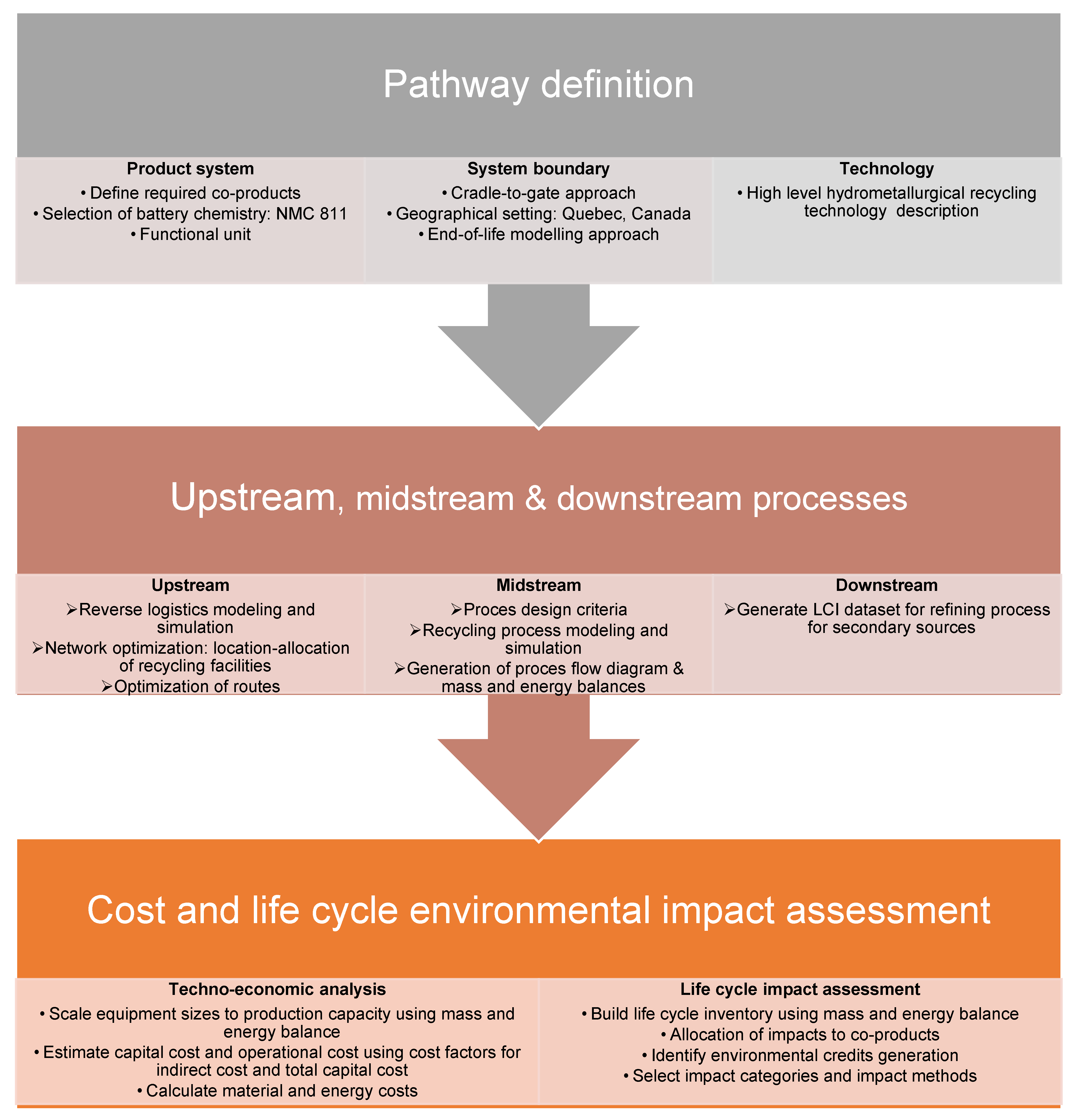
3.1. Cost Accounting Methodology of the Midstream
To assess the impact of the recycled battery material cost, a scoping-level techno-economic analysis (TEA) is conducted to evaluate the capital costs and operating costs incurred in an EV LIB recycling facility to recover critical cathode materials using conventional solvent extraction to produce high-purity (battery-grade) metal salts of nickel, cobalt, and manganese. Also, lithium carbonate is recovered.
The different steps required to complete a TEA of the recycled battery materials of LIBs in the midstream are described below.
3.1.1. Process Design Criteria
The process design criteria (PDC) used in this study are based on preliminary bench-scale test work performed at National Research Council Canada, literature data, and process conditions typically employed in similar process areas in conventional hydrometallurgical plants. Solvent extraction performance is estimated based on the expected properties of known extractants (i.e., Cyanex 272 and DEHPA). It should be noted that solvent extraction parameters frequently vary on a case-by-case basis, and thus test work is required to validate these inputs.
The PDC used in this study is used as the basis for a mass and energy balance model of the process plant, which was constructed using SysCAD simulation software [
28]. Additionally, the PDC serves as a basis for the development of process block flow diagrams, overall process flowsheet description, plant/process area throughputs, key process chemistries, and process parameters (retention time, pH, temperature).
Black mass, consisting of mainly anode (graphite) and cathode (metal oxides) materials, was selected as the feed material for this study. LIB cathode materials comprise a significant fraction of the mass composition and the material value of an LIB cell [
28,
34]. The process plant is designed for a nominal capacity of two dry tons of black mass cake per hour. With 20 wt.% cake moisture, an annual capacity throughput of approximately 16,560 dry tons is achievable using a 95% operating factor. The feed elemental composition is based on the projected LIB battery mix for 2030 containing 38.6 wt.% C, 5.14 wt.% Co, 23.2 wt.% Ni, and 3.97 wt.% Li, with an allowance for minor impurities [
28]. Based on the PDC employed, the overall metal recoveries for Ni, Co, Mn, and Li were calculated to be 92.4%, 92.3%, 30.1%, and 89.3%, respectively.
The estimated production, at 98 wt.% solids, of metal salts of manganese, cobalt, and nickel are 344 tpa (112 tpa Mn equivalent), 3749 tpa (786 tpa Co equivalent), and 15,766 tpa (3523 tpa Ni equivalent), respectively. The production of industrial-grade lithium carbonate is at 3120 dry tpa.
3.1.2. Process Flow Diagram
The LIB recycling plant uses a hydrometallurgical route to produce high-purity salts of manganese sulfate (MnSO
4·H
2O), cobalt sulfate (CoSO
4·7H
2O), and nickel sulfate (NiSO
4·6H
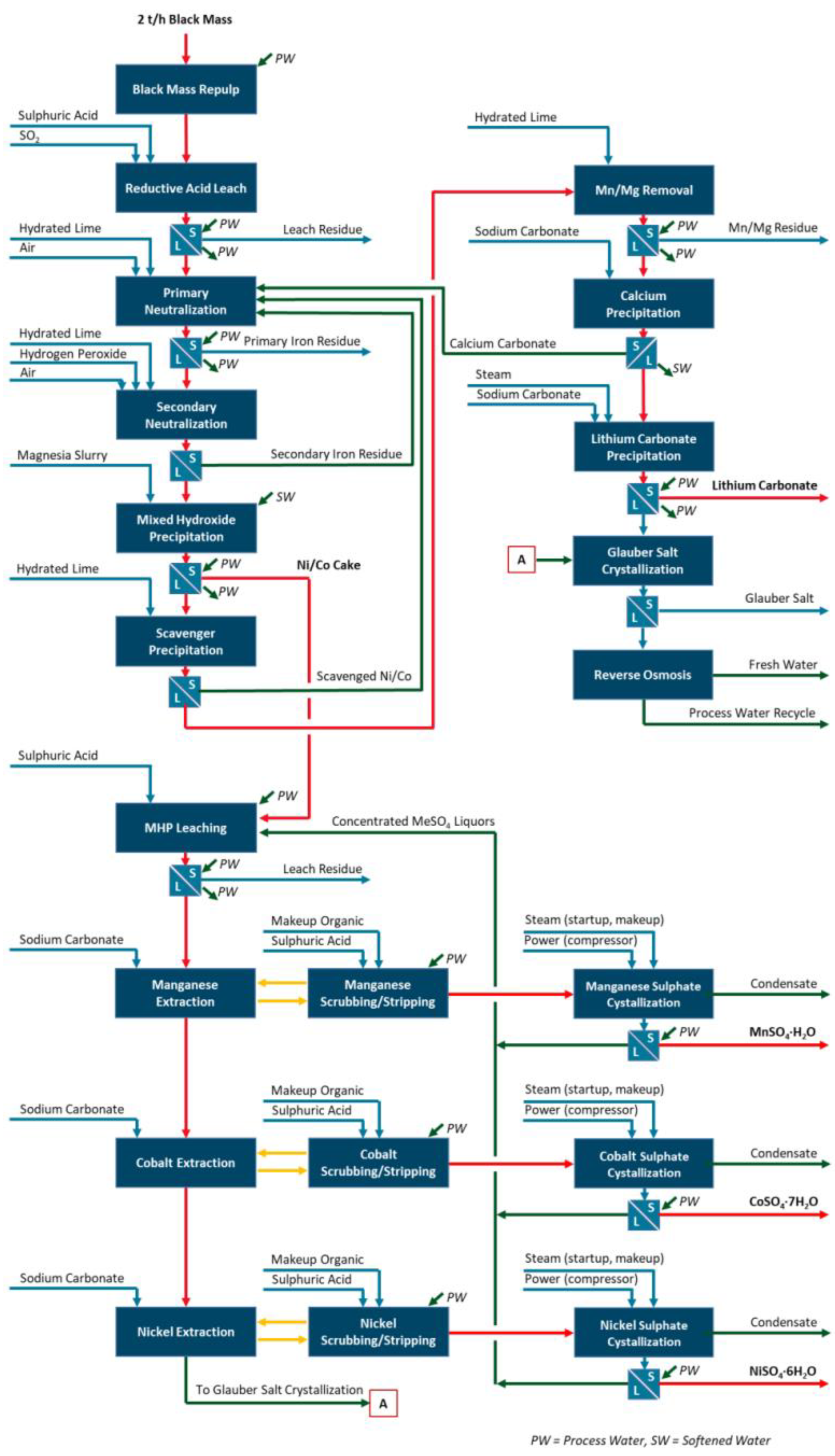
2O) products suitable for sale to battery cathode manufacturing. Conventional solvent extraction and evaporative crystallization steps were selected as a baseline. The process considers the following stages: a reductive acid leach, primary and secondary slurry neutralization steps, intermediate MHP, scavenger precipitation, manganese and magnesium removal, calcium carbonate precipitation, lithium carbonate precipitation, and sodium sulfate decahydrate (i.e., Glauber salt) crystallization. Further refinement is accomplished by MHP leaching, and sequential manganese, cobalt, and nickel solvent extraction and crystallization. Using this process, the intermediate MHP product could be upgraded into higher-value products and so re-introduced more directly into the battery supply chain. A reverse-osmosis step is required following Glauber salt removal to close the water balance. The overall process flow diagram (PFD) is presented in
Figure 5.
3.1.3. Mass Balance
The mass balance is generated as a result of the implementation of the PFD in the SysCAD process simulation software.
Table 2 provides the mass balance as an indicator of the process performance. The input and output streams indicate the flow amount of the input and output material flows through the system. Sulphuric acid, sodium carbonate, and lime represent all together 44% of total input materials; meanwhile, fresh water makeup and other consumables represent 42% and 14%, respectively. Metal sulfates and lithium carbonate, residues and material disposal, and Glauber salt account for 17%, 34%, and 49% of total output materials, respectively.
3.1.4. Capital Cost and Operating Cost
Based on the concept-level PDC specifications (
Section 3.1.1) and equipment sizing for the main process units, mass and energy balance data are obtained from the process modeling. Mass and energy balance for the hydrometallurgical solvent extraction process plant is used to estimate the overall recovery of critical battery materials, production rates, product compositions, and the selection and sizing of the major mechanical equipment items. This mass and energy balance is also used to calculate the operating costs of major reagents and consumables, energy, and fresh water. Based on the mass and energy balance, major equipment items were sized, and an AACE Class 5 estimate was produced [
50]. The capital and operating cost estimates will carry an anticipated accuracy of no better than +/−50%. The direct capital cost estimate is based on the sizing of major mechanical equipment and parametric factoring. In-house data [
36] will be used to cost major mechanical equipment items and estimate operating costs. A list of the direct capital costs for the process plant by process area is presented in
Table S1 in the Supplementary Information.
The total LIB recycling capital cost is presented in
Table 3 and estimated at kCAD (thousand Canadian dollars), including direct, indirect, and contingency costs. The capital and operating costs of this TEA study were originally estimated in US dollars, which were converted to CAD using the exchange rate USD to CAD provided by the Bank of Canada daily exchange rates [
51].
Total operating costs are shown in
Table 4. Based on the mass balance in
Table 2, the quantity of reagent materials can be estimated. These materials consist of sulphuric acid, sodium carbonate, lime, magnesia, sulphur dioxide, pellets of sodium hydroxide, and organic extractants and diluents. The prices of reagents are estimated using in-house and InfoMine data [
49,
52,
53,
54,
55]. The fresh water cost was estimated using an average of industrial water use rates from Canadian municipalities [
49]. Likewise, electricity cost was estimated using the average electricity rates from Canadian provinces. Labour costs were established considering 119 employees, considering a budget for management, administration, support services, and maintenance. Process waste streams are reductive acid leach residue, primary neutralization residue, manganese and magnesium precipitation residue, mixed hydroxide precipitation leaching residue, and Glauber salt. These washed solid residues containing mainly inert materials and small quantities of undissolved metals/metal oxides/metal hydroxides are trucked to a residue disposal facility (landfill).
Labour represents 20% of total operating costs due to the high labour rates in Canada and the relatively low production yield. As a group, the main reagents sulphuric acid, lime, sulphur dioxide, magnesia, and sodium carbonate account for 52% of the total operating cost; thus, an optimized consumption of reagents is needed to improve process economics. Sulphuric acid and sodium carbonate used during solvent extraction represent 20% and 19% of total operating costs, respectively.
All other potential operating costs associated with, but not limited to, marketing, royalties, corporate charges, taxes, depreciation and amortization, financing, legal, and cost incurred as a result of unforeseen circumstances (force majeure) are excluded.
3.1.5. Recycling Processing Cost
The overall recycling processing cost to operate a hydrometallurgical recycling facility is estimated by dividing the total recycled battery material production cost by the annual throughput of black mass. The total production cost is estimated as 96 MCAD/year, including an annual profit of 5% total capital cost and the annual black mass throughput of 16,560 tons per year. Therefore, the recycling processing cost is estimated as 5.8 CAD/kg of black mass (10.6 CAD/kg of CAM equivalent).
3.2. GHG Accounting Methodology of the Midstream
As the upstream stage methodology and calculation have been described in a recent publication [
13], we will focus on the midstream (battery recycling) and downstream (material refining). As shown in
Figure 2, GHG emission accounting is based on the life cycle assessment approach. According to ISO standards [
56,
57], the following methodological LCA components are evaluated in this study.
3.2.1. Definition of Functional Unit
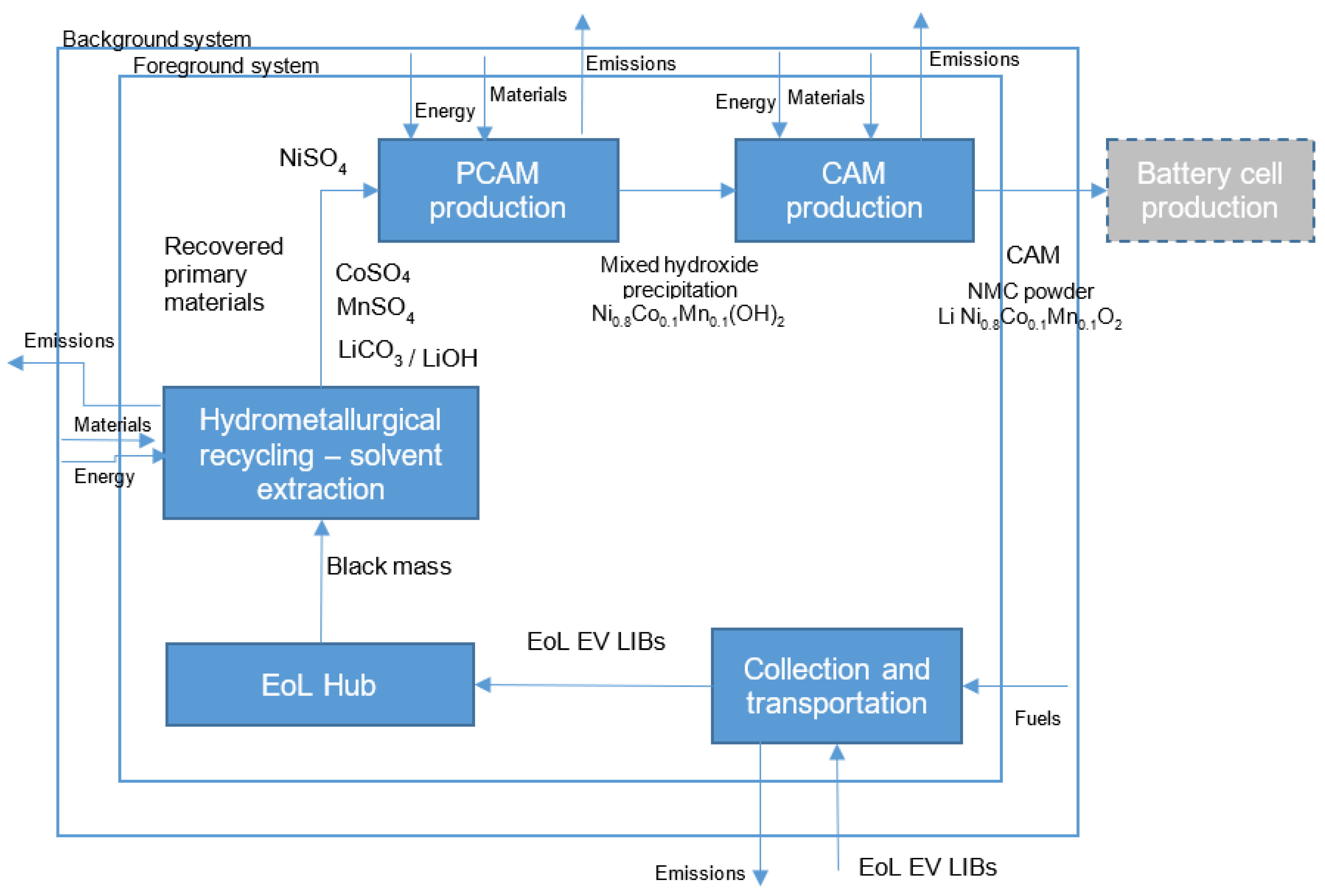
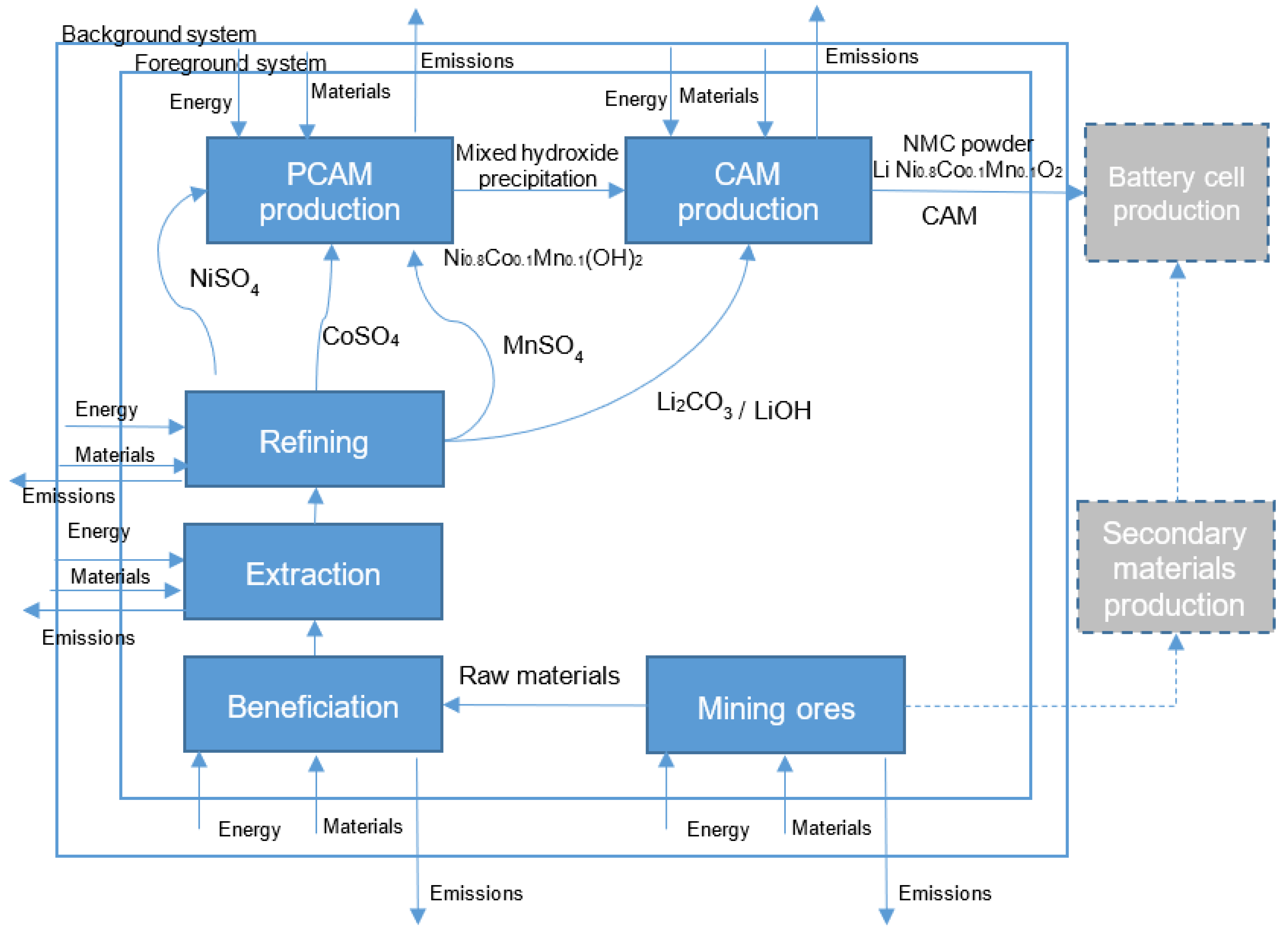
The goal of this LCA study is to estimate the life cycle environmental impact, global warming potential, of the supply chain of recycled and recovered EV LIB CAMs from grave-to-gate and evaluate the potential environmental credits associated with the recovery of primary material from recycling processes. These recovered primary materials in the form of sulfates (NiSO4, CoSO4, MnSO4) are precursor inputs for LIB cathode production that could be part of a closed-loop recycling scheme where LIB raw materials can be recycled and reused. The recycled sulfates could be considered as avoided products in the life cycle inventory of the battery cell cathode production process.
According to ISO 14044 [
56], the scope of a study is defined by the functional unit (FU) of the product system studied and the definition of the system boundary. In this LCA study, the product system considers the recycled cathode active material whose recovered materials come from a hydrometallurgical process of the black mass generated. This study considers two functional units: for the midstream stage, 1 kg of black mass and for the downstream processing and refining, 1 kg of EV LIB cathode active material.
3.2.2. Definition of System Boundary
The system boundary is described as a set of criteria to determine which unit processes are included or not in a product system [
56]. The system boundary of this LCA study of the supply chain of the EV LIB cathode active material from recycled and recovered materials to CAM manufacturing is illustrated in
Figure 3 in
Section 2.1.1.
3.2.3. Building the Life Cycle Inventory (LCI)
In accordance with Recharge [
44], the spent LIB pack is assumed to be dismantled and the main components separated to recover primary battery materials.
The life cycle inventory foreground system of the recovered LIB CAMs is developed with input and output materials and energy based on the mass balance resulted from the hydrometallurgical solvent extraction process simulation explained in
Section 3.1.3. The background system LCI data are obtained from the Ecoinvent environmental database v. 3.9.1.
Detailed LCI datasets are developed for the midstream stage of the CAM supply chain, i.e., the hydrometallurgical process with solvent extraction to recover sulfates and lithium from 1 kg of black mass. For the downstream stage of recycled battery materials, LCI datasets of the production of the precursor CAM (mixed hydroxide precipitation) and the cathode active material manufacturing via calcination are also developed. Two geographical scenarios for Canada with different electricity production mixes are considered: first, the province of Quebec consisting mostly of hydroelectricity and, second, the province of Ontario with a mix of hydroelectricity, nuclear, and natural gas energy sources.
The LCI datasets and metadata are presented in
Table S5 in the Supplementary Information. Datasets can be accessed online by using a free open-source tool called openLCA collaboration server, which supports import and export functions to download/upload the indicated datasets in JSON formats. The openLCA collaboration server is administered by the National Research Council Canada [
58].
Related to the LCI for the battery CAM production from virgin materials, the input materials were modelled as 100% virgin materials, which means that no environmental credits were considered to arise from recycled material content. Potential benefits from recycled battery materials are credited in terms of “recycled content material” in primary input material to manufacture battery cathode active material.
3.2.4. Allocation Method
According to ISO 14044 [
56], the input and output flows should be allocated to the different by-products based on established procedures. Global Battery Alliance and Nickel Institute have established the rules to apply for the allocation of metallic by-products. In the case of a base metal, which is a non-ferrous metal that is neither a precious nor noble metal, the following rule applies. If only base metals are produced as co-products, the best approach is mass allocation to define the environmental profile [
59,
60]. Then, NiSO
4, CoSO
4, MnSO
4, and lithium compounds could be classified as base metals. In this LCA study, the mass allocation factor for nickel sulfate, cobalt sulfate, manganese sulfate, and lithium carbonate is 68.7%, 16.3%, 1.4%, and 13.5%, respectively.
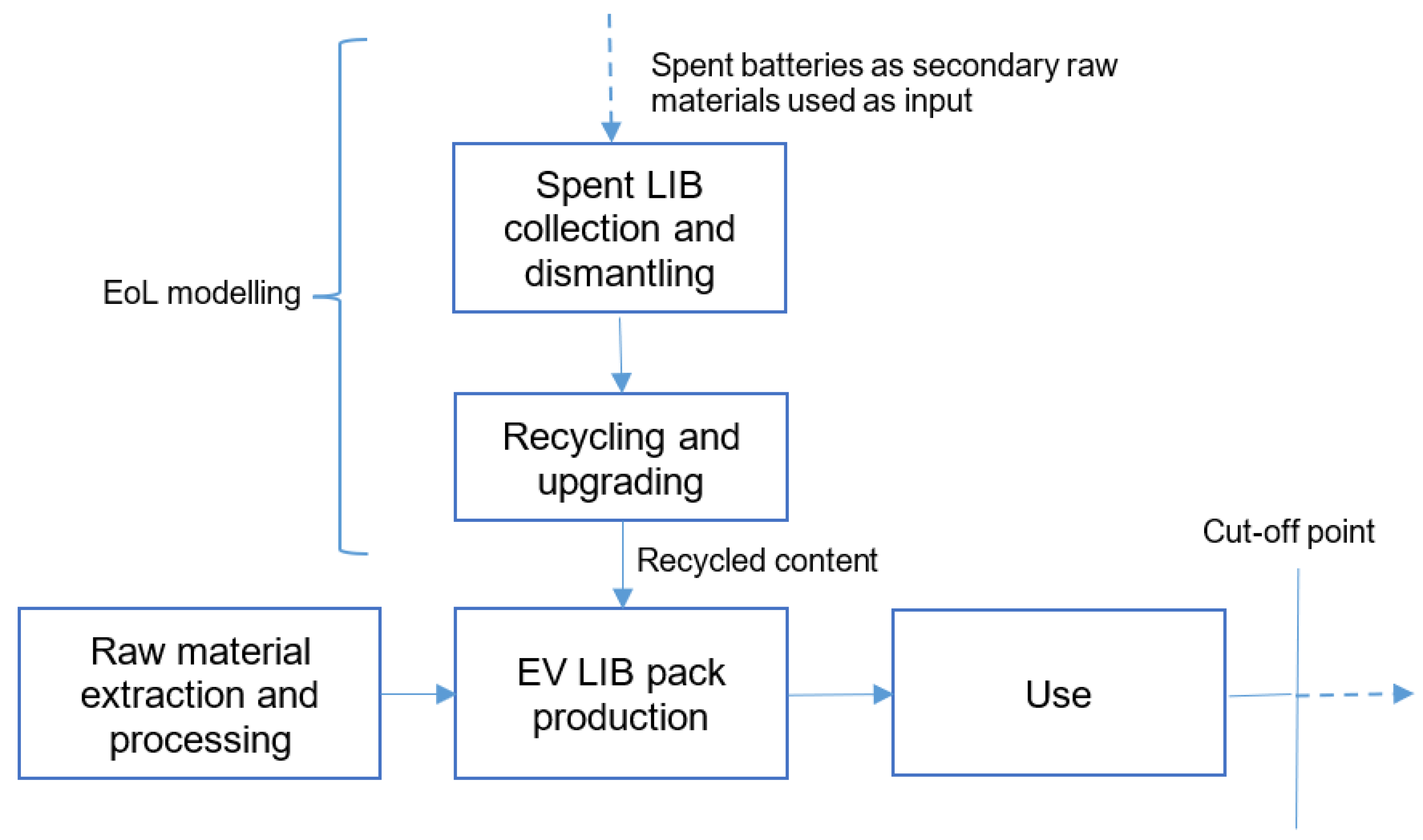
3.2.5. End-of-Life Approach
There are two main approaches in LCA to model the end-of-life (EoL) stage for EV LIBs. The cut-off approach, also known as the “recycled content approach”, does not give credits for material recovery, although it allows the possibility for secondary material input (recycled content). The EoL recycling approach, also known as “avoided burden”, gives credits for material recovery for primary material input [
45,
59]. In this LCA study, the EoL approach is a hybrid closed-loop cut-off recycling model by including recycling content, as well as modelling the material recovery processes in the EoL stage, but without giving any credits for avoided burdens. An attributional system model is used as an accounting type of LCA. The cut-off point is set just after the use phase has been completed as indicated in
Figure 6 showing the scope of the EoL modelling for spent lithium-ion batteries in a closed-loop approach. In this case, the spent batteries are burden-free.
3.2.6. Life Cycle Impact Assessment (LCIA)
This study conducted the LCIA by sorting the inventory materials in inputs and outputs according to the environmental selected impact category. The impact category selected in this study is the global warming potential (GWP) expressed in kg CO
2e, and the selected impact assessment method is the ReCiPe 2016 v1.1 midpoint method using the hierarchic version [
61]. The LCIA of the recycled battery CAMs was conducted using the open-source software openLCA version 1.11. The global warming potential impact of the hydrometallurgical recycling process located in Quebec (Canada) is estimated as 3.57 kgCO
2e/kg black mass (6.54 kg CO
2e/kg cathode active material). The GWP of the recycling process located in Ontario is 3.68 kg CO
2e/kg black mass (6.74 kg CO
2e/kg cathode active material).
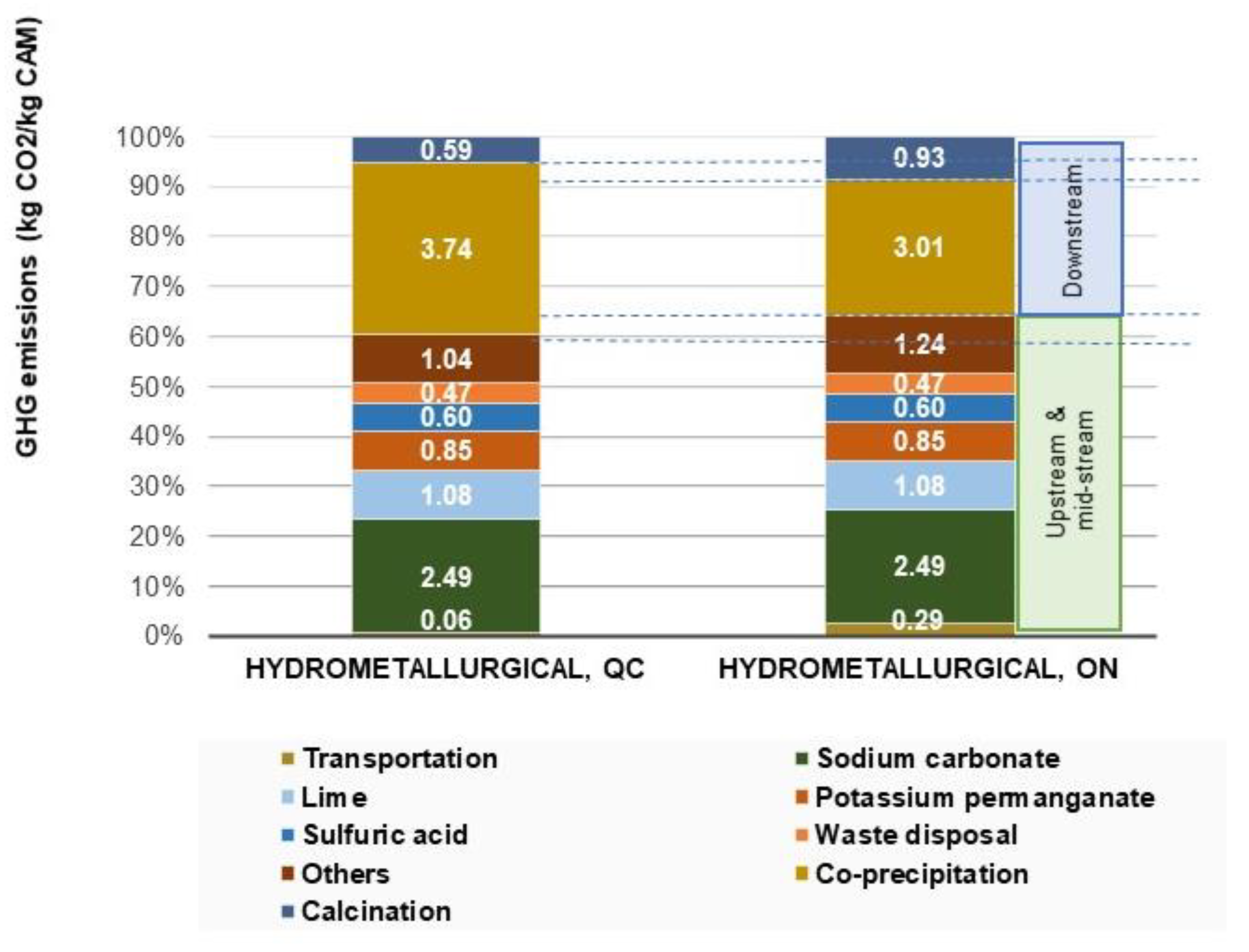
Figure 7 provides the breakdown of the different contributions to the overall GHG emissions for Quebec and Ontario. Overall, in both geographical locations, reagents (sodium carbonate, lime, sulphuric acid, and other chemicals) contribute the most to the total GHG emissions of the supply chain of cathode active material from recycled battery materials accounting for 51% of total emissions. Sodium carbonate contributes the most to the recycling processing GHG emissions accounting for 38.5% and 37% of total recycling emissions in Quebec and Ontario, respectively.
3.2.7. Environmental Credits
An environmental credit is assigned to each recovered material, based on the process it displaces. Since the bottom line of closed-loop recycling is to reuse any recovered material for its original purpose, it is assumed in this LCA study that all recovered materials replace the production of their virgin counterparts.
The life cycle environmental credits are obtained as the difference between the total recycling credits from CAM recovered in the overall recycling process and the life cycle environmental impact of recycling and recovering LIB CAM components, according to Equation (1) below.
where
ECi,k is the life cycle environmental credit impact category
k result for the battery component
i,
EIrecycling i,k is the life cycle environmental impact category
k result for recycling battery component
i, and
RCi,k is the life cycle recycling credit category
k result for battery component
i.
Potential life cycle recycling credits as a result of avoiding the production of primary materials (CoSO
4, NiSO
4, and MnSO
4) and Li
2CO
3/Li
2OH to manufacture NMC 811 LIB cathode active materials are estimated based on Equation (2).
where
mj denotes the mass in kilograms of recovered material
j, and
eij,k is the result of environmental impact/emission category
k for 1 kg of virgin material
j. It was assumed that the metal sulfates and lithium carbonate can be recycled back to produce NMC battery cathode active materials based on the closed-loop recycling approach.
Recycling credits from these avoided products represent the environmental impacts avoided/saved from recycling processes against the primary material production.
In our Quebec case study, the life cycle environmental credit as a result of recovering metals at battery EoL to produce CAM with 100% recycled content is −6.46 kgCO2e/kg CAM.
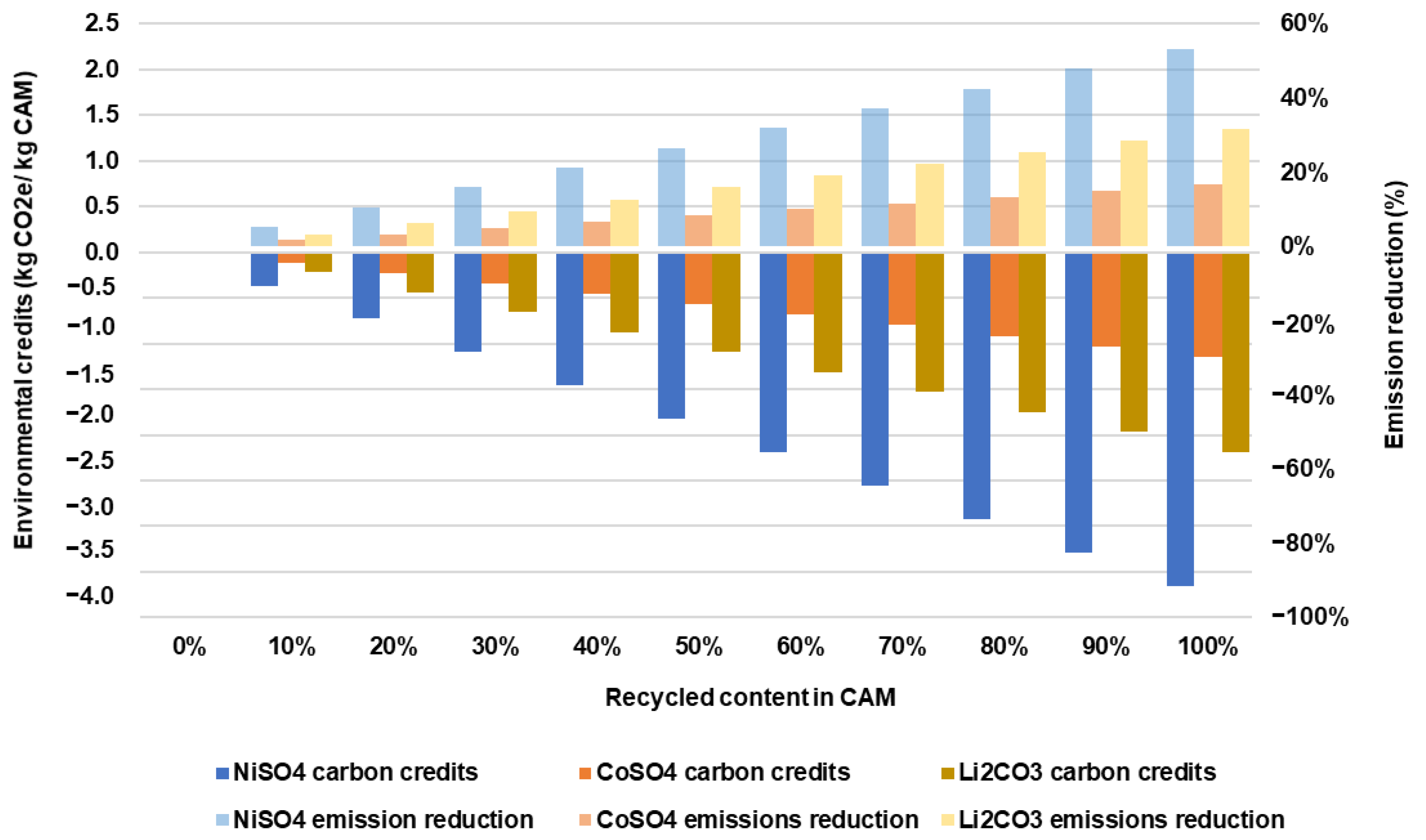
Figure 8 shows the environmental credits and GHG emission reduction rate of nickel sulfate, cobalt sulfate, and lithium carbonate at different recycled content rates. Nickel sulfate and lithium carbonate provide the highest potential for emission reduction at 48% and 28%, respectively, when the recycled content of these materials is 100%.
3.2.8. Comparison with Previous Studies
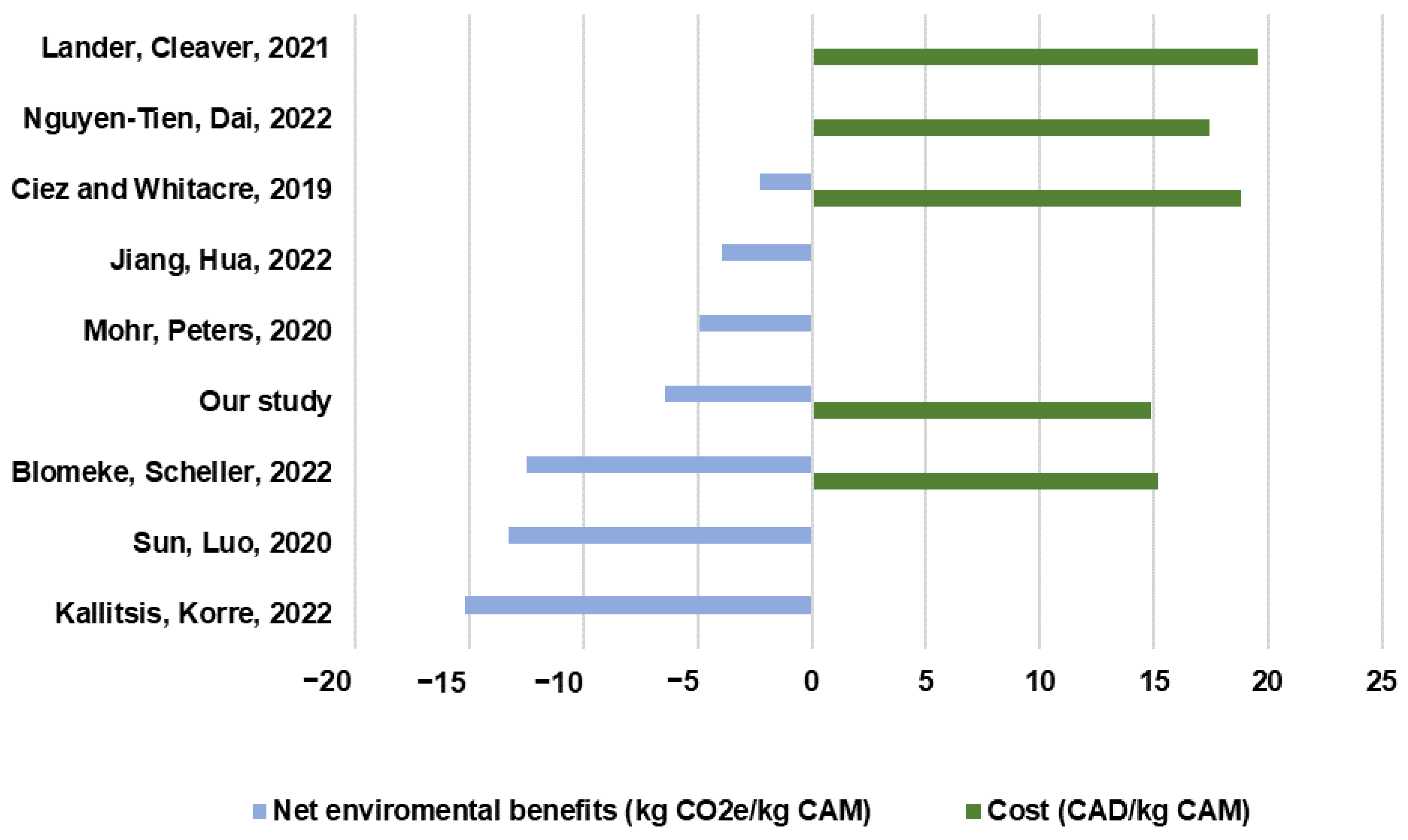
A comparison of our study’s environmental credit result, i.e., net environmental benefits, with the results reported in the literature is indicated in
Figure 9. The outcomes from recent life cycle assessment publications of lithium-ion batteries are reviewed, including net environmental benefits and recycling costs for recovering CAMs from spent NMC batteries [
19,
20,
21,
22,
23,
24,
27,
30]. The large variability of the reported results in the literature is attributed to the differentiations in the hydrometallurgical process route, system boundaries, functional units, data source of the supply chain process, and assumption scopes (geographical and supply chain processes included); therefore, transparency among LCAs on LIB recycling is needed [
19,
21]. Since our study scope is focused on the hydrometallurgical recycling process and does not include the recycling processing of aluminum, cooper, and steel by mechanical pretreatment, for comparative purposes, we harmonized the net environmental benefits reported in most studies by excluding the effect of recycling aluminum, cooper, and steel from the battery pack.
Our result of environmental credits of −6.46 kg CO
2e/kg of cathode material is in the midrange of literature estimates for hydrometallurgical processing (−2.31 to −12.5 kg CO
2e/kg of cathode material). Meanwhile, our estimate of the recycling cost for NMC 811 recycling (of 14.9 CAD/kg cathode material) is at the lower end of the existing results (15.18–19.5 CAD/kg cathode material). For example, Ciez and Whitacre [
20] present the lowest estimate of net environmental benefits due to the upstream LCI data sources they used; for instance, the energy inputs are from the GREET model while we used background data from Ecoinvent database 3.9.1. In addition, they assumed that the electrode materials (aluminum and cooper foil) not recovered through the recycling process, or collected as scrap, are incinerated. Mohr, Peters [
23] evaluated a parametrized model of mechanical-hydrometallurgical recycling processing at the cell level and estimated an overall recycling benefit considering aluminum, copper, and CAM metals. In regards to the net environmental credits for recovering metal sulfates, these are in line with the results of our model. Kallitsis, Korre [
19], Sun, Luo [
22], and Blömeke, Scheller [
24] present the highest net environmental benefits due to the differentiation in the cobalt sulfate inventory used in their studies. Blömeke, Scheller [
24] modelled a European recycling supply chain while Kallitsis, Korre [
19] and Sun, Luo [
22] modelled a Chinese one, thus partially explaining the variability together with the different chemistries modelled in each study. Regarding the recycling cost estimates, Lander, Cleaver [
30] provide an estimation of the total cost of recycling a full battery pack and assumes a supply chain configuration that includes the UK as the origin of the end of life and recycling locations in Belgium, China, South Korea, and the US, which increments essentially the transportation costs.
3.3. Overall Impact Assessment
In our performed LCA, recovered CAM is considered as avoided primary CAM and thus provides positive environmental credits to the overall impact. Since a closed-loop recycling process is assumed, the recovered product quality must comply with the battery grade. That means the supply chain of the recovered CAM modeled in our study considers the downstream stage for further refining of the output metal compounds from the recycling process to obtain a CAM at battery grade.
Assessment of the costs and GHG emissions of the supply chain of NMC 811 LIB CAMs using recycled battery materials (benchmark scenario) considers three stages, upstream, midstream, and downstream. For the upstream stage, the cost and GHG emissions of transporting spent EV LIBs from collection centers through dismantling hubs to recycling facilities in the province of Quebec, Canada, are estimated and scaled from Gonzales-Calienes, Yu’s [
13] LIB reverse logistics study. The costs and GHG emissions of the midstream related to the recycling processing of black mass to recover precursor material (metal sulfates and lithium) are estimated in
Section 3.1 and
Section 3.2 of this study. The downstream stage considers two processes, the co-precipitation as the conversion of the recycled battery materials into an NMC 811 precursor CAM, i.e., the mixed hydroxide precipitation, and the calcination process of the precursor to produce the LIB cathode active material at battery grade.
The benchmark scenario is compared to a scenario that considers evaluating the costs and GHG emissions of the supply chain of the NMC 811 LIB CAMs using virgin materials. For this route, costs and GHG emissions are estimated using open-source EverBatt developed by the Argonne National Laboratory [
62]. The geographical location of the upstream and midstream stages is assumed to be located in China, where lithium compounds (LiOH·H
20 or Li
2CO
3) are produced from concentrated ores after mining extraction and beneficiation. Metal sulfates are also produced and refined in China. The downstream stage processes also take place in China based on a study.
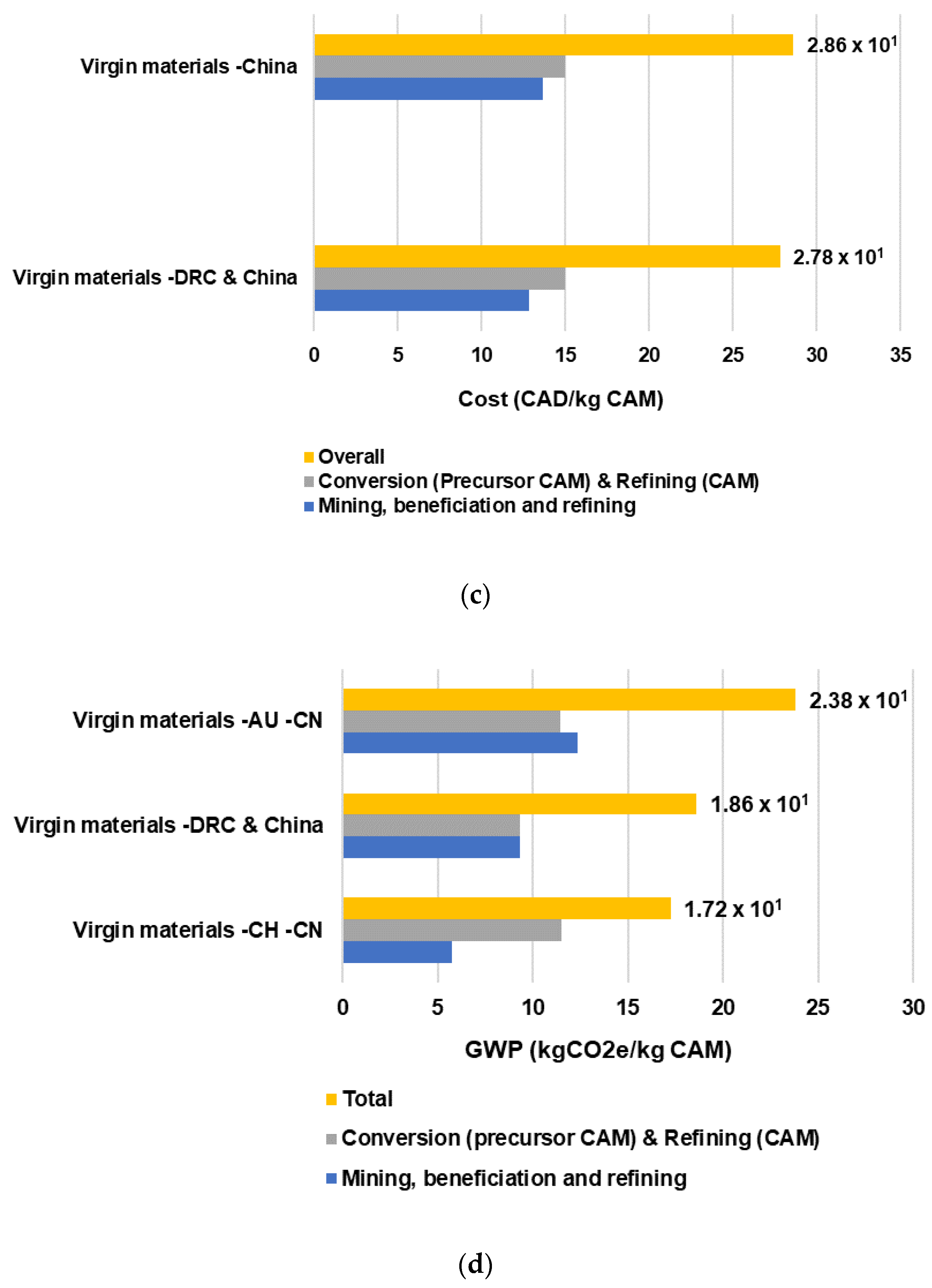
Table 5 and
Table 6 provide a comparison of cost and GHG emissions between the recycled battery material supply chain and the virgin material supply chain. The overall cost of the recycled battery material supply chain is 48% less expensive than the virgin material route. The downstream stage of the virgin battery material supply chain accounts for 52% and 15% of total costs and GHE emissions, respectively. In the case of the recycling processing cost, including spent battery collection and transportation, this is 21% less expensive than the mining, extraction, beneficiation, and refining of virgin raw materials.
Regarding the GHG emission comparison, the recycled battery material route polluted 54% less than the virgin material one. The upstream and downstream recycled material supply chain represents a 47% emission reduction with respect to the mining, beneficiation, and refining stages of the virgin materials. The main drivers of this difference are the local transportation and precursor CAM obtained from the hydrometallurgical process, which is less energy-intensive. The production of the PCAM and CAM (downstream stage) from recycled battery materials also represents an emission reduction of 62% due to the main source of energy in China being coal, which increments the carbon intensities of heat and electricity required to produce the LIB CAM. An important quantity of coal is consumed for process heat, as fuel, and for steam production [
42]. In the Quebec case study, the energy source is natural gas to provide heat, whereas electricity is obtained from hydroelectricity representing ninety-four percent of Quebec’s electricity generation mix. Quebec’s electricity rates are among the lowest in North America [
63].
In
Table 7, environmental impact in terms of the GHG emissions of individual recycled battery precursor materials, including nickel sulfate, cobalt sulfate, manganese sulfate, and lithium carbonate, are compared with the GHG emissions of the production of these precursor materials from virgin materials. Important emission reductions can be achieved when nickel sulfate and lithium carbonate from ore are recovered and recycled from spent EV LIBs.
The GHG emissions of recycled sulfates and lithium compounds are estimated using the mass allocation method for the calculated GWP of the hydrometallurgical process of 1 kg of black mass. The recovered sulfates, NiSO4·6H2O, CoSO4·7H2O, MnSO4·7H2O, and lithium carbonate (Li2CO3) are co-products with the following GHG emission allocation factors: 0.6875, 0.1630, 0.0149, and 0.1346, respectively.
Nickel is a major component of the active cathode material. Nickel sulfate hexahydrate battery-grade obtained from virgin materials is mostly produced from either oxidic (lateritic) or sulphide ore [
64]. Regionally, ore mining and processing are located in Russia, Canada, and Australia [
65]. China represents around 31% of global nickel refining production. Nickel Institute’s LCA study indicates a GWP of 5.4 kg CO
2e/kg nickel sulfate, where primary extraction and refining processes contribute 42% and 35%, respectively, to the GWP for the production of nickel sulfate [
66].
Cobalt sulfate is essentially mined in the Democratic Republic of Congo (DRC) and then concentrated and converted into raw Co(OH)
2. The further refining process, into CoSO
4, is essentially energy-intensive and generally occurs in China [
67]. In the case of NMC811 cathodes, these are aligned with the trend in the EV LIB industry with higher energy density and lower cobalt content [
42]. GREET© software provides a GWP of 9.7 kg CO
2e/kg cobalt sulfate using economic allocation [
68].
Manganese sulfate is used in NMC cathode active material in the form of high-purity electrolytic manganese metal which is produced mainly in China. This direct ore processing route involves beneficiation, leaching, precipitation, crystallization, and drying [
69]. GREET© provides a GWP of 0.869 kg CO
2e/kg manganese sulfate [
68].
Table 7.
Environmental impact comparison of recycled battery cathode active material and battery primary material production from virgin materials. Recycling processing values include transportation.
Table 7.
Environmental impact comparison of recycled battery cathode active material and battery primary material production from virgin materials. Recycling processing values include transportation.
| Battery Precursor CAM | Recycling Processing (kg CO2e/kg CAM) 1 | Mining, Beneficiation, Extraction, and Refining | Emission Reductions |
|---|
| (kg CO2e/kg CAM) |
|---|
| NiSO4·6H2O | 4.5000 | 6.8700 2 | −35% |
| CoSO4·7H2O | 1.0700 | 1.5500 3 | −31% |
| MnSO4·7H2O | 0.0973 | 0.0134 4 | −28% |
| Li2CO3—brine | 0.8810 | 1.3500 5 | −35% |
| Li2CO3—ore | 8.7300 5 | −90% |
Lithium carbonate is a chemical component used in the refining and conversion of the precursor CAM into cathode active material. For this study, NMC 811 CAM, two pathways for lithium carbonate extraction are considered: ore and brine. Ore-based lithium compounds are available from Western Australia and represent a major market share of global lithium production. This form of lithium is spodumene from mined ore, which can be refined to obtain Li
2CO
3 or LiOH [
70]. Chile represents a substantial market share of lithium carbonate or lithium hydroxide extracted from brine. Kelly et al.’s study [
42] concludes that the brine-based route largely consumes renewable energy to concentrate lithium up to 6%; meanwhile, the ore-based route depends greatly on diesel for ore mining and processing and coal for refining to get an equivalent 6% lithium oxide, which has less than 3% of lithium. This, mixed with the low-carbon electricity generated in Chile versus China, contributes to less carbon emissions from lithium compounds extracted from brine [
42]. Further refining to produce CAM is located in China. The GWP of lithium carbonate from brine is 2.7 kg CO
2e/kg lithium carbonate, and from ore, it is 20.4 kg CO
2e/kg lithium carbonate [
42].
3.5. Further Research
Although the costs and GHG emissions are critical indicators to evaluate the viability of battery cathode material recycling, the potential drawbacks of recycled battery materials should be also discussed in more detail through further research. Some studies have discussed the impact of recycling on battery performance, active material quality, and associated costs. Zheng, Chen [
72] compared battery performance using NMC 111 recycled battery materials versus virgin materials. The experimental results obtained showed that recovered NMC 111s obtained via the hydrometallurgical process have similar rate capability at low C rates (0.2, 0.5, 1, and 2 C) as cells manufactured with virgin materials and considerably better rate performance at high rates (5 and 10 C). Ma, Chen [
73] also found that recycled NMC 111 enables 4200 cycles and 11,600 cycles at 80% and 70% capacity retention, which is 33% and 53% better than the state-of-the-art, commercial NMC 111 cathode. Meanwhile, its rate performance is 88.6% better than commercial powders at 5C. Wagner-Wenz, van Zuilichem [
74] evaluated the recycled active material quality in terms of the purity and cyclability of recovered cathode material. After hydrometallurgical processing, the recovered transition metals and lithium salts correspond to the precursors from the production of LIBs. High-purity degrees of up to 99.9% could be achieved and used directly for the production of new cathode materials [
74]. Regarding the purity of recycled active materials from pyrometallurgical recycling routes, these are low-purity cathode precursors that need further refinement processes to obtain a battery-grade material [
75]. However, there is still a complex effect of impurities such as Cu, Fe, and Mg on recovered NMC cathode material electrochemistry performance, material stability, and lifetime after the hydrometallurgical process. In some cases, these can contribute to an improvement in the electrochemical performance, while in others, they damage the cell. It depends on the form (metal, ion) and concentration limits [
76,
77]. In the case that the recovered active material does not reach battery grade or high energy density or cycling performance, it is recommended to apply additional treatment to the recovered materials, for example, combining them with virgin materials before being reintroduced to battery production [
76,
78].