Piezoelectric-Based Energy Conversion and Storage Materials
Abstract
1. Introduction
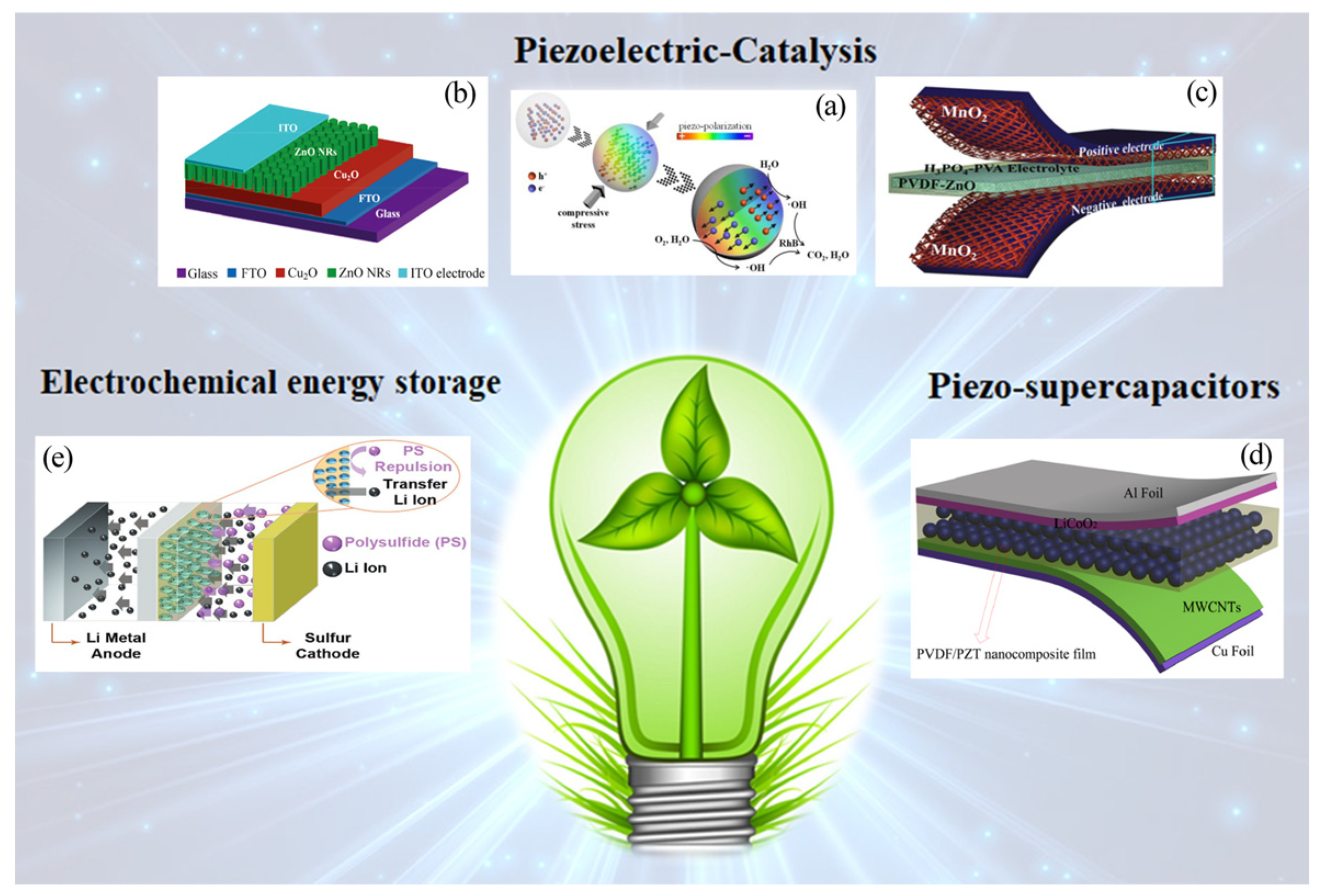
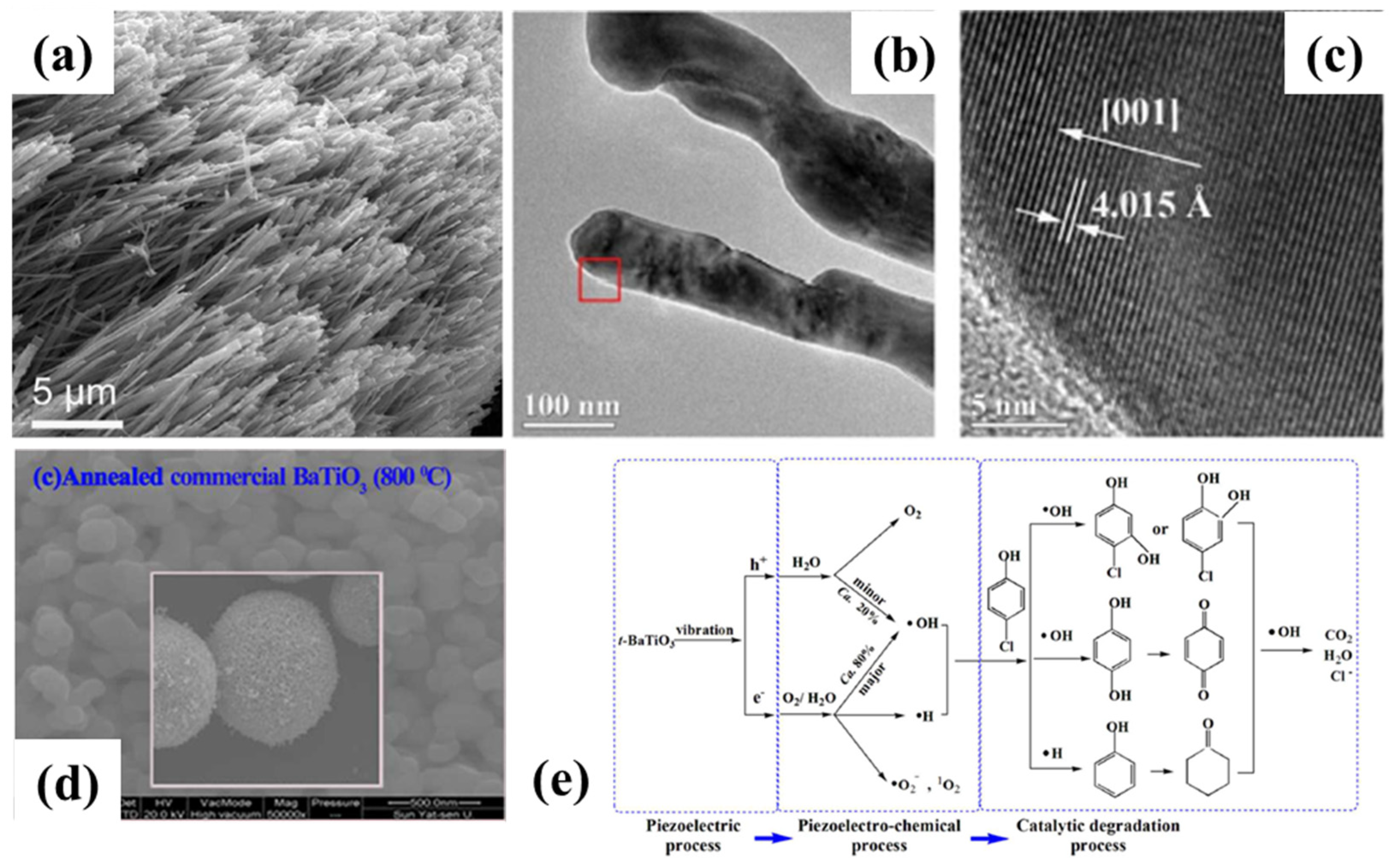
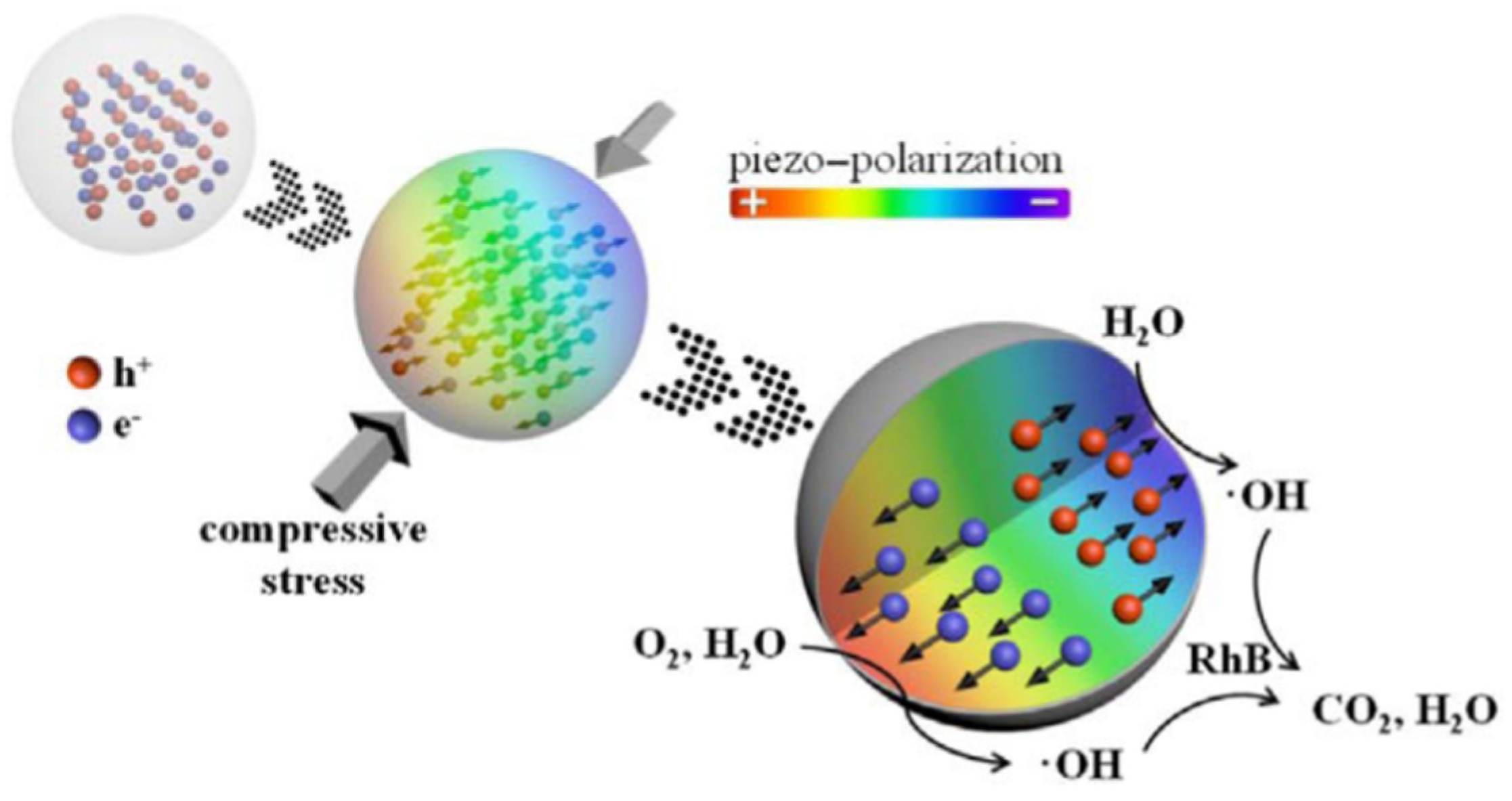
2. Piezoelectric Catalysis for Hybrid Energy Devices
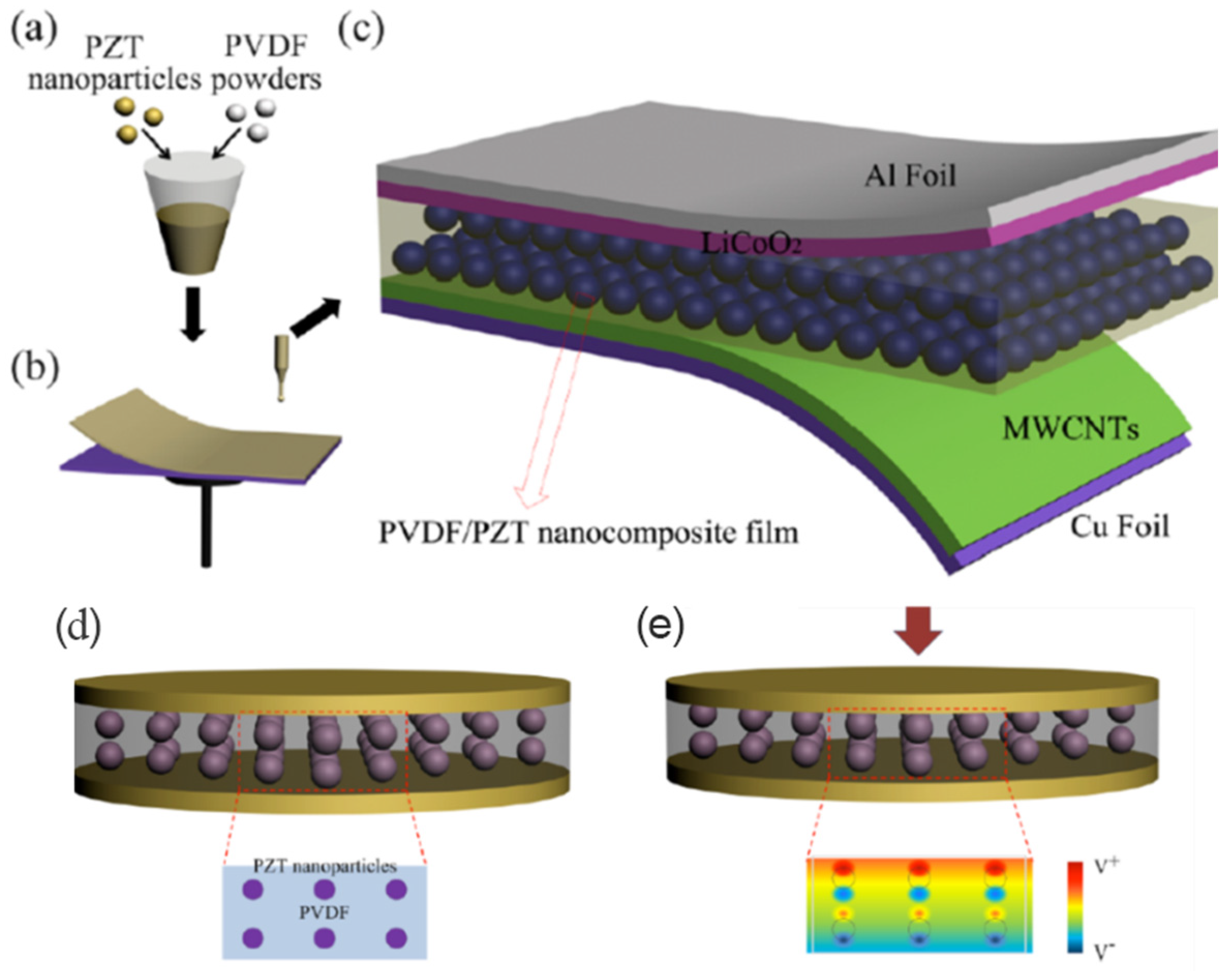
3. Piezo Supercapacitors
4. Piezoelectric-Based Self-Charging Devices
4.1. Self-Charging Efficiency
4.2. Flexibility
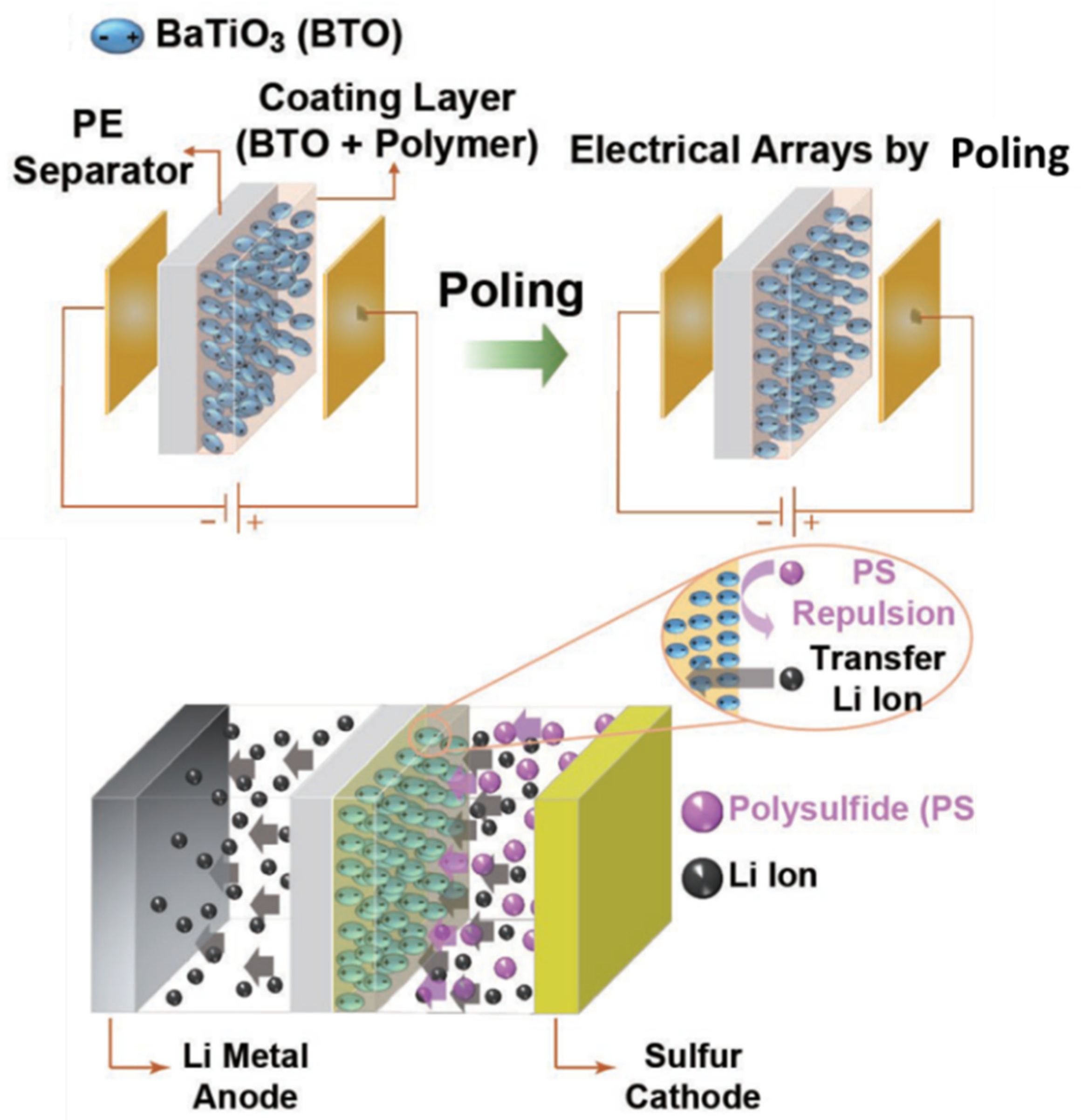
5. Piezoelectric Materials in Electrochemical Energy Storage
6. Conclusions
7. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiang, C.L.; Tseng, S.M.; Chen, C.T.; Hsu, C.P.; Shu, C.F. Influence of molecular dipoles on the photoluminescence and electroluminescence of dipolar spirobifluorenes. Adv. Funct. Mater. 2008, 18, 248–257. [Google Scholar] [CrossRef]
- Hu, Y.F.; Wang, Z.L. Recent progress in piezoelectric nanogenerators as a sustainable power source in self-powered systems and active sensors. Nano Energy 2015, 14, 3–14. [Google Scholar] [CrossRef]
- Wankhade, S.H.; Tiwari, S.; Gaur, A.; Maiti, P. PVDF-PZT nanohybrid based nanogenerator for energy harvesting applications. Energy Rep. 2020, 6, 358–364. [Google Scholar] [CrossRef]
- Gheibi, A.; Latifi, M.; Merati, A.A.; Bagherzadeh, R. Piezoelectric electrospun nanofibrous materials for self-powering wearable electronic textiles applications. J. Polym. Res. 2014, 21, 469. [Google Scholar] [CrossRef]
- Park, T.; Kim, B.; Kim, Y.; Kim, E. Highly conductive PEDOT electrodes for harvesting dynamic energy through piezoelectric conversion. J. Mater. Chem. A 2014, 2, 5462–5469. [Google Scholar] [CrossRef]
- Bae, S.H.; Kahya, O.; Sharma, B.K.; Kwon, J.; Cho, H.J.; Ozyilmaz, B.; Ahn, J.H. Graphene-P(VDF-TrFE) Multilayer Film for Flexible Applications. Acs Nano 2013, 7, 3130–3138. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.N.; Yang, W.Q.; Jing, Q.S.; Wang, Z.L. Harvesting Broadband Kinetic Impact Energy from Mechanical Triggering/Vibration and Water Waves. Acs Nano 2014, 8, 7405–7412. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.Y.; Wang, S.H.; Guo, W.X.; Zhang, Y.; Wang, Z.L. Hybridizing Energy Conversion and Storage in a Mechanical-to-Electrochemical Process for Self-Charging Power Cell. Nano Lett. 2012, 12, 5048–5054. [Google Scholar] [CrossRef]
- Kim, Y.S.; Xie, Y.N.; Wen, X.N.; Wang, S.H.; Kim, S.J.; Song, H.K.; Wang, Z.L. Highly porous piezoelectric PVDF membrane as effective lithium ion transfer channels for enhanced self-charging power cell. Nano Energy 2015, 14, 77–86. [Google Scholar] [CrossRef]
- Platt, S.R.; Farritor, S.; Garvin, K.; Haider, H. The use of piezoelectric ceramics for electric power generation within orthopedic implants. IEEE-Asme Trans. Mechatron. 2005, 10, 455–461. [Google Scholar] [CrossRef]
- Qi, Y.; Kim, J.; Nguyen, T.D.; Lisko, B.; Purohit, P.K.; McAlpine, M.C. Enhanced Piezoelectricity and Stretchability in Energy Harvesting Devices Fabricated from Buckled PZT Ribbons. Nano Lett. 2011, 11, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Kim, D.I.; Duy, L.T.; Nguyen, M.T.; Muhammad, S.; Yoon, W.S.; Lee, N.E. High-performance flexible lead-free nanocomposite piezoelectric nanogenerator for biomechanical energy harvesting and storage. Nano Energy 2015, 15, 177–185. [Google Scholar] [CrossRef]
- Cao, Y.S.; Zhang, F.; Sha, A.M.; Liu, Z.Z.; Li, J.R.; Hao, Y. Energy harvesting performance of a full-pressure piezoelectric transducer applied in pavement structures. Energy Build. 2022, 266, 112143. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric Nanogenerators as New Energy Technology for Self-Powered Systems and as Active Mechanical and Chemical Sensors. Acs Nano 2013, 7, 9533–9557. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Feng, Y.W.; Ling, L.L.; Wang, Y.X.; Xu, Z.M.; Cao, F.L.; Li, H.X.; Bian, Z.F. Engineering spherical lead zirconate titanate to explore the essence of piezo-catalysis. Nano Energy 2017, 40, 481–486. [Google Scholar] [CrossRef]
- Lin, P.; Chen, X.; Yan, X.Q.; Zhang, Z.; Yuan, H.G.; Li, P.F.; Zhao, Y.G.; Zhang, Y. Enhanced photoresponse of Cu2O/ZnO heterojunction with piezo-modulated interface engineering. Nano Res. 2014, 7, 860–868. [Google Scholar] [CrossRef]
- Ramadoss, A.; Saravanakumar, B.; Lee, S.W.; Kim, Y.S.; Kim, S.J.; Wang, Z.L. Piezoelectric-Driven Self-Charging Supercapacitor Power Cell. Acs Nano 2015, 9, 4337–4345. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.J.; Xue, X.Y.; Cui, C.X.; He, B.; Nie, Y.X.; Deng, P.; Wang, Z.L. PVDF-PZT nanocomposite film based self-charging power cell. Nanotechnology 2014, 25, 105401. [Google Scholar] [CrossRef]
- Yim, T.; Han, S.H.; Park, N.H.; Park, M.S.; Lee, J.H.; Shin, J.; Choi, J.W.; Jung, Y.; Jo, Y.N.; Yu, J.S.; et al. Effective Polysulfide Rejection by Dipole-Aligned BaTiO3 Coated Separator in Lithium-Sulfur Batteries. Adv. Funct. Mater. 2016, 26, 7817–7823. [Google Scholar] [CrossRef]
- Wang, X.D. Piezoelectric nanogenerators-Harvesting ambient mechanical energy at the nanometer scale. Nano Energy 2012, 1, 13–24. [Google Scholar] [CrossRef]
- Zhang, P.C.; Chen, L.; Xu, T.L.; Liu, H.L.; Liu, X.L.; Meng, J.X.; Yang, G.; Jiang, L.; Wang, S.T. Programmable Fractal Nanostructured Interfaces for Specific Recognition and Electrochemical Release of Cancer Cells. Adv. Mater. 2013, 25, 3566–3570. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Qian, F.; Wang, G.M.; Jiao, Y.Q.; He, Z.; Li, Y. Self-Biased Solar-Microbial Device for Sustainable Hydrogen Generation. Acs Nano 2013, 7, 8728–8735. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.Y.; Feng, J.X.; Xiong, Y.; Tian, S.H.; Liu, S.W.; Kong, L.J. Performance and Mechanism of Piezo-Catalytic Degradation of 4-Chlorophenol: Finding of Effective Piezo-Dechlorination. Environ. Sci. Technol. 2017, 51, 6560–6569. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.S.; Xu, H.F.; Konishi, H.; Li, X.C. Direct Water Splitting Through Vibrating Piezoelectric Microfibers in Water. J. Phys. Chem. Lett. 2010, 1, 997–1002. [Google Scholar] [CrossRef]
- Hong, K.S.; Xu, H.F.; Konishi, H.; Li, X.C. Piezoelectrochemical Effect: A New Mechanism for Azo Dye Decolorization in Aqueous Solution through Vibrating Piezoelectric Microfibers. J. Phys. Chem. C 2012, 116, 13045–13051. [Google Scholar] [CrossRef]
- Anton, S.R.; Sodano, H.A. A review of power harvesting using piezoelectric materials (2003–2006). Smart Mater. Struct. 2007, 16, R1–R21. [Google Scholar] [CrossRef]
- Wang, X.D.; Song, J.H.; Liu, J.; Wang, Z.L. Direct-current nanogenerator driven by ultrasonic waves. Science 2007, 316, 102–105. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J.H. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Wu, J.; Qin, N.; Bao, D.H. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration. Nano Energy 2018, 45, 44–51. [Google Scholar] [CrossRef]
- Lin, E.Z.; Kang, Z.H.; Wu, J.; Huang, R.; Qin, N.; Bao, D.H. BaTiO3 nanocubes/cuboids with selectively deposited Ag nanoparticles: Efficient piezocatalytic degradation and mechanism. Appl. Catal. B-Environ. 2021, 285, 119823. [Google Scholar] [CrossRef]
- Xu, X.L.; Jia, Y.M.; Xiao, L.B.; Wu, Z. Strong vibration-catalysis of ZnO nanorods for dye wastewater decolorization via piezo-electro-chemical coupling. Chemosphere 2018, 193, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Ujjan, Z.A.; Bhatti, M.A.; Shah, A.A.; Tahira, A.; Shaikh, N.M.; Kumar, S.; Mugheri, A.Q.; Medany, S.S.; Nafady, A.; Alnjiman, F.; et al. Simultaneous doping of sulfur and chloride ions into ZnO nanorods for improved photocatalytic properties towards degradation of methylene blue. Ceram. Int. 2022, 48, 5535–5545. [Google Scholar] [CrossRef]
- Zhang, Y.; Phuong, P.T.T.; Duy, N.P.H.; Roake, E.; Khanbareh, H.; Hopkins, M.; Zhou, X.F.; Zhang, D.; Zhou, K.C.; Bowen, C. Polarisation tuneable piezo-catalytic activity of Nb-doped PZT with low Curie temperature for efficient CO2 reduction and H-2 generation. Nanoscale Adv. 2021, 3, 1362–1374. [Google Scholar] [CrossRef]
- Kohtani, S.; Hiro, J.; Yamamoto, N.; Kudo, A.; Tokumura, K.; Nakagaki, R. Adsorptive and photocatalytic properties of Ag-loaded BiVO4 on the degradation of 4-n-alkylphenols under visible light irradiation. Catal. Commun. 2005, 6, 185–189. [Google Scholar] [CrossRef]
- Wu, J.M.; Kao, W.T. Heterojunction Nanowires of AgxZn1-xO-ZnO Photocatalytic and Antibacterial Activities under Visible-Light and Dark Conditions. J. Phys. Chem. C 2015, 119, 1433–1441. [Google Scholar] [CrossRef]
- Wu, J.M.; Hsu, G.K.; Yeh, H.H.; Lin, H.C. Metallic Zinc Nanowires Effect in High-Performance Photoresponsive and Photocatalytic Properties of Composite Zinc Stannate Nanowires. J. Electrochem. Soc. 2012, 159, H497–H501. [Google Scholar] [CrossRef]
- Wu, J.M.; Tsay, L.Y. ZnO quantum dots-decorated ZnO nanowires for the enhancement of antibacterial and photocatalytic performances. Nanotechnology 2015, 26, 395704. [Google Scholar] [CrossRef]
- Wu, M.H.; Lee, J.T.; Chung, Y.J.; Srinivaas, M.; Wu, J.M. Ultrahigh efficient degradation activity of single- and few-layered MoSe2 nanoflowers in dark by piezo-catalyst effect. Nano Energy 2017, 40, 369–375. [Google Scholar] [CrossRef]
- Jin, H.Y.; Peng, Z.H.; Tang, W.M.; Chan, H.L.W. Controllable functionalized carbon fabric for high-performance all-carbon-based supercapacitors. Rsc Adv. 2014, 4, 33022–33028. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, K.; Xie, J.Y. From spinel Mn3O4 to layered nanoarchitectures using electrochemical cycling and the distinctive pseudocapacitive behavior. Appl. Phys. Lett. 2007, 90, 104102. [Google Scholar] [CrossRef]
- Li, W.Y.; Liu, Q.; Sun, Y.G.; Sun, J.Q.; Zou, R.J.; Li, G.; Hu, X.H.; Song, G.S.; Ma, G.X.; Yang, J.M.; et al. MnO2 ultralong nanowires with better electrical conductivity and enhanced supercapacitor performances. J. Mater. Chem. 2012, 22, 14864–14867. [Google Scholar] [CrossRef]
- Tuukkanen, S.; Julin, T.; Rantanen, V.; Zakrzewski, M.; Moilanen, P.; Lilja, K.E.; Rajala, S. Solution-processible electrode materials for a heat-sensitive piezoelectric thin-film sensor. Synthetic Metals 2012, 162, 1987–1995. [Google Scholar] [CrossRef]
- Wen, L.; Chen, J.; Liang, J.; Li, F.; Cheng, H.M. Flexible batteries ahead. Natl. Sci. Rev. 2017, 4, 20–23. [Google Scholar] [CrossRef]
- Tuukkanen, S.; Julin, T.; Rantanen, V.; Zakrzewski, M.; Moilanen, P.; Lupo, D. Low-Temperature Solution Processable Electrodes for Piezoelectric Sensors Applications. Jpn. J. Appl. Phys. 2013, 52, 05da06. [Google Scholar] [CrossRef]
- Lan, L.Y.; Xiong, J.Q.; Gao, D.C.; Li, Y.; Chen, J.; Lv, J.; Ping, J.F.; Ying, Y.B.; Lee, P.S. Breathable Nanogenerators for an On-Plant Self-Powered Sustainable Agriculture System. Acs Nano 2021, 15, 5307–5315. [Google Scholar] [CrossRef]
- Wu, W.J.; Wickenheiser, A.M.; Reissman, T.; Garcia, E. Modeling and experimental verification of synchronized discharging techniques for boosting power harvesting from piezoelectric transducers. Smart Mater. Struct. 2009, 18, 55012. [Google Scholar] [CrossRef]
- Wickenheiser, A.M.; Reissman, T.; Wu, W.J.; Garcia, E. Modeling the Effects of Electromechanical Coupling on Energy Storage through Piezoelectric Energy Harvesting. IEEE-Asme Trans. Mechatron. 2010, 15, 400–411. [Google Scholar] [CrossRef]
- Bagheri, S.; Wu, N.; Filizadeh, S. Modeling of capacitor charging dynamics in an energy harvesting system considering accurate electromechanical coupling effects. Smart Mater. Struct. 2018, 27, 65026. [Google Scholar] [CrossRef]
- Bagheri, S.; Wu, N.; Filizadeh, S. Numerical modeling and analysis of self-powered synchronous switching circuit for the study of transient charging behavior of a vibration energy harvester. Smart Mater. Struct. 2019, 28, 105056. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Xiang, H.J.; Tang, L.H. Modeling, analysis and comparison of four charging interface circuits for piezoelectric energy harvesting. Mech. Syst. Signal Process. 2021, 152, 107476. [Google Scholar] [CrossRef]
- Yang, P.H.; Qu, X.P.; Liu, K.; Duan, J.J.; Li, J.; Chen, Q.; Xue, G.B.; Xie, W.K.; Xu, Z.M.; Zhou, J. Electrokinetic Supercapacitor for Simultaneous Harvesting and Storage of Mechanical Energy. Acs Appl. Mater. Interfaces 2018, 10, 8010–8015. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.Q.; Yang, W.Y.; Zhang, Y.; Bowen, C.R.; Yang, Y. Piezoelectric Materials for Controlling Electro-Chemical Processes. Nano-Micro Lett. 2020, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Peddigari, M.; Park, J.H.; Han, J.H.; Jeong, C.K.; Jang, J.; Min, Y.; Kim, J.W.; Ahn, C.W.; Choi, J.J.; Hahn, B.D.; et al. Flexible Self-Charging, Ultrafast, High-Power-Density Ceramic Capacitor System. Acs Energy Lett. 2021, 6, 1383–1391. [Google Scholar] [CrossRef]
- Xie, Y.Z.; Liu, Y.; Zhao, Y.D.; Tsang, Y.H.; Lau, S.P.; Huang, H.T.; Chai, Y. Stretchable all-solid-state supercapacitor with wavy shaped polyaniline/graphene electrode. J. Mater. Chem. A 2014, 2, 9142–9149. [Google Scholar] [CrossRef]
- Wang, S.Y.; Dryfe, R.A.W. Graphene oxide-assisted deposition of carbon nanotubes on carbon cloth as advanced binder-free electrodes for flexible supercapacitors. J. Mater. Chem. A 2013, 1, 5279–5283. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Mariappan, V.K.; Sahoo, S.; Manoharan, S.; Kim, S.J. A High Efficacy Self-Charging MoSe2 Solid-State Supercapacitor Using Electrospun Nanofibrous Piezoelectric Separator with Ionogel Electrolyte. Adv. Mater. Interfaces 2018, 5, 1800055. [Google Scholar] [CrossRef]
- Parida, K.; Bhavanasi, V.; Kumar, V.; Wang, J.X.; Lee, P.S. Fast charging self-powered electric double layer capacitor. J. Power Sources 2017, 342, 70–78. [Google Scholar] [CrossRef]
- Song, R.B.; Jin, H.Y.; Li, X.; Fei, L.F.; Zhao, Y.D.; Huang, H.T.; Chan, H.L.W.; Wang, Y.; Chai, Y. A rectification-free piezo-supercapacitor with a polyvinylidene fluoride separator and functionalized carbon cloth electrodes. J. Mater. Chem. A 2015, 3, 14963–14970. [Google Scholar] [CrossRef]
- Xing, L.L.; Nie, Y.X.; Xue, X.Y.; Zhang, Y. PVDF mesoporous nanostructures as the piezo-separator for a self-charging power cell. Nano Energy 2014, 10, 44–52. [Google Scholar] [CrossRef]
- Maitra, A.; Karan, S.K.; Paria, S.; Das, A.K.; Bera, R.; Halder, L.; Si, S.K.; Bera, A.; Khatua, B.B. Fast charging self-powered wearable and flexible asymmetric supercapacitor power cell with fish swim bladder as an efficient natural bio-piezoelectric separator. Nano Energy 2017, 40, 633–645. [Google Scholar] [CrossRef]
- He, H.X.; Fu, Y.M.; Zhao, T.M.; Gao, X.C.; Xing, L.L.; Zhang, Y.; Xue, X.Y. All-solid-state flexible self-charging power cell basing on piezo-electrolyte for harvesting/storing body-motion energy and powering wearable electronics. Nano Energy 2017, 39, 590–600. [Google Scholar] [CrossRef]
- Xue, X.Y.; Deng, P.; Yuan, S.; Nie, Y.X.; He, B.; Xing, L.L.; Zhang, Y. CuO/PVDF nanocomposite anode for a piezo-driven self-charging lithium battery. Energy Environ. Sci. 2013, 6, 2615–2620. [Google Scholar] [CrossRef]
- Xue, X.Y.; Deng, P.; He, B.; Nie, Y.X.; Xing, L.L.; Zhang, Y.; Wang, Z.L. Flexible Self-Charging Power Cell for One-Step Energy Conversion and Storage. Adv. Energy Mater. 2014, 4, 1301329. [Google Scholar] [CrossRef]
- Pu, X.; Hu, W.G.; Wang, Z.L. Toward Wearable Self-Charging Power Systems: The Integration of Energy-Harvesting and Storage Devices. Small 2018, 14, 1702817. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.X.; Hu, S.H.; Xia, M.J.; Li, P.W.; Hu, J.; Li, G.; Jiang, H.B.; Zhang, W.D. A Flexible Lead-Free BaTiO3/PDMS/C Composite Nanogenerator as a Piezoelectric Energy Harvester. Energy Technol. 2018, 6, 922–927. [Google Scholar] [CrossRef]
- Lin, Z.H.; Yang, Y.; Wu, J.M.; Liu, Y.; Zhang, F.; Wang, Z.L. BaTiO3 Nanotubes-Based Flexible and Transparent Nanogenerators. J. Phys. Chem. Lett. 2012, 3, 3599–3604. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.L.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Lee, B.S.; Yoon, J.; Jung, C.; Kim, D.Y.; Jeon, S.Y.; Kim, K.H.; Park, J.H.; Park, H.; Lee, K.H.; Kang, Y.S.; et al. Silicon/Carbon Nanotube/BaTiO3 Nanocomposite Anode: Evidence for Enhanced Lithium-Ion Mobility Induced by the Local Piezoelectric Potential. Acs Nano 2016, 10, 2617–2627. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.L.; Li, J.C.; Xiao, X.C.; Lott, A.; Lu, W.Q.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Yada, C.; Ohmori, A.; Ide, K.; Yamasaki, H.; Kato, T.; Saito, T.; Sagane, F.; Iriyama, Y. Dielectric Modification of 5V-Class Cathodes for High-Voltage All-Solid-State Lithium Batteries. Adv. Energy Mater. 2014, 4, 1301416. [Google Scholar] [CrossRef]
- Wen, L.; Wang, X.W.; Liu, G.Q.; Luo, H.Z.; Liang, J.; Dou, S.X. Novel surface coating strategies for better battery materials. Surf. Innov. 2018, 6, 13–18. [Google Scholar] [CrossRef]
- Teranishi, T.; Yoshikawa, Y.; Sakuma, R.; Hashimoto, H.; Hayashi, H.; Kishimoto, A.; Fujii, T. High-rate performance of ferroelectric BaTiO3-coated LiCoO2 for Li-ion batteries. Appl. Phys. Lett. 2014, 105, 143904. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Teranishi, T.; Hayashi, H.; Kishimoto, A. Loading effect of a barium titanate artificial interface on high voltage capabilities at high charge and discharge rates. Jpn. J. Appl. Phys. 2017, 56, 10pc01. [Google Scholar] [CrossRef]
- Teranishi, T.; Inohara, M.; Kano, J.; Hayashi, H.; Kishimoto, A.; Yoda, K.; Motobayashi, H.; Tasaki, Y. Synthesis of nano-crystalline LiNbO3-decorated LiCoO2 and resulting high-rate capabilities. Solid State Ion. 2018, 314, 57–60. [Google Scholar] [CrossRef]
- Takanashi, Y.; Orikasa, Y.; Mogi, M.; Oishi, M.; Murayama, H.; Sato, K.; Yamashige, H.; Takamatsu, D.; Fujimoto, T.; Tanida, H.; et al. Thickness estimation of interface films formed on Li1-xCoO2 electrodes by hard X-ray photoelectron spectroscopy. J. Power Sources 2011, 196, 10679–10685. [Google Scholar] [CrossRef]



| Electrode | Piezoelectric Materials | Electrolyte | Self-Charging Capacity | Self-Charging Performance | Ref. |
|---|---|---|---|---|---|
| LiCoO2 (coating) TiO2 nanotube (electrochemical anodizing) | PVDF | LiPF6/EC:DMC | 0.036 μAh | 327 to 395 mV in 240 s with 45 N at 2.3 Hz | [8] |
| MoSe2 nanosheets | NaNbO3/PVDF | PVDF-co-HFP/TEABF4 | 18.93 F/cm2 | Up to 708 mV in 100 s with 30 N | [57] |
| CNT | P(VDF-TrFE) | PMMA/PC/LiClO4 | 95 μF/cm2 | Up to 70 mV with 50 N at 10 Hz | [58] |
| FCC | PVDF | PVA/H2SO4 | 0.25 μAh | 0~100 mV in 40 s with 2000 s without any external forces at 4.5 Hz | [59] |
| LiCoO2/MWCNTs | PVDF-PZT | LiPF6/EC:DMC | ~0.010 μAh | 210 to 297.6 mV in 240 s with 10 N at 1.5 Hz | [19] |
| LiCoO2/graphite | PVDF-ZnO | LiPF6/EC:DMC | ~0.173 μAh | 160 to 300 mV in 240 s with 34 N at 1.8 Hz | [60] |
| NiCoOH-CuO@Cu RGO@Cu | Bio-piezoelectric separator | PVA-KOH | ~0.424 μAh | 130.1 to 281.3 mV in 80 s with finger imparting at 1.65 Hz | [61] |
| LiCoO2/graphite | PVDF/ZnO | LiPF6/EC:EMC | 3.04 μAh | 1335 at 1400 mV in 200 s with 282 mJ at 1 Hz | [9] |
| LiCoO2/graphite | PVDF | LiPF6/EC:DEC:DMC | 0.118 μAh | 105 to 220 mV in 300 s with 30 N at 1 Hz | [62] |
| LiCoO2 CuO | PVDF | LiPF6/EC: DMC | 0.0247 μAh | 90 mV increment in 240 s with 18 N at 1 Hz | [63] |
| LiCoO2 graphene | PVDF | LiPF6/EC: DMC | 0.266 μAh | 500 to 832 mV in 500 s with 34 N at 1 Hz | [64] |
| MnO2 nanowires | PVDF-ZnO | PVA/H3PO4 | / | 110 mV increment in 300 s under palm impact | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wen, L.; Gong, X.; Liang, J.; Hou, X.; Hou, F. Piezoelectric-Based Energy Conversion and Storage Materials. Batteries 2023, 9, 371. https://doi.org/10.3390/batteries9070371
Wang S, Wen L, Gong X, Liang J, Hou X, Hou F. Piezoelectric-Based Energy Conversion and Storage Materials. Batteries. 2023; 9(7):371. https://doi.org/10.3390/batteries9070371
Chicago/Turabian StyleWang, Sihui, Lei Wen, Xiaopeng Gong, Ji Liang, Xinggang Hou, and Feng Hou. 2023. "Piezoelectric-Based Energy Conversion and Storage Materials" Batteries 9, no. 7: 371. https://doi.org/10.3390/batteries9070371
APA StyleWang, S., Wen, L., Gong, X., Liang, J., Hou, X., & Hou, F. (2023). Piezoelectric-Based Energy Conversion and Storage Materials. Batteries, 9(7), 371. https://doi.org/10.3390/batteries9070371







