Electrolytes for Aqueous Zn-Ion Batteries Working in Wide-Temperature Range: Progress and Perspective
Abstract
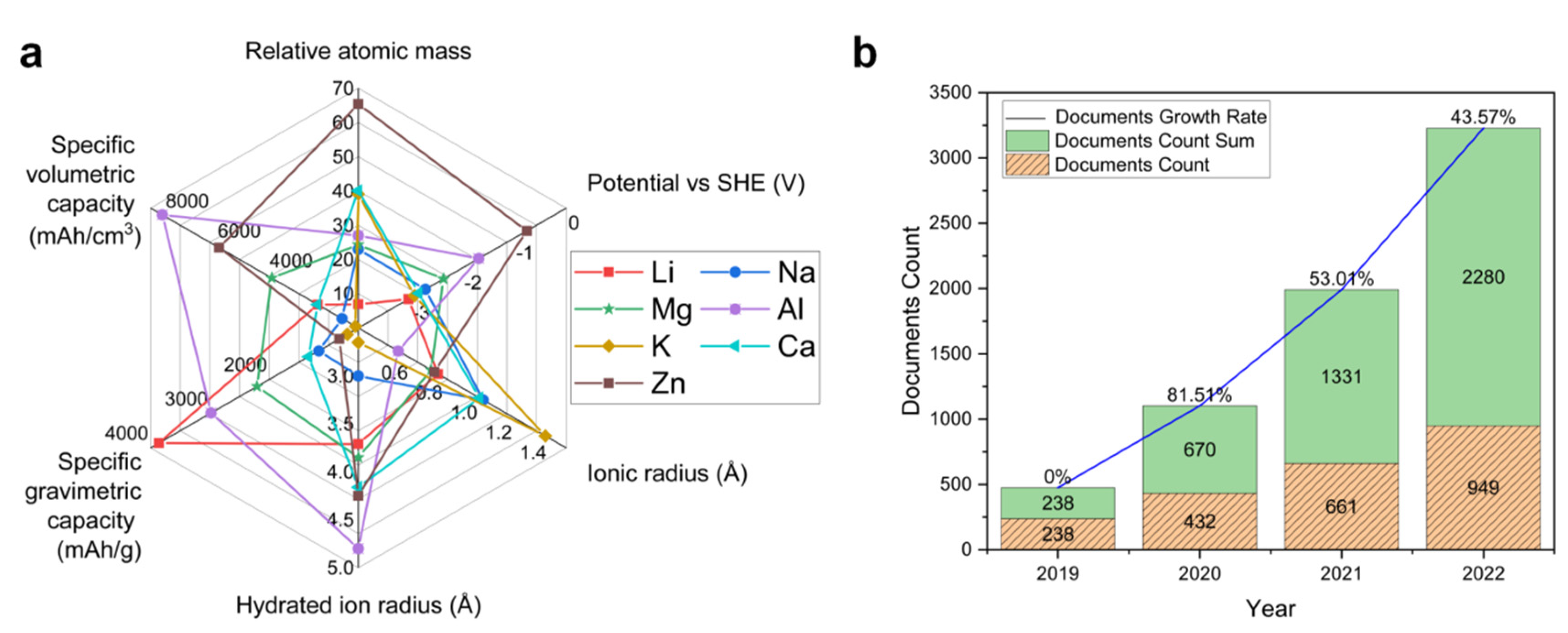
1. Introduction
2. Low Temperature Condition
2.1. Optimizing Aqueous Electrolytes
2.1.1. Water-in-Salt Electrolyte
2.1.2. Organic Additives
2.1.3. Hydrogel Electrolyte
3. High Temperature Condition
3.1. Optimizing Aqueous Electrolytes
3.1.1. Cosolvent Electrolytes
3.1.2. Colloidal Electrolytes
3.1.3. Hydrogel Electrolytes
4. Wide Temperature Condition
4.1. Quasi-Solid-State Electrolytes
4.2. Organic Additives
5. Summary and Outlook
- In addition to the development of wide-temperature electrolytes, self-protection represents another approach to enhance the wide-temperature performance of AZIBs [100,101]. Efficient thermal self-protection strategies for Zn-ion batteries using smart hygroscopic hydrogel electrolytes have been reported [97,102]. The reversible water evaporation and regeneration processes within the hydrogel are closely associated with temperature fluctuations, which can modulate ion migration in AZIB hydrogels. These findings present novel opportunities for creating environmentally adaptive aqueous energy storage devices with improved wide-temperature performance, driving future practical applications.
- While AZIBs’ operation at wide temperatures through intricate electrolyte design has been documented, the mechanisms underlying the entire battery system warrant a more comprehensive and systematic examination to better inform the practical application of AZIBs. Additionally, much of the current research on AZIBs remains confined to laboratory settings, with a considerable gap between these investigations and practical implementation. As such, achieving the real-world application of AZIBs necessitates the collective efforts of researchers worldwide.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Y.; Xing, G.; Chou, S.-L.; Tang, Y. Electrochemical energy storage devices working in extreme conditions. Energy Environ. Sci. 2021, 14, 3323–3351. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Li, W.; Ma, B.; Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 44, 5926–5940. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Hao, S.; Meng, L.; Xu, F.; Yang, J. Engineering Self-Adhesive Polyzwitterionic Hydrogel Electrolytes for Flexible Zinc-Ion Hybrid Capacitors with Superior Low-Temperature Adaptability. ACS Nano 2021, 15, 18469–18482. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X.; Liu, J.N.; Yao, N.; Wang, J.; Ren, D.; Chen, X.; Li, B.Q.; Zhang, Q. Can Aqueous Zinc-Air Batteries Work at Sub-Zero Temperatures? Angew. Chem.-Int. Ed. 2021, 60, 15281–15285. [Google Scholar] [CrossRef]
- Wang, M.; Emre, A.; Tung, S.; Gerber, A.; Wang, D.; Huang, Y.; Cecen, V.; Kotov, N.A. Biomimetic Solid-State Zn2+ Electrolyte for Corrugated Structural Batteries. ACS Nano 2019, 13, 1107–1115. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew. Chem.-Int. Ed. 2015, 54, 3431–3448. [Google Scholar] [CrossRef]
- Goodenough, J.B. How we made the Li-ion rechargeable battery. Nat. Electron. 2018, 1, 204. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.-G.; Yang, Z.; Lemmon, J.P.; Imhoff, C.; Graff, G.L.; Li, L.; Hu, J.; Wang, C.; Xiao, J.; et al. Materials Science and Materials Chemistry for Large Scale Electrochemical Energy Storage: From Transportation to Electrical Grid. Adv. Funct. Mater. 2013, 23, 929–946. [Google Scholar] [CrossRef]
- Yang, Q.; Qu, X.; Cui, H.; He, X.; Shao, Y.; Zhang, Y.; Guo, X.; Chen, A.; Chen, Z.; Zhang, R.; et al. Rechargeable Aqueous Mn-Metal Battery Enabled by Inorganic-Organic Interfaces. Angew. Chem. Int. Ed. Engl. 2022, 61, e202206471. [Google Scholar]
- Liang, G.; Li, X.; Wang, Y.; Yang, S.; Huang, Z.; Yang, Q.; Wang, D.; Dong, B.; Zhu, M.; Zhi, C. Building durable aqueous K-ion capacitors based on MXene family. Nano Res. Energy 2022, 1, e9120002. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Elia, G.A.; Marquardt, K.; Hoeppner, K.; Fantini, S.; Lin, R.; Knipping, E.; Peters, W.; Drillet, J.F.; Passerini, S.; Hahn, R. An Overview and Future Perspectives of Aluminum Batteries. Adv. Mater. 2016, 28, 7564–7579. [Google Scholar] [CrossRef]
- Liu, X.; Fan, X.; Liu, B.; Ding, J.; Deng, Y.; Han, X.; Zhong, C.; Hu, W. Mapping the Design of Electrolyte Materials for Electrically Rechargeable Zinc-Air Batteries. Adv. Mater. 2021, 33, e2006461. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Marcus, Y. Ionic radii in aqueous solutions. J. Solut. Chem. 1983, 12, 271–275. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Kang, H.; Liu, Y.; Cao, K.; Zhao, Y.; Jiao, L.; Wang, Y.; Yuan, H. Update on anode materials for Na-ion batteries. J. Mater. Chem. A 2015, 3, 17899–17913. [Google Scholar] [CrossRef]
- Mo, F.; Liang, G.; Wang, D.; Tang, Z.; Li, H.; Zhi, C. Biomimetic organohydrogel electrolytes for high-environmental adaptive energy storage devices. EcoMat 2019, 1, e12008. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, G.; Guo, Y.; Liu, Z.; Yan, B.; Wang, D.; Huang, Z.; Li, X.; Fan, J.; Zhi, C. Do Zinc Dendrites Exist in Neutral Zinc Batteries: A Developed Electrohealing Strategy to In Situ Rescue In-Service Batteries. Adv. Mater. 2019, 31, e1903778. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, L.; Hussain, T.; Wang, D.; Hui, L.; Guo, Y.; Liang, G.; Li, X.; Chen, Z.; Huang, Z.; et al. Stabilizing Interface pH by N-Modified Graphdiyne for Dendrite-Free and High-Rate Aqueous Zn-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202112304. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Feng, X.; Liu, D.; Zhang, Y. Recent advances and perspectives for Zn-based batteries: Zn anode and electrolyte. Nano Res. Energy 2023, 2, e9120039. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, X.; Qin, L.; Fang, G.; Liang, S. Progress and prospect of low-temperature zinc metal batteries. Adv. Powder Mater. 2022, 1, 100011. [Google Scholar] [CrossRef]
- Huang, J.; Dong, X.; Wang, N.; Wang, Y. Building low-temperature batteries: Non-aqueous or aqueous electrolyte? Curr. Opin. Electrochem. 2022, 33, 100949. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L. Advances in materials for all-climate sodium-ion batteries. EcoMat 2020, 2, e12043. [Google Scholar] [CrossRef]
- Holoubek, J.; Liu, H.; Wu, Z.; Yin, Y.; Xing, X.; Cai, G.; Yu, S.; Zhou, H.; Pascal, T.A.; Chen, Z.; et al. Tailoring Electrolyte Solvation for Li Metal Batteries Cycled at Ultra-Low Temperature. Nat. Energy 2021, 2021, 303–313. [Google Scholar] [CrossRef]
- Peng, H.-J.; Huang, J.-Q.; Cheng, X.-B.; Zhang, Q. Review on High-Loading and High-Energy Lithium-Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar] [CrossRef]
- Li, H.; Chao, D.; Chen, B.; Chen, X.; Chuah, C.; Tang, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Revealing Principles for Design of Lean-Electrolyte Lithium Metal Anode via In Situ Spectroscopy. J. Am. Chem. Soc. 2020, 142, 2012–2022. [Google Scholar] [CrossRef]
- Rodrigues, M.-T.F.; Babu, G.; Gullapalli, H.; Kalaga, K.; Sayed, F.N.; Kato, K.; Joyner, J.; Ajayan, P.M. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2017, 2, 17108. [Google Scholar] [CrossRef]
- Cho, Y.; Gabbar, H.A. Review of energy storage technologies in harsh environment. Saf. Extrem. Environ. 2019, 1, 11–25. [Google Scholar] [CrossRef]
- Yang, X.G.; Zhang, G.; Ge, S.; Wang, C.Y. Fast charging of lithium-ion batteries at all temperatures. Proc. Natl. Acad. Sci. USA 2018, 115, 7266–7271. [Google Scholar] [CrossRef]
- Abdellahi, A.; Urban, A.; Dacek, S.; Ceder, G. Understanding the Effect of Cation Disorder on the Voltage Profile of Lithium Transition-Metal Oxides. Chem. Mater. 2016, 28, 5373–5383. [Google Scholar] [CrossRef]
- Lee, J.; Kitchaev, D.A.; Kwon, D.H.; Lee, C.W.; Papp, J.K.; Liu, Y.S.; Lun, Z.; Clement, R.J.; Shi, T.; McCloskey, B.D.; et al. Reversible Mn2+/Mn4+ double redox in lithium-excess cathode materials. Nature 2018, 556, 185–190. [Google Scholar] [CrossRef]
- Cui, H.; Ma, L.; Huang, Z.; Chen, Z.; Zhi, C. Organic materials-based cathode for zinc ion battery. SmartMat 2022, 3, 565–581. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Yamauchi, Y.; Alothman, Z.A.; Kaneti, Y.V.; Wu, X. Enhanced Zinc Ion Storage Capability of V2O5 Electrode Materials with Hollow Interior Cavities. Batter. Supercaps 2021, 4, 1867–1873. [Google Scholar] [CrossRef]
- Kamenskii, M.A.; Eliseeva, S.N.; Kondratiev, V.V. The Electrochemical Performance of δ-MnO2 Cathode Material for Aqueous Zinc-Ion Batteries: The Role of Current Collector. ECS Trans. 2021, 105, 135–142. [Google Scholar] [CrossRef]
- Li, F.; Hu, X. Zinc Metal Energy Storage Devices under Extreme Conditions of Low Temperatures. Batter. Supercaps 2020, 4, 389–406. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, X.; Wang, K.; Zheng, S.; Shi, J.; Zhang, Q.; Cai, W.; Liang, J.; Tao, Z. An ultralow-temperature aqueous zinc-ion battery. J. Mater. Chem. A 2021, 9, 7042–7047. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Y.; Shi, L.; Wang, K.; Wang, B.; Li, L.; Ma, Y.; Li, Y.; Sun, Z.; Ali, W.; et al. An Overview and Future Perspectives of Rechargeable Zinc Batteries. Small 2020, 16, e2000730. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Liu, J.; Qu, S.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Electrodeposition of alloys and compounds from high-temperature molten salts. J. Alloys Compd. 2017, 690, 228–238. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Wang, Y.; Dong, S.; Cheng, L.; Li, Y.; Wang, Z.; Trabzon, L.; Wang, H. Working Aqueous Zn Metal Batteries at 100 °C. ACS Nano 2022, 16, 15770–15778. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. The low temperature performance of Li-ion batteries. J. Power Sources 2003, 115, 137–140. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Goodenough, J.B. Rechargeable batteries: Challenges old and new. J. Solid State Electrochem. 2012, 16, 2019–2029. [Google Scholar] [CrossRef]
- Zhu, G.; Wen, K.; Lv, W.; Zhou, X.; Liang, Y.; Yang, F.; Chen, Z.; Zou, M.; Li, J.; Zhang, Y.; et al. Materials insights into low-temperature performances of lithium-ion batteries. J. Power Sources 2015, 300, 29–40. [Google Scholar] [CrossRef]
- Nian, Q.; Wang, J.; Liu, S.; Sun, T.; Zheng, S.; Zhang, Y.; Tao, Z.; Chen, J. Aqueous Batteries Operated at -50 °C. Angew. Chem.-Int. Ed. 2019, 58, 16994–16999. [Google Scholar] [CrossRef]
- Matsumoto, M.; Saito, S.; Ohmine, I. Molecular dynamics simulation of the ice nucleation and growth process leading to water freezing. Nature 2002, 416, 409–413. [Google Scholar] [CrossRef]
- Moore, E.B.; Molinero, V. Structural transformation in supercooled water controls the crystallization rate of ice. Nature 2011, 479, 506–508. [Google Scholar] [CrossRef]
- Sosso, G.C.; Chen, J.; Cox, S.J.; Fitzner, M.; Pedevilla, P.; Zen, A.; Michaelides, A. Crystal Nucleation in Liquids: Open Questions and Future Challenges in Molecular Dynamics Simulations. Chem. Rev. 2016, 116, 7078–7116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, Y.; Lu, Y.; Li, L.; Wan, F.; Zhang, K.; Chen, J. Modulating electrolyte structure for ultralow temperature aqueous zinc batteries. Nat. Commun. 2020, 11, 4463. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hou, L.; Li, T.; Jiao, Y.; Wu, P. Antifreezing Hydrogel Electrolyte with Ternary Hydrogen Bonding for High-Performance Zinc-Ion Batteries. Adv. Mater. 2022, 34, e2110140. [Google Scholar] [CrossRef]
- Sun, T.; Zheng, S.; Du, H.; Tao, Z. Synergistic Effect of Cation and Anion for Low-Temperature Aqueous Zinc-Ion Battery. Nano-Micro. Lett. 2021, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, K.; Sun, T.; Shi, J.; Zhou, X.; Cai, W.; Tao, Z. Improving zinc anode reversibility by hydrogen bond in hybrid aqueous electrolyte. Chem. Eng. J. 2022, 427, 131705. [Google Scholar] [CrossRef]
- Chang, N.; Li, T.; Li, R.; Wang, S.; Yin, Y.; Zhang, H.; Li, X. An aqueous hybrid electrolyte for low-temperature zinc-based energy storage devices. Energy Environ. Sci. 2020, 13, 3527–3535. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, W.; Wang, A.; Huang, A.; Chen, J.; Xu, J.; Wong, C.-P. Anti-freezing flexible aqueous Zn–MnO2 batteries working at −35 °C enabled by a borax-crosslinked polyvinyl alcohol/glycerol gel electrolyte. J. Mater. Chem. A 2020, 8, 6828–6841. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y. A flexible zinc-ion battery based on the optimized concentrated hydrogel electrolyte for enhanced performance at subzero temperature. Electrochim. Acta 2021, 395, 139178. [Google Scholar] [CrossRef]
- Mo, F.; Liang, G.; Meng, Q.; Liu, Z.; Li, H.; Fan, J.; Zhi, C. A flexible rechargeable aqueous zinc manganese-dioxide battery working at −20 °C. Energy Environ. Sci. 2019, 12, 706–715. [Google Scholar] [CrossRef]
- Tamtogl, A.; Bahn, E.; Sacchi, M.; Zhu, J.; Ward, D.J.; Jardine, A.P.; Jenkins, S.J.; Fouquet, P.; Ellis, J.; Allison, W. Motion of water monomers reveals a kinetic barrier to ice nucleation on graphene. Nat. Commun. 2021, 12, 3120. [Google Scholar] [CrossRef]
- Roberts, A.J.; Danil de Namor, A.F.; Slade, R.C. Low temperature water based electrolytes for MnO2/carbon supercapacitors. Phys. Chem. Chem. Phys. 2013, 15, 3518–3526. [Google Scholar] [CrossRef]
- Tron, A.; Jeong, S.; Park, Y.D.; Mun, J. Aqueous Lithium-Ion Battery of Nano-LiFePO4 with Antifreezing Agent of Ethyleneglycol for Low-Temperature Operation. ACS Sustain. Chem. Eng. 2019, 7, 14531–14538. [Google Scholar] [CrossRef]
- Wu, Q.-L.; Zhao, S.-X.; Yu, L.; Zheng, X.-X.; Wang, Y.-F.; Yu, L.-Q.; Nan, C.-W.; Cao, G. Oxygen vacancy-enriched MoO3−x nanobelts for asymmetric supercapacitors with excellent room/low temperature performance. J. Mater. Chem. A 2019, 7, 13205–13214. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Liu, J.; Long, Y.; Fang, L.; Wang, Q.; Liu, T. Highly compressible and superior low temperature tolerant supercapacitors based on dual chemically crosslinked PVA hydrogel electrolytes. J. Mater. Chem. A 2020, 8, 6219–6228. [Google Scholar] [CrossRef]
- Yin, J.; Qi, L.; Wang, H. Anti-freezing aqueous electrolytes for electric double-layer capacitors. Electrochim. Acta 2013, 88, 208–216. [Google Scholar] [CrossRef]
- Peng, S.; Jiang, X.; Xiang, X.; Chen, K.; Chen, G.; Jiang, X.; Hou, L. High-performance and flexible solid-state supercapacitors based on high toughness and thermoplastic poly(vinyl alcohol)/NaCl/glycerol supramolecular gel polymer electrolyte. Electrochim. Acta 2019, 324, 134874. [Google Scholar] [CrossRef]
- Railanmaa, A.; Lehtimaki, S.; Keskinen, J.; Lupo, D. Non-toxic printed supercapacitors operating in sub-zero conditions. Sci. Rep. 2019, 9, 14059. [Google Scholar] [CrossRef]
- Yue, F.; Tie, Z.; Deng, S.; Wang, S.; Yang, M.; Niu, Z. An Ultralow Temperature Aqueous Battery with Proton Chemistry. Angew. Chem.-Int. Ed. 2021, 60, 13882–13886. [Google Scholar] [CrossRef]
- Jiang, H.; Shin, W.; Ma, L.; Hong, J.J.; Wei, Z.; Liu, Y.; Zhang, S.; Wu, X.; Xu, Y.; Guo, Q.; et al. A High-Rate Aqueous Proton Battery Delivering Power Below −78 °C via an Unfrozen Phosphoric Acid. Adv. Energy Mater. 2020, 10, 2000968. [Google Scholar] [CrossRef]
- Li, C.; Li, P.; Yang, S.; Zhi, C. Recently advances in flexible zinc ion batteries. J. Semicond. 2021, 42, 101603. [Google Scholar] [CrossRef]
- Liang, G.; Wang, Y.; Huang, Z.; Mo, F.; Li, X.; Yang, Q.; Wang, D.; Li, H.; Chen, S.; Zhi, C. Initiating Hexagonal MoO3 for Superb-Stable and Fast NH4+ Storage Based on Hydrogen Bond Chemistry. Adv. Mater. 2020, 32, e1907802. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Ji, Z.; Wang, P.; Wang, J.; Ling, W.; Huang, Y. Temperature adaptability issue of aqueous rechargeable batteries. Mater. Today Energy 2021, 19, 100577. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Wang, D.; Zhang, J. Fundamentals and Challenges of Lithium Ion Batteries at Temperatures between −40 and 60 °C. Adv. Energy Mater. 2020, 10, 1904152. [Google Scholar] [CrossRef]
- Xie, X.; Fu, H.; Fang, Y.; Lu, B.; Zhou, J.; Liang, S. Manipulating Ion Concentration to Boost Two-Electron Mn4+/Mn2+ Redox Kinetics through a Colloid Electrolyte for High-Capacity Zinc Batteries. Adv. Energy Mater. 2021, 12, 2102393. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Fang, G.; Shan, L.; Guo, J.; Zhang, W.; Wang, C.; Wang, L.; Zhou, J.; Liang, S. Li+ intercalated V2O5·nH2O with enlarged layer spacing and fast ion diffusion as an aqueous zinc-ion battery cathode. Energy Environ. Sci. 2018, 11, 3157–3162. [Google Scholar] [CrossRef]
- Liu, Q.; Ji, Z.; Mo, F.; Ling, W.; Wang, J.; Lei, H.; Cui, M.; Zhang, Z.; Liu, Y.; Cheng, L.; et al. Stable Thermochromic Hydrogel for a Flexible and Wearable Zinc-Ion Yarn Battery with High-Temperature Warning Function. ACS Appl. Energy Mater. 2022, 5, 12448–12455. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Qiu, X.; Dong, X.; Wang, Y.; Xia, Y. Stabilized Rechargeable Aqueous Zinc Batteries Using Ethylene Glycol as Water Blocker. ChemSusChem 2020, 13, 5556–5564. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, N.; Dong, X.; Xu, J.; Zhou, K.; Li, W.; Wang, Y. A High-Voltage Zn-Organic Battery Using a Nonflammable Organic Electrolyte. Angew. Chem. Int. Ed. 2021, 60, 21025–21032. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Zhang, Y.; Li, Y.; Sun, R.; Wang, Z.; Wang, H. Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries. Energy Storage Mater. 2021, 35, 19–46. [Google Scholar] [CrossRef]
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Xi, B.; Chen, W.; Jia, Y.; Feng, J.; Xiong, S. Advances and Perspectives of Cathode Storage Chemistry in Aqueous Zinc-Ion Batteries. ACS Nano 2021, 15, 9244–9272. [Google Scholar] [CrossRef]
- Verma, V.; Kumar, S.; Manalastas, W.; Srinivasan, M. Undesired Reactions in Aqueous Rechargeable Zinc Ion Batteries. ACS Energy Lett. 2021, 6, 1773–1785. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Sun, X.; Li, J.; Liu, Y.-N. Flexible Wide-Temperature Zinc-Ion Battery Enabled by an Ethylene Glycol-Based Organohydrogel Electrolyte. ACS Appl. Energy Mater. 2021, 4, 12718–12727. [Google Scholar] [CrossRef]
- Deng, W.; Zhou, Z.; Li, Y.; Zhang, M.; Yuan, X.; Hu, J.; Li, Z.; Li, C.; Li, R. High-Capacity Layered Magnesium Vanadate with Concentrated Gel Electrolyte toward High-Performance and Wide-Temperature Zinc-Ion Battery. ACS Nano 2020, 14, 15776–15785. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, J.; Zhou, W.; Han, X.; Yao, Y.; Wong, C.P. Realizing an All-Round Hydrogel Electrolyte toward Environmentally Adaptive Dendrite-Free Aqueous Zn-MnO2 Batteries. Adv. Mater. 2021, 33, e2007559. [Google Scholar] [CrossRef]
- Liu, C.; Xu, W.; Mei, C.; Li, M.-C.; Xu, X.; Wu, Q. Highly stable H2V3O8/Mxene cathode for Zn-ion batteries with superior rate performance and long lifespan. Chem. Eng. J. 2021, 405, 126737. [Google Scholar] [CrossRef]
- Hou, Z.; Lu, Z.; Chen, Q.; Zhang, B. Realizing wide-temperature Zn metal anodes through concurrent interface stability regulation and solvation structure modulation. Energy Storage Mater. 2021, 42, 517–525. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, H.; Li, J.; Wei, W.; Li, Y.; Wang, J.; Cheng, L.; Zhang, D.; Ding, Y.; Chen, D.; et al. Highly reversible and stable Zn metal anode under wide temperature conditions enabled by modulating electrolyte chemistry. Chem. Eng. J. 2022, 442, 136218. [Google Scholar] [CrossRef]
- Chan, C.Y.; Wang, Z.; Jia, H.; Ng, P.F.; Chow, L.; Fei, B. Recent advances of hydrogel electrolytes in flexible energy storage devices. J. Mater. Chem. A 2021, 9, 2043–2069. [Google Scholar] [CrossRef]
- Chen, F.; Xu, Z.; Wang, H.; Handschuh-Wang, S.; Wang, B.; Zhou, X. Bioinspired Tough Organohydrogel Dynamic Interfaces Enabled Subzero Temperature Antifrosting, Deicing, and Antiadhesion. ACS Appl. Mater. Interfaces 2020, 12, 55501–55509. [Google Scholar] [CrossRef]
- Lu, W.; Xie, C.; Zhang, H.; Li, X. Inhibition of Zinc Dendrite Growth in Zinc-Based Batteries. ChemSusChem 2018, 11, 3996–4006. [Google Scholar] [CrossRef]
- Li, Q.; Cui, X.; Pan, Q. Self-Healable Hydrogel Electrolyte toward High-Performance and Reliable Quasi-Solid-State Zn-MnO(2) Batteries. ACS Appl. Mater. Interfaces 2019, 11, 38762–38770. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Wang, J.; Hu, M.; Mo, F.; Liang, G.; Zhi, C. An Intrinsically Self-Healing NiCo||Zn Rechargeable Battery with a Self-Healable Ferric-Ion-Crosslinking Sodium Polyacrylate Hydrogel Electrolyte. Angew. Chem. Int. Ed. Engl. 2018, 57, 9810–9813. [Google Scholar] [CrossRef]
- Huang, S.; Wan, F.; Bi, S.; Zhu, J.; Niu, Z.; Chen, J. A Self-Healing Integrated All-in-One Zinc-Ion Battery. Angew. Chem. Int. Ed. Engl. 2019, 58, 4313–4317. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Liang, S.; Wu, Z.; Fang, G.; Cao, X.; Wang, C.; Lin, T.; Pan, A.; Zhou, J. Transition metal ion-preintercalated V2O5 as high-performance aqueous zinc-ion battery cathode with broad temperature adaptability. Nano Energy 2019, 61, 617–625. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, J.; Chen, M.; Wang, A.; Huang, A.; Xu, X.; Xu, J.; Wong, C.-P. An environmentally adaptive quasi-solid-state zinc-ion battery based on magnesium vanadate hydrate with commercial-level mass loading and anti-freezing gel electrolyte. J. Mater. Chem. A 2020, 8, 8397–8409. [Google Scholar] [CrossRef]
- Lu, H.; Hu, J.; Wang, L.; Li, J.; Ma, X.; Zhu, Z.; Li, H.; Zhao, Y.; Li, Y.; Zhao, J.; et al. Multi-Component Crosslinked Hydrogel Electrolyte toward Dendrite-Free Aqueous Zn Ion Batteries with High Temperature Adaptability. Adv. Funct. Mater. 2022, 32, 2112540. [Google Scholar] [CrossRef]
- Han, D.; Cui, C.; Zhang, K.; Wang, Z.; Gao, J.; Guo, Y.; Zhang, Z.; Wu, S.; Yin, L.; Weng, Z.; et al. A non-flammable hydrous organic electrolyte for sustainable zinc batteries. Nat. Sustain. 2021, 5, 205–213. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Zhang, C.; Ji, W.; Qian, J.; Cao, Y.; Yang, H.; Ai, X. A temperature-sensitive poly(3-octylpyrrole)/carbon composite as a conductive matrix of cathodes for building safer Li-ion batteries. Energy Storage Mater. 2019, 17, 275–283. [Google Scholar] [CrossRef]
- Chen, Z.; Hsu, P.-C.; Lopez, J.; Li, Y.; To, J.W.F.; Liu, N.; Wang, C.; Andrews, S.C.; Liu, J.; Cui, Y.; et al. Fast and reversible thermoresponsive polymer switching materials for safer batteries. Nat. Energy 2016, 1, 15009. [Google Scholar] [CrossRef]
- Yang, P.; Feng, C.; Liu, Y.; Cheng, T.; Yang, X.; Liu, H.; Liu, K.; Fan, H.J. Thermal Self-Protection of Zinc-Ion Batteries Enabled by Smart Hygroscopic Hydrogel Electrolytes. Adv. Energy Mater. 2020, 10, 2002898. [Google Scholar] [CrossRef]
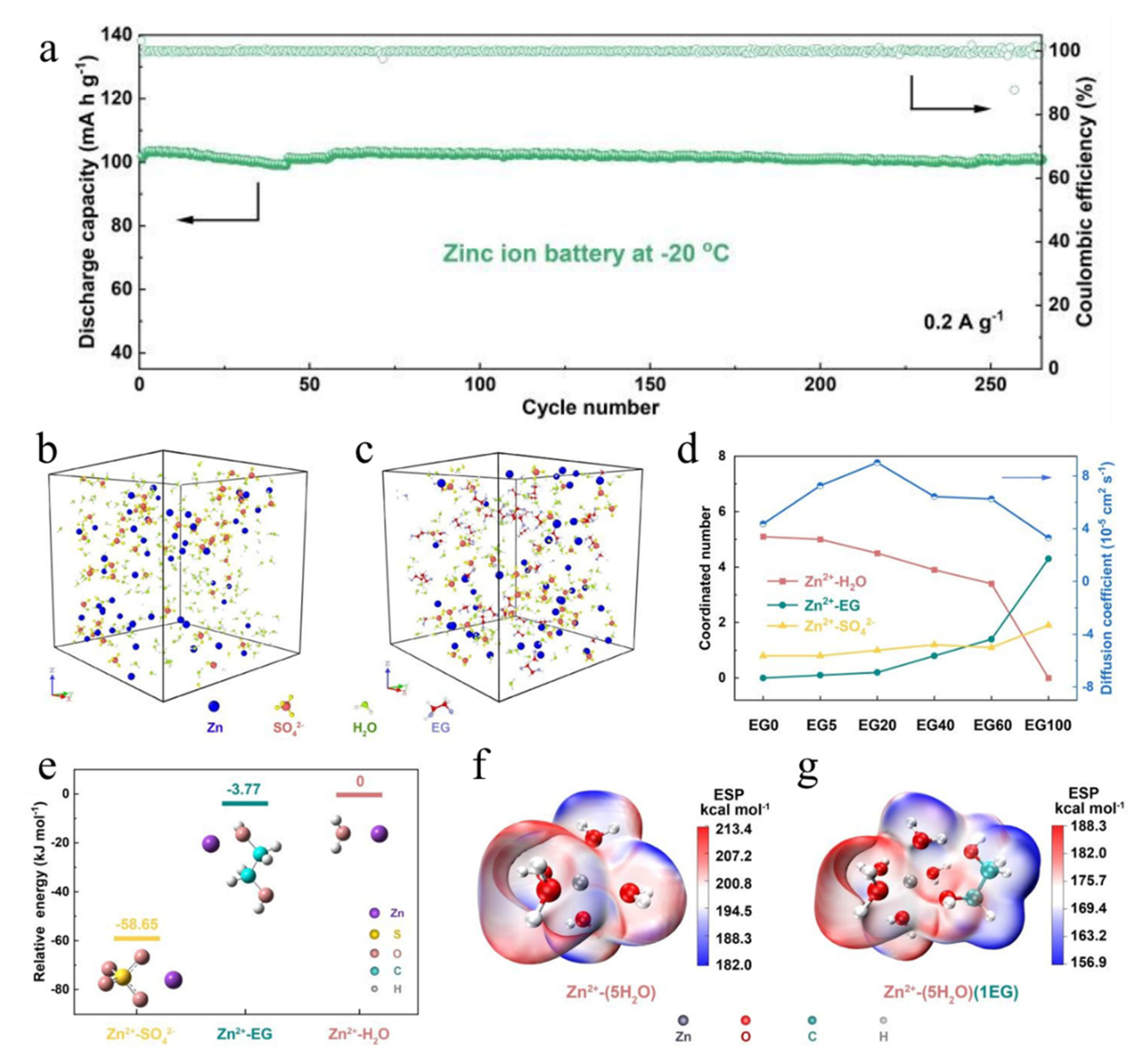
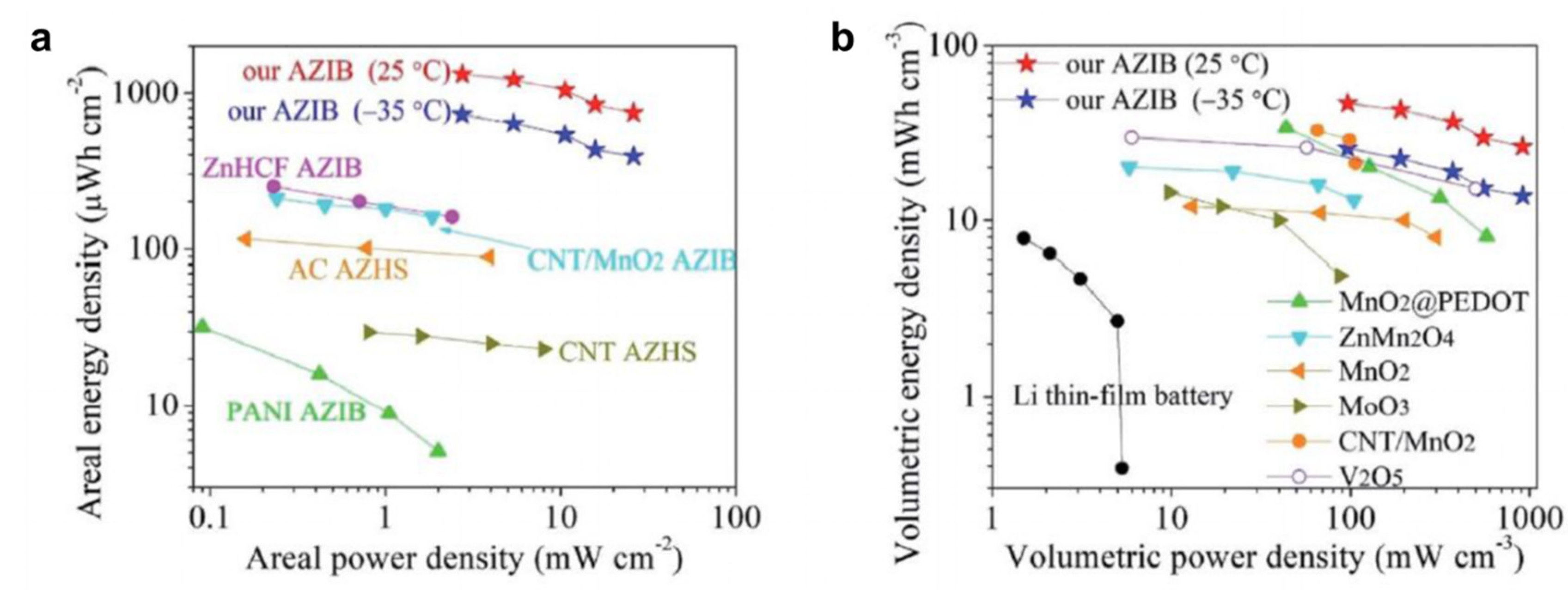

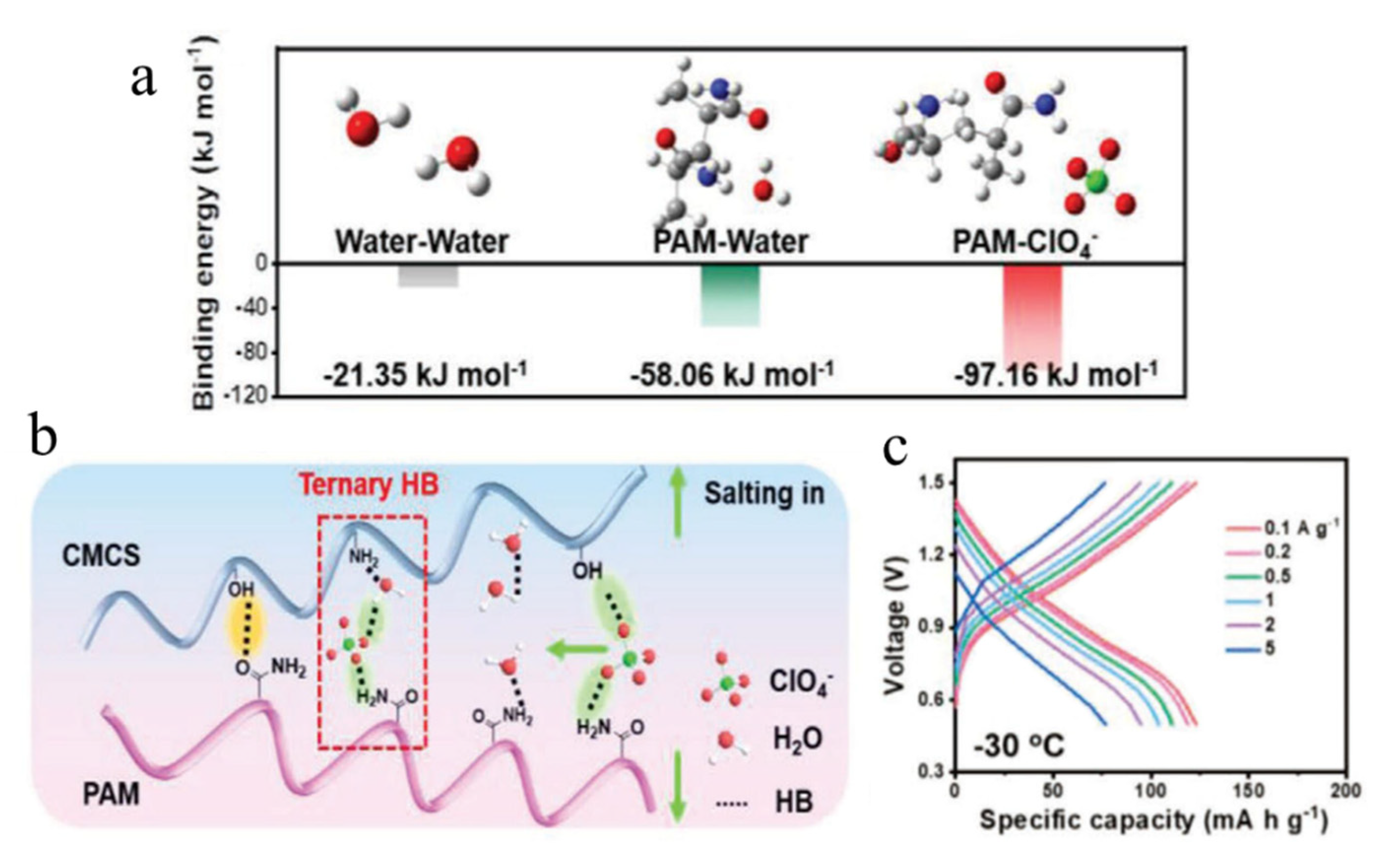
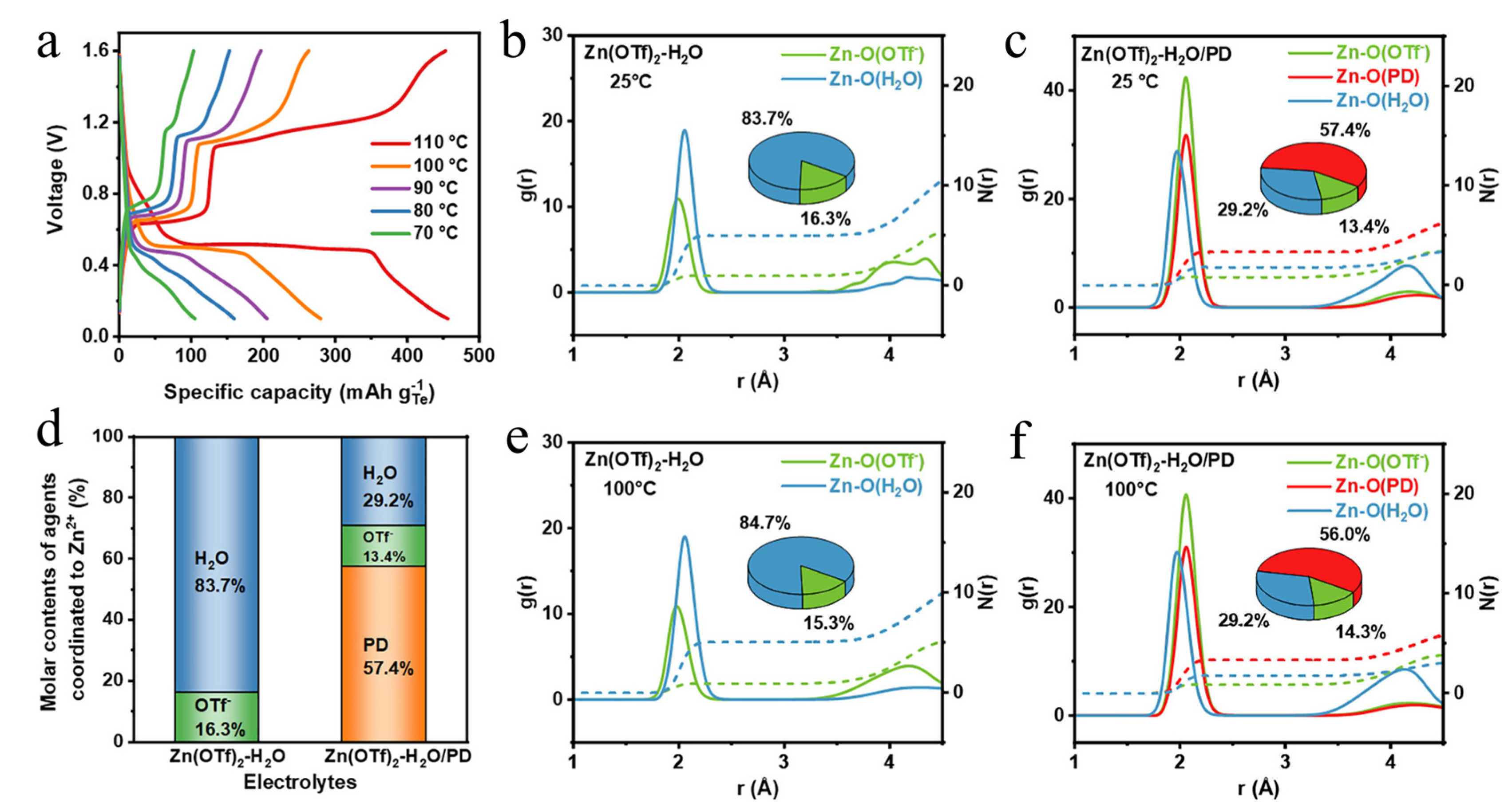
| Cathodes | Electrolytes | Temperature | Electrochemical Performance | Ref. |
|---|---|---|---|---|
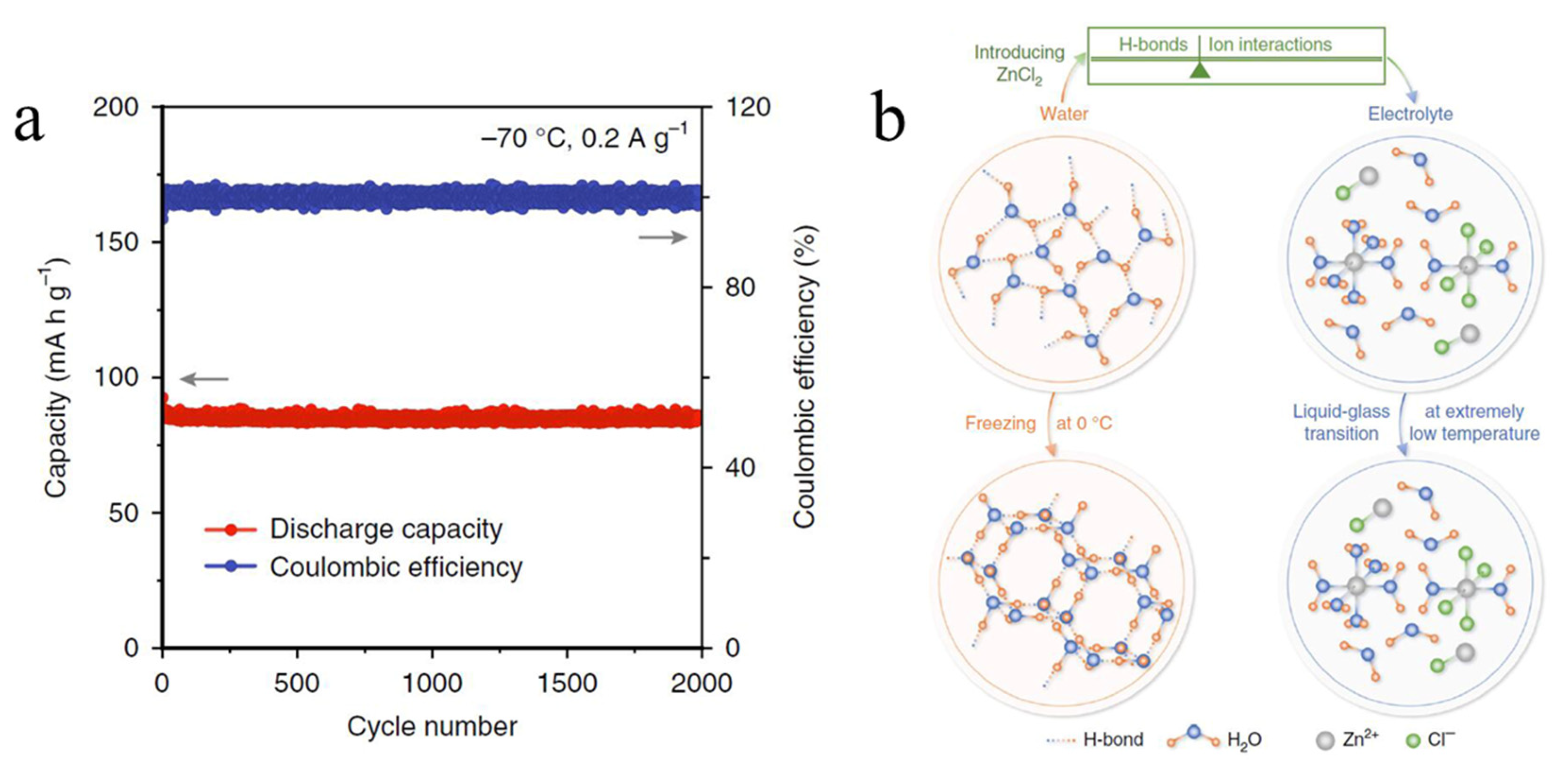
| polyaniline | ZnCl2 | −70 °C | 85 mAh/g after 2000 cycles at 0.2 A/g | [52] |
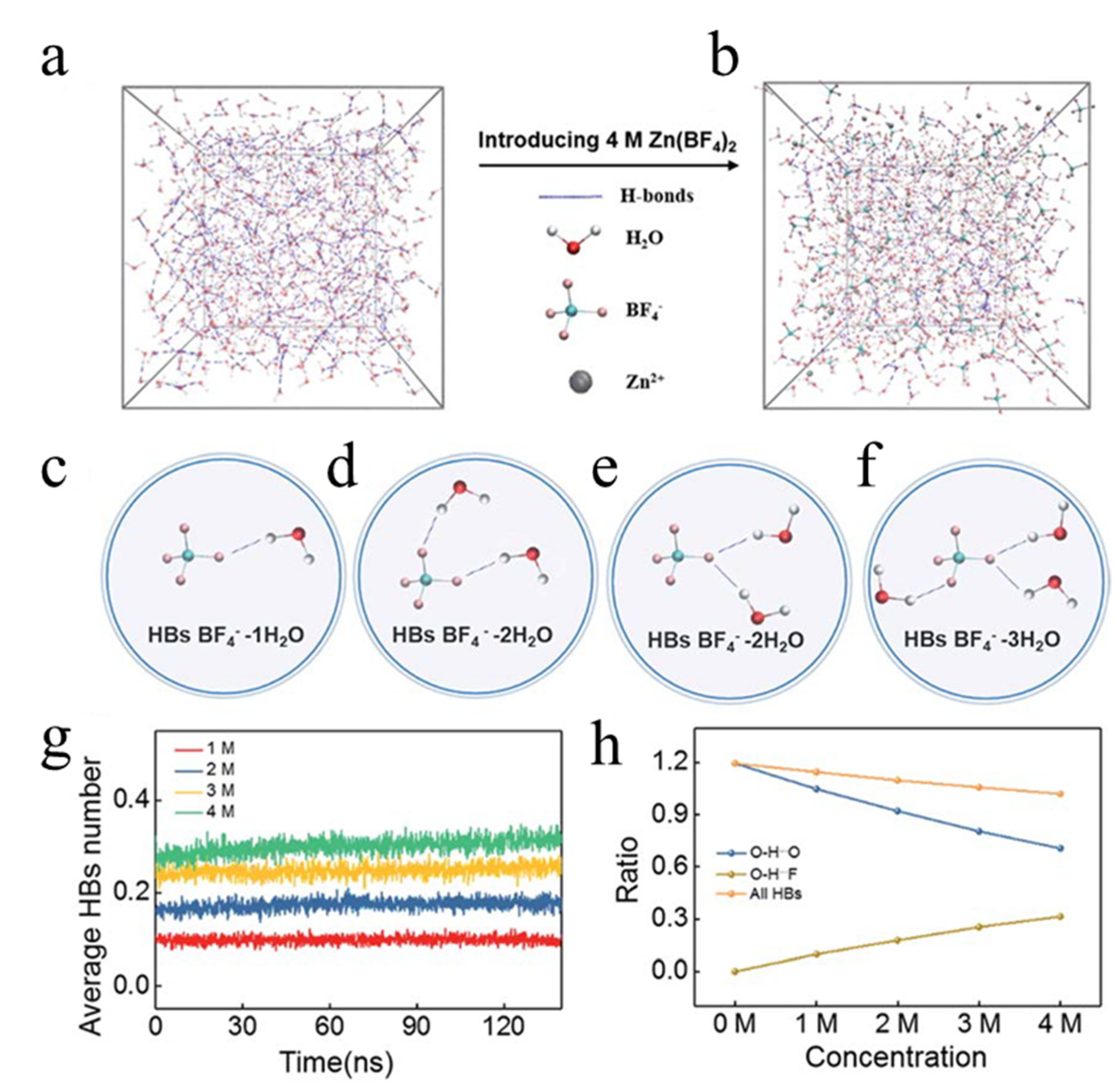
| tetrachlorobenzoquinone | Zn(BF4)2 | −60 °C −80 °C −95 °C | 86.1 mAh/g at 0.1 C 71.8 mAh/g at 0.1 C 63.5 mAh/g at 0.1 C | [40] |
| polyaniline | Zn(ClO4)2 | −30 °C | 64 mAh/g after 2500 cycles at 5 A/g | [53] |
| Pyrene4,5,9,10-tetraone | Mg(ClO4)2-Zn(ClO4)2 | −70 °C | 101.5 mAh/g at 0.2 A/g | [54] |
| Phenazine | Mg(ClO4)2-Zn(ClO4)2 | −70 °C | 71 mAh/g at 1.2 A/g | [54] |
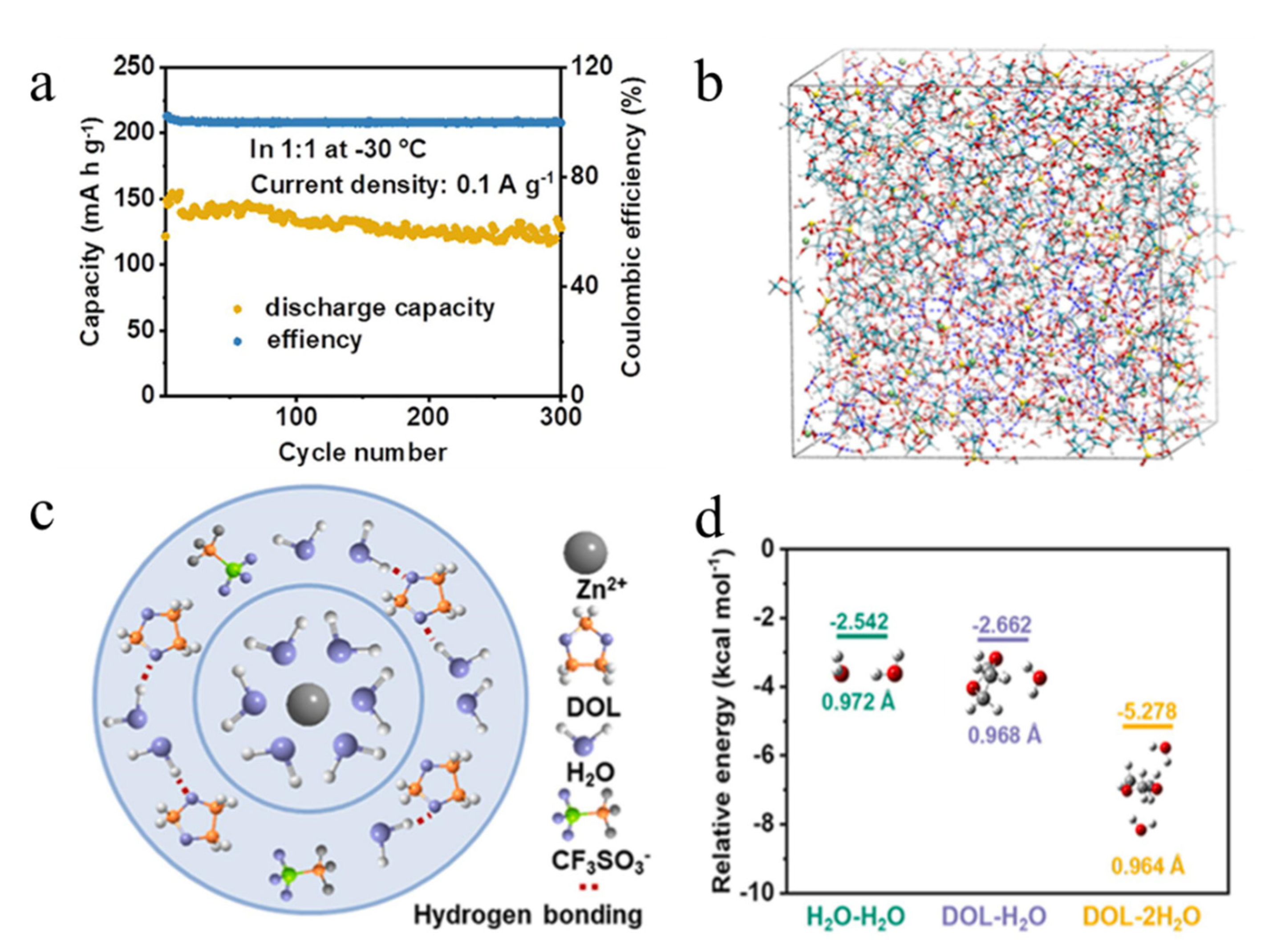
| V2O5 | (Zn(OTf)2)-DOL-H2O a | −30 °C | 131 mAh/g after 300 cycles at 0.1 A/g | [55] |
| PANI-V2O5 b | EG-H2O c | −20 °C | 100 mAh/g after 250 cycles at 0.2 A/g | [56] |
| MnO2 | polyvinyl alcohol (PVA)/glycerol gel | −35 °C | 25.8 mWh/cm 732 Wh/cm | [57] |
| NH4V3O8·1.9H2O | xanthan-ZnCl2 | −20 °C −40 °C | 201 mAh/g at 0.2 A/g 83 mAh/g at 0.2 A/g | [58] |
| MnO2 | EG-waPUA d | −20 °C | 196 mAh/g at 0.3 A/g | [59] |
| Cathodes | Electrolytes | Temperature | Electrochemical Performance | Ref. |
|---|---|---|---|---|
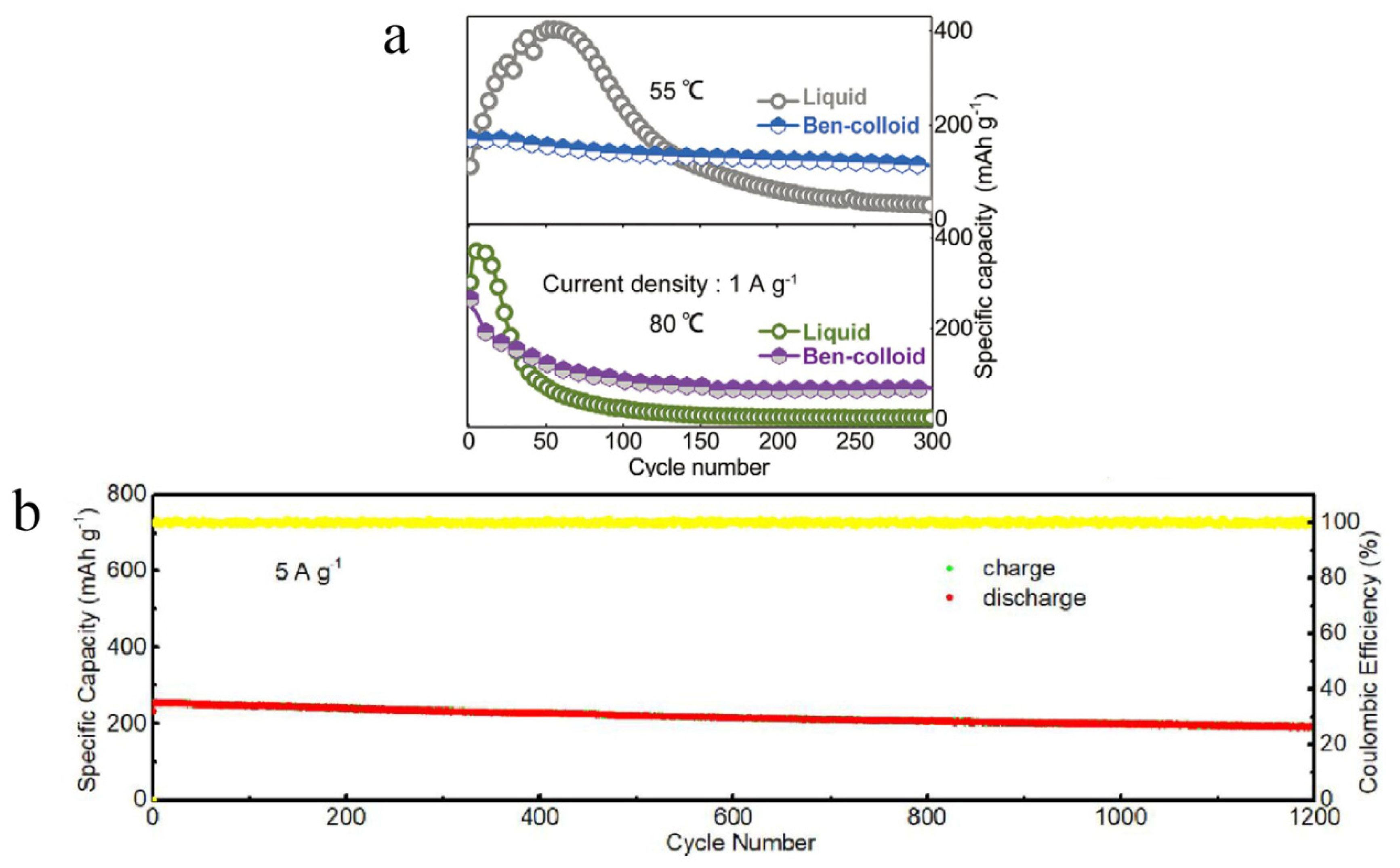
| MnO2 | bentonite-colloidal | 55 °C | 114.9 mAh/g after 300 cycles at 2 A/g | [74] |
| LVO-250 a | ZnSO4 | 50 °C | 232 mAh/g after 500 cycles at 5 A/g 192 mAh/g after 1000 cycles at 10 A/g | [75] |
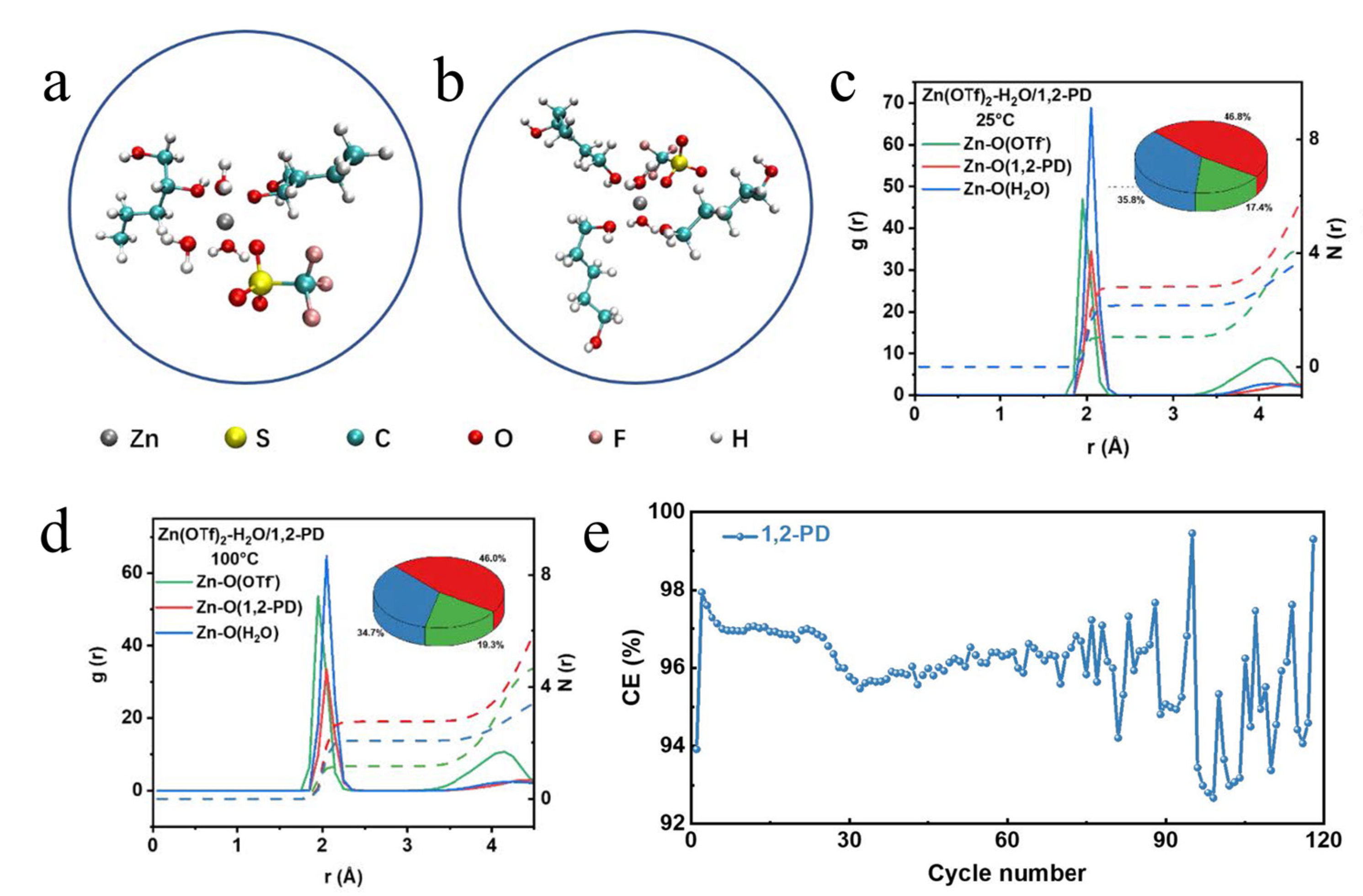
| Te | Zn(OTf)2−H2O/PD b | 100 °C | 195.7 mAh/gTe after 100 cycles at 2 C (850 mA/gTe) | [43] |
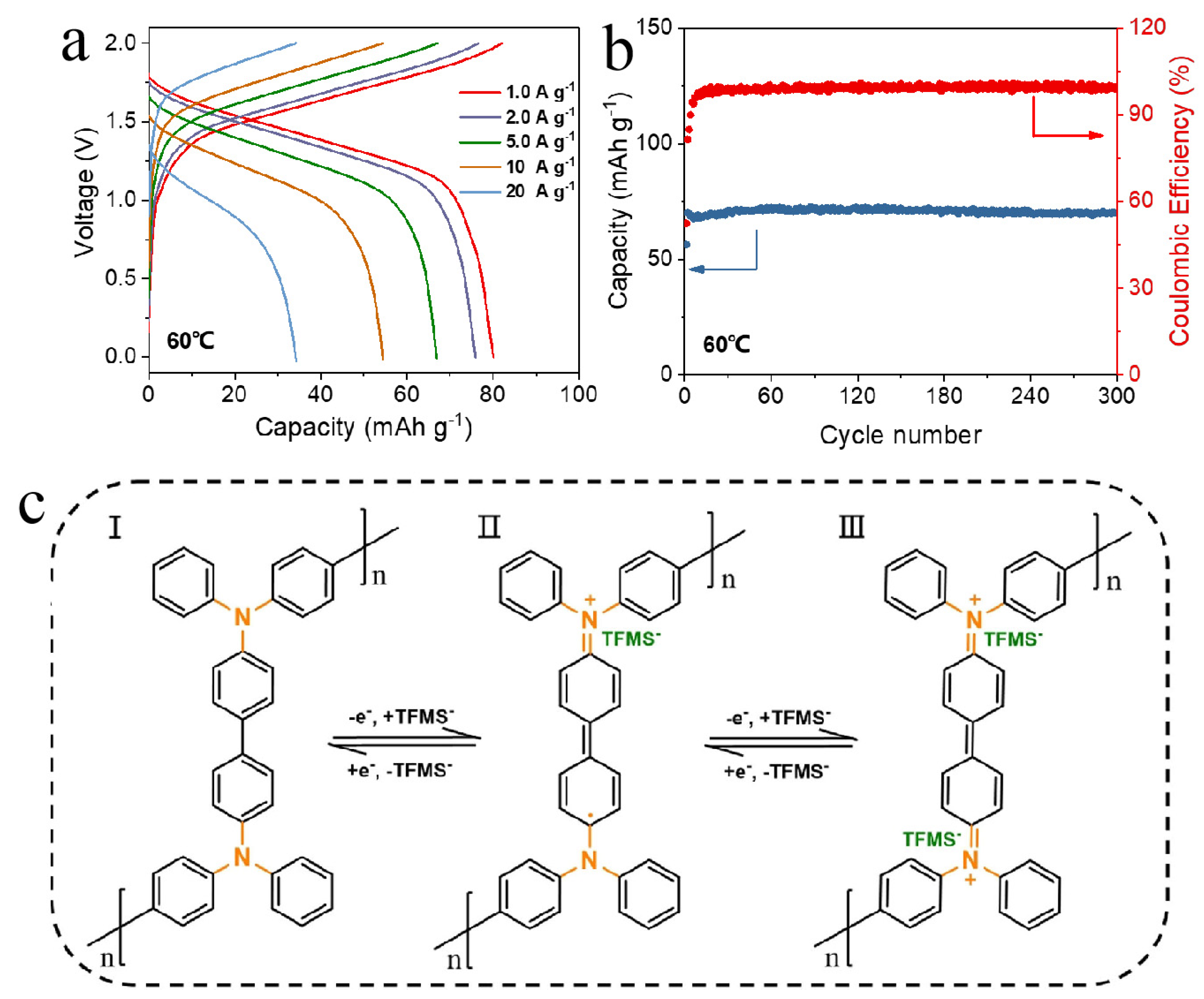
| CuVO c | PNMT d | 60 °C | 200 mAh/g after 1200 cycles at 5A/g | [76] |
| phenanthrenequinone macrocyclic trimer | ZnSO4/H2O-50% EG | 60 °C | 88 mAh/g after 900 cycles at 0.2 A/g | [77] |
| polytriphenylamine | Zn-TFMS/ (TEP:PC = 1:2) e | 60 °C | 300 cycles at 2 A/g | [78] |
| Cathodes | Electrolytes | Temperature | Electrochemical Performance | Ref. |
|---|---|---|---|---|
| CuVO-300 a | ZnSO4 | 50 °C to 0 °C | 410 mAh/g at 0.5 A/g at 50 °C 320 mAh/g at 0.5 A/g at 0 °C | [96] |
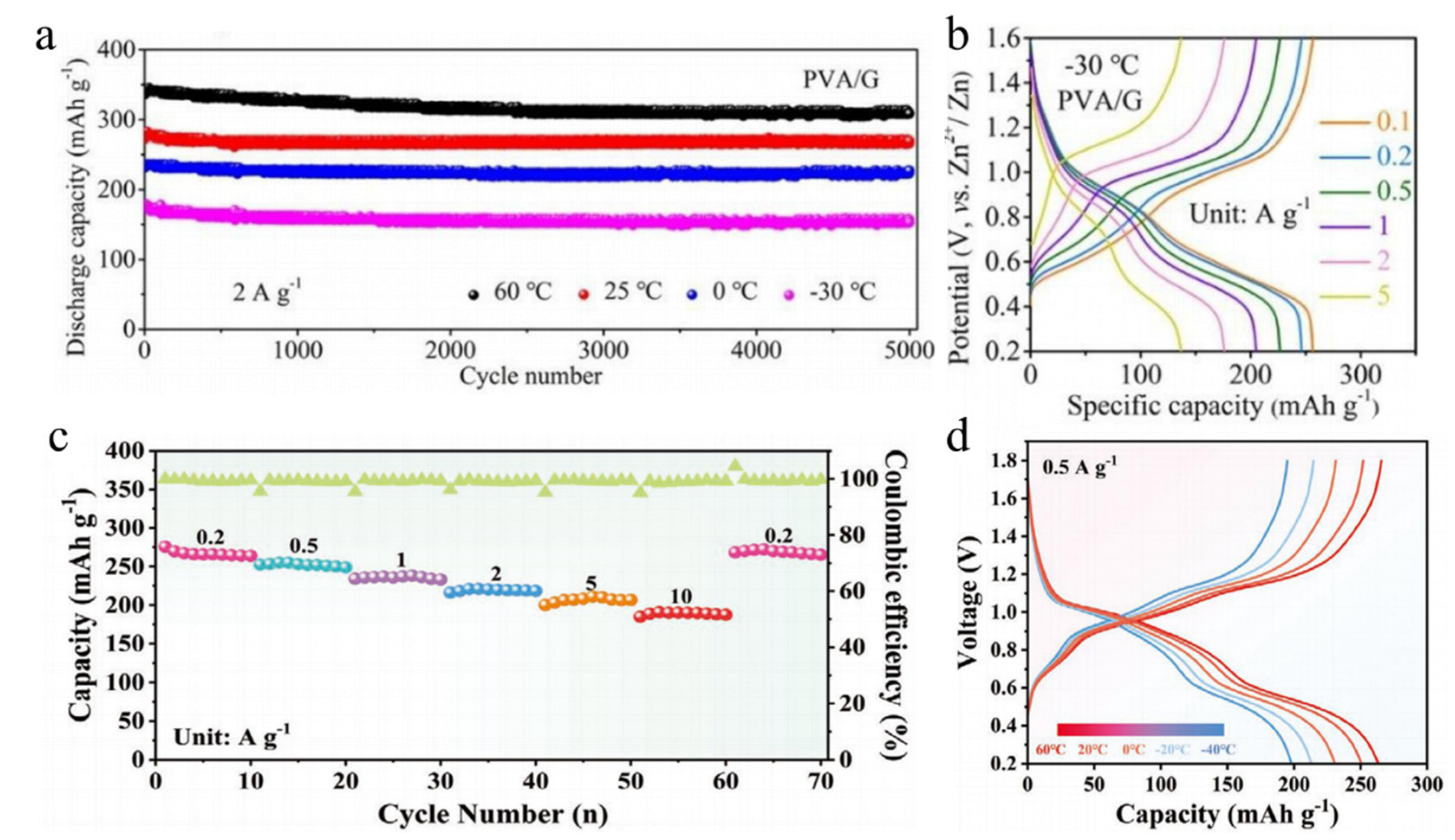
| δ-MgVO b | polyvinyl alcohol/glycerol gel-Zn(CF3SO3)2 | 60 °C to −30 °C | 308.7 mAh/g 153 mAh/g after 5000 cycles at 2A/g | [97] |
| Zn3V2O8 | PAAm/DMSO/Zn(CF3SO3)2 c | 60 °C to −40 °C | 265.2 mAh/g after 3000 cycles at 0.2 A/g | [98] |
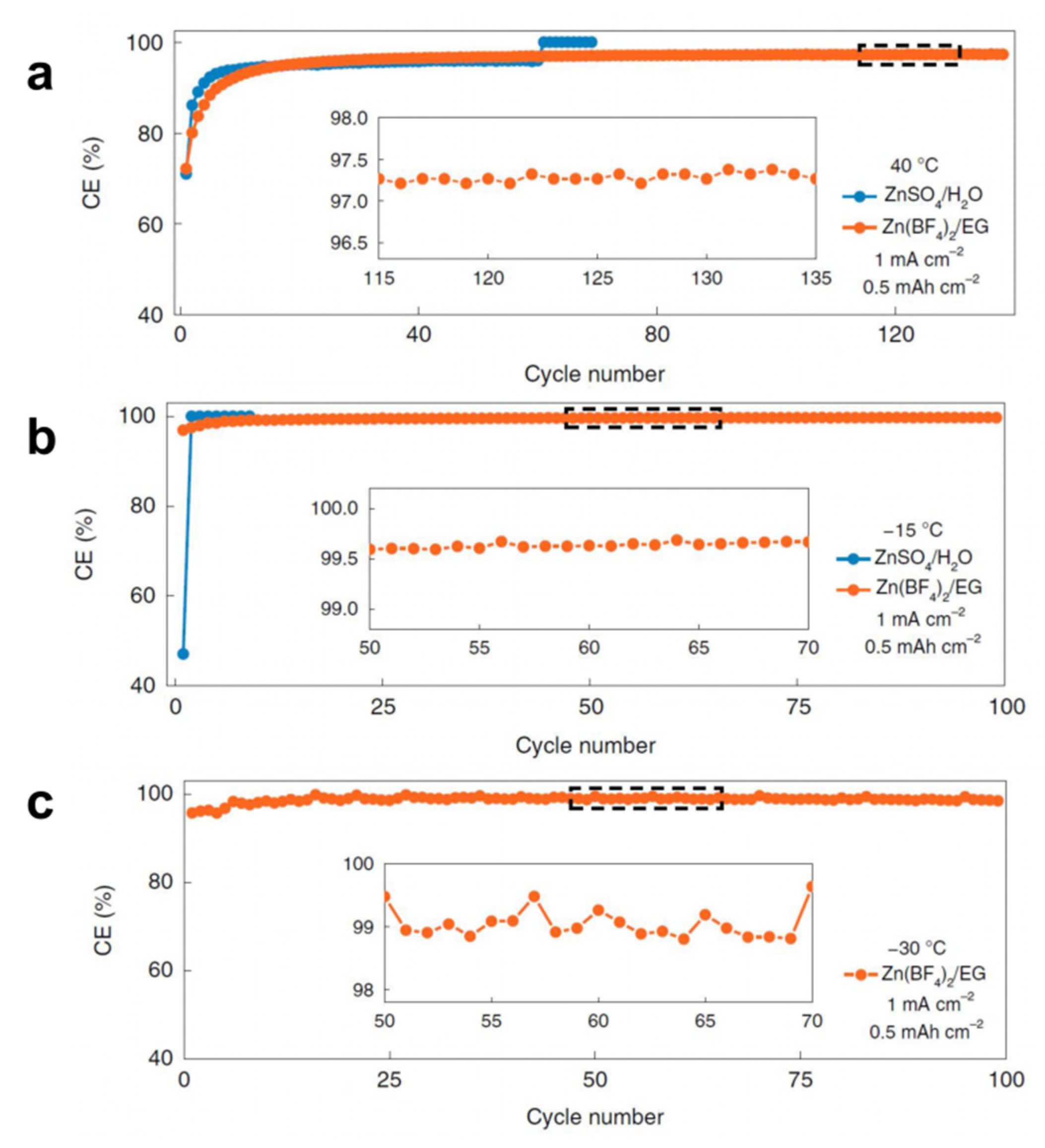
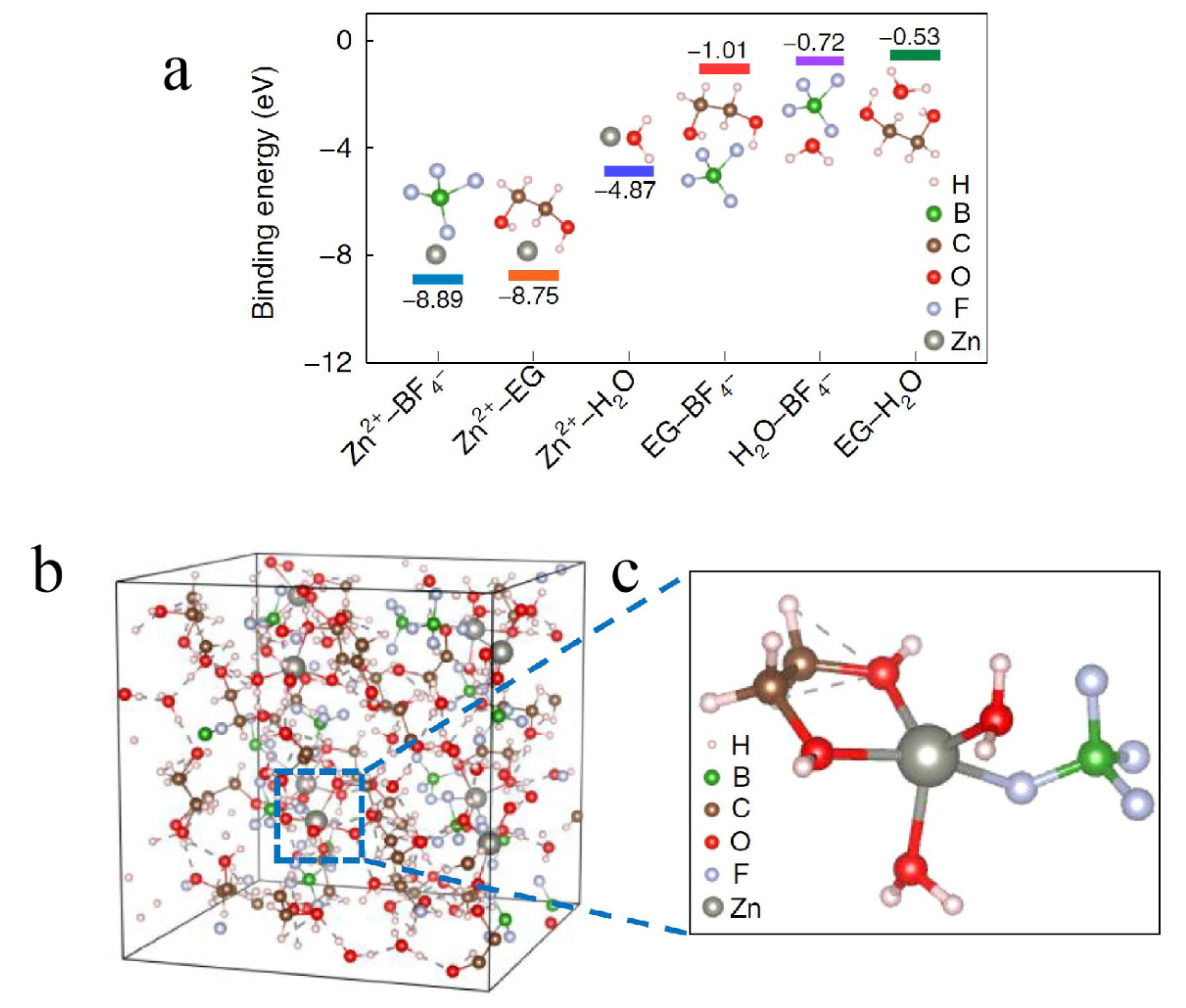
| Cu | Zn(BF4)2/EG | 40 °C to −30 °C | CE of 97.3%, 96.9% and 95.7% with good cycling stability (over 135, 100 and 100 cycles) | [99] |
| LiMn2O4 | BM-gel d | 80 °C to −20 °C | 105 mAh/g after 150 cycles at 2.0 A/g at 80 °C 165 mAh/g at 0.2 A/g at −20 °C | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Song, Z.; Deng, C.; Wang, Q.; Mo, F.; Hu, H.; Liang, G. Electrolytes for Aqueous Zn-Ion Batteries Working in Wide-Temperature Range: Progress and Perspective. Batteries 2023, 9, 386. https://doi.org/10.3390/batteries9070386
Sun L, Song Z, Deng C, Wang Q, Mo F, Hu H, Liang G. Electrolytes for Aqueous Zn-Ion Batteries Working in Wide-Temperature Range: Progress and Perspective. Batteries. 2023; 9(7):386. https://doi.org/10.3390/batteries9070386
Chicago/Turabian StyleSun, Lixia, Zhongcheng Song, Chao Deng, Qiang Wang, Funian Mo, Haibo Hu, and Guojin Liang. 2023. "Electrolytes for Aqueous Zn-Ion Batteries Working in Wide-Temperature Range: Progress and Perspective" Batteries 9, no. 7: 386. https://doi.org/10.3390/batteries9070386
APA StyleSun, L., Song, Z., Deng, C., Wang, Q., Mo, F., Hu, H., & Liang, G. (2023). Electrolytes for Aqueous Zn-Ion Batteries Working in Wide-Temperature Range: Progress and Perspective. Batteries, 9(7), 386. https://doi.org/10.3390/batteries9070386








