MOF–Derived N–Doped C @ CoO/MoC Heterojunction Composite for Efficient Oxygen Reduction Reaction and Long-Life Zn–Air Battery
Abstract
1. Introduction
2. Experimental Section
2.1. Material Preparation
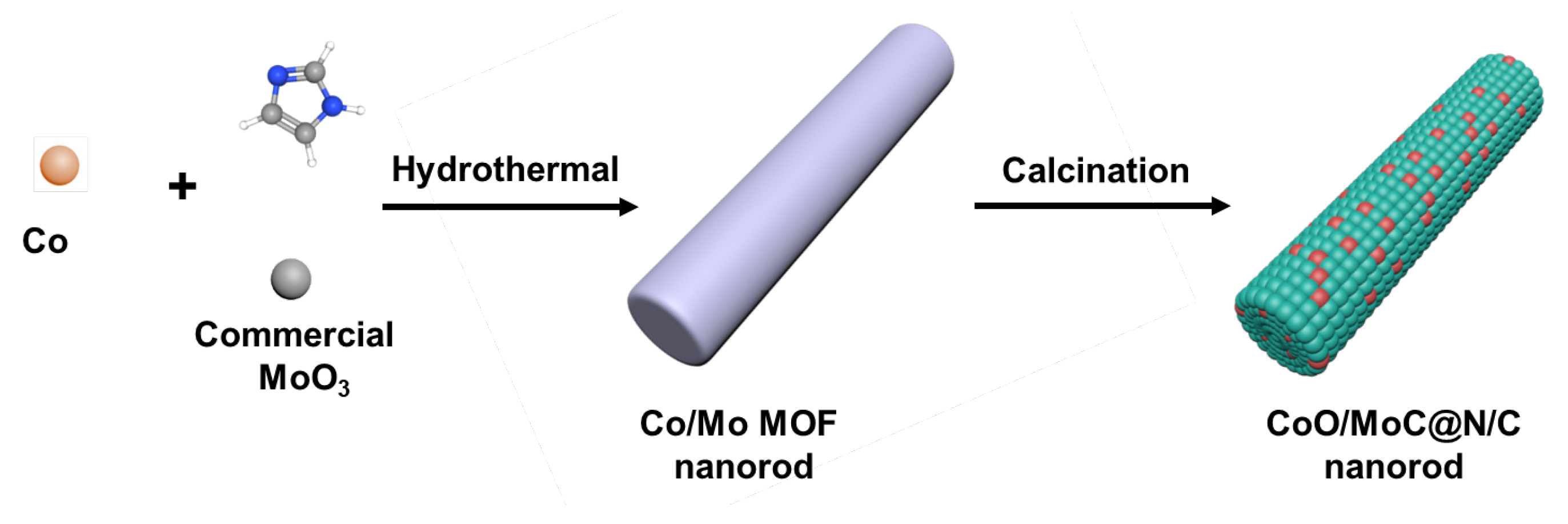
2.1.1. Synthesis of Co/Mo MOF
2.1.2. Synthesis of N–Doped Porous C @ CoO/MoC (NCCM) Heterostructure Composite
2.1.3. Synthesis of Co MOF–Derived CoO
2.1.4. Synthesis of Mo MOF–Derived MoC
2.2. Material Characterization
2.3. Electrochemical Measurement
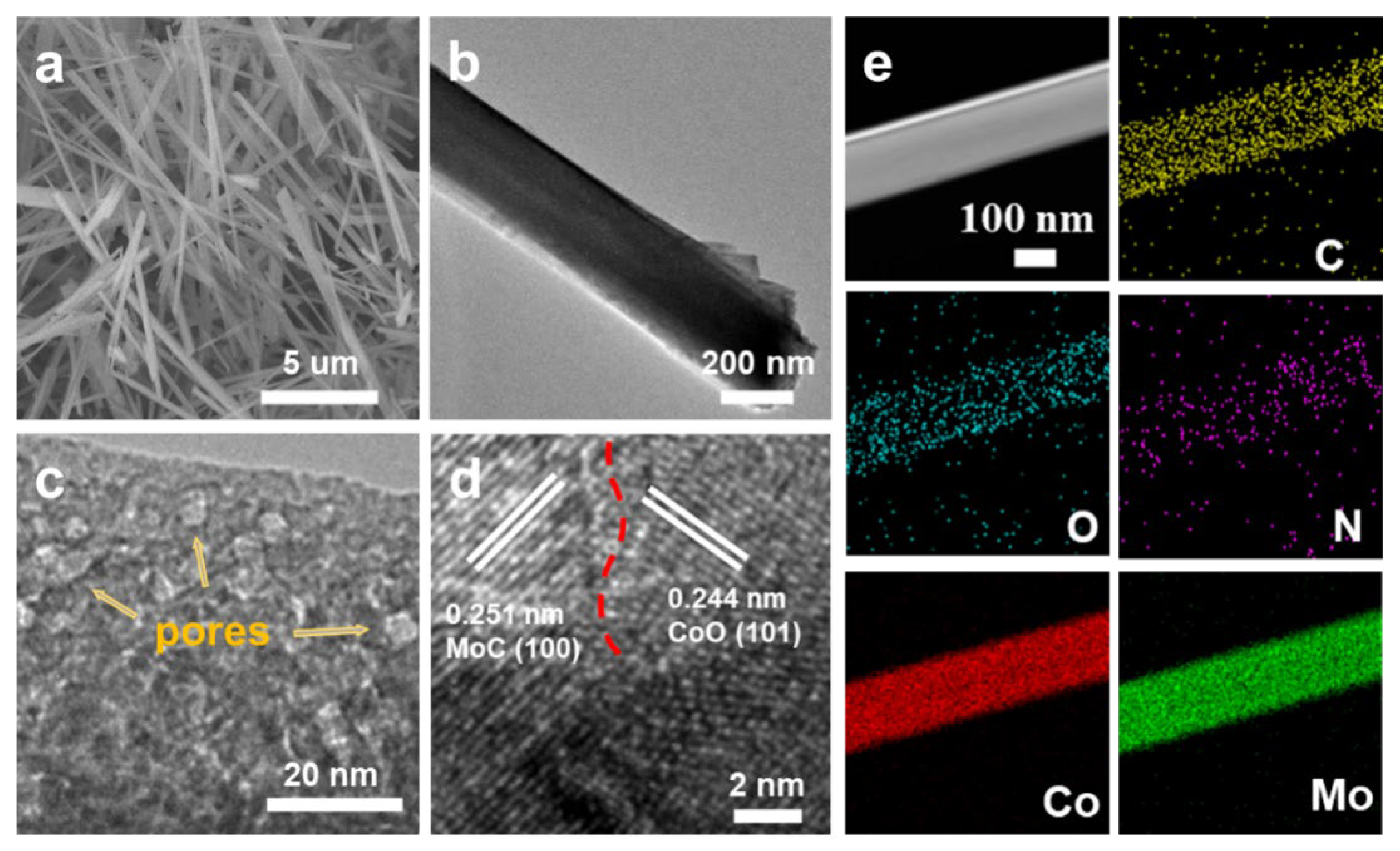
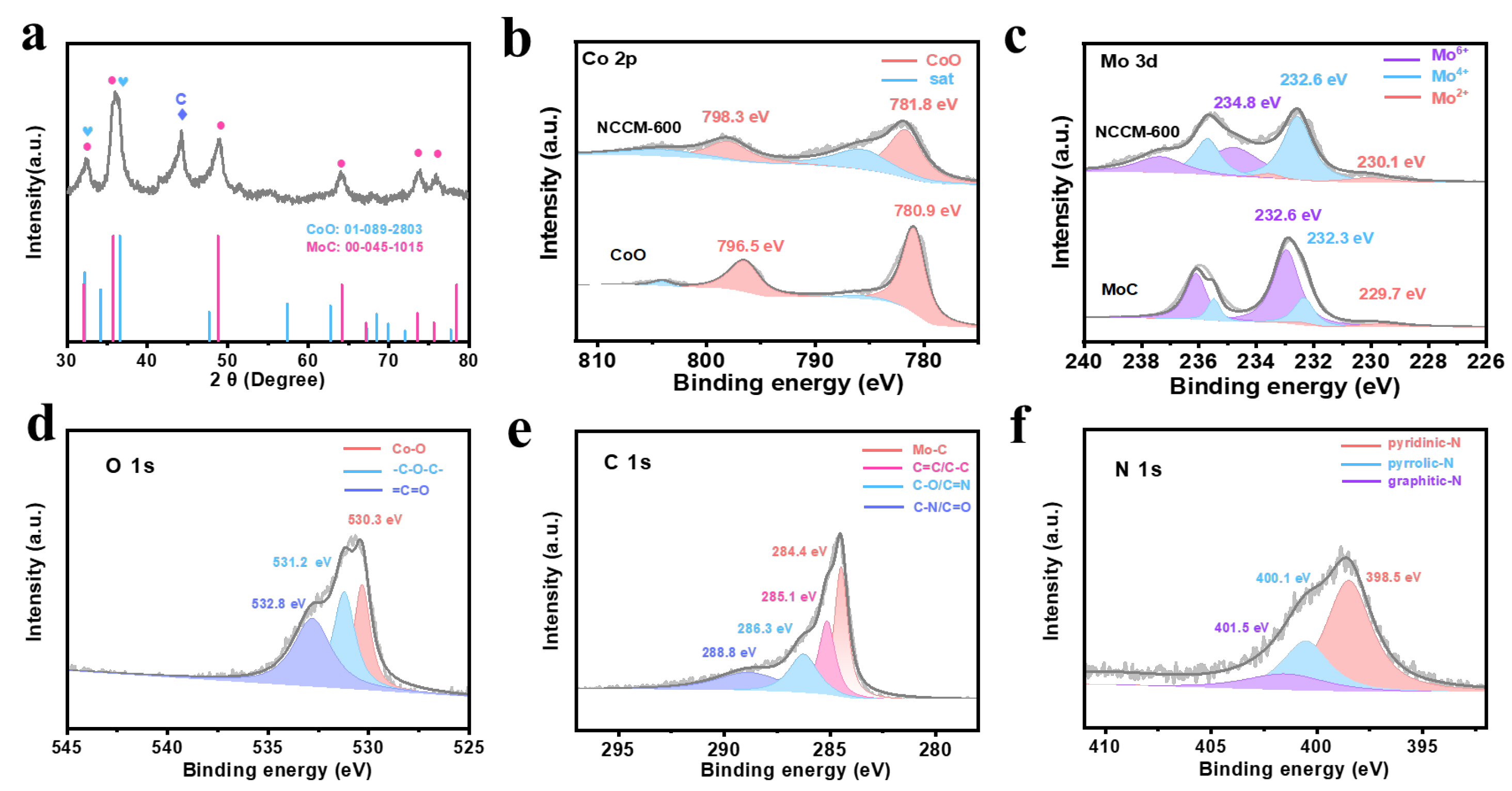
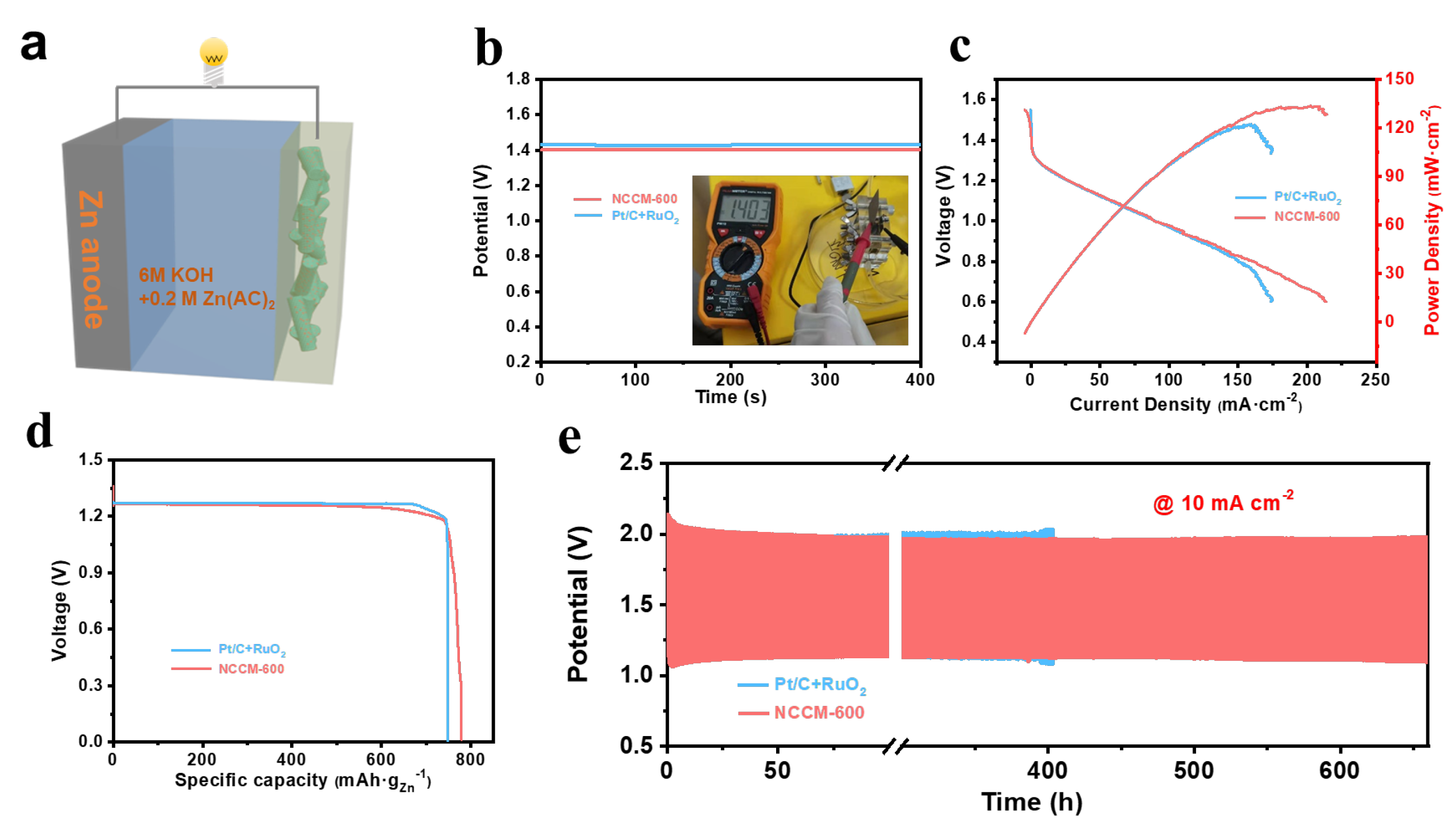
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ling, Y.Y.; Ma, Q.L.; Yu, Y.F.; Zhang, B. Optimization Strategies for Selective CO2 Electroreduction to Fuels. Trans. Tianjin Univ. 2021, 27, 180–200. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced architectures and relatives of air electrodes in Zn–air batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef]
- O’donnell, L.F.; Greenbaum, S.G. Review of Multivalent Metal Ion Transport in Inorganic and Solid Polymer Electrolytes. Batteries 2021, 7, 3. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Liu, Q.; Yu, P.; Luo, J.; Hu, G.; Liu, X. CoSe2 nanocrystals embedded into carbon framework as efficient bifunctional catalyst for alkaline seawater splitting. Inorg. Chem. Commun. 2022, 146, 110170. [Google Scholar] [CrossRef]
- Liu, W.; Feng, J.; Wei, T.; Liu, Q.; Zhang, S.; Luo, Y.; Luo, J.; Liu, X. Active-site and interface engineering of cathode materials for aqueous Zn-gas batteries. Nano Res. 2022, 16, 2325–2346. [Google Scholar] [CrossRef]
- Gao, S.; Wei, T.; Sun, J.; Liu, Q.; Ma, D.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. Atomically Dispersed Metal-Based Catalysts for Zn–CO2 Batteries. Small Struct. 2022, 3, 2200086. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Chu, P.K.; Liu, Q.; Liu, X.; Zhang, S.; Luo, J.; Wang, X.; Hu, G. Recent advances in non-noble metal-based bifunctional electrocatalysts for overall seawater splitting. J. Alloys Compd. 2022, 922, 166113. [Google Scholar] [CrossRef]
- Ding, J.; Yang, H.; Zhang, S.; Liu, Q.; Cao, H.; Luo, J.; Liu, X. Advances in the electrocatalytic hydrogen evolution reaction by metal nanoclusters-based materials. Small 2022, 18, 2204524. [Google Scholar] [CrossRef]
- Lee, D.; Kim, H.-W.; Kim, J.-M.; Kim, K.-H.; Lee, S.-Y. Flexible/rechargeable Zn–air batteries based on multifunctional heteronanomat architecture. ACS Appl. Mater. Interfaces 2018, 10, 22210–22217. [Google Scholar] [CrossRef]
- Chen, X.; Pu, J.; Hu, X.; An, L.; Jiang, J.; Li, Y. Confinement synthesis of bimetallic MOF-derived defect-rich nanofiber electrocatalysts for rechargeable Zn-air battery. Nano Res. 2022, 15, 9000–9009. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Q.; Guan, C.; Cheng, C. Recent advances on self-supported arrayed bifunctional oxygen electrocatalysts for flexible solid-state Zn–air batteries. Small 2020, 16, 2002902. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Jin, T.; Meng, J.; Jiao, L.; Zhu, M.; Chen, J. Electrospun thin-walled CuCo2O4@ C nanotubes as bifunctional oxygen electrocatalysts for rechargeable Zn–air batteries. Nano Lett. 2017, 17, 7989–7994. [Google Scholar] [CrossRef]
- Liu, W.; Que, W.; Yin, R.; Dai, J.; Zheng, D.; Feng, J.; Xu, X.; Wu, F.; Shi, W.; Liu, X.; et al. Ferrum-molybdenum dual incorporated cobalt oxides as efficient bifunctional anti-corrosion electrocatalyst for seawater splitting. Appl. Catal. B Environ. 2023, 328, 122488. [Google Scholar] [CrossRef]
- Ying, J.-P.; Zheng, D.; Meng, S.-B.; Yin, R.-L.; Dai, X.-J.; Feng, J.-X.; Wu, F.-F.; Shi, W.-H.; Cao, X.-H. Advanced design strategies for multi-dimensional structured carbon materials for high-performance Zn-air batteries. N. Carbon Mater. 2022, 37, 641–657. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, L.; Li, M.; Yuan, D.; Liu, X.; Qian, J.; Dou, Y.; Qiu, J.; Zhang, S. Interface engineering of CoS/CoO@ N-doped graphene nanocomposite for high-performance rechargeable Zn–Air batteries. Nano-Micro Lett. 2021, 13, 3. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, T.; Qiu, Y.; Zhang, S.; Liu, Q.; Hu, G.; Luo, J.; Liu, X. Recent progress in metal phosphorous chalcogenides: Potential high-performance electrocatalysts. Small 2023, 19, 2207249. [Google Scholar] [CrossRef]
- Gu, Y.; Yan, G.; Lian, Y.; Qi, P.; Mu, Q.; Zhang, C.; Deng, Z.; Peng, Y. MnIII-enriched α-MnO2 nanowires as efficient bifunctional oxygen catalysts for rechargeable Zn-air batteries. Energy Storage Mater. 2019, 23, 252–260. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Lv, F.; Fan, Q.; Zhao, Y.-Q.; Zhang, Q.; Wang, W.; Cheng, F.; Xi, P.; Guo, S. NiO/CoN porous nanowires as efficient bifunctional catalysts for Zn–air batteries. ACS Nano 2017, 11, 2275–2283. [Google Scholar] [CrossRef]
- Liu, P.; Ran, J.; Xia, B.; Xi, S.; Gao, D.; Wang, J. Bifunctional oxygen electrocatalyst of mesoporous Ni/NiO nanosheets for flexible rechargeable Zn–Air batteries. Nano-Micro Lett. 2020, 12, 68. [Google Scholar] [CrossRef]
- Tan, J.; Thomas, T.; Liu, J.; Yang, L.; Pan, L.; Cao, R.; Shen, H.; Wang, J.; Liu, J.; Yang, M. Rapid microwave-assisted preparation of high-performance bifunctional Ni3Fe/Co-NC for rechargeable Zn-air battery. Chem. Eng. J. 2020, 395, 125151. [Google Scholar] [CrossRef]
- Hu, G.; Zou, G.; Qiao, M.; Wågberg, T.; Mamat, X.; Hu, X.; Wa, Y. Ni–Co bimetallic coordination effect for long lifetime rechargeable Zn–air battery. J. Energy Chem. 2020, 47, 146–154. [Google Scholar]
- Luo, J.; Liu, Q.; Zhang, S.; Wei, T.; Liu, W.; Liu, X. A dual-functional Bi-doped Co3O4 nanosheets array towards high efficiency 5-hydroxymethylfurfural oxidation and hydrogen production. Chem. Commun. 2023, 59, 442–445. [Google Scholar]
- Chen, G.; Xu, Y.; Huang, L.; Douka, A.I.; Xia, B.Y. Continuous nitrogen-doped carbon nanotube matrix for boosting oxygen electrocatalysis in rechargeable Zn-air batteries. J. Energy Chem. 2021, 55, 183–189. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, C.; Liu, B.; Liu, Z.; Bi, X.; Zhao, N.; Han, X.; Deng, Y.; Lu, J.; Hu, W. Atomic layer Co3O4 nanosheets: The key to knittable Zn–air batteries. Small 2018, 14, 1702987. [Google Scholar] [CrossRef]
- Chen, X.; Pu, J.; Hu, X.; Yao, Y.; Dou, Y.; Jiang, J.; Zhang, W. Hollow nanofiber with bifunctional oxygen electrocatalyst for rechargeable Zn–Air battery. Small 2022, 18, 2200578. [Google Scholar] [CrossRef]
- Xu, X.; Shi, W.; Liu, W.; Ye, S.; Yin, R.; Zhang, L.; Xu, L.; Chen, M.; Zhong, M.; Cao, X. Preparation of two-dimensional assembled Ni–Mn–C ternary composites for high-performance all-solid-state flexible supercapacitors. J. Mater. Chem. A 2018, 6, 24086–24091. [Google Scholar] [CrossRef]
- Yuan, R.; Bi, W.; Zhou, T.; Zhang, N.; Zhong, C.; Chu, W.; Yan, W.; Xu, Q.; Wu, C.; Xie, Y. Two-dimensional hierarchical Fe–N–C electrocatalyst for Zn-Air batteries with ultrahigh specific capacity. ACS Mater. Lett. 2019, 2, 35–41. [Google Scholar] [CrossRef]
- Meng, G.; Jin, M.; Wei, T.; Liu, Q.; Zhang, S.; Peng, X.; Luo, J. MoC nanocrystals confined in N-doped carbon nanosheets toward highly selective electrocatalytic nitric oxide reduction to ammonia. Nano Res. 2022, 15, 8890–8896. [Google Scholar] [CrossRef]
- Liu, W.; Feng, J.; Yin, R.; Ni, Y.; Zheng, D.; Que, W.; Niu, X.; Dai, X.; Shi, W.; Wu, F.; et al. Tailoring oxygenated groups of monolithic cobalt-nitrogen-carbon frameworks for highly efficient hydrogen peroxide production in acidic media. Chem. Eng. J. 2022, 430, 132990. [Google Scholar] [CrossRef]
- Liu, Y.T.; Chen, X.; Yu, J.; Ding, B. Carbon-nanoplated CoS@ TiO2 nanofibrous membrane: An interface-engineered heterojunction for high-efficiency electrocatalytic nitrogen reduction. Angew. Chem. Int. Ed. 2019, 131, 19079–19083. [Google Scholar] [CrossRef]
- Luo, M.; Sun, W.; Xu, B.; Pan, H.; Jiang, Y. Interface engineering of air electrocatalysts for rechargeable zinc–air batteries. Adv. Energy Mater. 2021, 11, 2002762. [Google Scholar] [CrossRef]
- Zong, L.; Wu, W.; Liu, S.; Yin, H.; Chen, Y.; Liu, C.; Fan, K.; Zhao, X.; Chen, X.; Wang, F.; et al. Metal-free, active nitrogen-enriched, efficient bifunctional oxygen electrocatalyst for ultrastable zinc-air batteries. Energy Storage Mater. 2020, 27, 514–521. [Google Scholar] [CrossRef]
- Xu, W.; Yoon, D.; Yang, Y.; Xiong, Y.; Li, H.; Zeng, R.; Muller, D.A.; Abruña, H.D. MOF-derived bimetallic Pd–Co alkaline ORR electrocatalysts. ACS Appl. Mater. Interfaces 2022, 14, 44735–44744. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jiang, W.-J.; Niu, S.; Zhang, X.; Zhang, Y.; Yuan, L.-P.; He, C.; Hu, J.-S. Self-catalyzed growth of Co–N–C nanobrushes for efficient rechargeable Zn–Air Batteries. Small 2020, 16, 2001171. [Google Scholar] [CrossRef]
- Cai, X.; Lai, L.; Lin, J.; Shen, Z. Recent advances in air electrodes for Zn–air batteries: Electrocatalysis and structural design. Mater. Horiz. 2017, 4, 945–976. [Google Scholar] [CrossRef]
- Wang, H.F.; Tang, C.; Wang, B.; Li, B.-Q.; Zhang, Q. Bifunctional transition metal hydroxysulfides: Room-temperature sulfurization and their applications in Zn–air batteries. Adv. Mater. 2017, 29, 1702327. [Google Scholar] [CrossRef]
- Liu, W.; Yin, R.; Xu, X.; Zhang, L.; Shi, W.; Cao, X. Structural engineering of low-dimensional metal–organic frameworks: Synthesis, properties, and applications. Adv. Sci. 2019, 6, 1802373. [Google Scholar] [CrossRef]
- Yang, X.G.; Sun, X.; Gan, L.; Sun, L.; Mi, H.; Zhang, P.; Ren, X.; Li, Y. A CoOx/FeOx heterojunction on carbon nanotubes prepared by plasma-enhanced atomic layer deposition for the highly efficient electrocatalysis of oxygen evolution reactions. J. Mater. Chem. A 2020, 8, 15140–15147. [Google Scholar] [CrossRef]
- Huang, C.; Miao, X.; Pi, C.; Gao, B.; Zhang, X.; Qin, P.; Huo, K.; Peng, X.; Chu, P.K. Mo2C/VC heterojunction embedded in graphitic carbon network: An advanced electrocatalyst for hydrogen evolution. Nano Energy 2019, 60, 520–526. [Google Scholar] [CrossRef]
- Liu, W.; Yu, L.; Yin, R.; Xu, X.; Feng, J.; Jiang, X.; Zheng, D.; Gao, X.; Gao, X.; Que, W.; et al. Non-3d metal modulation of a 2D Ni–Co heterostructure array as multifunctional electrocatalyst for portable overall water splitting. Small 2020, 16, 1906775. [Google Scholar] [CrossRef]
- Liu, W.; Yin, R.; Shi, W.; Xu, X.; Shen, X.; Yin, Q.; Xu, L.; Cao, X. Gram-scale preparation of 2D transition metal hydroxide/oxide assembled structures for oxygen evolution and Zn-air battery. ACS Appl. Energy Mater. 2018, 2, 579–586. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, D.; Zhang, L.; Yin, R.; Xu, X.; Shi, W.; Wu, F.; Cao, X.; Lu, X. Bioinspired interfacial engineering of a CoSe2 decorated carbon framework cathode towards temperature-tolerant and flexible Zn–air batteries. Nanoscale 2021, 13, 3019–3026. [Google Scholar] [CrossRef]
- Wu, F.-F.; Gao, X.; Xu, X.; Jiang, Y.; Gao, X.; Yin, R.; Shi, W.; Liu, W.; Lu, G.; Cao, X. MnO2 nanosheet-assembled hollow polyhedron grown on carbon cloth for flexible aqueous zinc-ion batteries. ChemSusChem 2020, 13, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tan, C.; Sindoro, M.; Zhang, H. Correction: Hybrid micro-/nano-structures derived from metal–organic frameworks: Preparation and applications in energy storage and conversion. Chem. Soc. Rev. 2018, 47, 5997. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Nan, L.; He, J.; Li, Y.; Zhang, L.; Zhang, Q.; Ren, X.; He, C.; Zheng, L.; Sun, X. Tuning and understanding the electronic effect of Co-Mo-O sites in bifunctional electrocatalysts for ultralong-lasting rechargeable Zinc-air batteries. J. Mater. Chem. A 2021, 9, 21716–21722. [Google Scholar] [CrossRef]
- Cao, X.; Tan, C.; Zhang, X.; Zhao, W.; Zhang, H. Solution-processed two-dimensional metal dichalcogenide-based nanomaterials for energy storage and conversion. Adv. Mater. 2016, 28, 6167–6196. [Google Scholar] [CrossRef]
- Lim, J.; Jung, J.-W.; Kim, N.-Y.; Lee, G.Y.; Lee, H.J.; Lee, Y.; Choi, D.S.; Yoon, K.R.; Kim, Y.-H.; Kim, I.-D.; et al. N2-dopant of graphene with electrochemically switchable bifunctional ORR/OER catalysis for Zn-air battery. Energy Storage Mater. 2020, 32, 517–524. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Pu, Z.; Liu, X.; Owusu, K.A.; Monestel, H.G.R.; Boakye, F.O.; Zhang, H.; Mu, S. Multifunctional Mo–N/C@ MoS2 electrocatalysts for HER, OER, ORR, and Zn–air batteries. Adv. Funct. Mater. 2017, 27, 1702300. [Google Scholar] [CrossRef]
- He, B.; Wang, J.; Fan, Y.; Jiang, Y.; Zhai, Y.; Wang, Y.; Huang, Q.; Dang, F.; Zhang, Z.; Wang, N. Mesoporous CoO/Co–N–C nanofibers as efficient cathode catalysts for Li–O2 batteries. J. Mater. Chem. A 2018, 6, 19075–19084. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Y.; Luo, J.; Jin, B.; Wu, Z.; Ning, X.; Zhan, L.; Fan, X.; Zhou, T.; Zhang, S.; et al. MoC Quantum Dots@ N-Doped-Carbon for Low-Cost and Efficient Hydrogen Evolution Reaction: From Electrocatalysis to Photocatalysis. Adv. Funct. Mater. 2022, 32, 2201518. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Nie, Z.; Li, Z.; Ye, Y.; Li, L.; Hong, J.; Bi, Z.; Zhou, Y.; Hu, G. Co/MoC nanoparticles embedded in carbon nanoboxes as robust trifunctional electrocatalysts for a Zn–air battery and water electrocatalysis. ACS Nano 2021, 15, 13399–13414. [Google Scholar] [CrossRef]
- Chou, S.C.; Tso, K.C.; Hsieh, Y.C.; Sun, B.Y.; Lee, J.F.; Wu, P.W. Facile synthesis of Co3O4@ CoO@ Co gradient core@ shell nanoparticles and their applications for oxygen evolution and reduction in alkaline electrolytes. Materials 2020, 13, 2703. [Google Scholar] [CrossRef]
- Jin, W.; Chen, J.; Liu, B.; Hu, J.; Wu, Z.; Cai, W.; Fu, G. Oxygen Vacancy–Rich In-Doped CoO/CoP Heterostructure as an Effective Air Cathode for Rechargeable Zn–Air Batteries. Small 2019, 15, 1904210. [Google Scholar] [CrossRef]
- Chen, S.; Chen, S.; Zhang, B.; Zhang, J. Bifunctional oxygen electrocatalysis of N, S-codoped porous carbon with interspersed hollow CoO nanoparticles for rechargeable Zn–air batteries. ACS Appl. Mater. Interfaces 2019, 11, 16720–16728. [Google Scholar] [CrossRef]
- Guo, C.; Zheng, Y.; Ran, J.; Xie, F.; Jaroniec, M.; Qiao, S.Z. Engineering high-energy interfacial structures for high-performance oxygen-involving electrocatalysis. Angew. Chem. Int. Ed. 2017, 56, 8539–8543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Han, C.; Cao, W.Q.; Cao, M.S.; Yang, H.J.; Yuan, J. A nano-micro engineering nanofiber for electromagnetic absorber, green shielding and sensor. Nano-Micro Lett. 2021, 13, 1–12. [Google Scholar] [CrossRef]
- Defo, C.; Mishra, A.K.; Yerima, B.P.K.; Mabou, P.B.; Ako, A.A.; Fonkou, T. Current conditions of groundwater resources development and related problems in the Republic of Cameroon, West Africa. Eur. Water 2016, 54, 43–68. [Google Scholar]
- Zhao, Y.; Lai, Q.; Zhu, J.; Zhong, J.; Tang, Z.; Luo, Y.; Liang, Y. Controllable construction of core–shell polymer@ zeolitic imidazolate frameworks fiber derived heteroatom-doped carbon nanofiber network for efficient oxygen electrocatalysis. Small 2018, 14, 1704207. [Google Scholar] [CrossRef] [PubMed]
- Tomon, C.; Sarawutanukul, S.; Duangdangchote, S.; Krittayavathananon, A.; Sawangphruk, M. Photoactive Zn–air batteries using spinel-type cobalt oxide as a bifunctional photocatalyst at the air cathode. Chem. Commun. 2019, 55, 5855–5858. [Google Scholar] [CrossRef] [PubMed]
- Sadighi, Z.; Liu, J.; Zhao, L.; Ciucci, F.; Kim, J.K. Metallic MoS 2 nanosheets: Multifunctional electrocatalyst for the ORR, OER and Li–O 2 batteries. Nanoscale 2018, 10, 22549–22559. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Artrith, N.; Lun, Z.; Jadidi, Z.; Kitchaev, D.A.; Ji, H.; Urban, A.; Ceder, G. Effect of Fluorination on Lithium Transport and Short-Range Order in Disordered-Rocksalt-Type Lithium-Ion Battery Cathodes. Adv. Energy Mater. 2020, 10, 1903240. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Mehdi, S.; Wu, X.; Liu, T.; Zhou, B.; Zhang, P.; Jiang, J.; Li, B. Surface Phosphorus-Induced CoO Coupling to Monolithic Carbon for Efficient Air Electrode of Quasi-Solid-State Zn-Air Batteries. Adv. Sci. 2021, 8, 2101314. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, R.; Ma, S.; Ying, J.; Lu, Z.; Niu, X.; Feng, J.; Xu, F.; Zheng, Y.; Liu, W.; Cao, X. MOF–Derived N–Doped C @ CoO/MoC Heterojunction Composite for Efficient Oxygen Reduction Reaction and Long-Life Zn–Air Battery. Batteries 2023, 9, 306. https://doi.org/10.3390/batteries9060306
Yin R, Ma S, Ying J, Lu Z, Niu X, Feng J, Xu F, Zheng Y, Liu W, Cao X. MOF–Derived N–Doped C @ CoO/MoC Heterojunction Composite for Efficient Oxygen Reduction Reaction and Long-Life Zn–Air Battery. Batteries. 2023; 9(6):306. https://doi.org/10.3390/batteries9060306
Chicago/Turabian StyleYin, Ruilian, Suli Ma, Jiaping Ying, Zhentao Lu, Xinxin Niu, Jinxiu Feng, Feng Xu, Yifan Zheng, Wenxian Liu, and Xiehong Cao. 2023. "MOF–Derived N–Doped C @ CoO/MoC Heterojunction Composite for Efficient Oxygen Reduction Reaction and Long-Life Zn–Air Battery" Batteries 9, no. 6: 306. https://doi.org/10.3390/batteries9060306
APA StyleYin, R., Ma, S., Ying, J., Lu, Z., Niu, X., Feng, J., Xu, F., Zheng, Y., Liu, W., & Cao, X. (2023). MOF–Derived N–Doped C @ CoO/MoC Heterojunction Composite for Efficient Oxygen Reduction Reaction and Long-Life Zn–Air Battery. Batteries, 9(6), 306. https://doi.org/10.3390/batteries9060306





