Photothermal-Conversion-Enhanced LiMn2O4 Pouch Cell Performance for Low-Temperature Resistance: A Theoretical Study
Abstract
1. Introduction
2. Model Description
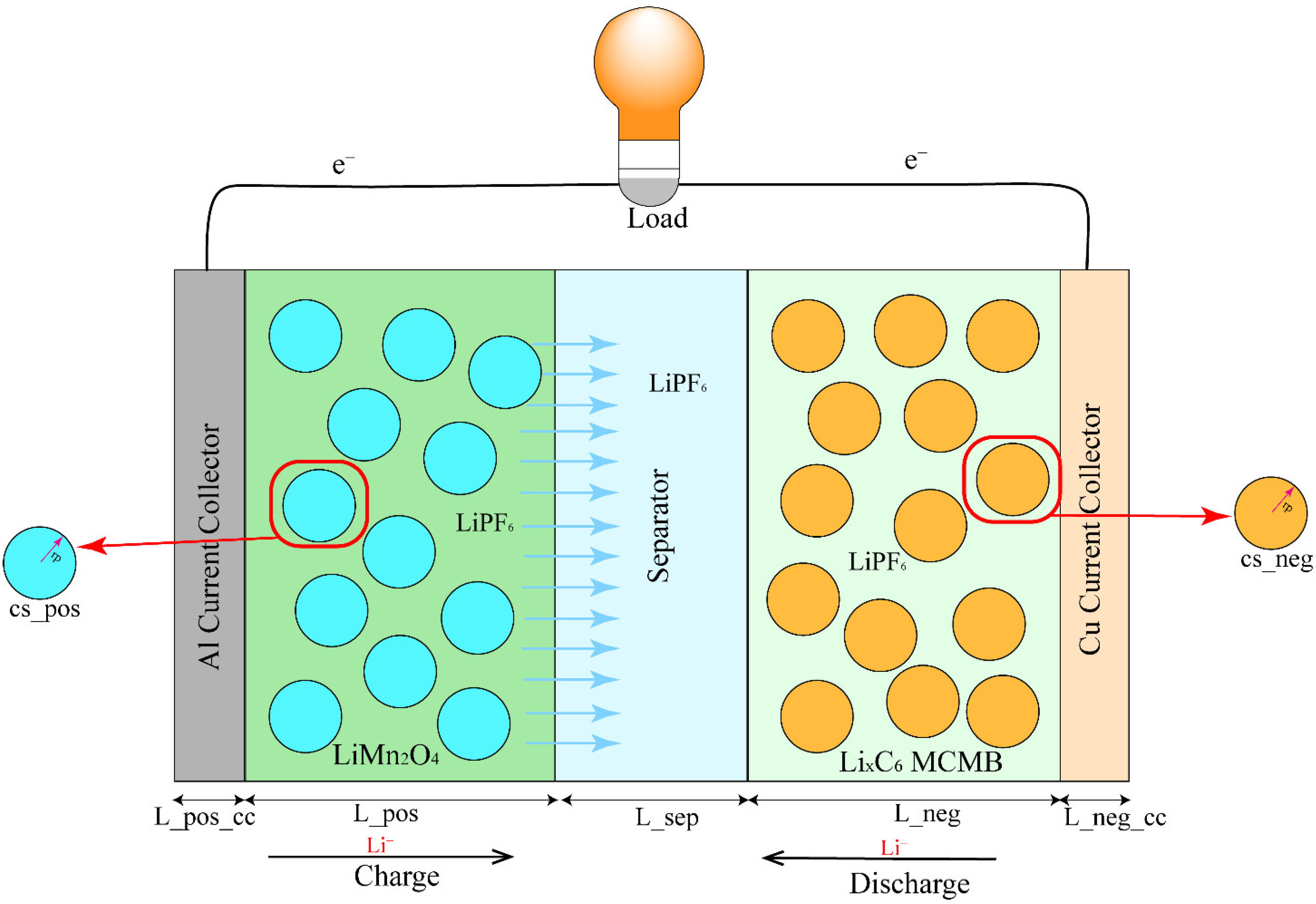
2.1. Electrochemical–Thermal Coupling Model
2.2. Mathematic Models
Anode: xLi+ + xe− + 6C ⇔ LixC6
2.2.1. Mass Conservation Equation
2.2.2. Charge Conservation Equation
2.2.3. Transfer Equation
2.2.4. Heat Transfer Equation
2.2.5. External Radiation Equation
3. Results and Discussion
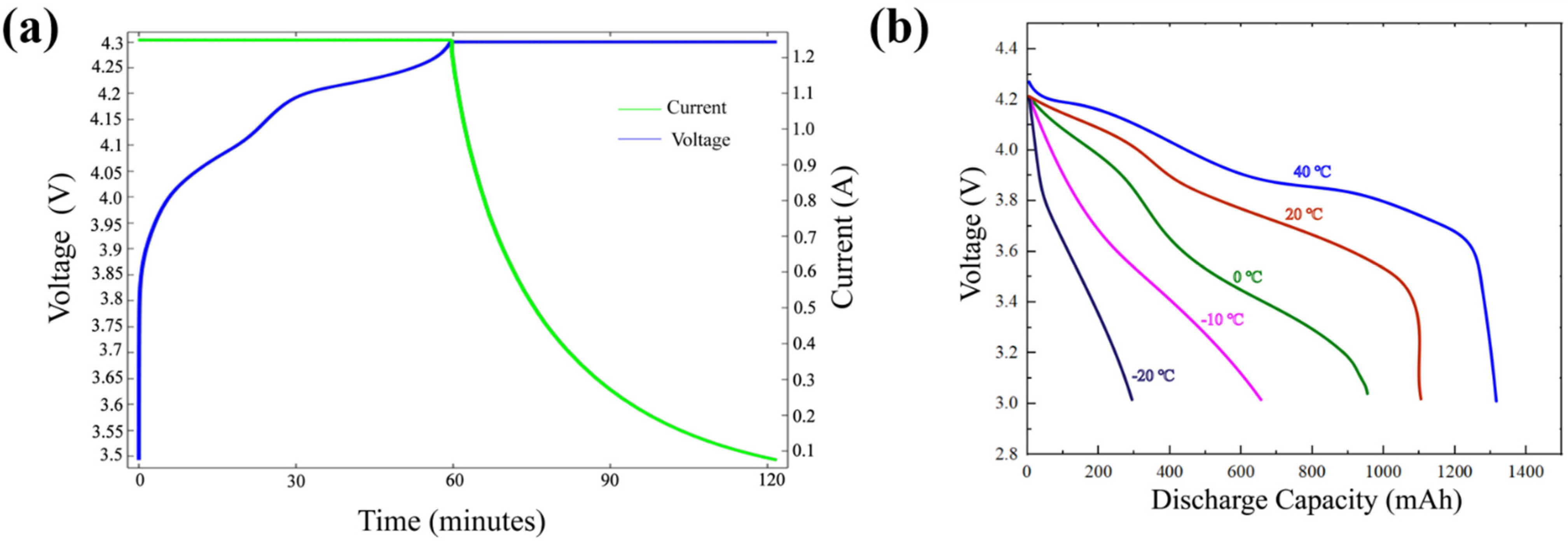
- The established battery model includes relevant electrochemical and thermal parameters, and comparisons of the battery charge and discharge process, to verify the accuracy of the model.
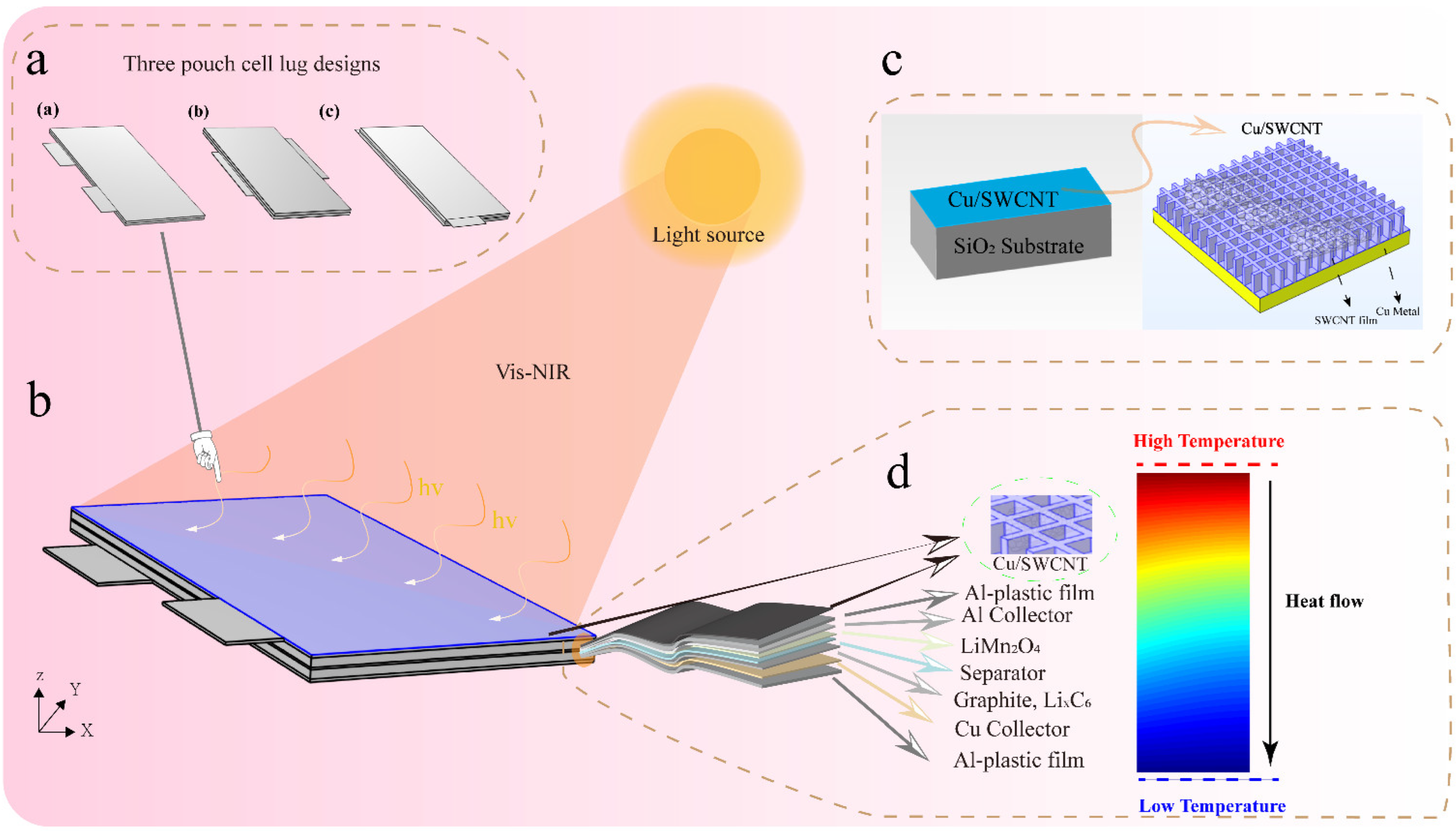
- After verifying the model’s accuracy, the battery temperature uniformity and charge/discharge characteristics improve the battery dissipation. Three lug designs are shown in Figure 1, to analyze the LIB temperature distribution and performance changes before and after illumination. The heat flux boundary conditions of the six external surfaces are established. The external natural convection heat dissipation is considered to simulate heat dissipation during the experiment, and the heat production is included for different areas of the battery.
- A radiation beam irradiates the pouch cell for preheating, with a radiation power of 10 W and 1 W, in two stages. The heat production and temperature distribution are analyzed, and the reversible heat and irreversible thermal changes of the battery under different levels of radiation intensity are analyzed.
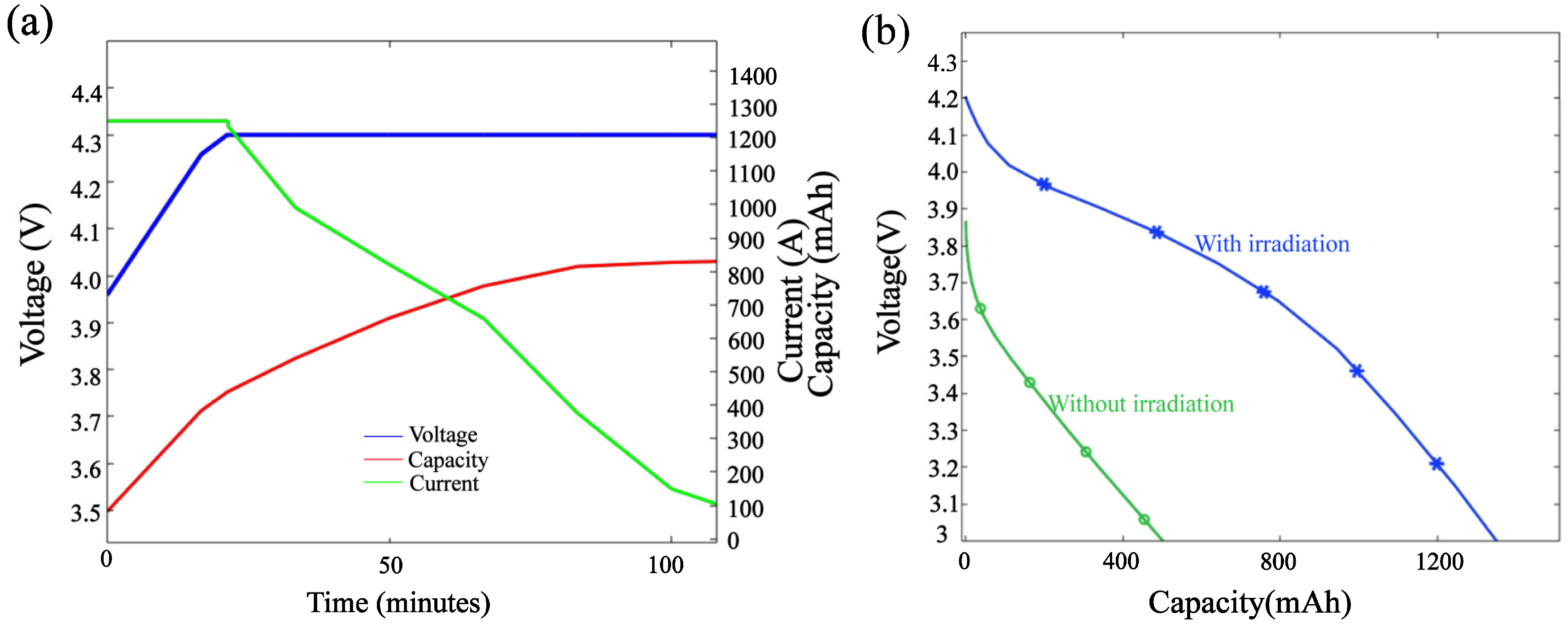
3.1. Model Validation
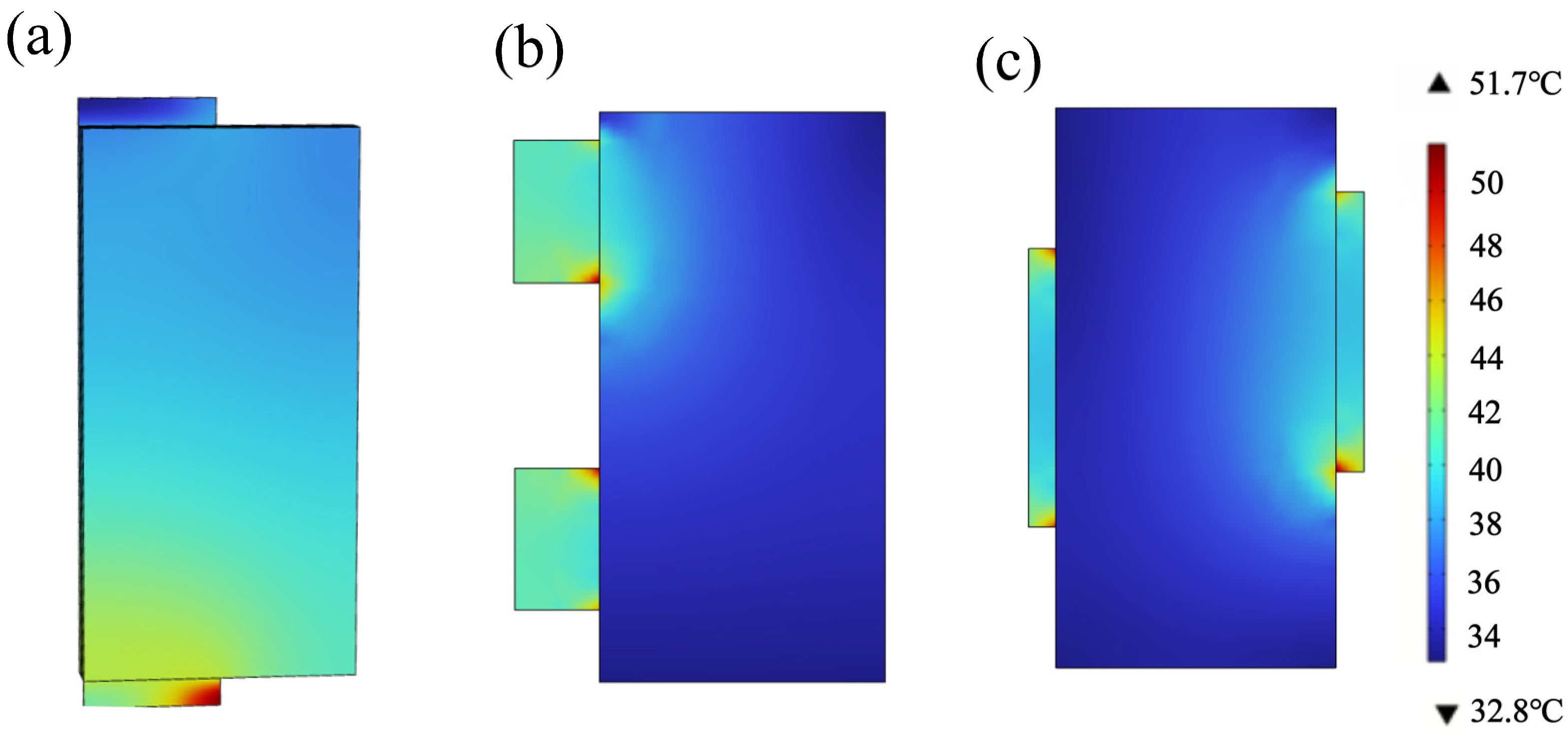
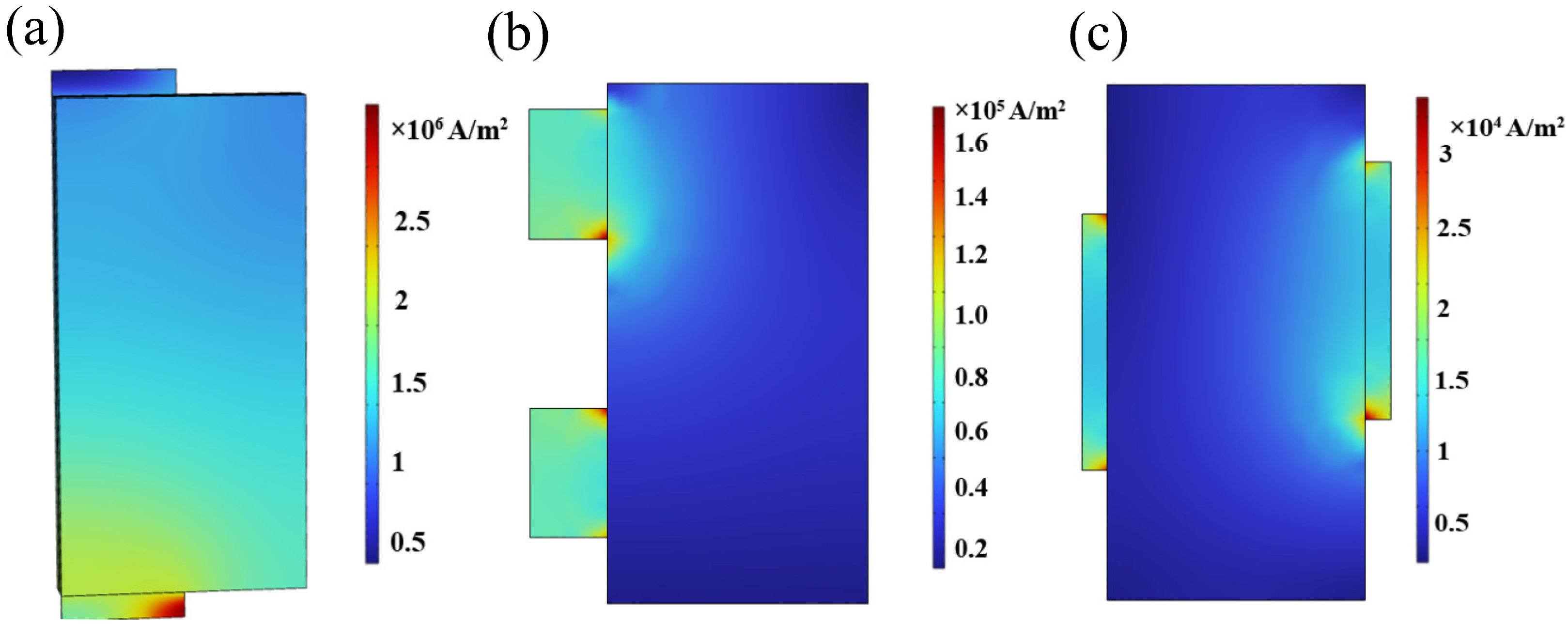
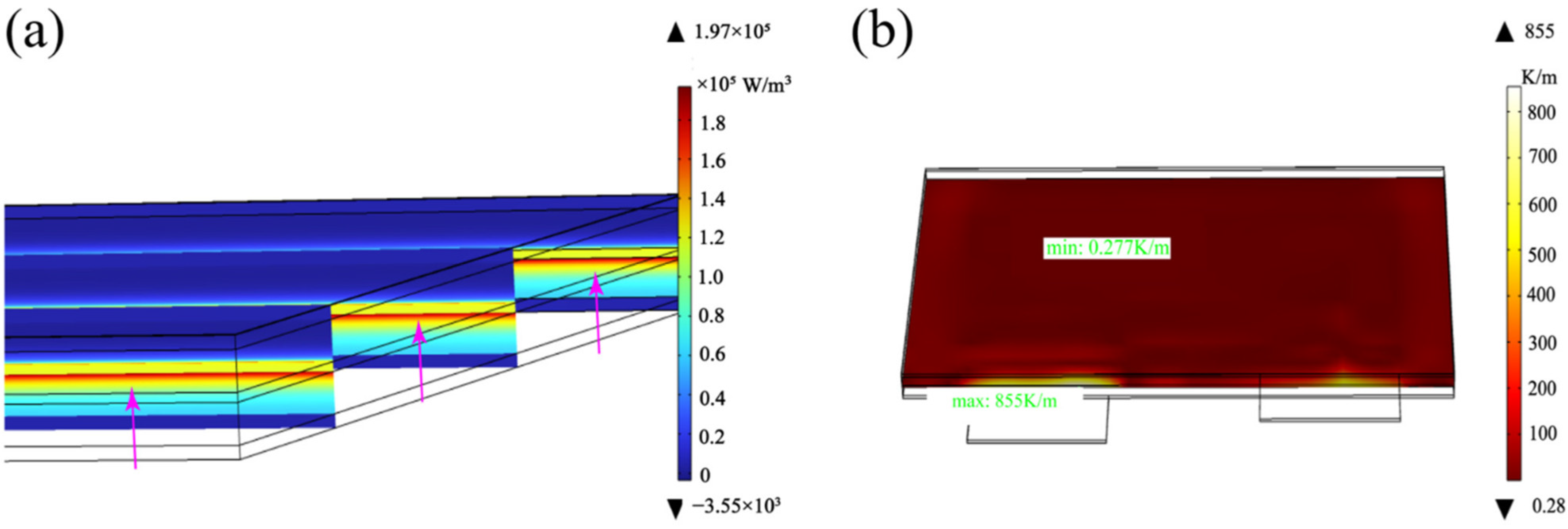
3.2. Lug Design Optimization and Analysis
3.2.1. Optimization of the Lug Design
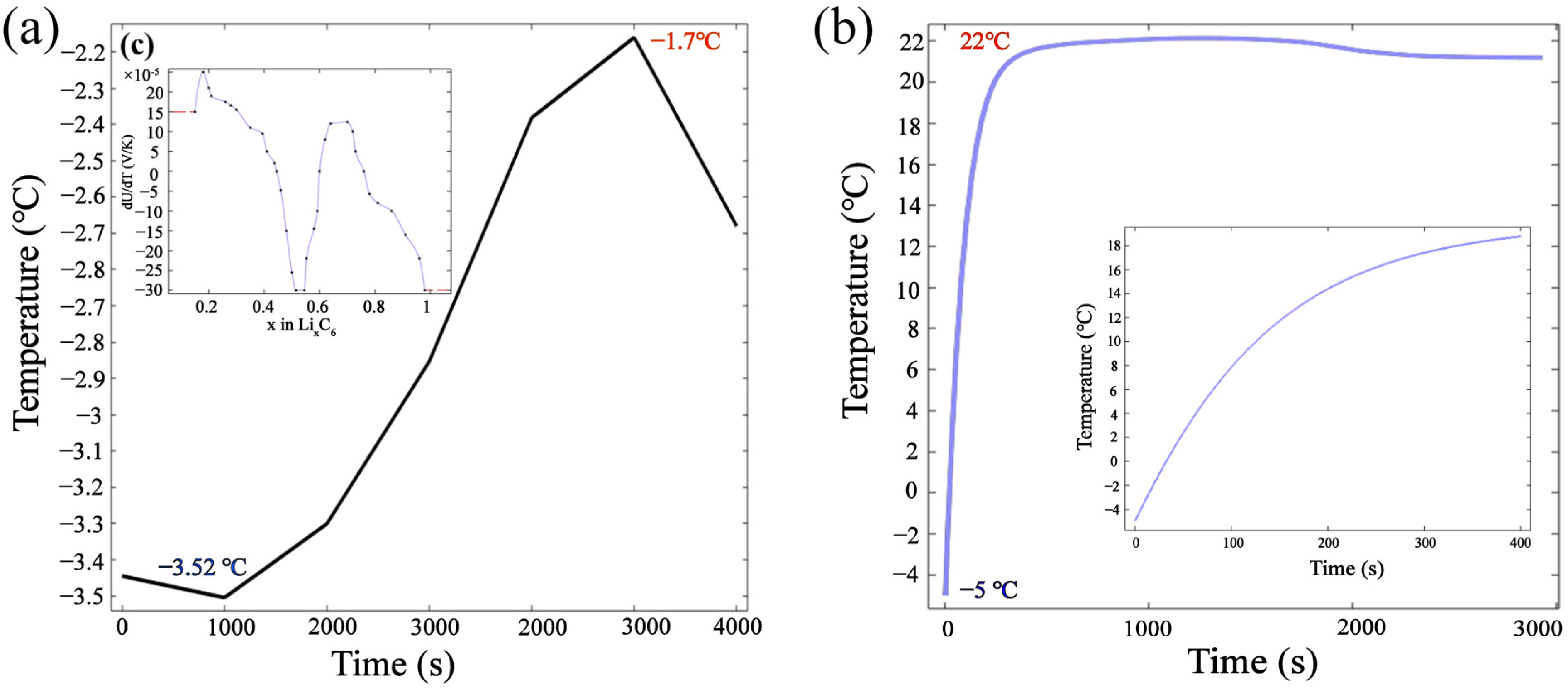
3.2.2. Optimal Lug Design Radiation Preheating Temperature Field
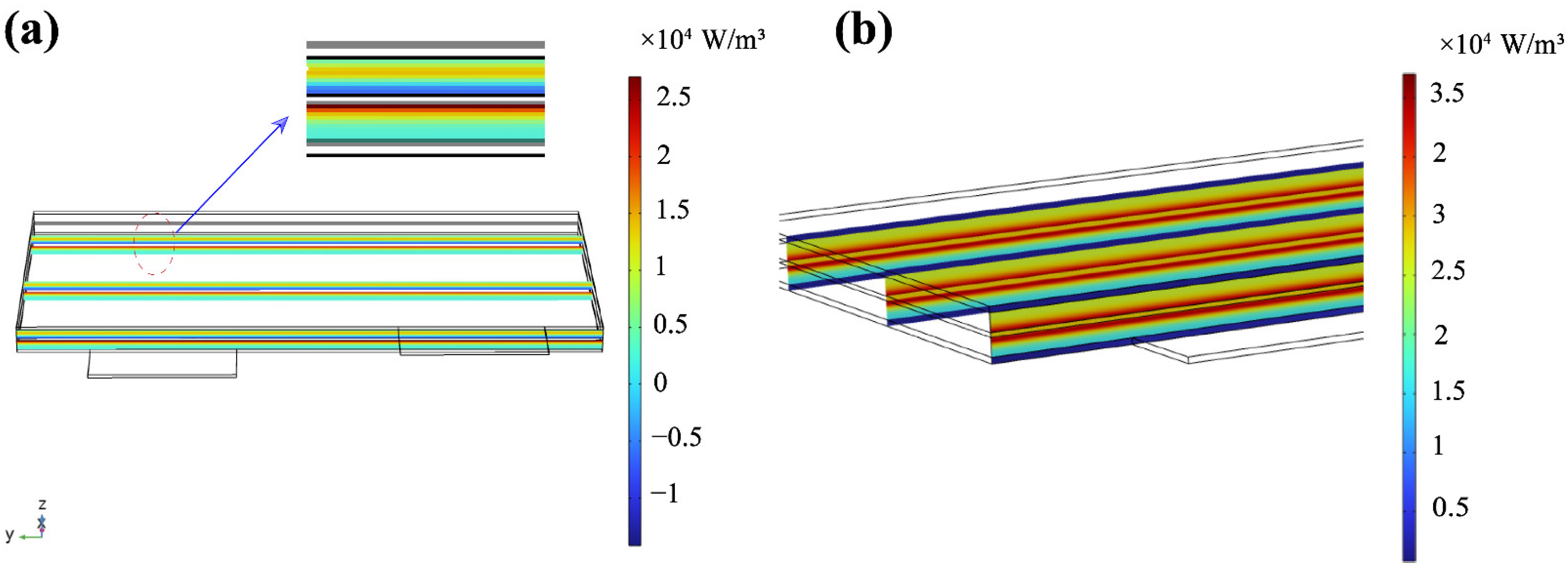
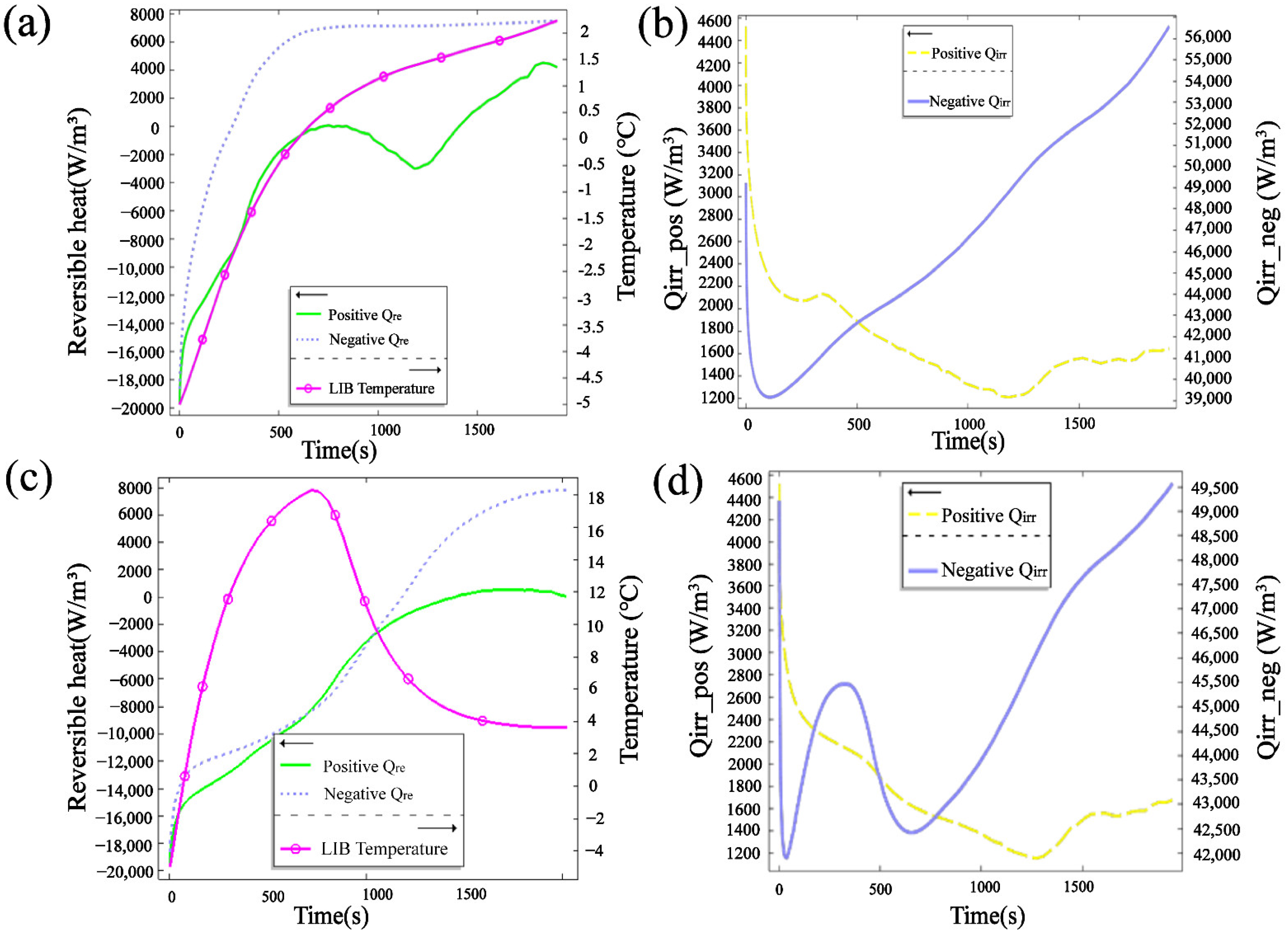
3.3. Reversible Heat Distribution
3.4. Analysis of Radiation Heating Heat Performance
3.4.1. Radiation Preheating Temperature Field Changes
3.4.2. Thermal Behavior under Radiation Preheating
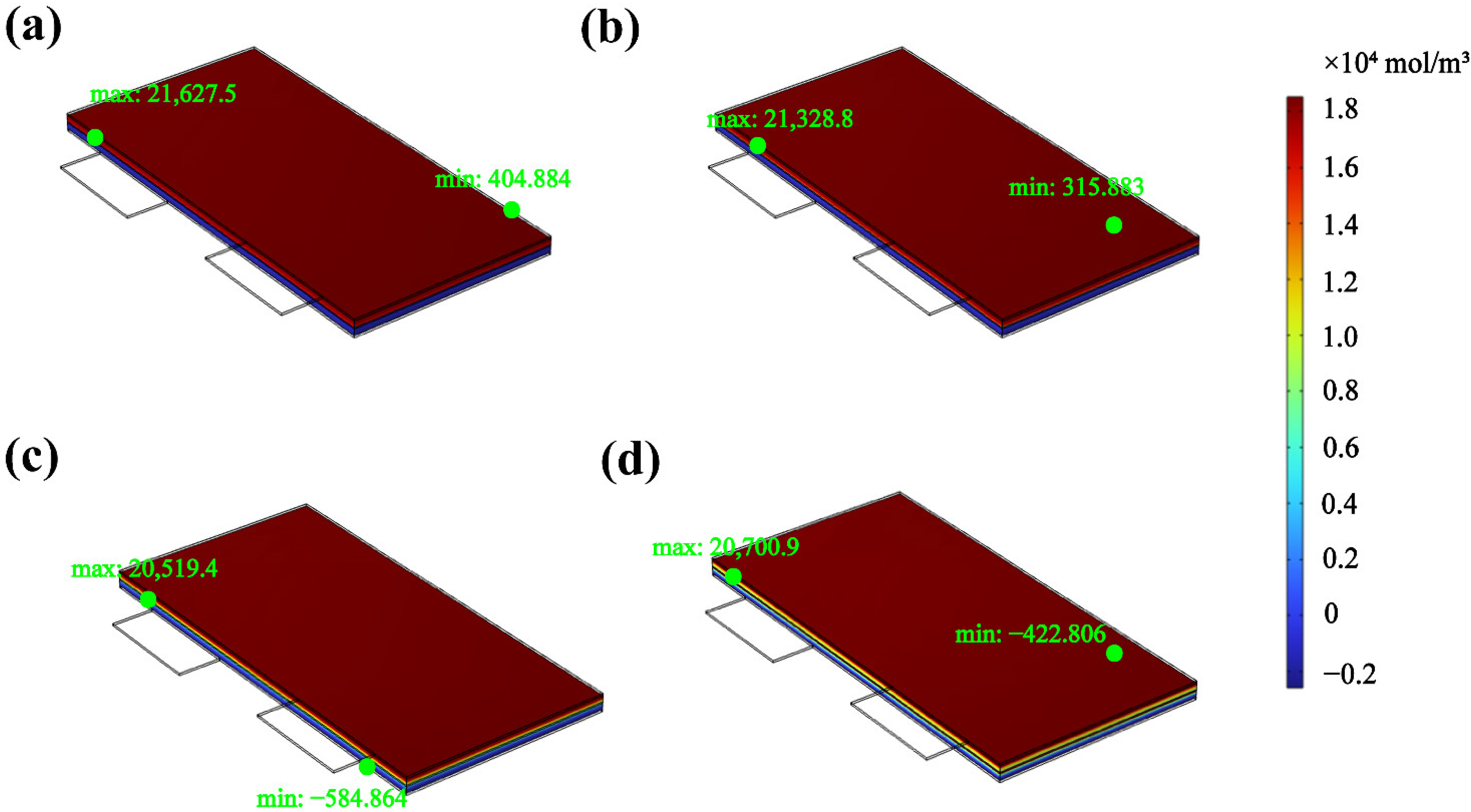
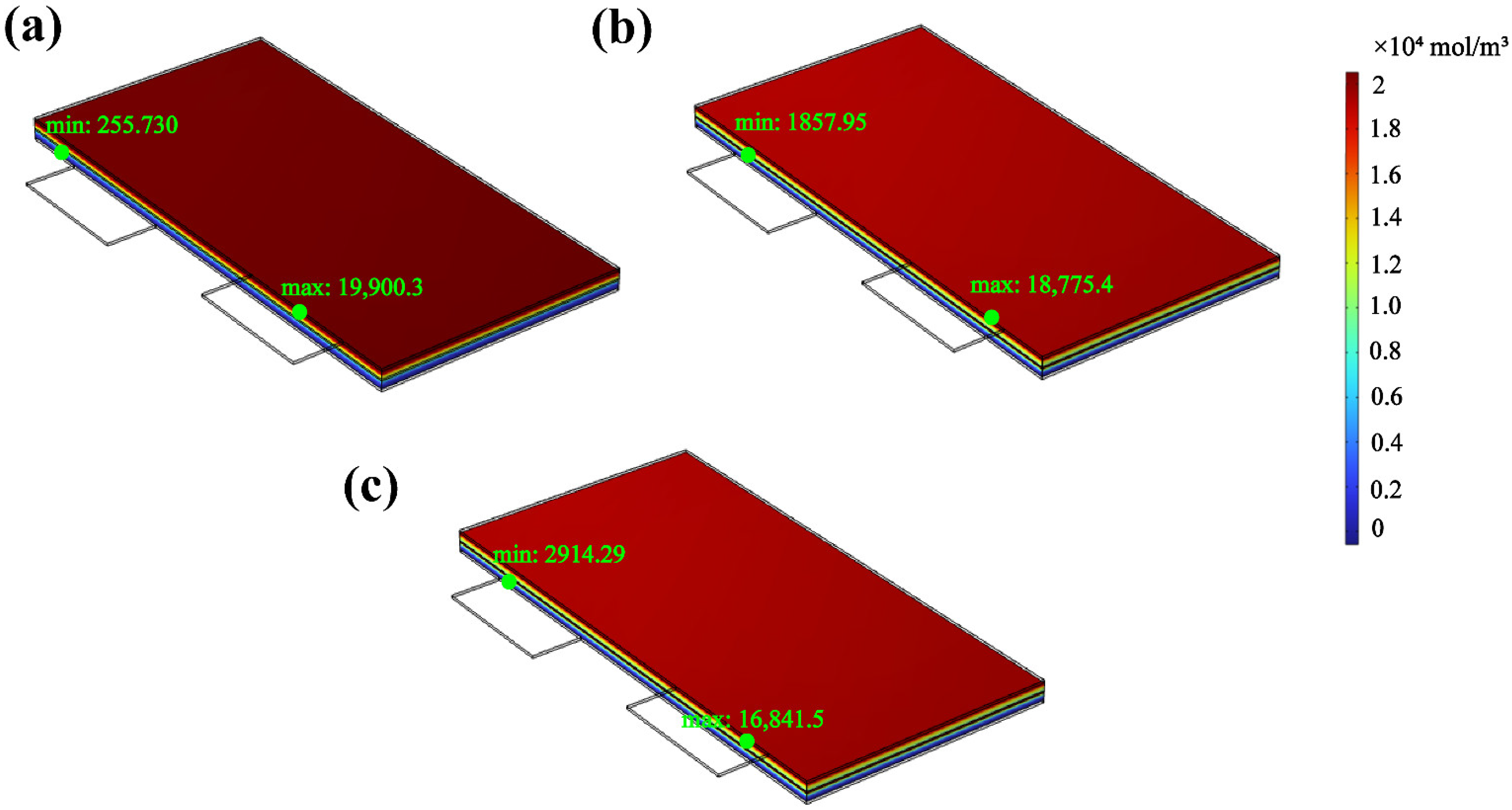
3.4.3. Change in the Lithium-Ion Flow

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, S.-C.; Xu, J.-H.; Fan, Y. Characteristics and key trends of global electric vehicle technology development: A multi-method patent analysis. J. Clean. Prod. 2022, 338, 130502. [Google Scholar] [CrossRef]
- Piao, N.; Gao, X.; Yang, H.; Guo, Z.; Hu, G.; Cheng, H.-M.; Li, F. Challenges and development of lithium-ion batteries for low temperature environments. eTransportation 2022, 11, 100145. [Google Scholar] [CrossRef]
- Zhang, G.; Ge, S.; Xu, T.; Yang, X.-G.; Tian, H.; Wang, C.-Y. Rapid self-heating and internal temperature sensing of lithium-ion batteries at low temperatures. Electrochim. Acta 2016, 218, 149–155. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, C.Y. Heating strategies for Li-ion batteries operated from subzero temperatures. Electrochim. Acta 2013, 107, 664–674. [Google Scholar] [CrossRef]
- Shang, Y.; Zhu, C.; Lu, G.; Zhang, Q.; Cui, N.; Zhang, C. Modeling and analysis of high-frequency alternating-current heating for lithium-ion batteries under low-temperature operations. J. Power Sources 2020, 450, 227435. [Google Scholar] [CrossRef]
- Wu, M.; Lin, X.; Wang, T.; Qiu, J.; Ma, T. Low-cost dye-sensitized solar cell based on nine kinds of carbon counter electrodes. Energy Environ. Sci. 2011, 4, 2308–2315. [Google Scholar] [CrossRef]
- Jakubinek, M.B.; Johnson, M.B.; White, M.A.; Guan, J.; Simard, B. Novel method to produce single-walled carbon nanotube films and their thermal and electrical properties. J. Nanosci. Nanotechnol. 2010, 10, 8151–8157. [Google Scholar] [CrossRef]
- Chen, K.; Gao, W.; Emaminejad, S.; Kiriya, D.; Ota, H.; Nyein, H.Y.; Takei, K.; Javey, A. Printed Carbon Nanotube Electronics and Sensor Systems. Adv. Mater. 2016, 28, 4397–4414. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Nieto de Castro, C.A. Superior thermal features of carbon nanotubes-based nanofluids—A review. Renew. Sustain. Energy Rev. 2014, 37, 155–167. [Google Scholar] [CrossRef]
- Zhang, S.; Pang, J.; Li, Y.; Yang, F.; Gemming, T.; Wang, K.; Wang, X.; Peng, S.; Liu, X.; Chang, B.; et al. Emerging Internet of Things driven carbon nanotubes-based devices. Nano Res. 2022, 15, 4613–4637. [Google Scholar] [CrossRef]
- Yavari, F.; Koratkar, N. Graphene-Based Chemical Sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, M.; Wang, Y.L.; Wang, Q.Y.; Lv, C.; Liu, K.X.; Suresh, S.; Yin, Y.H.; Hu, Y.Y.; Li, Y.S.; et al. Engineering the Surface/Interface of Horizontally Oriented Carbon Nanotube Macrofilm for Foldable Lithium-Ion Battery Withstanding Variable Weather. Adv. Energy Mater. 2018, 8, 1802349. [Google Scholar] [CrossRef]
- Yang, X.-S. Modelling heat transfer of carbon nanotubes. Model. Simul. Mater. Sci. Eng. 2005, 13, 893–902. [Google Scholar] [CrossRef]
- Jin, X.Z.; Li, H.; Wang, Y.; Yang, Z.Y.; Qi, X.D.; Yang, J.H.; Wang, Y. Ultraflexible PEDOT:PSS/Helical Carbon Nanotubes Film for All-in-One Photothermoelectric Conversion. ACS Appl. Mater. Interfaces 2022, 14, 27083–27095. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, J.; Jiang, Y.; Wang, L.; Yang, X.; Li, X.; Lü, W.; Sun, X. Boosting Low-Temperature Resistance of Energy Storage Devices by Photothermal Conversion Effects. ACS Appl. Mater. Interfaces 2022, 14, 23400–23407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Ngandjong, A.C.; Lombardo, T.; Primo, E.N.; Chouchane, M.; Shodiev, A.; Arcelus, O.; Franco, A.A. Investigating electrode calendering and its impact on electrochemical performance by means of a new discrete element method model: Towards a digital twin of Li-Ion battery manufacturing. J. Power Sources 2021, 485, 229320. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Shamlooei, M. Fe3O4–H2O nanofluid natural convection in presence of thermal radiation. Int. J. Hydrogen Energy 2017, 42, 5708–5718. [Google Scholar] [CrossRef]
- Dogonchi, A.S.; Nayak, M.K.; Karimi, N.; Chamkha, A.J.; Ganji, D.D. Numerical simulation of hydrothermal features of Cu–H2O nanofluid natural convection within a porous annulus considering diverse configurations of heater. J. Therm. Anal. Calorim. 2020, 141, 2109–2125. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ghasemi, A. Solidification heat transfer of nanofluid in existence of thermal radiation by means of FEM. Int. J. Heat Mass Transf. 2018, 123, 418–431. [Google Scholar] [CrossRef]
- Larruquert, J.I.; Rodriguez-de Marcos, L.V.; Mendez, J.A.; Martin, P.J.; Bendavid, A. High reflectance ta-C coatings in the extreme ultraviolet. Opt. Express 2013, 21, 27537–27549. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, Y.; Wu, N.; Zhao, S.; Jin, H.; Wang, G.; Li, X.; Liu, W.; Liu, J.; Zeng, Z. Bicontinuous, High-Strength, and Multifunctional Chemical-Cross-Linked MXene/Superaligned Carbon Nanotube Film. ACS Nano 2022, 16, 19293–19304. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Bahiraei, F.; Ghalkhani, M.; Fartaj, A.; Nazri, G.-A. A pseudo 3D electrochemical-thermal modeling and analysis of a lithium-ion battery for electric vehicle thermal management applications. Appl. Therm. Eng. 2017, 125, 904–918. [Google Scholar] [CrossRef]
- Yao, H.-R.; Yin, Y.-X.; Guo, Y.-G. Size effects in lithium ion batteries. Chin. Phys. B 2016, 25, 018203. [Google Scholar] [CrossRef]
- Goutam, S.; Nikolian, A.; Jaguemont, J.; Smekens, J.; Omar, N.; Van Dan Bossche, P.; Van Mierlo, J. Three-dimensional electro-thermal model of li-ion pouch cell: Analysis and comparison of cell design factors and model assumptions. Appl. Therm. Eng. 2017, 126, 796–808. [Google Scholar] [CrossRef]
- Yoon, Y.-H.; Song, J.-W.; Kim, D.; Kim, J.; Park, J.-K.; Oh, S.-K.; Han, C.-S. Transparent film heater using single-walled carbon nanotubes. Adv. Mater. 2007, 19, 4284–4287. [Google Scholar] [CrossRef]
- Gong, F.; Li, H.; Wang, W.; Huang, J.; Xia, D.; Liao, J.; Wu, M.; Papavassiliou, D.V. Scalable, eco-friendly and ultrafast solar steam generators based on one-step melamine-derived carbon sponges toward water purification. Nano Energy 2019, 58, 322–330. [Google Scholar] [CrossRef]
- Richter, F.; Vie, P.J.S.; Kjelstrup, S.; Burheim, O.S. Measurements of ageing and thermal conductivity in a secondary NMC-hard carbon Li-ion battery and the impact on internal temperature profiles. Electrochim. Acta 2017, 250, 228–237. [Google Scholar] [CrossRef]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A General Energy Balance for Battery Systems. J. Electrochem. Soc. 2019, 132, 5–12. [Google Scholar] [CrossRef]
- Gerke, R.H. Temperature coefficient of electromotive force of galvanic cells and the entropy of reactions. J. Electrochem. Soc. 1922, 44, 1684–1704. [Google Scholar] [CrossRef]
- Rao, L.; Newman, J. Heat-Generation Rate and General Energy Balance for Insertion Battery Systems. J. Electrochem. Soc. 1997, 144, 2697–2704. [Google Scholar] [CrossRef]
- Gunnarshaug, A.F.; Vie, P.J.S.; Kjelstrup, S. Review—Reversible Heat Effects in Cells Relevant for Lithium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 050522. [Google Scholar] [CrossRef]
- Son, K.; Hwang, S.M.; Woo, S.-G.; Paik, M.; Song, E.H.; Kim, Y.-J. Thermal and chemical characterization of the solid-electrolyte interphase in Li-ion batteries using a novel separator sampling method. J. Power Sources 2019, 440, 227083. [Google Scholar] [CrossRef]
- Lee, A.; Voros, M.; Dose, W.M.; Niklas, J.; Poluektov, O.; Schaller, R.D.; Iddir, H.; Maroni, V.A.; Lee, E.; Ingram, B.; et al. Photo-accelerated fast charging of lithium-ion batteries. Nat. Commun. 2019, 10, 4946. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Liu, Z.; Yang, Q.; Xu, N.; Li, X.; Wang, D.; Liu, R.; Lü, W. Photothermal-Conversion-Enhanced LiMn2O4 Pouch Cell Performance for Low-Temperature Resistance: A Theoretical Study. Batteries 2023, 9, 366. https://doi.org/10.3390/batteries9070366
Tian S, Liu Z, Yang Q, Xu N, Li X, Wang D, Liu R, Lü W. Photothermal-Conversion-Enhanced LiMn2O4 Pouch Cell Performance for Low-Temperature Resistance: A Theoretical Study. Batteries. 2023; 9(7):366. https://doi.org/10.3390/batteries9070366
Chicago/Turabian StyleTian, Shuo, Zhifeng Liu, Qiang Yang, Na Xu, Xiang Li, Dejun Wang, Runru Liu, and Wei Lü. 2023. "Photothermal-Conversion-Enhanced LiMn2O4 Pouch Cell Performance for Low-Temperature Resistance: A Theoretical Study" Batteries 9, no. 7: 366. https://doi.org/10.3390/batteries9070366
APA StyleTian, S., Liu, Z., Yang, Q., Xu, N., Li, X., Wang, D., Liu, R., & Lü, W. (2023). Photothermal-Conversion-Enhanced LiMn2O4 Pouch Cell Performance for Low-Temperature Resistance: A Theoretical Study. Batteries, 9(7), 366. https://doi.org/10.3390/batteries9070366






