Recent Advances and Perspectives in Single-Ion COF-Based Solid Electrolytes
Abstract
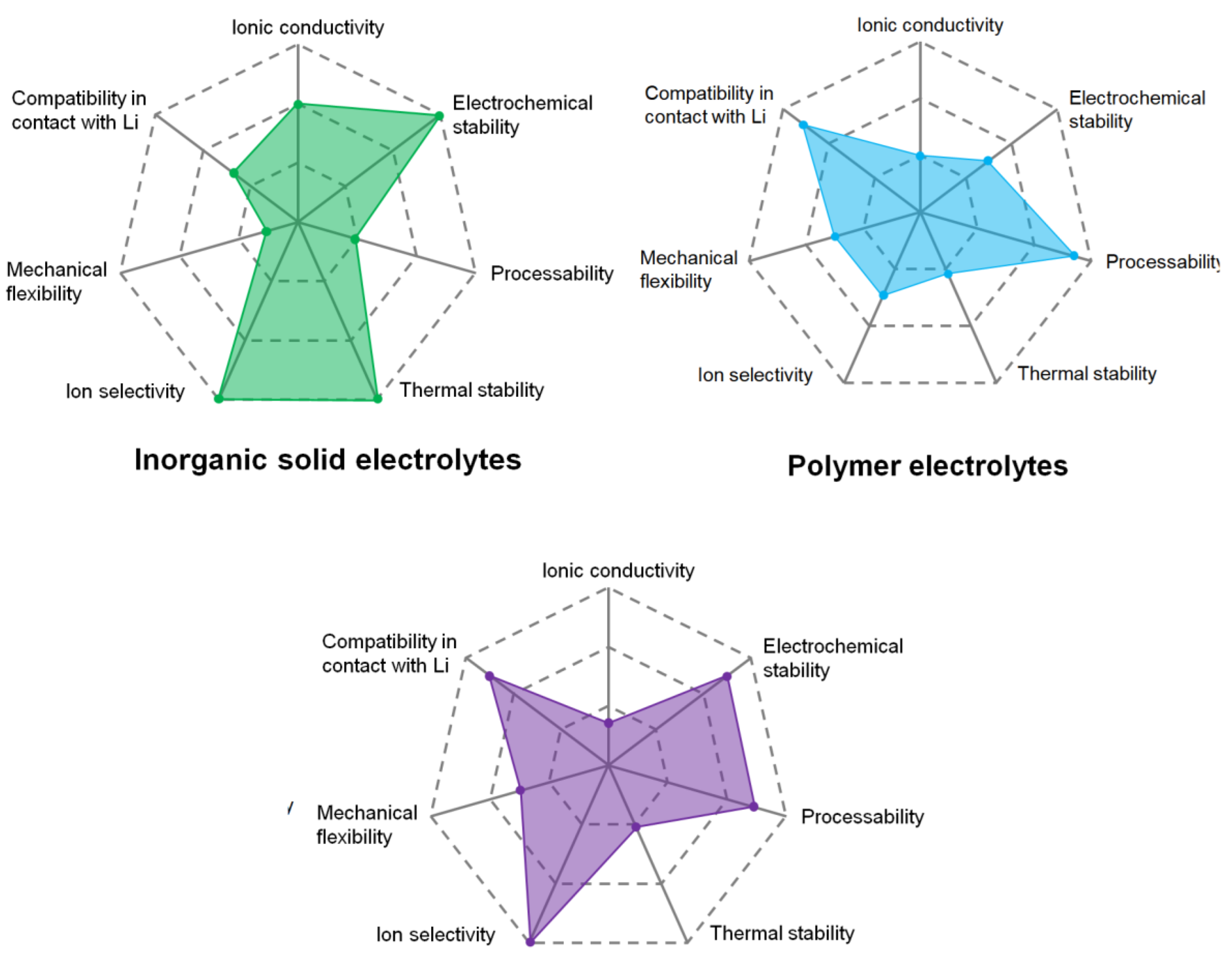
:1. Introduction
2. Single-Ion Conducting Solid Electrolyte
2.1. Single-Ion Conductor
2.2. Advantages of Single-Ion Conductors
3. Single-Ion COF-Based Electrolytes
3.1. Li-Ion Transport Mechanisms in COFs
3.2. Single-Ion COF-Based Solid Electrolytes
3.2.1. Anionic Single-Ion COF Conducting Electrolytes
3.2.2. Cationic Single-Ion COF Conducting Electrolytes
3.2.3. Hybrid Single-Ion COF Conducting Electrolytes
3.2.4. Performance of the Cells with Single-Ion COF-Based Solid Electrolytes
3.3. Other Organic Single-Ion Conducting Polymers
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, F.; Westover, A.S.; Yue, J.; Fan, X.; Wang, F.; Chi, M.; Leonard, D.N.; Dudney, N.J.; Wang, H.; Wang, C. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 2019, 4, 187–196. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Banerjee, A.; Wang, X.; Fang, C.; Wu, E.A.; Meng, Y.S. Interfaces and Interphases in All-Solid-State Batteries with Inorganic Solid Electrolytes. Chem. Rev. 2020, 120, 6878–6933. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, H.; Zhu, X.; He, P.; Zhou, H. A stable quasi-solid electrolyte improves the safe operation of highly efficient lithium-metal pouch cells in harsh environments. Nat. Commun. 2022, 13, 1510. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Wan, J.; Xie, J.; Kong, X.; Liu, Z.; Liu, K.; Shi, F.; Pei, A.; Chen, H.; Chen, W.; Chen, J.; et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat. Nanotechnol. 2019, 14, 705–711. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. Challenges in speeding up solid-state battery development. Nat. Energy 2023, 8, 230–240. [Google Scholar] [CrossRef]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium Batteries and the Solid Electrolyte Interphase (SEI)—Progress and Outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide Solid Electrolyte with Favorable Mechanical Property for All-Solid-State Lithium Battery. Sci. Rep. 2013, 3, 2261. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Chi, M.; Liang, C.; Dudney, N.J. Solid Electrolyte: The Key for High-Voltage Lithium Batteries. Adv. Energy Mater. 2015, 5, 1401408. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Shen, L.; Liu, Q.; Ma, J.; Lv, W.; He, Y.; Yang, Q. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef]
- Hodge, M.I.; Ingram, A. West, Impedance and modulus spectroscopy of polycrystalline solid electrolytes. J. Electroanal. Chem. Interfacial Electrochem. 1976, 74, 125–143. [Google Scholar] [CrossRef]
- Gao, J.; Wang, C.; Han, D.-W.; Shin, D.-M. Single-ion conducting polymer electrolytes as a key jigsaw piece for next-generation battery applications. Chem. Sci. 2021, 12, 13248–13272. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, Z.; Zhou, R.; Wang, D.; Guo, X.; Zhu, Y.; Zhang, B. Critical Roles of Mechanical Properties of Solid Electrolyte Interphase for Potassium Metal Anodes. Adv. Funct. Mater. 2022, 32, 2112399. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kuwajima, H.; Masaki, T. Phase change and mechanical properties of ZrO2-Y2O3 solid electrolyte after ageing. Solid State Ionics 1981, 3–4, 489–493. [Google Scholar] [CrossRef]
- Armand, M. Polymer solid electrolytes—An overview. Solid State Ionics 1983, 9–10, 745–754. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, L.; Huang, B.; Xu, B.; Shao, G.; Wang, H.; Li, Y.; Wang, C.-A. Enhanced performance of Li6. 4La3Zr1. 4Ta0. 6O12 solid electrolyte by the regulation of grain and grain boundary phases. ACS Appl. Mater. Interfaces 2020, 12, 56118–56125. [Google Scholar] [CrossRef]
- Yu, S.; Siegel, D.J. Grain boundary contributions to Li-ion transport in the solid electrolyte Li7La3Zr2O12 (LLZO). Chem. Mater. 2017, 29, 9639–9647. [Google Scholar] [CrossRef]
- Hou, W.; Guo, X.; Shen, X.; Amine, K.; Yu, H.; Lu, J. Solid electrolytes and interfaces in all-solid-state sodium batteries: Progress and perspective. Nano Energy 2018, 52, 279–291. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.; van Eck, E.R.H.; Wang, C.; Ganapathy, S.; Wagemaker, M. Improving Li-ion interfacial transport in hybrid solid electrolytes. Nat. Nanotechnol. 2022, 17, 959–967. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, T.; Zhao, B.; Shen, F.; Jin, H.; Han, X. Recent advances in organic-inorganic composite solid electrolytes for all-solid-state lithium batteries. Energy Storage Mater. 2021, 34, 388–416. [Google Scholar] [CrossRef]
- Bo, X.; Wang, L.; Zhao, H.; Almardi, J.M.; Li, W.; Daoud, W.A. A Stretchable Solid Ionic Electrode-Based Triboelectric Nanogenerator for Biomechanical Energy Harvesting and Self-Powered Sensors. Small 2023. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, Z.; Gray, J.L.; He, X.; Wang, D.; Chen, T.; Huang, Q.; Li, Y.C.; Wang, H.; Kim, S.H.; et al. Polymer–Inorganic solid–electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 2019, 18, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Peled, E.; Golodnitsky, D.; Ardel, G. Advanced Model for Solid Electrolyte Interphase Electrodes in Liquid and Polymer Electrolytes. J. Electrochem. Soc. 1997, 144, L208–L210. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L.M.; Armand, M.; Zhou, Z. Single lithium-ion conducting solid polymer electrolytes: Advances and perspectives. Chem. Soc. Rev. 2017, 46, 797–815. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Bin Yong, A.X.; Gustafson, W.J.; Reedy, C.N.; Ertekin, E.; Krogstad, J.A.; Perry, N.H. Toward design of cation transport in solid-state battery electrolytes: Structure-dynamics relationships. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100875. [Google Scholar] [CrossRef]
- Hu, B.; Xu, J.; Fan, Z.; Xu, C.; Han, S.; Zhang, J.; Ma, L.; Ding, B.; Zhuang, Z.; Kang, Q. Covalent Organic Framework Based Lithium–Sulfur Batteries: Materials, Interfaces, and Solid-State Electrolytes. Adv. Energy Mater. 2023, 13, 2203540. [Google Scholar]
- Kang, T.; Sun, C.; Li, Y.; Song, T.; Guan, Z.; Tong, Z.; Nan, J.; Lee, C. Dendrite-Free Sodium Metal Anodes Via Solid Electrolyte Interphase Engineering With a Covalent Organic Framework Separator. Adv. Energy Mater. 2023, 13, 2204083. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, L.; Liu, Q.; Liu, Q.; Chen, Z.; Zhao, W.; Kong, X.; Bin Wu, H. Segmental molecular dynamics boosts Li-ion conduction in metal-organic solid electrolytes for Li-metal batteries. Energy Storage Mater. 2023, 54, 854–862. [Google Scholar] [CrossRef]
- Hu, Q.; Osswald, S.; Daniel, R.; Zhu, Y.; Wesel, S.; Ortiz, L.; Sadoway, D.R. Graft copolymer-based lithium-ion battery for high-temperature operation. J. Power Sources 2011, 196, 5604. [Google Scholar] [CrossRef]
- Ahmadi, M.; Asadinezhad, A. Synthesis and characterization of azodianiline covalent organic frameworks intended for energy storage. J. Mol. Struct. 2023, 1286, 135647. [Google Scholar] [CrossRef]
- Lee, J.; Lim, H.; Park, J.; Kim, M.S.; Jung, J.W.; Kim, J.; Kim, I.D. Fluorine-Rich Covalent Organic Framework to Boost Electrochemical Kinetics and Storages of K+ Ions for Potassium-Ion Battery. Adv. Energy Mater. 2023, 13, 2300442. [Google Scholar] [CrossRef]
- Guan, X.; Fang, Q.; Yan, Y.; Qiu, S. Functional Regulation and Stability Engineering of Three-Dimensional Covalent Organic Frameworks. Accounts Chem. Res. 2022, 55, 1912–1927. [Google Scholar] [CrossRef]
- Kang, T.W.; Lee, J.H.; Lee, J.; Park, J.H.; Shin, J.H.; Ju, J.M.; Lee, H.; Lee, S.U.; Kim, J.H. Ion Channel-Restructured Zwitterionic Covalent Organic Framework Solid Electrolyte for All-Solid-State Lithium Metal Batteries. Adv. Mater. 2023, 2301308. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Guo, L.; Wei, G.; Xian, D.; Zhang, B.; Wang, L. Enhancing the safety and cyclic performance of lithium-ion batteries using heat resistant and wettable separator based on covalent organic framework and polybenzimidazole. Chem. Eng. J. 2022, 443, 136480. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, P.; Yan, D.; Liu, J.; Chen, Y.; Liu, Q.; Cheng, P.; Zaworotko, M.J.; Zhang, Z. Thermally rearranged covalent organic framework with flame-retardancy as a high safety Li-ion solid electrolyte. eScience 2022, 2, 311–318. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, B.; Yang, Z.; Yang, X.; Yu, X.; Xing, G.; Zhang, Y.; Chen, L. Stable 2D Heteroporous Covalent Organic Frameworks for Efficient Ionic Conduction. Angew. Chem. Int. Ed. 2019, 58, 15742–15746. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yuan, J.; Wu, H.; Su, Y.; Yang, H.; You, X.; Zhang, R.; He, X.; Khan, N.A.; Kasher, R.; et al. Ultrathin nanofiltration membrane with polydopamine-covalent organic framework interlayer for enhanced permeability and structural stability. J. Membr. Sci. 2019, 576, 131–141. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, H.; Yildirim, T. Structural stability and elastic properties of prototypical covalent organic frameworks. Chem. Phys. Lett. 2010, 499, 103–107. [Google Scholar] [CrossRef]
- Zhao, H.; Lam, W.-Y.A.; Wang, L.; Xu, H.; Daoud, W.A.; He, X. The significance of detecting imperceptible physical/chemical changes/reactions in lithium-ion batteries: A perspective. Energy Environ. Sci. 2022, 15, 2329–2355. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hou, J.; Jiang, L.; Wang, H. Angstrom-scale ion channels towards single-ion selectivity. Chem. Soc. Rev. 2022, 51, 2224–2254. [Google Scholar] [CrossRef] [PubMed]
- Gritzner, G. Single-ion transfer properties: A measure of ion-solvation in solvents and solvent mixtures. Electrochim. Acta 1998, 44, 73–83. [Google Scholar] [CrossRef]
- Ge, Y.; Li, J.; Meng, Y.; Xiao, D. Tuning the structure characteristic of the flexible covalent organic framework (COF) meets a high performance for lithium-sulfur batteries. Nano Energy 2023, 109, 108297. [Google Scholar] [CrossRef]
- Wei, C.; Wang, Y.; Zhang, Y.; Tan, L.; Qian, Y.; Tao, Y.; Xiong, S.; Feng, J. Flexible and stable 3D lithium metal anodes based on self-standing MXene/COF frameworks for high-performance lithium-sulfur batteries. Nano Res. 2021, 14, 3576–3584. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Sakaushi, K.; Antonietti, M. Carbon-and nitrogen-based organic frameworks. Acc. Chem. Res. 2015, 48, 1591–1600. [Google Scholar] [CrossRef]
- Yang, H.; Liu, B.; Bright, J.; Kasani, S.; Yang, J.; Zhang, X.; Wu, N. A Single-Ion Conducting UiO-66 Metal–Organic Framework Electrolyte for All-Solid-State Lithium Batteries. ACS Appl. Energy Mater. 2020, 3, 4007–4013. [Google Scholar] [CrossRef]
- Chi, X.; Li, M.; Di, J.; Bai, P.; Song, L.; Wang, X.; Li, F.; Liang, S.; Xu, J.; Yu, J. A highly stable and flexible zeolite electrolyte solid-state Li–air battery. Nature 2021, 592, 551–557. [Google Scholar] [CrossRef]
- Zhao, H.; Sheng, L.; Wang, L.; Xu, H.; He, X. The opportunity of metal organic frameworks and covalent organic frameworks in lithium (ion) batteries and fuel cells. Energy Storage Mater. 2020, 33, 360–381. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, M.; Chen, Q.; Duan, H.; Liu, S. Recent Progress in Framework Materials for High-Performance Lithium-Sulfur Batteries. Chem. Rec. 2023, e202200278. [Google Scholar] [CrossRef]
- Hu, Y.; Wayment, L.J.; Haslam, C.; Yang, X.; Lee, S.-H.; Jin, Y.; Zhang, W. Covalent organic framework based lithium-ion battery: Fundamental, design and characterization. Energychem 2021, 3, 100048. [Google Scholar] [CrossRef]
- Li, J.; Jing, X.; Li, Q.; Li, S.; Gao, X.; Feng, X.; Wang, B. Bulk COFs and COF nanosheets for electrochemical energy storage and conversion. Chem. Soc. Rev. 2020, 49, 3565–3604. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Loh, K.P. Recent Progress in Covalent Organic Frameworks as Solid-State Ion Conductors. ACS Mater. Lett. 2019, 1, 327–335. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Q.; Zhao, G.; Sun, Y.; Guo, H. Covalent organic frameworks for solid-state electrolytes of lithium metal batteries. J. Mater. Chem. A 2022, 10, 7497–7516. [Google Scholar] [CrossRef]
- Newman, J.; Balsara, N.P. Electrochemical Systems; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Diederichsen, K.M.; McShane, E.J.; McCloskey, B.D. Promising Routes to a High Li+ Transference Number Electrolyte for Lithium Ion Batteries. ACS Energy Lett. 2017, 2, 2563–2575. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.-P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.; Fuller, T.F.; Newman, J. The importance of the lithium ion transference number in lithium/polymer cells. Electrochimica Acta 1994, 39, 2073–2081. [Google Scholar] [CrossRef]
- Doyle, M.; Newman, J. The use of mathematical modeling in the design of lithium/polymer battery systems. Electrochimica Acta 1995, 40, 2191–2196. [Google Scholar] [CrossRef]
- Bannister, D.; Davies, G.; Ward, I.; McIntyre, J. Ionic conductivities for poly(ethylene oxide) complexes with lithium salts of monobasic and dibasic acids and blends of poly(ethylene oxide) with lithium salts of anionic polymers. Polymer 1984, 25, 1291–1296. [Google Scholar] [CrossRef]
- Fong, K.D.; Self, J.; Diederichsen, K.M.; Wood, B.M.; McCloskey, B.D.; Persson, K.A. Ion Transport and the True Transference Number in Nonaqueous Polyelectrolyte Solutions for Lithium Ion Batteries. ACS Central Sci. 2019, 5, 1250–1260. [Google Scholar] [CrossRef]
- Zhang, Z.; Roy, P.-N.; Li, H.; Avdeev, M.; Nazar, L.F. Coupled Cation–Anion Dynamics Enhances Cation Mobility in Room-Temperature Superionic Solid-State Electrolytes. J. Am. Chem. Soc. 2019, 141, 19360–19372. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yao, Y.; Li, R.; Guo, Z.H.; Li, L.; Pan, C.; Hu, W.; Pu, X. Self-healing single-ion-conductive artificial polymeric solid electrolyte interphases for stable lithium metal anodes. Nano Energy 2022, 93, 106871. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Zhai, T. Reviving Lithium-Metal Anodes for Next-Generation High-Energy Batteries. Adv. Mater. 2017, 29, 1700007. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Suzuki, Y.; Nishimoto, A. Single ion conduction in polyether electrolytes alloyed with lithium salt of a perfluorinated polyimide. Electrochim. Acta 2000, 45, 1187–1192. [Google Scholar] [CrossRef]
- Watanabe, M.; Tokuda, H.; Muto, S. Anionic effect on ion transport properties in network polyether electrolytes. Electrochim. Acta 2001, 46, 1487–1491. [Google Scholar] [CrossRef]
- Lee, S.-Y. Organic Single-Ion Conductors for All-Solid-State Lithium Metal Batteries, Electrochemical Society Meeting Abstracts Prime2020; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2020; p. 793. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, A.; Liu, X.; Luo, J. Dendrites in lithium metal anodes: Suppression, regulation, and elimination. Acc. Chem. Res. 2019, 52, 3223–3232. [Google Scholar] [CrossRef]
- Chazalviel, J.-N. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 1990, 42, 7355–7367. [Google Scholar] [CrossRef]
- Brissot, C.; Rosso, M.; Chazalviel, J.-N.; Lascaud, S. Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 1999, 81–82, 925–929. [Google Scholar] [CrossRef]
- Rosso, M.; Brissot, C.; Teyssot, A.; Dollé, M.; Sannier, L.; Tarascon, J.-M.; Bouchet, R.; Lascaud, S. Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim. Acta 2006, 51, 5334–5340. [Google Scholar] [CrossRef]
- Martinez-Ibañez, M.; Sanchez-Diez, E.; Qiao, L.; Zhang, Y.; Judez, X.; Santiago, A.; Aldalur, I.; Carrasco, J.; Zhu, H.; Forsyth, M.; et al. Unprecedented Improvement of Single Li-Ion Conductive Solid Polymer Electrolyte Through Salt Additive. Adv. Funct. Mater. 2020, 30, 2000455. [Google Scholar] [CrossRef]
- Stolz, L.; Hochstädt, S.; Röser, S.; Hansen, M.R.; Winter, M.; Kasnatscheew, J. Single-Ion versus Dual-Ion Conducting Electrolytes: The Relevance of Concentration Polarization in Solid-State Batteries. ACS Appl. Mater. Interfaces 2022, 14, 11559–11566. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, N. Ionic conductivity and ion transport mechanisms of solid-state lithium-ion battery electrolytes: A review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, Y.-Y. New Insights into the Compositional Dependence of Li-Ion Transport in Polymer–Ceramic Composite Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 4113–4120. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Molina, D.A.; Mohammad-Pour, G.S.; Lee, C.; Logan, M.W.; Duan, X.; Harper, J.K.; Uribe-Romo, F.J. Mechanically Shaped Two-Dimensional Covalent Organic Frameworks Reveal Crystallographic Alignment and Fast Li-Ion Conductivity. J. Am. Chem. Soc. 2016, 138, 9767–9770. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Suh, K.; Rohman, R.; Hwang, W.; Yoon, M.; Kim, K. Solid lithium electrolytes based on an organic molecular porous solid. Chem. Commun. 2015, 51, 9313–9316. [Google Scholar] [CrossRef]
- Xu, Q.; Tao, S.; Jiang, Q.; Jiang, D. Ion conduction in polyelectrolyte covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 7429–7432. [Google Scholar] [CrossRef]
- Hou, T.; Xu, W.; Pei, X.; Jiang, L.; Yaghi, O.M.; Persson, K.A. Ionic Conduction Mechanism and Design of Metal–Organic Framework Based Quasi-Solid-State Electrolytes. J. Am. Chem. Soc. 2022, 144, 13446–13450. [Google Scholar] [CrossRef]
- Farina, M.; Duff, B.B.; Tealdi, C.; Pugliese, A.; Blanc, F.; Quartarone, E. Li+ Dynamics of Liquid Electrolytes Nanoconfined in Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 53986–53995. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, B.; Weng, M.; Zheng, J.; Li, S.; Pan, F. Lithium ion diffusion mechanism in covalent organic framework based solid state electrolyte. Phys. Chem. Chem. Phys. 2019, 21, 9883–9888. [Google Scholar] [CrossRef]
- Zhao, G.; Mei, Z.; Duan, L.; An, Q.; Yang, Y.; Zhang, C.; Tan, X.; Guo, H. COF-Based single Li+ solid electrolyte accelerates the ion diffusion and restrains dendrite growth in quasi-solid-state organic batteries. Carbon Energy 2023, 5, e248. [Google Scholar] [CrossRef]
- Sun, M.; Feng, J.; Feng, Y.; Xin, X.; Ding, Y.; Sun, M. Preparation of ionic covalent organic frameworks and their applications in solid-phase extraction. TrAC Trends Anal. Chem. 2022, 157, 116829. [Google Scholar] [CrossRef]
- Hu, Y.; Dunlap, N.; Wan, S.; Lu, S.; Huang, S.; Sellinger, I.; Ortiz, M.; Jin, Y.; Lee, S.-H.; Zhang, W. Crystalline Lithium Imidazolate Covalent Organic Frameworks with High Li-Ion Conductivity. J. Am. Chem. Soc. 2019, 141, 7518–7525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ren, Y.; Bo, X.; Si, L.; Wei, Z.; Daoud, W.A. A novel study on COF-based semi-solid electrolyte for spinel LiNi0.5Mn1.5O4 targeting transition metals migration. Scr. Mater. 2023, 223, 115101. [Google Scholar] [CrossRef]
- Jeong, K.; Park, S.; Jung, G.Y.; Kim, S.H.; Lee, Y.-H.; Kwak, S.K.; Lee, S.-Y. Solvent-free, single lithium-ion conducting covalent organic frameworks. J. Am. Chem. Soc. 2019, 141, 5880–5885. [Google Scholar] [CrossRef]
- Ren, Z.; Li, J.; Cai, M.; Yin, R.; Liang, J.; Zhang, Q.; He, C.; Jiang, X.; Ren, X. An in situ formed copolymer electrolyte with high ionic conductivity and high lithium-ion transference number for dendrite-free solid-state lithium metal batteries. J. Mater. Chem. A 2023, 11, 1966–1977. [Google Scholar] [CrossRef]
- Li, X.; Hou, Q.; Huang, W.; Xu, H.-S.; Wang, X.; Yu, W.; Li, R.; Zhang, K.; Wang, L.; Chen, Z.; et al. Solution-Processable Covalent Organic Framework Electrolytes for All-Solid-State Li–Organic Batteries. ACS Energy Lett. 2020, 5, 3498–3506. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.-Q.; Li, F.; Wu, Z.; Ma, C.; Xu, Q.; Wang, P.; Zhang, X.-M. A pre-synthetic strategy to construct single ion conductive covalent organic frameworks. Chem. Commun. 2020, 56, 2747–2750. [Google Scholar] [CrossRef]
- Du, Y.; Yang, H.; Whiteley, J.M.; Wan, S.; Jin, Y.; Lee, S.H.; Zhang, W. Ionic covalent organic frameworks with spiroborate linkage. Angew. Chem. Int. Ed. 2016, 55, 1737–1741. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Dong, Y.; Li, J.; Li, S.; Shao, P.; Feng, X.; Wang, B. Fast Ion Transport Pathway Provided by Polyethylene Glycol Confined in Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 1923–1927. [Google Scholar] [CrossRef]
- Chen, H.; Tu, H.; Hu, C.; Liu, Y.; Dong, D.; Sun, Y.; Dai, Y.; Wang, S.; Qian, H.; Lin, Z.; et al. Cationic Covalent Organic Framework Nanosheets for Fast Li-Ion Conduction. J. Am. Chem. Soc. 2018, 140, 896–899. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.-W.; Mu, Z.-J.; Cao, C.; Li, Z.; Wang, T.-X.; Li, Y.; Ding, X.; Han, B.-H.; Feng, W. Cationic covalent organic framework based all-solid-state electrolytes. Mater. Chem. Front. 2020, 4, 1164–1173. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Li, Z.; Wang, T.; Zhao, F.; Ding, X.; Feng, W.; Han, B. Defective 2D Covalent Organic Frameworks for Postfunctionalization. Adv. Funct. Mater. 2020, 30, 1909267. [Google Scholar] [CrossRef]
- Shan, Z.; Wu, M.; Du, Y.; Xu, B.; He, B.; Wu, X.; Zhang, G. Covalent Organic Framework-Based Electrolytes for Fast Li+ Conduction and High-Temperature Solid-State Lithium-Ion Batteries. Chem. Mater. 2021, 33, 5058–5066. [Google Scholar] [CrossRef]
- Cho, S.K.; Kim, H.I.; An, J.W.; Jung, K.; Bae, H.; Kim, J.H.; Yim, T.; Lee, S.Y. Printable solid electrolyte interphase mimic for Antioxidative Lithium metal electrodes. Adv. Funct. Mater. 2020, 30, 2000792. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, K.-H.; Cho, S.-J.; Yoo, J.; Lee, S.-S.; Lee, S.-Y. Flexible/shape-versatile, bipolar all-solid-state lithium-ion batteries prepared by multistage printing. Energy Environ. Sci. 2018, 11, 321–330. [Google Scholar] [CrossRef]
- Guo, D.; Shinde, D.B.; Shin, W.; Abou-Hamad, E.; Emwas, A.H.; Lai, Z.; Manthiram, A. Foldable Solid-State Batteries Enabled by Electrolyte Mediation in Covalent Organic Frameworks. Adv. Mater. 2022, 34, 2201410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Niu, C.; Yu, C.; Zhang, L.; Xu, Y. Highly crystalline vinylene-linked covalent organic frameworks enhanced solid polycarbonate electrolyte for dendrite-free solid lithium metal batteries. Nano Res. 2022, 15, 8083–8090. [Google Scholar] [CrossRef]
- Niu, C.; Luo, W.; Dai, C.; Yu, C.; Xu, Y. High-voltage-tolerant covalent organic framework electrolyte with holistically oriented channels for solid-state lithium metal batteries with nickel-rich cathodes. Angew. Chem. Int. Ed. 2021, 60, 24915–24923. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.-C.; Cheng, T.; Liu, Y.-Y.; Li, Q.; Bai, M.; Liu, X.; Geng, H.; Lai, W.-Y.; Huang, W. Highly conjugated three-dimensional covalent organic frameworks with enhanced Li-ion conductivity as solid-state electrolytes for high-performance lithium metal batteries. J. Mater. Chem. A 2022, 10, 8761–8771. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, G.; Fu, Y.; Yang, Y.; Zhang, C.; An, Q.; Guo, H. Understanding a Single-Li-Ion COF Conductor for Being Dendrite Free in a Li-Organic Battery. Research 2022, 2022, 9798582. [Google Scholar] [CrossRef]
- An, Q.; Wang, H.E.; Zhao, G.; Wang, S.; Xu, L.; Wang, H.; Fu, Y.; Guo, H. Understanding Dual-Polar Group Functionalized COFs for Accelerating Li-Ion Transport and Dendrite-Free Deposition in Lithium Metal Anodes. Energy Environ. Mater. 2023, 6, e12345. [Google Scholar] [CrossRef]
- Guzmán-González, G.; Vauthier, S.; Alvarez-Tirado, M.; Cotte, S.; Castro, L.; Guéguen, A.; Casado, N.; Mecerreyes, D. Single-Ion Lithium Conducting Polymers with High Ionic Conductivity Based on Borate Pendant Groups. Angew. Chem. 2022, 134, e202114024. [Google Scholar] [CrossRef]
- Porcarelli, L.; Manojkumar, K.; Sardon, H.; Llorente, O.; Shaplov, A.S.; Vijayakrishna, K.; Gerbaldi, C.; Mecerreyes, D. Single Ion Conducting Polymer Electrolytes Based On Versatile Polyurethanes. Electrochim. Acta 2017, 241, 526–534. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, J.; Wang, C.; Ma, X.; Jiang, K.; Kim, E.; Li, C.; Liu, H.; Xu, L.; Shum, H.C.; et al. Engineered networking in a family of solvent-free single-ion conducting borate network polymer electrolytes for Li-metal battery applications. Chem. Eng. J. 2022, 450, 138407. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. A glimpse on all-solid-state Li-ion battery (ASSLIB) performance based on novel solid polymer electrolytes: A topical review. J. Mater. Sci. 2020, 55, 6242–6304. [Google Scholar] [CrossRef]
- Porcarelli, L.; Sutton, P.; Bocharova, V.; Aguirresarobe, R.H.; Zhu, H.; Goujon, N.; Leiza, J.R.; Sokolov, A.; Forsyth, M.; Mecerreyes, D. Single-Ion Conducting Polymer Nanoparticles as Functional Fillers for Solid Electrolytes in Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 54354–54362. [Google Scholar] [CrossRef]
- Zhang, G.; Hong, Y.-L.; Nishiyama, Y.; Bai, S.; Kitagawa, S.; Horike, S. Accumulation of Glassy Poly(ethylene oxide) Anchored in a Covalent Organic Framework as a Solid-State Li+ Electrolyte. J. Am. Chem. Soc. 2018, 141, 1227–1234. [Google Scholar] [CrossRef]
- Xu, Q.; Tao, S.; Jiang, Q.; Jiang, D. Designing Covalent Organic Frameworks with a Tailored Ionic Interface for Ion Transport across One-Dimensional Channels. Angew. Chem. 2020, 132, 4587–4593. [Google Scholar] [CrossRef]
- Peng, J.; Yi, X.; Fan, L.; Zhou, J.; Lu, B. Molecular extension engineering constructing long-chain organic elastomeric interphase towards stable potassium storage. Energy Lab 2023, 1, 220014. [Google Scholar]
- Zhao, H.; Lam, W.A.; Sheng, L.; Wang, L.; Bai, P.; Yang, Y.; Ren, D.; Xu, H.; He, X. Cobalt-Free Cathode Materials: Families and their Prospects. Adv. Energy Mater. 2022, 12, 2103894. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Bo, X.; Wang, X.; Ren, Y.; Wei, Z.; Daoud, W.A. Recent Advances and Perspectives in Single-Ion COF-Based Solid Electrolytes. Batteries 2023, 9, 432. https://doi.org/10.3390/batteries9090432
Zhao H, Bo X, Wang X, Ren Y, Wei Z, Daoud WA. Recent Advances and Perspectives in Single-Ion COF-Based Solid Electrolytes. Batteries. 2023; 9(9):432. https://doi.org/10.3390/batteries9090432
Chicago/Turabian StyleZhao, Hong, Xiangkun Bo, Xiucai Wang, Yaqi Ren, Zhaohuan Wei, and Walid A. Daoud. 2023. "Recent Advances and Perspectives in Single-Ion COF-Based Solid Electrolytes" Batteries 9, no. 9: 432. https://doi.org/10.3390/batteries9090432
APA StyleZhao, H., Bo, X., Wang, X., Ren, Y., Wei, Z., & Daoud, W. A. (2023). Recent Advances and Perspectives in Single-Ion COF-Based Solid Electrolytes. Batteries, 9(9), 432. https://doi.org/10.3390/batteries9090432








