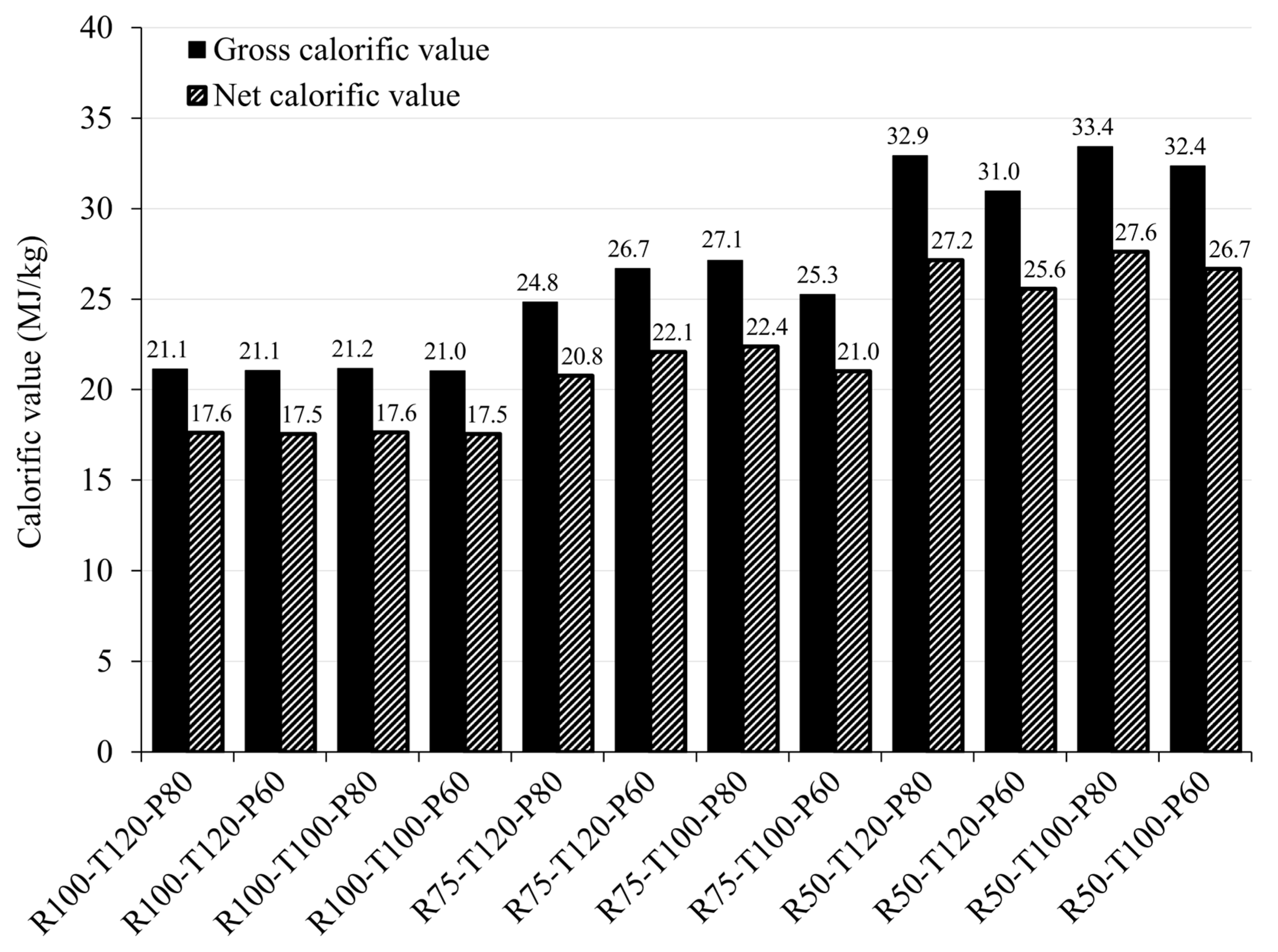
3.2.2. Calorific Value
3.2.3. Chemical Composition
3.2.4. Intensity of Heavy Metals
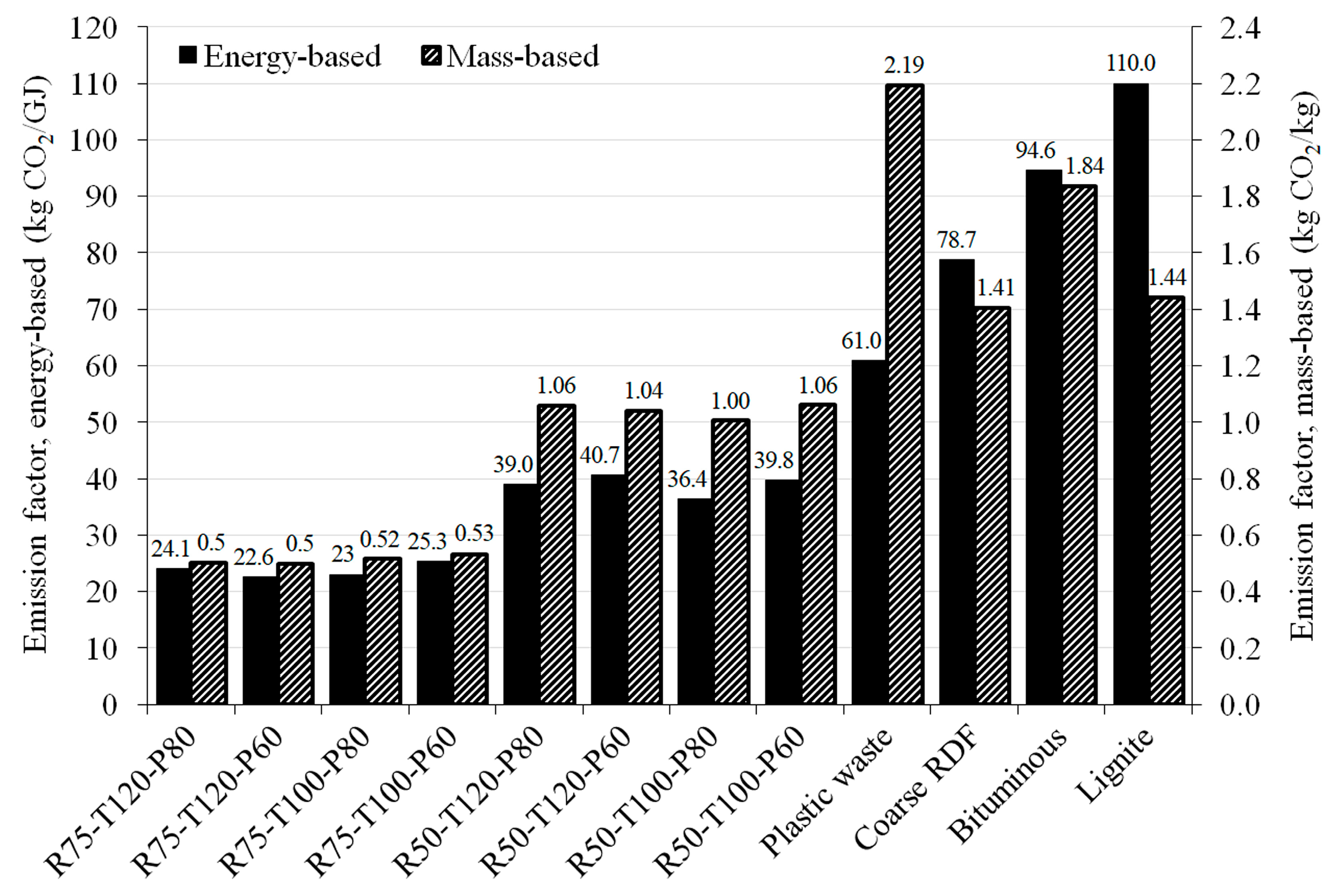
3.3. Emission Factors of Solid Fuels
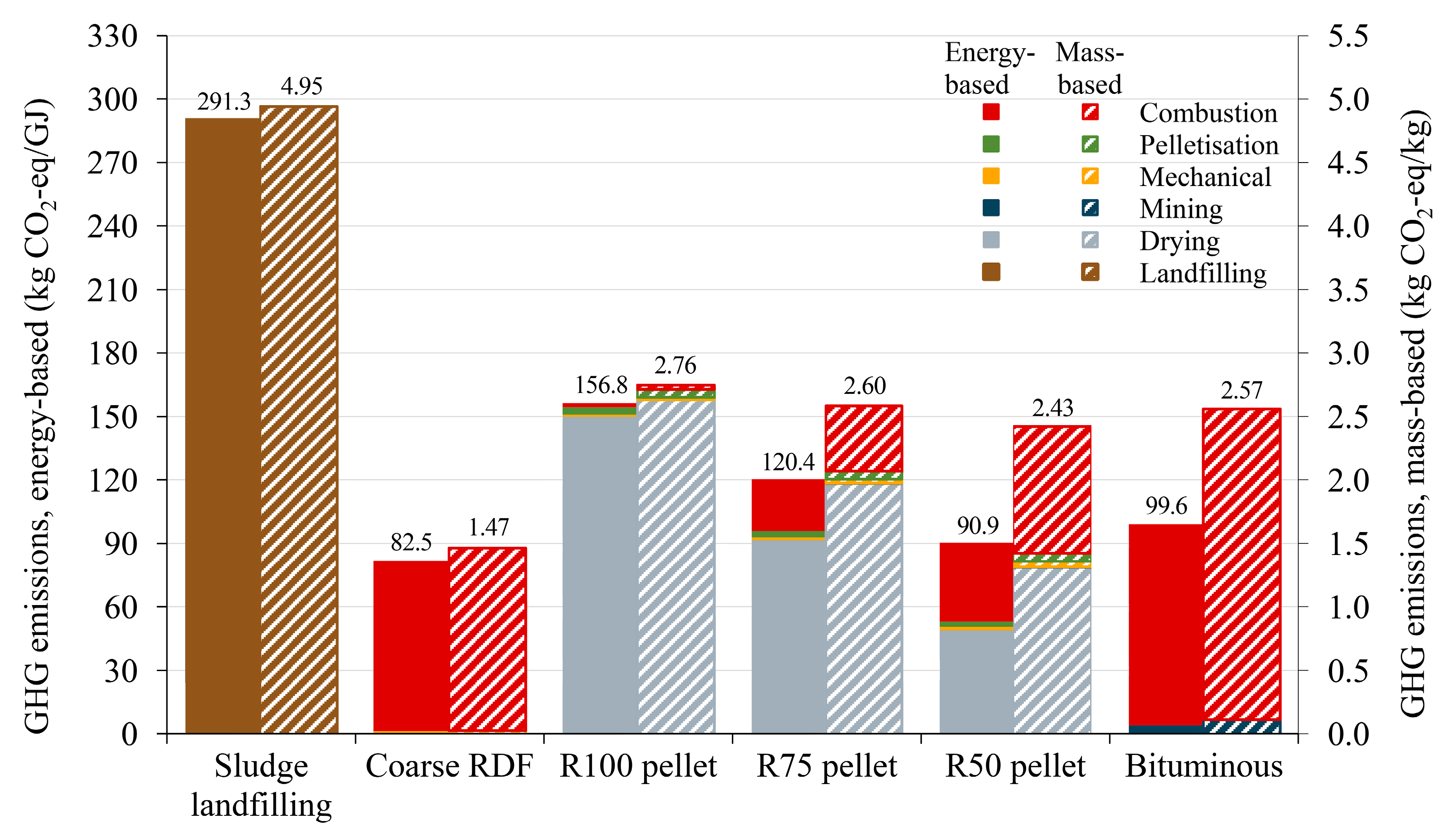
3.4. GHG Emissions from Treatment and Disposal Scenarios
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thai Tuna Industry Association. Thai Tuna Statistics of Import & Export in 2021–2023; Thai Tuna Industry Association: Bangkok, Thailand, 2024; pp. 11–13. [Google Scholar]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Tian, H.Z.; Lu, L.; Hao, J.M.; Gao, J.J.; Cheng, K.; Liu, K.Y.; Qiu, P.P.; Zhu, C.Y. A review of key hazardous trace elements in Chinese coals: Abundance, occurrence, behavior during coal combustion and their environmental impacts. Energy Fuels 2013, 27, 601–614. [Google Scholar] [CrossRef]
- Altikulac, A.; Turhan, S.; Kurnaz, A.; Goren, E.; Duran, C.; Hancerliogullari, A.; Ugur, F.A. Assessment of the enrichment of heavy metals in coal and its combustion residues. ACS Omega 2022, 7, 21239–21245. [Google Scholar] [CrossRef]
- Pollution Control Department. Thailand State of Pollution Report 2023; AP CONNEX Co., Ltd.: Bangkok, Thailand, 2024; pp. 126–129. [Google Scholar]
- Muttaraid, A.; Towprayoon, S.; Chiemchaisri, C.; Silalertruksa, T.; Wangyao, K. Enhanced landfill mining in Thailand: Policy implications from qualitative case study analysis. Sustainability 2024, 16, 11181. [Google Scholar] [CrossRef]
- Laosena, R.; Palamanit, A.; Luengchavanon, M.; Kittijaruwattana, J.; Nakason, C.; Lee, S.H.; Chotikhun, A. Characterization of mixed pellets made from rubberwood (Hevea brasiliensis) and refuse-derived fuel (RDF) waste as pellet fuel. Materials 2022, 15, 3093. [Google Scholar] [CrossRef]
- Rezaei, H.; Yazdan Panah, F.; Lim, C.J.; Sokhansanj, S. Pelletization of refuse-derived fuel with varying compositions of plastic, paper, organic and wood. Sustainability 2020, 12, 4645. [Google Scholar] [CrossRef]
- ASTM D3174-12; Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM D5630-13; Standard Test Method for Ash Content in Plastics. ASTM International: West Conshohocken, PA, USA, 2013. [CrossRef]
- ASTM D3175-20; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- ISO 22167:2019; Solid Recovered Fuels–Determination of the Content of Volatile Matter. International Organization for Standardization: Geneva, Switzerland, 2021.
- ASTM D4208-19; Standard Test Method for Total Chlorine in Coal by the Oxygen Vessel Combustion/Ion Selective Electrode Method. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Thipkhunthod, P.; Meeyoo, V.; Rangsunvigit, P.; Kitiyanan, B.; Siemanond, K.; Rirksomboon, T. Predicting the heating value of sewage sludges in Thailand from proximate and ultimate analyses. Fuel 2005, 84, 849–857. [Google Scholar] [CrossRef]
- ISO 21654:2019; Solid Recovered Fuels – Determination of Calorific Value. International Organization for Standardization: Geneva, Switzerland, 2019.
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Intergovernmental Panel on Climate Change: Hayama, Japan, 2006. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2015. [Google Scholar]
- EPPO. Energy Statistics of Thailand 2023; Energy Policy and Planning Office, Ministry of Energy: Bangkok, Thailand, 2023; p. 228. [Google Scholar]
- TGO. Grid Emission Factor; Thailand Greenhouse Gas Management Organization: Bangkok, Thailand, 2023; p. 2. [Google Scholar]
- Wulyapash, W.; Phongphiphat, A.; Towprayoon, S.; Johann, F. Characterisation and Carbon Intensity of RDF from Landfill Mining: A Case Study in Samut Prakan, Thailand. In Proceedings of the Sixth Symposium on Circular Economy and Urban Mining, Capri, Italy, 18–20 May 2022. [Google Scholar]
- Rigamonti, L.; Grosso, M.; Møller, J.; Sanchez, V.M.; Magnani, S.; Christensen, T.H. Environmental evaluation of plastic waste management scenarios. Resour. Conserv. Recycl. 2014, 85, 42–53. [Google Scholar] [CrossRef]
- Alves, O.; Calado, L.; Panizio, R.M.; Goncalves, M.; Monteiro, E.; Brito, P. Techno-economic study for a gasification plant processing residues of sewage sludge and solid recovered fuels. Waste Manag. 2021, 131, 148–162. [Google Scholar] [CrossRef]
- TGO. Emission Factor of Industrial Sector; Thailand Greenhouse Gas Management Organization: Bangkok, Thailand, 2022; p. 63. [Google Scholar]
- Wulyapash, W.; Phongphiphat, A.; Towprayoon, S. Comparative study of hot air drying and microwave drying for dewatered sludge. Clean Technol. Environ. Policy 2021, 24, 423–436. [Google Scholar] [CrossRef]
- CEN/TR 15508:2007; Key Properties on Solid Recovered Fuels to be Used for Establishing a Classification System. European Committee for Standardization: Brussels, Belgium, 2006.
- Onukak, I.; Mohammed-Dabo, I.; Ameh, A.; Okoduwa, S.; Fasanya, O. Production and characterization of biomass briquettes from tannery solid waste. Recycling 2017, 2, 17. [Google Scholar] [CrossRef]
- Chiemchaisri, C.; Charnnok, B.; Visvanathan, C. Recovery of plastic wastes from dumpsite as refuse-derived fuel and its utilization in small gasification system. Bioresour. Technol. 2010, 101, 1522–1527. [Google Scholar] [CrossRef]
- Nithikul, J.; Karthikeyan, O.P.; Visvanathan, C. Reject management from a mechanical biological treatment plant in Bangkok, Thailand. Resour. Conserv. Recycl. 2011, 55, 417–422. [Google Scholar] [CrossRef]
- Canopoli, L.; Fidalgo, B.; Coulon, F.; Wagland, S.T. Physico-chemical properties of excavated plastic from landfill mining and current recycling routes. Waste Manag. 2018, 76, 55–67. [Google Scholar] [CrossRef]
- Bouzar, B.; Mamindy-Pajany, Y. Manufacture and characterization of carbonated lightweight aggregates from waste paper fly ash. Powder Technol. 2022, 406, 117583. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Zhang, J.; Zhao, J.; Liu, Y.; Sun, L.; Liu, B.; Mao, H.; Lin, Y.; Li, W.; et al. Evaluation of the potential of pelletized biomass from different municipal solid wastes for use as solid fuel. Waste Manag. 2018, 74, 260–266. [Google Scholar] [CrossRef]
- Viczek, S.A.; Aldrian, A.; Pomberger, R.; Sarc, R. Origins of major and minor ash constituents of solid recovered fuel for co-processing in the cement industry. Waste Manag. 2021, 126, 423–432. [Google Scholar] [CrossRef]
- ISO 21640:2020; Solid Recovered Fuels—Specifications and Classes. International Organization for Standardization: Geneva, Switzerland, 2020.
- Okot, D.K.; Bilsborrow, P.E.; Phan, A.N. Briquetting characteristics of bean straw-maize cob blend. Biomass Bioenerg. 2019, 126, 150–158. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Li, H.; Chen, X.; Xiao, Z.; Liang, J.; Leng, L.; Guo, Z.; Zeng, G. Co-pelletization of sewage sludge and biomass: Thermogravimetric analysis and ash deposits. Fuel Process. Technol. 2016, 145, 109–115. [Google Scholar] [CrossRef]
- Withatanang, K.; Silapanuntakul, S.; Sihabut, T.; Pandii, W. Production of briquette fuel using wastewater sludge and banana peel waste. Asia-Pac. J. Sci. Technol. 2017, 22, 1–7. [Google Scholar] [CrossRef]
- Gilvari, H.; de Jong, W.; Schott, D.L. Breakage behavior of biomass pellets: An experimental and numerical study. Comput. Part. Mech. 2020, 8, 1047–1060. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Xiao, Z.; Liang, J.; Li, H.; Cao, L.; Wang, H.; Chen, X.; Zeng, G. A comparative study of biomass pellet and biomass-sludge mixed pellet: Energy input and pellet properties. Energy Convers. Manag. 2016, 126, 509–515. [Google Scholar] [CrossRef]
- Okot, D.K.; Bilsborrow, P.E.; Phan, A.N. Effects of operating parameters on maize COB briquette quality. Biomass Bioenerg. 2018, 112, 61–72. [Google Scholar] [CrossRef]
- Bui, N.K.; Satomi, T.; Takahashi, H. Recycling woven plastic sack waste and PET bottle waste as fiber in recycled aggregate concrete: An experimental study. Waste Manag. 2018, 78, 79–93. [Google Scholar] [CrossRef]
- Zinchik, S.; Xu, Z.; Kolapkar, S.S.; Bar-Ziv, E.; McDonald, A.G. Properties of pellets of torrefied U.S. waste blends. Waste Manag. 2020, 104, 130–138. [Google Scholar] [CrossRef]
- Bahij, S.; Omary, S.; Feugeas, F.; Faqiri, A. Fresh and hardened properties of concrete containing different forms of plastic waste—A review. Waste Manag. 2020, 113, 157–175. [Google Scholar] [CrossRef]
- Kannan, S.; Gariepy, Y.; Raghavan, G.S.V. Optimization and characterization of hydrochar produced from microwave hydrothermal carbonization of fish waste. Waste Manag. 2017, 65, 159–168. [Google Scholar] [CrossRef]
- Yilmaz, E.; Wzorek, M.; Akcay, S. Co-pelletization of sewage sludge and agricultural wastes. J. Environ. Manag. 2018, 216, 169–175. [Google Scholar] [CrossRef]
- Yang, W.S.; Lee, Y.J.; Kang, J.G.; Shin, S.K.; Jeon, T.W. Assessment of quality test methods for solid recovered fuel in South Korea. Waste Manag. 2020, 103, 240–250. [Google Scholar] [CrossRef]
- Gisi, S.D.; Chiarelli, A.; Tagliente, L.; Notarnicola, M. Energy, environmental and operation aspects of a SRF-fired fluidized bed waste-to-energy plant. Waste Manag. 2018, 73, 271–286. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.Q.; Zhou, Y.Y.; Liu, J.W.; Zhang, X.D.; Hu, G.W. Effect of microwave chlorine depleted pyrolyzate on the combustion characteristics of refuse derived fuel derived from package waste. Waste Manag. 2018, 82, 1–8. [Google Scholar] [CrossRef]
- Eriksen, M.K.; Pivnenko, K.; Olsson, M.E.; Astrup, T.F. Contamination in plastic recycling: Influence of metals on the quality of reprocessed plastic. Waste Manag. 2018, 79, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Waldheim, L. Gasification of Waste for Energy Carriers; International Energy Agency—Bioenergy: Paris, France, 2018; p. 23. [Google Scholar]
- Schwarzbock, T.; Rechberger, H.; Cencic, O.; Fellner, J. Determining national greenhouse gas emissions from waste-to-energy using the Balance Method. Waste Manag. 2016, 49, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Obermoser, M.; Fellner, J.; Rechberger, H. Determination of reliable CO2 emission factors for waste-to-energy plants. Waste Manag. Res. J. Sustain. Circ. Econ. 2009, 27, 907–913. [Google Scholar] [CrossRef]
- Chojnacka, K.; Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Witek-Krowiak, A.; Moustakas, K.; Ludwig, W.; Kułażyński, M. Improvements in drying technologies—Efficient solutions for cleaner production with higher energy efficiency and reduced emission. J. Clean. Prod. 2021, 320, 128706. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Yao, L.; Xu, Z. Auto-sorting commonly recovered plastics from waste household appliances and electronics using near-infrared spectroscopy. J. Clean. Prod. 2020, 246, 118732. [Google Scholar] [CrossRef]



| Scenario and Treatment | Activity Conditions | Parameters |
|---|---|---|
| Sludge scenario | ||
| Landfilling of sludge | Dewatered sludge was dealt with managed solid waste disposal sites with covered material under moist and wet tropical conditions | DOC: 0.06 DOCf: 0.7 [16] MCF: 1 [16] OF: 0.1 [16] |
| IPCC defaults for other parameters | ||
| There is no CH4 recovery. It was not collected or flared | ||
| Coarse RDF scenario | ||
| Landfill mining of MSW | Diesel consumption by excavators and loaders during excavation of landfilled MSW | FCmining, diesel: 1.03 liter/ton RDF NCVdiesel: 36.42 MJ/liter [18] EFdiesel: 0.0741 kg CO2/MJ [16] |
| Mechanical treatment for RDF production | Electricity consumption by machinery | ECRDF prod.: 58.78 kWh/ton RDF EFEC: 0.4857 kg CO2/kWh [19] |
| Diesel consumption of loaders | FCRDF prod, diesel: 0.69 liter/ton RDF | |
| Combustion of coarse RDF | N2O and CH4 emissions | NCVcoarse RDF: 17.86 GJ/ton EFN2O, RDF: 0.004 kg N2O/GJ [16] EFCH4, RDF: 0.03 kg CH4/GJ [16] |
| CO2 emissions following Equation (6) | OF: 1 [16] CFi; FCFi [16] WFi; dmi [20] | |
| Treatment of rejected materials was not included | ||
| Pellet scenario | ||
| Landfill mining of MSW | Refer to landfill mining emissions from Coarse RDF scenario | |
| Mechanical treatment for RDF production | Refer to the emissions from mechanical treatment of the Coarse RDF scenario | |
| Plastic waste preparation | Coarse RDF was further separated and milled | ECseparate: 17.7 kWh/ton RDF [21] ECmilling: 35.51 kWh/ton [22] |
| Sludge preparation | Sludge was dried using an electric dryer and then milled | ECdrying: 5445.68 kWh/ton dried sludge [20] |
| Pelletization | The prepared sludge and plastic waste were mixed, following the mixing ratios, and pelletized | ECmixing: 3.97 kWh/ton [22] ECpelletizing: 127.98 kWh/ton [22] WFsludge: WFplastic are 100:0, 75:25, 50:50 |
| Combustion of pellets | Calculating the same method as the RDF scenario | |
| Coal scenario | ||
| Coal mining | Emissions of coal production at the surface mine | EFcoal mining: 0.1187 kg CO2-eq/kg [23] |
| Combustion of coal | Emissions of bituminous coal combustion | EFN2O, bituminous: 0.0015 kg N2O/GJ [16] EFCH4, bituminous: 0.001 kg CH4/GJ [16] EFCO2, bituminous: 94.6 kg CO2/GJ [16] NCVbituminous: 0.0258 GJ/kg [16] |
| Characteristics | Sludge | Plastic Waste | ||
|---|---|---|---|---|
| Mean | RSD% | Mean | RSD% | |
| Glass transition temperature (°C) | – | – | 79 | – |
| Melting point (°C) | 183 | – | 124 | – |
| GCV (MJ/kg) | 20 | 0.1 | 36 | 0.2 |
| Proximate analysis (wt%, dry basis) | ||||
| Ash | 15 | 0.8 | 11 | 17.2 |
| FC | 16 | – | 3 | – |
| VM | 69 | 2.4 | 86 | 7.1 |
| Elemental analysis (mg/kg, dry basis) | ||||
| C | 448,000 | 0.6 | 587,000 | 1.2 |
| O | 309,000 | 6.5 | 86,800 | 7.5 |
| H | 62,800 | 1.0 | 90,000 | 1.9 |
| N | 67,400 | 0.3 | 4900 | 6.0 |
| S | 16,400 | 0.6 | 3600 | 1.5 |
| Cl | 23,000 | – | 89,200 | – |
| Ca | 30,900 | 1.4 | 108,000 | 0.8 |
| P | 17,600 | 1.5 | 2350 | 5.9 |
| Fe | 6350 | 1.4 | 5600 | 1.6 |
| Al | 4780 | 14.6 | 2800 | 26.7 |
| Na | 4400 | 0.7 | 1030 | 1.2 |
| K | 4100 | 0.4 | 900 | 1.5 |
| Si | 3400 | 9.9 | 10,300 | 4.4 |
| Zn | 895 | 2.0 | 360 | 3.4 |
| Ti | 437 | 8.9 | 4300 | 2.0 |
| Sr | 97 | 2.5 | 42 | 4.2 |
| Cu | 96 | 0.3 | 140 | 1.0 |
| Cr | 71 | 1.6 | 100 | 1.0 |
| Sc | 69 | 26.4 | 120 | 23.3 |
| Mn | 59 | 0.2 | 50 | 1.2 |
| Ni | 46 | 1.4 | 22 | 3.1 |
| Se | 35 | 7.3 | – | – |
| Cd | 11 | 0.6 | 1 | 17.0 |
| As | 10 | 33.9 | 2 | 23.0 |
| Rb | 8 | 13.5 | 8 | 15.3 |
| Mo | 7 | 19.7 | 9 | 16.9 |
| Zr | 7 | 20.6 | 12 | 12.1 |
| V | 6 | 8.3 | 2 | 7.2 |
| U | 5 | 41.0 | – | – |
| Pb | 4 | 58.1 | 40 | 1.9 |
| Nb | 4 | 30.7 | 8 | 17.6 |
| Co | 2 | 15.4 | 5 | 3.2 |
| Hg | 1 | 4.1 | 1 | 10.0 |
| Characteristics (mg/kgdb) | R100 | R75 | R50 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T120-P80 | T120-P60 | T100-P80 | T100-P60 | T120-P80 | T120-P60 | T100-P80 | T100-P60 | T120-P80 | T120-P60 | T100-P80 | T100-P60 | |
| C | 407,000 | 418,000 | 417,000 | 398,000 | 443,000 | 441,000 | 456,000 | 471,000 | 505,000 | 496,000 | 479,000 | 506,000 |
| O | 263,000 | 272,000 | 270,000 | 256,000 | 205,000 | 200,000 | 206,000 | 222,000 | 136,000 | 160,000 | 118,000 | 146,000 |
| H | 56,300 | 57,800 | 58,500 | 54,000 | 62,000 | 79,400 | 86,600 | 72,500 | 104,000 | 97,000 | 95,700 | 104,000 |
| N | 61,800 | 67,200 | 66,000 | 60,200 | 47,100 | 47,400 | 44,000 | 49,000 | 25,000 | 29,400 | 19,000 | 26,000 |
| S | 13,500 | 14,500 | 14,000 | 14,000 | 11,500 | 10,600 | 10,000 | 11,000 | 6700 | 7870 | 6250 | 6880 |
| Cl | 108,000 | 80,500 | 81,100 | 122,000 | 106,000 | 131,000 | 107,000 | 86,400 | 108,000 | 95,000 | 171,000 | 110,000 |
| Ca | 44,200 | 43,000 | 44,000 | 45,600 | 64,700 | 49,200 | 49,000 | 47,000 | 83,600 | 77,300 | 84,100 | 65,000 |
| P | 17,700 | 17,700 | 18,000 | 18,000 | 19,100 | 14,000 | 13,800 | 14,3000 | 10,000 | 11,900 | 8720 | 11,900 |
| Fe | 12,300 | 12,000 | 12,100 | 12,400 | 10,600 | 11,000 | 9730 | 11,100 | 6650 | 10,300 | 5850 | 10,000 |
| Al | 3700 | 3730 | 4300 | 4660 | 4900 | 3400 | 3480 | 3000 | 3500 | 3860 | 2900 | 3840 |
| Na | 3600 | 4040 | 4730 | 5000 | 2590 | 3200 | 2830 | 2860 | 1000 | 1800 | 1320 | 510 |
| K | 3400 | 3950 | 4600 | 4460 | 2500 | 3200 | 2510 | 2770 | 850 | 1630 | 1110 | 420 |
| Si | 3000 | 3000 | 3000 | 2980 | 5280 | 3000 | 3540 | 3400 | 3000 | 4170 | 3300 | 4250 |
| Zn | 1200 | 1300 | 1300 | 1290 | 1390 | 1000 | 930 | 1040 | 800 | 925 | 630 | 870 |
| Ti | 627 | 631 | 632 | 700 | 2200 | 2000 | 1830 | 2000 | 2900 | 2350 | 2700 | 4030 |
| Cu | 97 | 102 | 132 | 132 | 90 | 93 | 90 | 120 | 30 | 55 | 48 | 10 |
| Cr | 165 | 91 | 122 | 130 | 116 | 123 | 111 | 114 | 37 | 81 | 50 | 19 |
| Mn | 70 | 70 | 82 | 86 | 72 | 60 | 60 | 70 | 15 | 37 | 25 | 10 |
| Ni | 50 | 51 | 70 | 65 | 60 | 58 | 50 | 54 | 12 | 26 | 23 | 8 |
| Cd | 10 | 11 | 12 | 11 | 9 | 9 | 7 | 8 | 2 | 4 | 3 | 1 |
| As | 11 | 12 | 15 | 12 | 9 | 8 | 9 | 9 | 6 | 8 | 5 | 6 |
| V | 4 | 5 | 6 | 6 | 4 | 4 | 4 | 4 | 3 | 4 | 3 | 4 |
| Pb | 5 | 5 | 6 | 5 | 11 | 10 | 10 | 10 | 9 | 12 | 13 | 5 |
| Co | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 1 |
| Hg | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wulyapash, W.; Phongphiphat, A.; Fellner, J.; Towprayoon, S. Exploring Refuse-Derived Fuel Production from Seafood-Processing Sludge and Landfill-Mined Plastic Waste Co-Pelletization. Recycling 2025, 10, 52. https://doi.org/10.3390/recycling10020052
Wulyapash W, Phongphiphat A, Fellner J, Towprayoon S. Exploring Refuse-Derived Fuel Production from Seafood-Processing Sludge and Landfill-Mined Plastic Waste Co-Pelletization. Recycling. 2025; 10(2):52. https://doi.org/10.3390/recycling10020052
Chicago/Turabian StyleWulyapash, Wittawat, Awassada Phongphiphat, Johann Fellner, and Sirintornthep Towprayoon. 2025. "Exploring Refuse-Derived Fuel Production from Seafood-Processing Sludge and Landfill-Mined Plastic Waste Co-Pelletization" Recycling 10, no. 2: 52. https://doi.org/10.3390/recycling10020052
APA StyleWulyapash, W., Phongphiphat, A., Fellner, J., & Towprayoon, S. (2025). Exploring Refuse-Derived Fuel Production from Seafood-Processing Sludge and Landfill-Mined Plastic Waste Co-Pelletization. Recycling, 10(2), 52. https://doi.org/10.3390/recycling10020052







