Abstract
Multivalent metal ion (Mg2+, Zn2+, Ca2+, and Al3+) batteries (MMIBs) emerged as promising technologies for large-scale energy storage systems in recent years due to the abundant metal reserves in the Earth’s crust and potentially low cost. However, the lack of high-performance electrode materials is still the main obstacle to the development of MMIBs. As a newly large family of two-dimensional transition metal carbides, nitrides, and carbonitrides, MXenes have attracted growing focus in the energy storage field because of their large specific surface area, excellent conductivity, tunable interlayer spaces, and compositional diversity. In particular, the multifunctional chemistry and superior hydrophilicity enable MXenes to serve not only as electrode materials but also as important functional components for heterojunction composite electrodes. Herein, the advances of MXene-based materials since its discovery for MMIBs are summarized, with an emphasis on the rational design and controllable synthesis of MXenes. More importantly, the fundamental understanding of the relationship between the morphology, structure, and function of MXenes is highlighted. Finally, the existing challenges and future research directions on MXene-based materials toward MMIBs application are critically discussed and prospected.
1. Introduction
Driven by the global carbon peaking and carbon neutrality goals, more and more attention has been focused on abundant and clean renewable energy sources, such as wind energy and solar energy [1,2]. However, these energy resources suffer from intermittent volatility; thus, they cannot meet the stability and reliability requirements of the rigid grid [3]. The lithium-ion battery is widely accepted as the suitable energy storage device to bridge renewable energy power generation to the power grid due to its high-energy density, long lifetime, and fast response capability, but it is plagued by increasing costs and safety issues [4]. In this regard, exploring alternative electrochemical energy storage technologies is particularly urgent. Magnesium, zinc, calcium, and aluminum metals are all Earth-abundant elements, and their redox reactions are always accompanied by a two or three electron transfer; hence, batteries based on these metal anodes in theory can offer much higher capacity than lithium-ion batteries [5,6]. Inspired by the above advantages, the research of multivalent metal ion (Mg2+, Zn2+, Ca2+, and Al3+) batteries (MMIBs) has flourished. However, the development of MMIBs is hindered by several critical issues. Firstly, the large radius of hydrated ions slows the solid-phase ion diffusion kinetics of carriers in the electrode material, resulting in severe attenuation of battery performance [7]. Secondly, the high electrostatic repulsion between the inserted cations and the host structure often leads to drastic structural deterioration and capacity decay [8]. Therefore, on the basis of good conductivity, small loss and easy modeling of general electrode materials, MMIB electrode materials also need to have a wide ion diffusion channel and sufficient structural stability to adapt to the enhanced electrostatic effect.
Two-dimensional (2D) materials have exotic electronic, chemical, mechanical, and optical properties that distinguish them from three-dimensional (3D) materials [9]. These properties are mainly derived from the thin layer structure of atoms, with strong intralayer bonds and weak interlaminar bonds. Their relatively large layer spacings allow for rapid ionic (de)intercalating, while the highly anisotropic 2D structure imparts the ability to transfer charges [10]. At the same time, two-dimensional (2D) materials provide a platform that allows the creation of heterogeneous structures with a variety of properties [11]. Therefore, synthesizing 2D materials containing various components can give us access to a wider range of properties and technical applications. As a newly large family of 2D transition metal carbides, nitrides, and carbonitrides, MXenes have attracted growing focus because of their large and adjustable layer spacing, rich specific surface area, good bending property, high electrical conductivity, hydrophilicity and rich surface functional groups (-F, -OH, -O, etc.) [12,13]. Naturally, since MXene was discovered by Yury Gogotsi’s group in 2011 [14], it has been applied in various fields such as sensing, photocatalysis, electrocatalysis, and energy. In terms of energy storage, Gogotsi unfolded the lithium storage activity of MXene through DFT calculations at the beginning of its discovery. Because both Na and K have similar structures and chemical properties to Li, much extensive research has been made on MXene for alkali metal battery applications [15].
To date, many review papers have summarized the advances of MXene in alkali metal-ion batteries [16]. However, MMIBs undergo quite different redox chemistry from alkali metal-ion batteries; hence, the role of MXenes in MMIBs will be different from that in alkali metal-ion batteries. Unfortunately, the study of MXene for MMIBs is still in its infancy, and the compatibility between MXenes and MMIBs is being investigated. Therefore, it is urgent to summarize the latest research progress of functionalized MXene for MMIBs. Herein, this review will first briefly introduce the synthesis and properties of MXene materials. Then, the application of MXenes in MMIBs is categorically overviewed separately in terms of magnesium ion battery, aluminum ion battery, calcium ion battery, and zinc-ion battery. Finally, the advantages, the remained challenges, and the future direction toward the practical applications of MXenes in MMIBs are deeply discussed.
2. MXene
MXene is a class of compounds with a two-dimensional layered structure. Its general formula can be written as Mn+1XnTx, in which M refers to some transition metal elements, such as Sc, Ti, V, Cr, Mo, Hf, Nb, Ta, W, etc. More in-depth studies show that M can be expanded to binary or even multiple metallic elements. X contains the C and/or N element (Figure 1a); T is the surface terminal functional group, generally -F, -OH, -O, etc., which is closely related to the synthesis method [17]. Among them, the early n value is 1~3 [18], and the discovery of Mo4VC4Tx extends the scope of research to n = 4 [19]. Later, with the diversification of synthesis mechanisms, the discovery of Mo1.33CTx [20], V2−xC (x ≤ 0.05) [21], and solid solution structures [22] greatly enriched the members of the MXene family (Figure 1b).
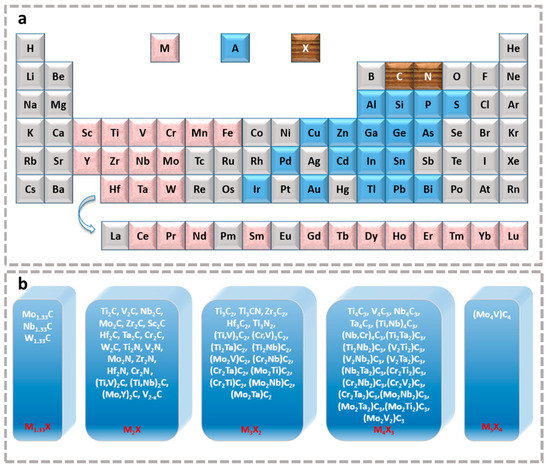
Figure 1.
(a) Periodic table fragments marked with the “M”, “A” and “X” elements in known MAX and MXene phases. (b) Currently known MXene compositions, ignoring the terminal here.
2.1. Synthesis of MXene
The most ordinary method for preparing MXene is etching the MAX precursor. MAX [23] can be expressed as Mn+1AXn (n ranges from 1 to 4), where M and X are the same compositions as mentioned above; A is mainly from the elements of the main group 13–15, such as Si, Al, Ge, Sn, etc. (Figure 1b). The MAX phase exists as three different types of unit cells with a six-square tightly packed structure of the space group P63/mmc. The A atomic layer in the MAX phase is sandwiched between the densely packed M layers, and the octahedral position is occupied by the X atom. In accordance with the crystal structure of MAX, Mn+1AXn is also recognized with a layered structure, in which the two-dimensional Mn+1Xn layer is connected by the A layer. M-A is a metal bond, while M-X has both covalent and ionic bond properties, which are more stable than the M-A bond [24]. This feature makes it possible to remove the A atoms from the MAX phase to obtain MXene.
2.1.1. Fluorine-Containing Etching Method
- HF Acid Etching
Unlike the van der Waals forces between black phosphorus [25], the strong metal bonds make directional removal of the A atomic layer still challenging. Until 2011, Gogotsi et al. [14] successfully prepared accordion-like multilayer Ti3C2Tx by chemical etching the Al atomic layers in Ti3AlC2 with HF acid, which opened the prelude of etching the A-layer structure from the MAX phase by fluorine-containing etchants (Figure 2a). Thereafter, lots of MXenes have successfully synthesized with different M elements (such as (V0.5Cr0.5)3C2, Nb2C, V2C, TiNbC), different X elements (such as Ti3CNx) and different compositions (such as Ti2C, Ta4C3) by HF acid etching [18,26], which promotes the universality of HF acid etching in MXene synthesis [14].
Taking the synthesis of Ti3AlC2 as an example, the reaction route of HF acid etching is as follows:
Ti3AlC2 + 3 HF → AlF3 + 3/2 H2 + Ti3C2
Ti3C2 + 2 H2O → Ti3C2(OH)2 + H2
Ti3C2 + 2 HF → Ti3C2F2 + H2
After the reaction of HF acid with the Al layer, each Mn+1Xn group was exposed to two highly active Ti atomic sites, which spontaneously combined with -OH and -F in the solution, so the surface terminal of the HF acid etching method is randomly distributed in -OH and -F groups. As for HF etching, the HF acid concentration, etching time, and etching temperature need to be appropriately adjusted for different precursors [27,28,29]. Excessive etching time and HF acid concentration will cause defects in the synthesized MXene and even lead to the complete collapse of the structure. Conversely, insufficient etching time or HF acid concentration results in incomplete etching.
- In Situ Generation of HF
Although HF acid etching methods are easy to operate and have a wide range of applications, hydrogen fluoride and its aqueous solutions are toxic and harmful. To avoid the use of HF acid, a method of in situ generating HF acids by strong acids (HCl/H2SO4) and fluorine-containing salts (LiF/NaF/KF/CsF/CaF2) has emerged. In 2014, Gogotsi et al. [30] successfully prepared Ti3C2Tx by etching Ti3AlC2 at 40 °C for 45 h with HCl/LiF. Liu et al. [31] etched V2AlC with NaF/HCl at 90 °C for more than 48 h to obtain V2C. This synthesis method is milder than HF acid etching, thus greatly reducing the defect content [32]. The prepared MXene is rich in the -F/-OH terminal group, which is easy to form hydrogen bonds, showing hydrophilicity and negative electricity. Due to the spontaneous intercalation of metal cations and H2O molecules, the prepared MXene exhibits a larger layer spacing and fewer layers than that obtained by the HF acid preparation method, eliminating the subsequent peeling step.
- Other Fluoride Salts
In addition to the (in situ) HF acid method, some fluoride salts and their mixtures can also be used for etching purposes to avoid the use of strong acids. Gogotsi et al. [33] prepared Ti3C2 by etching 1 M NH4HF2 at room temperature, and the embedding of NH4+ increased the layer spacing from 18.6 Å to 24.7 Å. Nitride MXene has higher conductivity than carbide, but because of the stronger Al bonding force compared to Tin+1AlCn in the Tin+1AlNn phase which is soluble in HF solution, the traditional synthesis method is not suitable for nitride MXene. Gogotsi et al. [34] mixed Ti4AlN3 powder with molten fluorine salts (59 wt% KF, 29 wt% LiF, 12 wt% NaF) and heated at 550 °C for 30 min under argon gas protection to synthesize Ti4N3Tx (Figure 2b), opening up the research field of nitride MXene.
MXene prepared by chemical etching is usually multilayered, it is usually followed by a peeling procedure for better application (Figure 2c). In addition to the metal cation [35] spontaneously interpolated in the etching above, the layer spacing can also be extended by dimethyl sulfoxide (DMSO) [36], tetrabutylammonium hydroxide (TBAOH) [37], isopropylamine [38], tetramethylammonium hydroxide (TMAOH) [39], the water molecule (H2O), cetyltrimethylammonium bromide (CTAB) [40], etc., and subsequent sonication can mechanically peel off the MXene to form a few-layer suspension.
Although the fluorine-containing etching method can well break the M-A bond to complete the synthesis of a variety of MXene materials, the MXene terminal group etched by the fluorine-containing method is single and the fluoride pollutes the environment, which is also very harmful to the human body. Therefore, it is urgent to find a feasible fluorine-free etching method to prepare MXene.
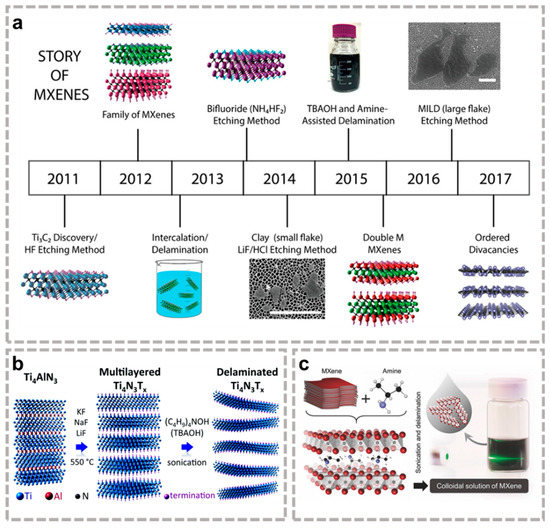
Figure 2.
(a) The history of MXene synthesis [41]. (a) Reproduced with permission from the American Chemical Society. (b) Schematic diagram of molten fluorine salt etching Ti4N3Tx [34]. (b) Reproduced with permission from RSC Pub. (c) Nb2CTx MXene delamination process [38]. (c) Reproduced with permission from WILEY-VCH.
2.1.2. Fluorine-Free Etching Method
Although the fluorine-containing etching method can break the M-A bond to synthesize a variety of MXene materials, the MXene terminal group etched by the fluorine-containing method is single. Therefore, with the amphoteric elements characteristics of Al in the middle of Ti3AlC2, Li et al. [42] designed a fluorine-free NaOH-assisted hydrothermal method etching Ti3AlC2 to prepare Ti3C2Tx (T=OH, O). This method does not produce -F termination groups, but there are special application conditions: (1) alkali concentration and hydrothermal temperature should be screened according to the precursor; (2) Phase A in the original MAX phase needs to be amphoteric/acidic atoms. In 2018, Yang et al. [43] demonstrated a high-efficiency fluorine-free etching method based on Ti3AlC2 anodic corrosion in binary aqueous electrolyte (1 M NH4Cl +0.2 M TMAOH). During this process, NH4+ is constantly intercalated to ensure an ongoing reaction, resulting in more than 90% of the single and double layers of MXene. Sun et al. [44] prepared monolayer and few-layer fluorine-free Ti3C2Tx (T=O or OH) by intercalation-alloying-expansion-micro-explosion mechanism. Huang et al. [45] first discovered that Lewis acid molten salts undergo a displacement reaction through redox to produce MXene structures, and Figure 3a shows the etching route using CuCl2 as an example. The capacity of Lewis acids to extract electrons from element A in the MAX phase depends on their respective electrochemical redox potentials in halide fusion. According to thermodynamic calculations (Figure 3b) [46], A atoms in the MAX phase can be engraved by means of molten Lewis acid that has a higher redox potential. Therefore, their group was successful in extending this synthesis strategy to various Lewis acid molten salts (CuCl2, ZnCl2, FeCl2, and AgCl) and more MAX family members with different A elements (Zn, Al, Ga, Si). This method also enables the successful preparation of MXene for specific groups (O, S, Se, Te, Cl, Br, I, NH) [47] by displacement and elimination reactions (Figure 3c,d), extending the boundaries of MAX precursors and providing significant opportunities to tune the surface chemistry of Mxene.
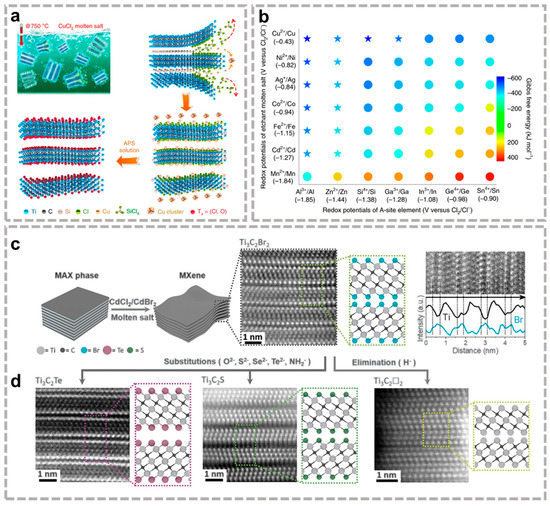
Figure 3.
(a) Preparation process of Ti3C2Tx MXene by reaction between Ti3SiC2 and CuCl2; (b) Diagram of redox potential and Gibbs free energy (700 °C) [46]. (a,b) Reproduced with permission from MDPI AG. (c) Schematics for etching MAX phases with Lewis acidic molten salts and high-angle annular dark-field (HAADF) image of Ti3C2Br2 MXene; (d) HAADF diagram of changing the end group by displacement and elimination reactions [47]. (c,d) Reproduced with permission from AAAS.
2.1.3. Non-Etching Methods
For the method of preparing MXene by means of the etching method, the etching conditions (etchant concentration, temperature, time, etc.) are greatly dependent on the strength of the M-A bond and n value. The prepared MXene is often inevitably defective, and the topography and number of layers are uncontrollable. Dimoulas et al. [48] and Zhao et al. [49] synthesized high-purity bare Mo2C without terminal groups in one step by means of the chemical vapor deposition method (CVD), which can regulate the size and morphology of materials through synthetic parameters. However, whether other MXene materials can be synthesized through this method is still unknown.
Table 1 summarizes the reagents and surface terminals in different preparation methods of MXene. Through comparison, it is found that although the fluorine-containing etching method has many unavoidable shortcomings, it is still the most popular and widely used etching method. In addition, researchers are also accelerating the research of fluorine-free preparation methods, which not only abandon toxic fluoride reagents but also greatly enrich the surface functional groups of MXene. All in all, each preparation method has its own advantages and disadvantages, so it is necessary to choose the appropriate etching method according to the desired performance of the material.

Table 1.
Summary of etching methods.
2.2. Properties of MXene
To select MXene synthesis methods according to the purpose of the experiment, understanding the influence of MXene composition and structure on properties can help us better design experiments.
2.2.1. Structural Stability
Considering the application of a material, including laboratory and industrial applications, the first thing that must be considered is the stability of the material, which is the cornerstone of the performance and application of the material in all aspects. MXene prepared by chemical etching is very similar to its parent MAX. MXene has a hexagonal dense structure in which X atoms are filled as solid solutions to octahedral interstitial sites in the tightly packed structure of M atoms. M2X-M2X crystals are typically packed hexagonal stacks with high stability [50]. Rosen et al. [51] experimentally confirmed that Mo2CTx has a stable, tightly packed hexagonal structure. In particular, Ti3C2 also has a densely packed hexagonal structure, so it is the first to discover and the most studied MXene material. The M4X3-M4X3 structure and the M3X2-M3X2 structure are typical face-centered cubic crystal structures, making these two materials unstable [52]. This structure can be stabilized by introducing another transition metal element, M’ (such as Ti) [53].
2.2.2. Environmental Stability
The bare MXene surface has abundant reactive sites and excellent conductivity due to the presence of transition metal-free electrons, but this also brings challenges to the long-term stable existence of MXene in the environment. Zhang et al. [54] exposed the Ti3C2Tx colloidal solution to air, which was completely oxidized to TiO2 in 30 days. The stability of MXene materials under water and oxygen conditions is a key factor restricting their development, and the introduction of surface terminals, surface modification, or the construction of MXene composites to occupy the active site can effectively slow down the oxidation rate and improve the stability of MXene. For example, in Ti3C2Tx, the -O-containing MXene is more stable than the -F/-OH containing group [55].
2.2.3. Interlayer Stability
In MXene, the layers rely on the van der Waals force between -F/-O and the hydrogen bonds between -F/-O and -OH to link, and the strength of the hydrogen bonds between layers is determined by the number and distribution of -OH and -F/-O on the sheet [56]. The weak interlayer adhesion is conducive to peeling by various intercalators. However, it generates stack tendency due to its large surface energy, leading to hydrophilicity and conductivity attenuation in the practical application. Currently, stacking between layers can be avoided by increasing layer spacing, building three-dimensional (3D) network structures, or building composite materials. Layer spacing is influenced by a combination of surface termination, synthesis method, precursor, n value, and intercalator. The originator of MXene [14] proposed that the geometric optimization of hydroxylated and fluorinated structures led to the expansion of the original Ti3AlC2 lattice by 5% and 16%, respectively. Conversely, simply removing the Al atom without being replaced by a functional group would result in a 19% contraction of the structure. Therefore, the presence of surface terminals will occupy the exposed Ti atomic active site, increasing the stability of the material. At the same time, the tendency to stack can be suppressed by reducing the surface contact with the material. The vacuum filtration step of MXene during preparation is subjected to atmospheric pressure, resulting in reduced layer spacing and increased stacking. Starting from the material synthesis method, Xu et al. [57] replaced vacuum filtration with natural sedimentation, and the prepared layer spacing changed from the original 14.06 Å to 14.76 Å, showing good stability (Figure 4e). In addition, the layer spacing of MXene materials can also be adjusted by intercalation reaction or by regulating the precursor MAX phase. Typical intercalators have been described in the previous synthesis section and will not be repeated here. Zhou et al. [58] also obtained an increased layer spacing by etching the precursor V4AlC3 after ball milling (c value changed from 2.534 to 3.210 nm, Figure 4f). At the same time, some researchers have shown that for the same type of MXene, the larger the n value, the larger the layer spacing [5]. The increased layer spacing not only contributes to the stability of MXene materials but also exposes more active sites, broadens ion transport channels, and further activates more potential of MXene.
The construction of a three-dimensional network structure can effectively avoid stacking between layers and build an ion transport network, so as to better utilize the high conductivity of MXene material, and also has a miraculous effect in alleviating some high-volume strain materials. The template method and freeze-drying method are common methods for constructing 3D network structures. Xu et al. [59] in situ grew S particles on the surface of Ti3C2 by chemical reduction, after which the S particles were removed as templates at a high temperature of 300 °C to obtain flexible MXene foam with a well-developed 3D structure (Figure 4g). Gogotsi and colleagues [60] prepared porous Ti3C2 (p-Ti3C2) by rapid and simple chemical etching using in situ oxidation TiO2 as a template at room temperature (Figure 4h), which is used as an electrode material for lithium-ion batteries with long lifetime and excellent rate performance (330 mAh g−1 at 10 C). Yang et al. [61] freeze-dried the Ti3C2Tx/CNT nanosheet colloidal solution, the specific procedure is illustrated in Figure 4a, and the SEM of the resulting parallel arrangement of Ti3C2Tx/CNT (PA-MXene/CNT) structure is depicted in Figure 4b–d. It can be seen that numerous pores are formed between the nanosheets, avoiding the stacking of Ti3C2Tx nanosheets. Because of the large specific surface area and rich active sites of MXene, it is easy to connect with many electronic active materials by ultrasound, hydrothermal and other methods. The introduction of composite materials is often accompanied by an increase in layer spacing, and they can also be seen as special intercalators in this regard. Specific applications are presented in “Applications in Multivalent Metal-Ion Batteries (MMIBs)”.

Figure 4.
(a) PA-MXene/CNT preparation scheme using unidirectional freeze-drying; (b–d) SEM image of PA-MXene/CNT [61]. (a–d) Reproduced with permission from WILEY-VCH. (e) Comparison of MXene films naturally sedimented and conventional filtered under vacuum [57]. (e) Reproduced with permission from Springer Nature. (f) Schematic showing the two different processes of V4C3Tx MXene [58]. (f) Reproduced with permission from ELSEVIER BV. (g) Diagram showing the chemical engraving of Ti3C2Tx flakes to produce a porous structure [60]. (g) Reproduced with permission from John Wiley and Sons. (h) Diagram of the porous, free-standing, and flexible 3D MXene foam prepared with an S template [59]. (h) Reproduced with permission from WILEY VCH.
2.2.4. Mechanical Properties
MXene inevitably carries terminal groups Tx (-F, -OH, -O) during synthesis, which is conducive to stabilizing bare MXene materials. It can also avoid the collapse of the atomic layer on the surface of bare MXene materials due to tensile strain, improving the mechanical flexibility of materials [62]. It is therefore urgent to examine the impact of terminal groups on performance. Many studies have found that the terminal group greatly reduces the elastic coefficient of intrinsic MXene [63,64], but the elastic coefficient is still higher than the original MAX phase and other two-dimensional materials (such as MoS2) excluding graphene [65]. The elastic coefficient relationship of typical terminal groups is -O > -F > -OH, which is proved by nanoindentation experiments to measure Young’s modulus of Ti3C2Tx [66]. In addition, the presence of surface terminal groups can also greatly enhance the bending properties of MXene, making MXene materials show great application prospects in composite structures and flexible electronics.
2.2.5. Electronic Performance
The introduction of surface terminals can improve MXene stability but may come with a certain sacrifice in electronic performance (Figure 5). Theoretical calculations suggest that bare MXene exhibits metallic properties [67], although most bare MXenes have not been experimentally synthesized. The introduction of terminal groups turns MXene into a semiconductor or even a topological insulator. DFT calculations show that most M2CTx compounds with functional groups on their surfaces are semiconductors with bandgap values that typically increment according to the atomic number of “M” and with the order of -O > -F > -OH with the terminal group [68,69]. For example, after converting Sc2CT2 (T=F, OH, O), Ti2CO2, Zr2CO2, and Hf2CO2 into semiconductors, the band gap of Sc2CT2 (T=F, OH, O) is 1.03, 0.45 and 1.8 eV, respectively, and the band gap values of Ti2CO2, Zr2CO2, and Hf2CO2 are 0.24 eV, 0.88 eV, and 1.0 eV, respectively [70]. Further, electronic performance is also related to M and X types. “M” is Cr, W, and Mo, which may be topological insulators [71], while Ti3C2, Ti4C3, V2C, and Nb4C3 maintain metallic properties [72]. It was also found that nitride and carbonitride MXenes have higher metallicity than carbide MXene due to the fact that nitrogen has more electrons [73].
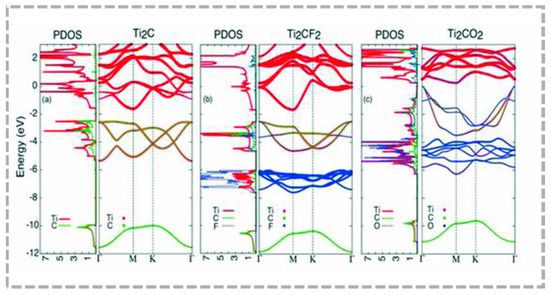
Figure 5.
Band structure diagram of the -OH, -F terminations and bare Ti2C MXene monolayers, showing that MXene changes from metal to semiconductor due to changes in surface chemistry [70]. Reproduced with permission from WILEY VCH.
In addition to theoretical studies, there are also studies that measure the resistivity of some MXenes experimentally. We summarize the results in Table 2. It can be seen that the sheet resistance of some MXenes is in the same order of magnitude as graphene, reflecting excellent conductivity.

Table 2.
Resistivity of some MXenes and graphene.
2.2.6. Surface Terminals
In addition to affecting the stability of MXene materials, surface terminals of special groups have some specific uses. The capacity of the -O terminal is significantly higher than that of -F/-OH in energy storage performance [75]. -S terminal has an adsorption effect on dissolved polysulfides and the cycling performance of the battery can be obvious when used in Li-S batteries [76]. The tunable fit of surface terminal groups begins in the material synthesis phase. When etching using fluorine-containing methods, the terminals are mainly -O, -OH, and -F [77]. -F content also depends on the type of etchant used. For example, the HF acid etching -F terminal is four times LiF+HCl [77]. Fully chlorinated MXene (T=Cl) is obtained using ZnCl2 etching [46]. Secondly, the MXene terminal group can also be changed by post-treatment such as displacement reaction, annealing treatment, chemical method, or reaction with alkali metal hydroxide. Kamysbayev et al. [47] successfully prepared Ti3C2Tx containing S, Se, Te, Cl, Br, and NH terminals by displacement reaction, and a variety of groups brought different stability and electronic properties from traditional -F/-O/-OH. However, the discovery time of these groups is short, so the current research on their performance is not in-depth enough. Persson et al. [78] completed the transition from -F to -O by thermal annealing at 750 °C, and Xie et al. [55] completed the transition from -OH to -O. Although the -O band gap is slightly higher than -F, the stability of the -O-terminal group is greatly improved, and it has been proven to have the highest storage capacity, so the O-terminal group may be advantageous in energy storage batteries. The post-treatment method also expands the scope of the application of fluorine-containing etching.
However, MXene also has some challenges. As we mentioned earlier, The M4X3-M4X3 structure and the M3X2-M3X2 belong to unstable face-centered cubic structures. However, some of these structures MXene exist (Zr3C2, Hf3C2, V4C3, Nb4C3, Ta4C3), while others do not exist with this chemical formula at all (V3C2, Mo3C2, Cr3C2, Mo4C3, Cr4C3). In addition, M2C-M2C MXene is more active than other types and is susceptible to environmental oxidation. The stability of N-based MXene is lower than its carbide counterpart. Therefore, when using M2C-M2C or N-based MXene, strict oxidation resistance or rational utilization of oxidation is necessary.
Therefore, choosing the right MXene and appropriate etching method is primarily for the experiment. If used as battery electrode materials, Ti3C2, V2C, Nb2C, etc. with good conductivity are preferred. Once the type and number of layers are determined, MXene is more of a surface chemical. Terminal groups can improve the stability of the material, improve layer spacing, and affect electronic and mechanical properties. The modification of the terminal group by the post-treatment method widens the access channels of the individual terminals. In addition, the trend of MXene being stackable can be improved by many treatments, offering more potential for MXene applications in the energy storage area [79].
3. Applications in Multivalent Metal-Ion Batteries (MMIBs)
The MMIBs currently studied mainly include Mg2+, Al3+, Ca2+, and Zn2+ batteries. Because of their abundant reserves and significantly lower vivacity than lithium, the corresponding metal is commonly used as an anode in MMIBs [80,81,82]. However, due to the high charge and large hydration radius of polyvalent ions, the ion has a strong binding force with electronic materials and slow diffusion kinetics, which is not conducive to the reversibility of the battery [83]. Therefore, new challenges are posed to the electrode materials of MMIBs.
3.1. Magnesium Ion Batteries (MIBs)
Magnesium [84] is a reactive metal with a density of 1.74 g cm−3, a high theoretical volumetric energy density (∼3833 mAh cm−3), rich reserves, low price (1/24 of Li), good thermal and electrical conductivity, and low potential (Mg2+/Mg redox potential −2.37 V). In the periodic table, magnesium is diagonal to lithium and has similar chemical properties. Therefore, the research of MIBs as an alternative to lithium-ion batteries is of great significance for the exploration and utilization of future energy.
However, MIBs lack suitable cathode materials with high ion diffusion/intercalation rates [85] hindered by the common problems of polyvalent ions. It is expected that MXene will store magnesium because of its wide and adjustable layer spacing, high conductivity, and abundant surfactant sites. Based on theoretical calculations, Ti2CO2 has a theoretical magnesium storage capacity of 570 mAh g−1 (comparable to the alloy anode) [55], demonstrating the magnesium storage activity of MXene material. Xie et al. [55] calculated the reaction mechanism of Mg2+ in MXene materials. When MXene is used as a cathode material for MIBs, Mg2+ will first be adsorbed to negatively charged O-MXene through electrostatic interaction due to negative surface adsorption energy. Herein, because of the positive adsorption energy of OH-MXene and the instability of F-MXene, these two are not considered. Because of the small repulsion of Mg2+ and the transition metal atoms in MXene and the strong shielding of negative electron clouds, Mg2+ is the only polyvalent metal ion that can form two intact adsorption layers. After that, the lower adsorbed Mg2+ is intercalated/deintercalated between the MXene layers as the reaction progressed, and the upper adsorbed Mg2+ is released/stored by similar deposition/dissolution mechanisms, which greatly increases the magnesium storage capacity of MXene. It is worth noting that Mg2+ on the adsorption layer does not form a passivation layer like magnesium metal during deposition, which is conducive to the reaction. Finally, in the presence of Mg, O-MXene undergoes a transformation reaction to decompose into bare MXene and magnesium oxide. Although the ion diffusion of bare MXene is better than that of O-MXene, in fact, due to the lack of support of stable electrolyte, the final conversion reaction has not been experimentally demonstrated, and even the dissolution/deposition reaction of the second adsorption layer does not necessarily occur. It is inevitable that the prepared MXene material contains terminals, and the polyvalent metal-ion diffusion performance in Tx-MXene is far inferior to that of alkali metal ions, which make the rate of performance slightly worse. Therefore, promoting Mg2+ intercalation/deintercalation by improving the adsorption and diffusion processes is a key driver for enhancing the utilization of MXene materials.
Xu et al. [40] demonstrated the ability of CTAB intercalated Ti3C2Tx (Ti3C2Tx/CTAB) for the storage of magnesium ions, whereas the original Ti3C2Tx did not function well (Figure 6a). The main performance improvement factor is the reduction of the diffusion barrier of Mg2+ on the surface of Ti3C2Tx/CTAB (about 0.19 eV, Figure 6c–h), which endows the prepared Ti3C2Tx/CTAB cathode an elevated capacity of 300 mAh cm−3 at 50 mA g−1 (Figure 6b). Additionally, the intercalation of CTAB prevents Ti3C2Tx from self-stacking, improving the overall stability. Besides that, the pre-intercalated layers of Mg2+ [86], K+ [87], and nanocarbon materials [88] can also realize the magnesium storage properties of MXene materials.
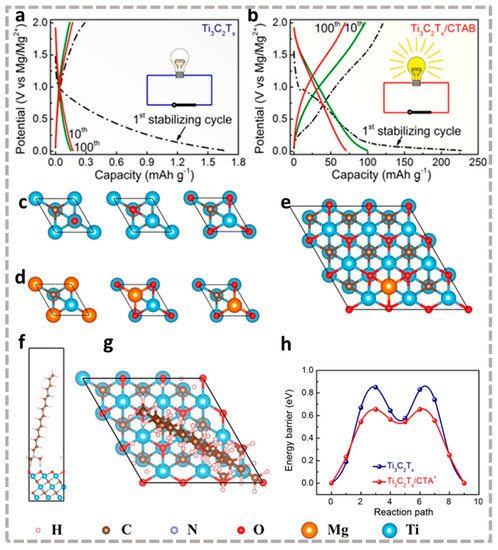
Figure 6.
Galvanostatic charge/discharge (GCD) curves of (a) Ti3C2Tx and (b) Ti3C2Tx/CTAB electrode at different cycles at 0.05 A g−1; Top view of the structures for (c) an O atom and (d) a Mg atom adsorbed on 1 × 1 Ti3C2 surface at the top site, body-centered cubic (bcc) site, and face-centered cubic (fcc) site, respectively. Top view of (e) Mg2+ and (g) CTA+ as well as (f) side view of CTA+ adsorbed on the 3 × 3 Ti3C2O surface; (h) Diffusion profile of Mg2+ on Ti3C2O and CTA+/Ti3C2O surface in nudged elastic band calculations [40]. Reproduced with permission from ACS Nano.
Apart from being directly used as an active electrode material, MXene is an excellent substrate material due to its good laminated structure and electrical conductivity. The combination with magnesium cathode active material can stimulate the maximum potential for both of them. As a typical magnesium storage cathode material, MoS2 is plagued by low capacity and poor cycle stability. Xu et al. [89] introduced Ti3C2Tx as an “enhancer” into MoS2 by in situ hydrothermal method to prepare the MoS2/Ti3C2Tx composite structure. MXene adsorbed Mo4+ as a nucleation site, and the flaky MoS2 gradually grew to obtain thin petal-like MoS2/Ti3C2Tx (Figure 7a–d). This particular topography provides more exposed active sites for Mg2+ storage while providing more channels for improving Mg2+ diffusion kinetics. In addition, MXene’s excellent electrical conductivity gives the composite excellent electron transport properties. At the same time, MoS2 between MXene layers further inhibits the stacking of MXene materials, and the confinement effect of MXene structure synergistically alleviates the volume expansion during cycling, jointly maintaining the stability of the composite material. For these reasons, MoS2/Ti3C2Tx composite reached a reinforced capacity of 165 mAh g−1 at 50 mA g−1, which was much higher than other MoS2 (Figure 7e,f). Li et al. [90] synthesized TiS2/Ti3C2 composites by hydrothermal assembly using Ti3C2 MXene as an ideal substrate. Compared with pure TiS2 cathode material, TiS2/Ti3C2 has a cumulative capacity of 97 mAh g−1 for MIBs at 50 mA g−1. Liu et al. [91] prepared Ti3C2/CoSe2 heterojunctions by in situ growth of MOF(ZIF-67) structure on Ti3C2 combined with selenization (Figure 7g). Similar to the mechanism proposed by Xu et al. [89], Ti3C2/CoSe2 as a cathode material for MIBs showed excellent cycle life (>500 cycles) and excellent rate performance (75.7 mA h g−1 at 1000 mA g−1, Figure 7h,i). During the in situ process, MXene is more susceptible to interference from external unstable factors (such as high temperature) to accelerate the oxidation process. Zhu et al. [92] prepared VS4@Ti3C2/C by adding glucose to inhibit the oxidation of Ti3C2 by providing a reducing environment with the in situ synthetic of VS4@Ti3C2. Glucose is anchored on the surface of Ti3C2 by forming a hydrogen bond with the oxygen-containing terminal, and finally forms a carbon film uniformly coated on the surface of Ti3C2 through pyrolysis, which not only achieves the purpose of inhibiting oxidation but also increases the overall conductivity of the system. VS4@Ti3C2/C as a cathode has a smaller impedance than VS4, showing a capacity of 492 mAh g−1 at 50 mA g−1, as well as over 900 cycles stability and excellent rate performance. Nevertheless, the in situ generation method is highly susceptible to oxidation of MXene, so the development of simpler composite combination methods is also a favorable solution. Yang et al. [93] prepared Li4Ti5O12 nanosheets/d-Ti3C2 flexible film(LTO NSs@d-Ti3C2) by simple ultrasonic mixed filtration, which perfectly utilized the excellent kinetic properties, abundant active sites, and mutually supportive stability provided by the composite structure to achieve a self-supporting and non-current collecting flexible electrode.
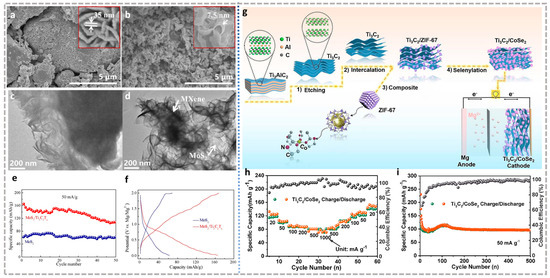
Figure 7.
FESEM and TEM images of (a,b) MoS2 and (c,d) MoS2/Ti3C2Tx composite. The illustrations show the corresponding image with different magnifications. (e) Cycle performance and (f) charge/discharge curves of MoS2 and MoS2/Ti3C2Tx electrodes in MIBs after stabilization [89]. (a–f) Reproduced with permission from Elsevier. (g) Schematic illustration of the Ti3C2/CoSe2 synthesis process and half-cell mechanism. (h) Rate capability at different current densities and (i) cycling performance at 50 mAg−1 of Ti3C2/CoSe2 [91]. (g–i) Reproduced with permission from Elsevier BV.
In composite materials, the proportion between the constituents is vital. Too little MXene leads to excessive filling between MXene layers, which has a negative impact on ion transport; too much MXene challenges the uniformity of combinations. Li et al. [94] demonstrated that in MnO2/V2C composites with MnO2 content of 20%, MnO2 and V2C achieve complementary advantages, where the uniform combinations facilitate the mobility of ions. Therefore, for the composite of different materials with MXene, the first thing to prove is the feasibility of compounding, in other words, to consider the stability after compounding. Followed immediately, the optimal compound ratio needed to be determined according to the actual situation.
It can be found that the preparation of MXene composites is an important part of MXene applications. Therefore, here is a brief summary of composite synthesis methods. In addition to the in situ generation method similar to MoS2/Ti3C2Tx [89], in situ conversion (partial oxidation [95], vulcanization [96] or selenization [97]) and ex situ methods (electrostatic action or van der Waals self-assembly by ball milling [98], ultrasound [93], spray [99,100], chemical vapor deposition [101], etc.) can also successfully prepare MXene composites.
In order to increase MXene’s magnesium storage capacity, the researchers also turned to magnesium-lithium hybrid batteries (HMLBs). In such batteries, the cathode material needs to have good dual-ion (Mg2+ and Li+) compatibility. The storability of MXene to single ions (Li+, Mg2+), respectively, makes it a favorable candidate for HMLB electrode materials. Gogotsi et al. [102] report a free-standing flexible Ti3C2Tx/carbon nanotube (Ti3C2Tx/CNT) composite “paper” HMLB electrode that provides only 105 mAh g−1 reversible capacity at 10 mA g−1. Therefore, the researchers hope to increase the layer spacing by selecting other intercalators or compounding some “pillar effect” materials to achieve high-capacity HMLBs. Prelithiated-V2C [103] as a cathode can achieve a fascinating reversible capacity of about 230 mA h g−1 at a current density of 0.02 A g−1. The increased interlayer spacing influenced by Li+ pre-embedding unlocked additional ion diffusion channels, which provides insight into the rapid reaction kinetics and high Mg2+ storage capacity of MXene. Similarly, Li et al. [104] obtained Ti3C2Tx with different layer spacing through different alkyl chain surfactant intercalation layers, indicating that Mg2+ intercalating/de-intercalating is only possible if the layer spacing is greater than 1.83 nm. They also pointed out that the N element in the CTAB is tightly bound to Ti3C2Tx, which can be regarded as N doping, further neutralizing the electronegativity of Ti3C2Tx so that Ti3C2/CTAB exhibits a reversible capacity of 115.9 mA h g−1 at 0.1 A g−1 in an HMLB. This method has also been experimentally proven to be applied to Mo2C MXene [102].
At present, the application of MXene in MIBs mainly focuses on the cathode. The biggest challenge of MXene when storing Mg2+ independently is the slow Mg2+ diffusion, so various intercalation methods are often used to improve MXene’s Mg2+ storage performance, which is also reflected in HMLBs. Some studies on transition metal chalcogenides as cathodes for MIBs have been added to Table 3. Compared with other cathode materials, the magnesium storage capacity of Ti3C2Tx/CTAB can be compared with some classical MIBs cathode materials (50 mAh g−1 for V2O5 [105], 130 mAh g−1 for Mo6S8 [106], 85 mAh g−1 for MnO2 [107]). In composites, with the addition of MXene-Ti3C2, the storage capacity of magnesium ions of MoS2 and TiS2 is improved. MXene-Ti3C2 nanosheets with good electrical conductivity provide a looser structure and more active sites for the entry and exit of magnesium ions. Of course, intercalation and composite can cooperate to achieve a win-win situation, so we briefly summarize some composite synthesis methods above.
3.2. Aluminum Ion Batteries (AIBs)
Aluminum [108] is the most abundant metal element in Earth’s crust. The specific volume capacity of aluminum metal is 8046 mAh cm−3, which is up to four times that of lithium metal, and its mass-specific capacity is 2980 mAh g−1, which is also relatively high. In addition, the low cost and environmental friendliness advantages of AIBs further promote its research progress. However, the stable cycle of Al3+ is closely related to the electrolyte. It was found that only the ionic electrolyte (anhydrous aluminum chloride (AlCl3) + 1-ethyl-3-methylimidazolium chloride ([EMIm]Cl)) can ensure the dissolution/deposition of the aluminum metal anode, in which the carriers exist in the form of AlCl4− or Al2Cl7− [109]. Therefore, the main research is divided into two categories: one focuses on AIBs where Al3+ is extracted from cathode materials, and the other focuses on the two-ion reaction of AlCl4− or Al2Cl7− as carriers at the cathode. At present, the transition metal chalcogenides (TMCs) are mainly used as cathode materials for AIBs, but their extraction process of Al3+ is the result of the combined action of intercalation and phase transition, thus resulting in poor cycle stability [110]. This is because of the difficulty of extracting Al3+ from the electrolyte and the strong interaction between Al3+ and the electrode material that the performance of AIBs cathode materials still needs to be further improved.
Similar to the storage mechanism of Mg2+ in MXene, the adsorption energy of Al3+ on O-MXene is slightly higher than that of Mg2+ because of the strong repulsion between Al3+ and the transition metal atoms. As a result, during the adsorption process, the adsorption layer only has a 2/3 coverage, so the intercalation/deintercalation storage mechanism of Al3+ is revealed [55]. However, thanks to the charge contribution of each atom (1.09 e−/Al, higher than 0.76 e−/Mg, 0.79 e−/Ca), MXene still has a high aluminum storage capacity. For instance, theoretical calculations show that Ti2CO2 has a theoretical aluminum storage capacity of 552 mAh g−1 [55].
Beidaghi et al. [111] confirmed that Al3+ can be reversibly inserted into the V2CTx host. With the help of TBAOH, the expanded layer spacing improved electrochemical performance (Figure 8a,b). The optimized MXene provides a high capacity of over 300 mAh g−1 at of 100 mA g−1, along with an approximately 50% capacity remaining after 100 cycles at 200 mA g−1 (Figure 8c–e). The embedding mechanism is revealed as follows: during the discharge process, Al3+ generated by dissociation of Al2Cl7− at the electrode/electrolyte interface is inserted into the MXene interlaminar region. At the same time, the anode undergoes the process of Al3+ generated by Al dissolution contacting AlCl4− to form Al2Cl7−. The charging process is the opposite. Although continuous capacity deterioration was observed, the specific capacity and cycling performance reported here were the best at that time. With the deepening of the research of AIBs, Lee et al. [112] found that the Fe2CS2 obtained by replacing Ti with a late transition metal Fe and replacing O-terminal groups with S-terminal groups has higher capacity and kinetic properties than Ti2CO2. This is attributed to the redistribution of charge under the action of Fe and -S, along with the reduction of the Al3+ intercalation barrier by -S.
Recently, Li et al. [113] found that Ti3C2 possesses a 455.5 mAh g−1 capacity at 100 mA g−1 when used as a cathode in AIBs, but the stability is extremely poor due to the adsorption of polychloride anions by the -OH and -F terminals. Therefore, they improve the performance by building composite materials. They first ultrasonicated the mixture of CTAB and Ti3C2 to enlarge the layer spacing in Ti3C2 (Ti3C2@CTAB). Then, after being selenized, selenium was deposited between layers to prepare Ti3C2@CTAB-Se [113]. Through the intercalation of CTAB and selenium, Ti3C2@CTAB-Se electrode demonstrates 583.7 mAh g−1 discharge capacity at 100 mA g−1, and still 132.6 mAh g−1 maintained after 400 cycles. It is worth noting that this cathode composite relies on AlCl4− to achieve charge storage/release, in which selenium can not only improve the surface adsorption of AlCl4− but also improve the overall capacity as an active material. Li et al. [114] synthesized D-Ti3C2Tx@S@TiO2 with -S terminal by in situ deposition S into the DMSO stripped Ti3C2Tx layers and then high-temperature oxidation method (Figure 8f), whose working mechanism is also AlCl4− intercalation/deintercalation when used as the cathode of AIBs (Figure 8g). Unlike in the previous study, the residual S between the layers will form polysulfides dissolved in the electrolyte during the electrochemical activation process at a small current density and are not used as active materials. The D-Ti3C2Tx@S@TiO2 shows a discharge capacity of 151.3 mAh g−1 after 120 cycles (72.3% retention), which is about 80.0 mAh g−1 higher than Ti3C2Tx in AIBs (Figure 8h). The improvement is mainly due to the following reasons: the -S terminal generated by high temperatures avoids the capacity decay caused by the irreversible reaction of -OH and AlCl4−, and the TiO2 generated by partial oxidation attached to the surface to maintain the overall frame to further increase the stability of MXene material. It can be seen that scientists have noticed the exploration of MXene in AIBs. However, both bare and modified MXene have the problem of rapid deterioration of capacity, and the mechanism needs to be further explained to better guide the design of cathode materials for AIBs.
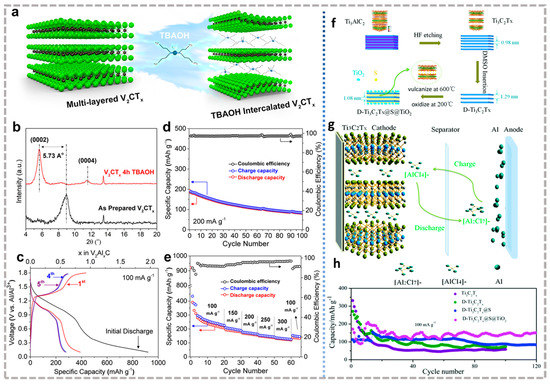
Figure 8.
(a) Schematic diagram of TBAOH intercalated ML-V2CTx for interlayer expansion; (b) XRD patterns of ML-V2CTx and TBAOH treated ML-V2CTx, showing an increased interlayer spacing of about 5.73 Å after TBAOH treatment; (c) Charge/discharge curves of TBAOH treated ML-V2CTx for the first five cycles; (d) Cycling performance of TBAOH treated ML-V2CTx cathode over 100 cycles at 200 mA g−1; (e) Rate performance of a TBAOH treated ML-V2CTx cathode [111]. (a–e) Reproduced with permission from ACS Nano. (f) Schematic diagram of the synthesis process of D-Ti3C2Tx@S@TiO2; (g) The working mechanism of Ti3C2Tx as a cathode material for AIBs; (h) Cycling performances of D-Ti3C2Tx@S@TiO2 [114]. (f–h) Reproduced with permission from RSC Pub.
3.3. Calcium Ion Batteries (CIBs)
Ca2+ carries two units of positive charge like Mg2+, and Ca is the fifth most abundant element in Earth’s crust, with low polarization and low reduction potential (−2.87 V), making it a promising energy system [115]. However, CIBs have developed slowly since their discovery. In the 1990s, Aurbach et al. [116] found that the dissolution/deposition of Ca2+ on calcium/precious metal electrodes is impossible due to the fact that the formed surface film has no Ca2+ transport properties. In 2016, Ponrouch et al. [117] achieved reversible dissolution/deposition of Ca2+ in Ca(TFSI)2, Ca(ClO4)2, or Ca(BF4)2 electrolytes using EC: PC as a solvent, that CIBs research showed signs of recovery. Therefore, the research of CIBs is in an embryonic state, and the report of MXene for CIBs is only in the theoretical research stage as well. Because the electronic shielding layer of Ca2+ is not as strong as Mg2+, Ca2+ partially forms a second adsorption layer on the basis of forming at least one stable adsorption layer on the surface of MXene [55]. Through theoretical calculations, it is shown that Ti2CO2 has a theoretical calcium storage capacity of 487 mAh g−1, which is slightly lower than Mg2+ since the increased relative atomic mass, but it can still be comparable to defective graphene materials [55]. Later, Dunda et al. [118] calculated that the V3C2/graphene composite structure has a calcium storage capacity of 598.63 mAh g−1. Demiroglu et al. [119] calculated that the theoretical calcium storage capacities of V2CO2/graphene and Ti2CO2/graphene were 411.31 mAh g−1 and 416.39 mAh g−1, respectively, which were significantly higher than their theoretical lithium storage capacity. Further altering the surface terminals, V2CSe2 [120] exhibited a maximum theoretical capacity of 394.12 mA h g−1 for Ca2+ storage.
The applications of MXene in MIBs, AIBs, and CIBs are summarized in Table 3. It can see that although MXenes’ ability to store calcium has been theoretically demonstrated, there is still a certain distance from practical application considering the adaptation of electrode materials to electrolytes.

Table 3.
Applications of MXene and MXene-based composites in MIBs/HMLBs, AIBs, CIBs.
Table 3.
Applications of MXene and MXene-based composites in MIBs/HMLBs, AIBs, CIBs.
| Material | Cycling Performance | Rate Performance Capacity (mAh g−1) | Ref. | |||
|---|---|---|---|---|---|---|
| Ion Storage System | Current Density (mA g−1) | Initial Capacity (mAh g−1) | ||||
| MXene | Ti2CO2 | MIB | / | 570 | / | [55] |
| Ti3C2Tx/CTAB | MIB | 50 | 100 | 42 at 1 A g−1 | [40] | |
| Mg0.21Ti3C2Tx | MIB | 50 | 210 | 55 at 0.5 A g−1 | [86] | |
| Ti3C2Tx@C | MIB | 10 | 198.7 | 123.3 at 0.2A g−1 | [88] | |
| Ti3C2Tx/CNT | HMLB | 10 | 105 | ∼50 at 1 A g−1 | [102] | |
| Prelithiated-V2C | HMLB | 20 | 230.3 | 260.7 at 1 A g−1 | [103] | |
| Ti3C2/CTAB | HMLB | 100 | 119.5 | 100.5 at 2 A g−1 | [104] | |
| Ti2CO2 | AIB | / | 552 | / | [55] | |
| V2CTx | AIB | 100 | 162 | ∼150 at 0.3 A g−1 | [111] | |
| Fe2CS2 | AIB | / | 642 | / | [112] | |
| Ti2CO2 | CIB | / | 487 | / | [55] | |
| V3C2/graphene | CIB | / | 598.63 | / | [118] | |
| V2CO2/graphene | CIB | / | 411.31 | / | [119] | |
| Ti2CO2/graphene | CIB | / | 416.39 | / | [119] | |
| V2CSe2 | CIB | / | 394.12 | / | [120] | |
| MXene-based composites | MoS2/Ti3C2Tx | MIB | 50 | 165 | 93 at 0.2A g−1 | [89] |
| TiS2/Ti3C2 | MIB | 50 | 97 | / | [90] | |
| Ti3C2/CoSe2 | MIB | 20 | 114.5 | 75.7 at 1A g−1 | [91] | |
| VS4@Ti3C2/C | MIB | 50 | 492 | 129 at 1A g−1 | [92] | |
| LTO NSs@d-Ti3C2 | MIB | 20 | 320 | 42 at 0.3A g−1 | [93] | |
| MnO2/V2C | MIB | 100 | 130 | 25 at 0.5A g−1 | [94] | |
| Ti3C2@CTAB-Se | AIB | 100 | 583.7 | 68.1 at 0.3A g−1 | [113] | |
| D-Ti3C2Tx@S@TiO2 | AIB | 100 | 209.2 | 51.8 at 0.5A g−1 | [114] | |
| Classic cathode material | MoS2 | MIB | 50 | 62 | / | [89] |
| TiS2 | MIB | 50 | 58 | / | [90] | |
3.4. Zinc-Ion Batteries (ZIBs)
3.4.1. Anode
Compared to other polyvalent ions, the high redox potential (−0.76 V) of Zn makes it possible to use water as an electrolyte solvent, greatly enhancing the safety of the battery [121]. As the hydrophilicity of MXene can promote the penetration of aqueous electrolytes, the application of MXene in aqueous ZIBs far exceeds other MMIBs. However, the commonly used zinc anode is prone to generate dendrites similar to Li during the dissolution/deposition in aqueous electrolytes and suffered from side reactions such as corrosion and hydrogen evolution, which seriously affects the safety and lifespan of the ZIBs [122]. The essence of dendrite formation lies in the uneven zinc nucleation site, and the “top effect” aggravates this phenomenon into a vicious circle [123]. At present, surface modification, structural design, alloyed anode, intercalated anode, and electrolyte modification methods have been used to improve the surface uniformity of zinc anode [122]. As a category of two-dimensional layered materials with excellent conductivity, hydrophilicity, and extensive specific surface area, MXene has the hope of promoting uniform zinc deposition in all these aspects.
The structural design of zinc anode is one of the effective methods to reduce dendrite growth. In aqueous ZIBs, the purpose of structural optimization is to alleviate the formation of zinc dendrites by reducing the local current density and further achieving the homogenization of the surface electric field [124]. The most common method is to load Zn onto a highly conductive substrate by electrodeposition to form a composite material. MXene’s high conductivity is considered a good conductive substrate. Tian et al. [125] prepared a Ti3C2Tx@Zn paper electrode by electrochemically depositing Zn onto Ti3C2Tx, which replaced Zn as the anode of ZIBs, showing a much better cycling performance. Good electrolyte infiltration of Ti3C2Tx@Zn guaranteed by Ti3C2Tx and fast electron transport channels and ion diffusion channels provided by the layered composite structures endow Ti3C2Tx@Zn with uniform charge distributions (Figure 9a,j). When 1 mAh cm−2 is deposited at 1 mA cm−2, the surface remains smooth and dendritic free (Figure 9b–i). On this basis, shifting the 2D structure to 3D can further promote the uniform Zn2+ concentration on the Zn surface by shortening the ion migration pathway. Chen et al. [126] first prepared a 3D porous structure (MGA) by freeze-drying MXene (Ti3C2Tx) and graphene aerogel and then used it as an electrodeposition matrix to prepare MGA@Zn anodes. Thanks to the formation of ZnF2-rich solid electrolyte interface (SEI) induced by the -F terminal and inhibited side reactions by composite structure, MGA@Zn has a flat zinc deposition layer even at 10 mA cm−2. Zinc powder with a large specific surface area and abundant electroactive sites has been widely used as the anode of ZIBs [127]. However, the increase of the surface area aggravates the thermodynamic and kinetic instability of zinc in the weak acidic electrolytes, which will produce a violent hydrogen evolution and corrosion reaction, so zinc powder is usually mixed with other materials to make a zinc powder composite anode. Zhi et al. [128] obtained the Ti3C2Tx@Zn electrode by Ti3C2Tx wrapping zinc powder through a solution mixing method.
Zinc surface modification is another strategy to improve the performance of zinc anodes. The aim is to achieve uniform zinc nucleation and a flat deposition layer on the surface of the zinc foil by constructing artificial interface layers [129]. Niu et al. [130] coated Ti3C2Tx on the surface of zinc foil(MZn) by self-assembly for the first time, inducing a homogeneous zinc deposition process. After this, various modified Mxene as a surface modification layer began to emerge. Zhi et al. [131] proposed a rare halogen-containing terminal Mxene as an artificial interface layer to regulate zinc deposition, and prepared (Ti3C2Cl2, Ti3C2Br2, and Ti3C2I2)@Zn composite anodes. Ti3C2@Zn heterogeneous structure, coupled with ion adsorption with orderly regulation of halogen terminals, jointly promote uniform deposition of zinc planes (Figure 9k,l). It is worth noting that good results can only be achieved when the lattice is matched between Mxene and metal anode, such as Ti3C2Cl2@Zn is the best performance here. It can cycle stably for 840 h at 2 mA cm−2 at a capacity of 1 mAh cm−2, and the polarization voltages are only 103 mV at 10 mA cm−2(Figure 9m,n). The emergence of this study has given researchers more inspiration. The zinc-ion full battery assembled with Zn/Co co-doped MnO/C (ZnCo-MnO/C) as the cathode, and Ti3C2Cl2@Zn as the anode attain at least 3000 cycles at 3.0 A g−1, showing good stability [132]. Qian et al. [133] constructed a composite protective film from MXene composite materials. First, S-loaded Ti3C2Tx (S@Ti3C2Tx) is combined with Zn, and then the S-doped Ti3C2Tx and ZnS composite film are obtained by high-temperature treatment, which is anchored on the zinc surface (S/Ti3C2Tx@ZnS@Zn) as a protective layer. S-Ti3C2Tx can effectively instruct uniform zinc deposition by uniform surface electric field distribution, reducing local current density, and mitigating volume changes during charge and discharge. The function of ZnS is to accelerate Zn2+ migration, further promote the homogenization of Zn2+ concentration, and inhibit side reactions (such as passivation, corrosion, H2 precipitation, etc.). The synergy between them additionally improves the stability of the surface protection film. This anode with a multifunctional composite interface coating is assembled into a symmetrical battery with good rate performance and cycle stability (up to 1600 h at 0.5 mA cm−2 under 0.5 mAh cm−2). Feng et al. [134] designed a flexible artificial protection interface based on self-assembled Ti3C2Tx nanosheets and chitosan (MX/CS) through a simple blade casting strategy, which achieved high reversibility of zinc metal anodes.
The zinc-preferred nucleation sites and uniformly interface electric field provided by the alloyed anode [127] make it a practical and effective solution for dendrite-free ZIBs. However, the violent volume expansion of such materials in the process of alloying/dealloying can easily lead to the crushing and collapse of the material structure, resulting in a sharp attenuation of material capacity. Qian et al. [135] obtained the Ti3C2Tx@Sb-300 composite backbone by annealing the product acquired through the substitution reaction of SbCl3 with Ti3C2Tx@Zn. The volume expansion during cycling is effectively mitigated by the introduction of flexible Ti3C2Tx, which additionally increases the overall integrity and conductivity of the material. The original Sb electrode exhibits rapid capacity degradation, with only 136 mA h g−1 capacity left after 100 cycles. Conversely, with similar initial capacities, the Ti3C2Tx@Sb-300 maintained 299.6 mAh g−1 even after 200 cycles, indicating a significant improvement in cycle performance.
The Zn2+ insertion/extraction potential of intercalation anodes is generally higher than that of zinc deposition/dissolution potential, which can effectively avoid dendrite problems. MXene is often used as a modified material to compound with other intercalation anodes to improve the performance of the original material. (NH4)2V10O25·8H2O@Ti3C2Tx (NHVO@Ti3C2Tx) [136] and Ti3C2Tx-TiS2 [137] are practically used in “rocking chair” ZIBs by Yuan’s group and Ni’s group. Ti3C2Tx’s conductive structure and stabilizing effect on the material give the composite a competitive rate performance and excellent long-cycle stability.
Zn2+ is carried by electrolyte, so the significance of the electrolyte is obvious. Thus, using of electrolyte additives to control the deposition/dissolution behavior of Zn2+ is also an irreplaceable strategy. Ti3C2Tx [138] was first used as an electrolyte additive to guide uniform Zn deposition by controlling the nucleation and growth process of Zn. Ti3C2Tx adsorbed on Zn foils, inducing uniform initial Zn deposition by providing abundant nucleation –O terminals and subsequently participating in the formation of an intact solid electrolyte interface (SEI) film. At the same time, ion transport can be accelerated to reduce the Zn2+ concentration gradient at the electrode/electrolyte interface. The symmetrical battery containing Ti3C2Tx is stable to 1180 cycles at 2 mA cm−2 under 1 mAh cm−2 capacity (only 118 cycles without Ti3C2Tx). Because of the problems in the aqueous solutions mentioned above, alternative non-liquid electrolytes (hydrogels and solid electrolytes, etc.) have been widely experimented with for ZIBs. Due to the low water content of the non-liquid electrolyte, the adverse side reactions (corrosion and passivation) caused by the presence of H2O molecules were avoided effectively. The key to high-performance non-liquid electrolytes is the conductive property, so high-ionic conductivity additives are often needed. Zhi et al. [139] added Ti3C2Tx of surface-grafted polymethyl acrylate (Ti3C2Tx-g-PMA) to the polyvinylidene fluoride-hexafluoropropylene (PVHF) solid polymer electrolyte (SPE), and the grafting of PMA was to improve the compatibility of PVHF with Ti3C2Tx. Ti3C2Tx greatly improves the ion conduction of SPE while reducing the crystallinity of the polymer. The SPE added by Ti3C2Tx can achieve a Zn2+ ion conductivity of 2.69 × 10−4 S cm−1 at room temperature, and reversible dendrite-free plating/stripping for over 1000 h.
Because of MXene’s large specific surface area and high conductivity, it can reduce the local current density as an electrodeposition substrate or electrolyte additive, make the electric field on the surface of Zn more uniform, and achieve dendrite-free zinc deposition. Its abundant surface activity makes it easy to be perfectly compatible with other materials. When it is used as a composite material for surface modification, accommodation of alloyed anodes, and construction of embedded anodes, it can achieve a more uniform Zn2+ distribution, giving the battery excellent zinc deposition stability.
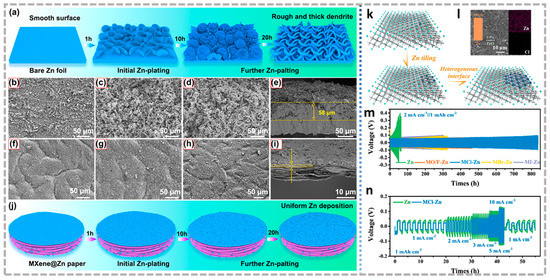
Figure 9.
Schematic diagram of Zn deposition on the (a) Zn foil and (j) Ti3C2Tx MXene@Zn paper; SEM images of Zn deposition after plating capacity up to (b,f) 1, (c,g) 10, (d,h) 20 mAh cm−2 on Zn foil and Ti3C2Tx MXene@Zn paper at 1 mA cm−2, respectively; Cross-sectional SEM images of (e) Zn foil and (i) Ti3C2Tx MXene@Zn paper corresponding to d and h, respectively [125]. (a–j) Reproduced with permission from ACS Nano. (k) Schematic illustration of the Zn deposition process on Ti3C2Cl2; (l) SEM images and EDX mapping of the Ti3C2Cl2-Zn electrode after cycling with a flat and smooth surface; (m) Long cycling performance of symmetric batteries; (n) Comparison of rate performance of bared Zn metal and Ti3C2Cl2–Zn symmetric batteries with a capacity of 1 mAh cm−2 [131]. (k–n) Reproduced with permission from ACS Nano.
3.4.2. Capacitive Electrode
In addition to solving the problem of zinc anodes, the researchers also explored MXene’s own zinc storage activity. Zeng’s group [140] used MnO2–CNTs as the cathode and Ti3C2Tx as the anode for charge/discharge tests, demonstrating the energy storage characteristics of hybrid capacitors. Furthermore, MXene is widely used in the field of flexible electronics because of its flexibility. Therefore, they further assembled δ-MnO2 on carbon cloth (δ-MnO2@CAC) and Ti3C2Tx on cotton cloth (MXene@COC) to prepare flexible zinc-ion hybrid capacitors(ZIHCs) [141]. The Ti3C2Tx, which exhibits the capacitance effect of zinc storage, keeps the power density of hybrid capacitors at a high level, even when used as cathodes in ZIHCs. Wang et al. [142] manufactured Ti3C2-Zn hybrid capacitors by combining Ti3C2 and Zn. To take the conductivity of MXene to the next level, N-doped Ti3C2 (N-Ti3C2) [143] was synthesized by a one-step hydrothermal method using urea as a N source. Compared with the C element, the additional electrons of N increase the conductivity of the material and increase the repulsion between layers, which inhibits the self-stacking trend of MXene material and gives the material good stability. The N-Ti3C2/Zn hybrid capacitors prepared with N-Ti3C2 as the cathode, Zn as the anode, and ZnSO4 as the electrolyte had a discharge capacitance of 247.9 F g−1 and an energy density of 45.54 Wh kg−1 at 0.1 A g−1. In addition to heteroatom doping, mixing with carbon materials is also a commonly used way to improve conductivity. Wang et al. [144] prepared Ti3C2Tx-reduced graphene oxide (Ti3C2Tx-rGO) as a cathode in ZIHCs, improving the conductivity of MXene by adding carbon materials. Ti3C2Tx-rGO shows a high capacitance of 128.6 F g−1 at 0.4 A g−1. Song et al. [145] prepared a DV2C@CNT cathode by adding carbon nanotubes (CNTs), which exhibit a high capacity of 190.2 F g−1 at 0.5 A g−1 with excellent rate performance and durability. Maughan et al. [146] prepared pillared Ti3C2 by in situ embedding CTAB, which showed higher capacity when assembled with a zinc anode to form a hybrid capacitor. The main reason is that the embedding CTAB increases the layer spacing of Ti3C2, which brings more adsorption site exposure. Fan et al. [147] also prepared Ti3C2-Zn hybrid capacitors, but he proposed for the first time that 3D printing technology improved the topography of Ti3C2, providing optimized carrier transport, simple electrolyte permeation, and sufficient porosity. The application of 3D printing technology in MXene provides the possibility for more MXene shapes. Although these hybrid capacitors have excellent performance, the application of zinc anodes still faces dendrite risks. Therefore, referring to the method mentioned above, Li et al. [148] showed a cycle life of more than 10,000 times at 3.3 A g−1 by alkalizing MXene-Zn composite (AMX-Zn) as the anode matched with the porous carbon as the cathode. Zhi et al. [149] used electrodeposited Zn@Ti3C2 anodes to improve the zinc anode cycling problem in the Ti3C2-Zn system.
3.4.3. Composite Redox Cathode
It can be seen that MXene’s storage of Zn2+ exhibits capacitive behavior, and the early application of MXene to ZIBs redox cathodes was achieved by constructing composite materials with known ZIBs active materials. In the composite system, the high conductivity of MXene can ensure the rapid transfer of electrons in the electrode and improve the rate characteristics and utilization rate of the active material. The active material is uniformly loaded on the MXene matrix, and the structural stability is effectively improved, which is conducive to improving cycling performance.
- Manganese-Based Material/MXene
Most of the cathode materials of ZIBs belong to transition metal oxides, such as redox materials based on manganese (Mn4+/Mn2+) and vanadium (such as V5+/V4+ in V2O5). Manganese oxides always suffer severe structural transformations, which will lead to large volume expansions and structural collapse during cycling. MXene can stabilize the structure by providing a stable support framework. Yan et al. [100] prepared 3D Ti3C2Tx@MnO2 composites by vapor spray drying a mixed solution of Ti3C2Tx and manganese nitrate. Ti3C2Tx protects MnO2 inside and forms a surface fold spherical shape under the impact of spray hot air flow, effectively constructing a solid and conductive 3D microflower structure (Figure 10a–c). The 3D structure improves the wettability of the electrolyte, and the high conductivity of Ti3C2Tx builds a three-dimensional conductive network, which greatly improves the dynamic properties of the material (Figure 10d,e). At the same time, the special 3D structure avoids the self-stacking of Ti3C2Tx in the cycle, and the material stability is improved. When used as ZIBs cathode, Ti3C2Tx@MnO2 microflowers demonstrate a high reversible capacity (∼301.2 mA h g−1 at 100 mA g−1), excellent rate capability and excellent cycle stability over 2000 cycles (Figure 10f,g). Luo et al. [150] obtained MnOx@Ti3C2Tx composite materials by uniformly growing manganese oxide (MnOx) on Ti3C2Tx two-dimensional surface by hydrothermal method. The MnOx@Ti3C2Tx composite retains Ti3C2Tx’s layered structure, similar to a parallel circuit. This parallel circuit distributes the current evenly in the layers to increase the upper limit of the electrode’s current tolerance, resulting in excellent zinc storage at high current densities. When the current density increases from 0.1 A g−1 to 10 A g−1, the capacity retention rate of the MnOx@Ti3C2Tx cathode is 50%. However, the specific capacity is only 82.5 mAh g−1 at 0.1 A g−1. In addition to manganese oxides, ZnMn2O4(ZMO), a zinc-rich cathode that realizes charge storage by reversible with MnO2, has been widely studied. However, during the cycle of ZMO, it is plagued by structural degradation (agglomeration and dissolution) and side reactions (ZnO generation), resulting in serious capacity loss during long cycles. Peng et al. [151] successfully synthesized ZMO@Ti3C2Tx composite structures by freeze-drying them after using the hydrothermal method. Ti3C2Tx inhibits the structural degradation and side reactions of ZMO while improving its kinetic performance and realizing long-cycle performance (92.4% capacity retention after 5000 cycles). In addition to compounding with Ti-based MXene, Zhu et al. [152] also uniformly anchored MnO2 to K-embedded V2CTx (K-V2C) by hydrothermal method. The K-V2C@MnO2 material remains 119.2 mAh g−1 capacity in long-term cycles (10,000 cycles) even at 10 A g−1.
- Vanadium-Based Materials/MXene
Similar to manganese oxide materials, vanadium oxide materials have poor intrinsic conductivity and vanadium dissolution problems, resulting in suboptimal cycle life and rate performance. As the first discovered MXene material, Ti3C2Tx was first used to study the composite with V-based zinc storage active materials. Pan et al. [153] prepared V2O5·nH2O/Ti3C2Tx (VOM) 3D flower-like composites by one-step hydrothermal method and freeze-drying. H2O embedding V2O5 and 3D structures provide rich active sites, laying the foundation for the realization of high capacity [154]. The excellent kinetic properties provided by Ti3C2Tx confer excellent rate properties on the composite cathode material (225 mAh g−1 at 2 A g−1). The alleviation of V2O5 deformation during cycling by the 3D structure was synergistic with the stabilization of the composite structure, achieving 60 mAh g−1 capacity, which remained after 500 cycles at 1 A g−1. At the same time, they also paid attention to the ratio of composites, and the lowest charge transfer impedance can be achieved with sufficient active sites at 20 wt% Ti3C2Tx. In addition, the high average valence state (4.67) of mixed V5+/V 4+ in H2V3O8 can accommodate many inserted Zn2+. Wu et al. [155] constructed H2V3O8/MXene composites by one-step hydrothermal method and freeze-drying. The composite structure provides a large number of feasible paths for the diffusion of Zn2+ and H2O, and in synergy with the high conductivity of MXene, the composite exhibits 365 mAh g−1 at 0.2 A g−1 (306 mAh g−1 for H2V3O8), higher rate performance of 73 mAh g−1 at 20 A g−1 (24 mAh g−1 for H2V3O8), and better cycling performance up to 5600 cycles at 5 A g−1 (only 900 cycles for H2V3O8). However, Wei et al. [156] synthesized a flexible, independent VO2/Ti3C2Tx hybrid film with a three-dimensional interleaved network using only ultrasound methods. Unlike constructing the 3D structure, Guo et al. [157] designed the Ti3C2Tx MXene layer on the surface of the V2O5 nanoplate (VPMX) by van der Waals self-assembly method under ultrasound to inhibit vanadium dissolution in the electrochemical process, thereby greatly improving its zinc-ion storage performance.
With the deepening of MXene research, the research of V-matrix active materials and V-based MXene composites began to appear. Interestingly, because it contains the same metallic elements, V-based MXene can be used not only as a frame structure but also as a precursor material. MXene’s easy oxidation allows it to partially or completely oxidize to form vanadium oxide active materials. The first to exploit the instability of MXene materials in ZIBs was Zhi et al. [158], who found a large capacity increase during cycling when they used V2CTx as the cathode of aqueous zinc/lithium-ion hybrid batteries. The co-embedding of Li+/Zn2+ increases the layer spacing, which in turn peels off V2CTx, exposing more active sites and exposing V to the electrolyte. During the cycling process, V2CTx is partially oxidized to V2O5, which is equivalent to in situ conversion to generate V2O5/V2CTx, greatly improving the capacity and rate performance of the material (Figure 10h,i). This discovery has inspired researchers, and a large number of research protocols have emerged to activate the zinc storage performance of V-based MXene in recent years. Chou et al. [159] achieved the transition from V2+/V3+ to V4+/V5+ on the surface of V2CTx by precisely controlling the voltage (1.8V) and activation time (2h), which is a process of electrochemical in situ oxidation to form a VOx/V2CTx composite structure. The activated VOx/V2CTx cathode showed a high capacity (423.5 mAh g−1 at 1 A g−1) and excellent rate performance (358 mAh g−1 at 30 A g−1). Later, Narayanasamy et al. [160] generated V2O5@V2C by partially oxidizing V2CTx through controlling hydrothermal conditions (180 °C, 12 h), achieving high-performance Zn2+ storage. Tian et al. [161] used V2CTx as a precursor to oxidize V2CTx by one-step annealing to prepare a porous two-dimensional V2O5 material. V2O5 inherits the V2CTx accordion-like structure and has good structural stability, which guarantees fast charge transport and reversible transport of zinc ions. In addition, Qin et al. [162] achieved the oxidation of V2CTx using a high temperature during in situ generation of HF. The V2Ox@V2CTx was directly synthesized by etching V2AlC at 90 °C by NaF+HCl.
Chen et al. [95] successfully synthesized VO2@V2COx by combining hydrothermal and electrochemical oxidation. They first prepared VO2@V2C heterostructures by hydrothermal reactions of V2C with H2O2 mixtures. Compared with traditional methods, the shorter length of the prepared VO2 nanorods shortens the diffusion path. Coupled with the good conductivity and wettability of the V2C frame, the VO2@V2C rate performance and stability are improved, but the performance in terms of capacity is not satisfactory. Therefore, they prepared a VO2@V2COx by increasing the electrode working voltage activation V2C with reference to the electrochemical activation method, and the capacity was greatly improved while maintaining the rate of performance (327 mAh g−1 at 5 A g−1).
When the V2CTx is oxidized, the ion intercalation can also be performed synchronously, so as to combine the advantages of the two modification methods to obtain a high zinc storage active cathode material. Shen et al. [163] soaked V2CTx powder in NaOH solution to perform Na+ intercalation of V2CTx MXene to increase the layer spacing. Afterwards, the V2CTx of the Na+ intercalation was hydrothermally treated in ZnCl2 and H2O2 solution, and Zn2+ intercalation and the oxidation of V2CTx were realized simultaneously. The generated ZnxV2O5 (ZVO) nanoribbons are uniformly distributed on the surface of V2CTx, and ZnxV2O5∙nH2O-V2CTx (VC-ZVO) hybrid materials are obtained with high conductivity and large lattice channels. Electrochemical kinetic tests show that the pre-embedded Zn2+ effectively increases the lattice spacing of vanadium oxides, while the V4+ generated by in situ oxidation can provide more d-electrons, thereby reducing the activation energy of charge transfer at the electrode/electrolyte interface. In addition, the charge redistribution at the heterogeneous interface between ZVO and V2CTx weakens the electrostatic interaction of Zn2+, which promotes its diffusion. Therefore, the VC-ZVO electrode shows amazing electrochemical performance, and 96.4% capacity retention can be achieved after 8000 cycles at 10 A g−1. Using a similar method, the same group synthesized pre-embedded composites MnxV10O24·nH2O@V2CTx (MVO@VC), LixV2O5·H2O@V2CTx (LVO@VC) and AlxV2O5· H2O@V2CTx [164]. As cathode materials for ZIBs, they exhibit improved performance.
In the process of studying the electrochemical in situ oxidation of V2CTx, Zhi et al. also found that the XRD peak strength of the residual precursor V2AlC weakened and disappeared during the cycling process. Therefore, they replaced the cathode material with V2AlC in the same electrolyte system (21 M LiTFSI + 1 M Zn (OTf)2) [165], with zinc metal as anode assembled into ZIB [166]. During the cycle, V2AlC is in situ etched into V2CTx MXene by a fluorine-rich electrolyte inside the battery. This method is a one-step green etching, peeling method without any acid/alkali to prepare MXene. ZIB always works normally during the etching process and exhibits a gradually increasing specific capacity with the formation of MXene and the oxidation product V2O5.
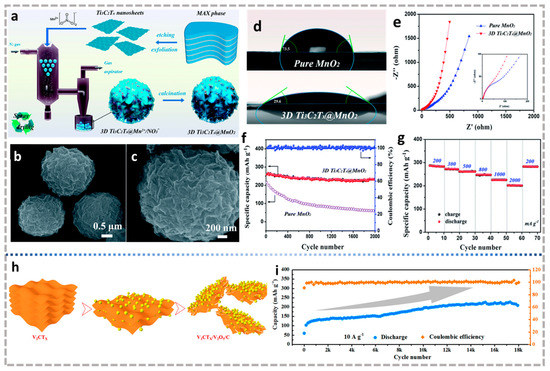
Figure 10.
(a) Schematic of 3D Ti3C2Tx@MnO2 microflower synthesis progress. (b,c) SEM images of 3D Ti3C2Tx@MnO2 microflowers with different magnifications. (d) Contact angles of droplets of electrolyte on the surface of MnO2 and 3D Ti3C2Tx@MnO2 microflower. (e) Comparison of EIS for MnO2 and 3D Ti3C2Tx@MnO2 microflower cathodes in aqueous ZIBs. (f) Long-term cycling stability at 500 mA g−1; (g) rate performance of 3D Ti3C2Tx@MnO2 microflowers in aqueous ZIBs [100]. (a–g) Reproduced with permission from the Royal Society of Chemistry. (h) Schematic diagram of possible structural changes in the V2CTx cathode during cycling. (i) Long-term cycling performance of V2CTX cathode at 10 A g−1 [158]. (h,i) Reproduced with permission from ACS Nano.
- Other Categories Materials/MXene
In addition to Mn-based and V-based cathode materials, MXene materials can also be compounded with other categories of ZIBs cathode materials. Layered transition metal chalcogenides (TMCs) are also potential candidates for ZIBs cathode materials, and low conductivity and collapse-prone layered structures are the main factors plaguing the cycling performance of TMCs. Long et al. [167] synthesized 1 T-MoS2/Ti3C2 heterojunctions by in situ hydrothermal method (Figure 11a), and used them as ZIBs cathode material to demonstrate high capacity, rate performance (284.3 mAh g−1 at 0.10 A g−1 and 105.2 mAh g−1 at 10.00 A g−1) and cycle stability (93.2% capacity retention after 3000 cycles, Figure 11b), all higher than pristine 1 T-MoS2. The elevated capacity is due to the richer ion storage space caused by the expansion layers of 1 T-MoS2. Excellent rate capability thanks to ultrafast electron and ion transport from Ti3C2. The outstanding long-term cycle stability is attributed to the synergy effect of 1 T-MoS2 and Ti3C2 in 3D interconnected networks. Gao et al. [168] also applied MXene to the MoO3 cathode with high capacity and low conductivity to prepare MoO3−x/Ti3C2Tx composites. MoO3−x/Ti3C2Tx, as the ZIBs cathode, provides an ultra-large capacity of 369.8 mAh g−1 at 0.20 A g−1, maintaining 46.7% capacity after 1600 cycles. Inspired by in situ oxidation of V-based MXene to produce vanadium oxides, Sun et al. [97] proposed a gentle one-step surface selenization strategy, which has successfully synthesized VSe2@V2CTx, TiSe2@Ti3C2Tx, and NbSe2@Nb2CTx. MXene’s surface metal atoms are converted into high-capacity transition metal selenides (TMSe2), while MXene’s inner layer is deliberately preserved to improve overall conductivity and structural stability. Typically, VSe2@V2CTx cathode provides a high-rate capability (132.7 mA h g−1 at 2.0 A g−1) and long-term cycling capability (93.1% capacity retention at 2.0 A g−1 after 200 cycles). Similarly, many cathode materials suffer from low conductivity, including two-dimensional layered double hydroxides (LDHs). Han et al. [169] obtained Co-doped NiMn-LDH@V2CTx (CNMV) by a one-step hydrothermal method. V2CTx as a frame structure improves the overall conductivity, and the specific capacity of CNMV as ZIBs cathode material is 322.7 mAh g−1 after 100 cycles at 0.2 A g−1. In addition to compounding with highly conductive materials, finding active materials with high intrinsic conductivity is also an option. Studies have found that many conductive polymers exhibit energy storage activity. Polyaniline (PANI) has been applied to ZIBs as a typical conductive polymer, but volumetric strain and protonation hinder its stable cycling. Huang et al. [170] used sulfonated group grafting Ti3C2Tx MXene (S-Ti3C2Tx) with high conductivity, good flexibility to composite with PANI. S-Ti3C2Tx continuously contributes to protons and locally delivers high acidity, keeping the polymer at a low pH, effectively inhibiting the deprotonation of PANI (Figure 11c). Consequently, high discharge capacity (262 mAh g−1 at 0.5 A g−1), excellent rate performance (160 mAh g−1 at 15 A g−1, Figure 11d) and cycle stability (more than 5000 cycles) are simultaneously demonstrated on the S-Ti3C2Tx-enhanced PANI cathode.
Figure 11.
(a) Synthesis schematic of 1 T-MoS2/Ti3C2 MXene. (b) Cycle performance at 1.00 A g−1 of 1 T-MoS2/Ti3C2 MXene and 1 T-MoS2 [167]. (a,b) Reproduced with permission from ELSEVIER BV. (c) Schematic of S-Ti3C2Tx/PANI preparation. (d) Rate performance of S-Ti3C2Tx/PANI [170]. (c,d) Reproduced with permission from ACS Nano.
3.4.4. Redox Cathode
Previous studies have shown that MXene itself exhibits capacitive behavior for Zn2+ storage. However, inspired by the conversion of V-based MXene, changes in voltage and surface terminal groups may stimulate the redox behavior of MXene. Nb2CTx/Zn battery exhibits capacitive behavior when the voltage is lower than 2.0 V, and Zhi et al. successfully activated the Nb2CTx zinc storage voltage platform at 1.55 V by extending the voltage window from 2.0 V to 2.4 V [171]. The results of molecular dynamics simulation tell us that sufficient energy can be assimilated to pierce the embedding barrier when the voltage reaches a certain value. Zn2+ is further embedded between layers from simple surface adsorption to exhibit a redox plateau. They also increased the voltage to activate the redox zinc storage behavior of Ti3C2Tx, which is expected to be applied to more MXene materials. Because the activation voltage is much higher than the electrochemical window of water (1.23 V), the implementation of this method requires the guarantee of high salt concentration.
The above research on multivalent ion batteries basically focuses on M materials in MXene, and little is known about X materials, let alone Tx, which makes the rich surface chemistry of MXene not reflected in multivalent ion batteries. In 2021, Zhi et al. [172] first studied the effect of terminal groups on the zinc storage performance of Ti3C2, and successfully activated the redox activity of Ti3C2 by changing the terminal group. As mentioned above, Ti3C2Cl2 and Ti3C2(OF) exhibit zinc storage capacitance behavior without an obvious plateau. Varying their terminal groups, Ti3C2Br2 and Ti3C2I2 successfully exhibited different discharge plates with capacities of 97.6 and 135 mAh g−1, respectively (Figure 12). Taking Ti3C2Br2 as an example to illustrate its zinc storage mechanism, there is a 2 Br− −2 e−↔ 2 Br0 redox reaction during the electrode charge/discharge process, followed by the following reaction Br− + (n − 1) Br0 ↔ Brn− (3 ≤ n ≤ 5), and finally Zn2+ achieves deintercalation/intercalation. The success of this experiment provides strong proof of the surface chemistry of MXene and will greatly increase the confidence of researchers in the exploration of MXene surface chemistry. The applications of MXene in ZIBs are summarized in Table 4.
Figure 12.
Cyclic voltammetry curve (CV) of (a) Ti3C2(OF), Ti3C2Cl2, (b) Ti3C2Br2, Ti3C2I2, (c) Ti3C2(BrI), and Ti3C2(ClBrI) at 1 mV s−1. (d) GCD curves of Ti3C2Tx with terminals corresponding to (a–c) at 0.5 A g−1 [172]. (a–d) Reproduced with permission from ACS Nano.

Table 4.
Applications of MXene and MXene-based composites in ZIBs.
In summary, MXene is used independently as an electrode material for MMIBs with the following challenges: slope curves that only reflect capacitive effects, such as the zinc storage process of Ti3C2; the low diffusivity brought by the large radius of polyvalent ions, which is most obvious in MIBs; and the problem of the collapse of the structure during cycling caused by the high charge of carriers, which is most obvious in AIBs. Nevertheless, the application of MXene in MMIBs is developing rapidly. The researchers not only explored the multivalent ion storage activity of MXene itself but also utilized the high conductivity and surface activity of MXene as a substrate material and artificial interface layer to achieve excellent cycling and rate performance. Although many excellent results have been achieved, they are generally concentrated in several classes of MXene materials, such as Ti-based (Ti3C2, Ti2C) and V-based (V2C). The calcium storage activity of MXene is only at the theoretical level, and there is no experimental study to prove it. Research on the surface chemistry of MXene by changing terminals is also limited, as is topography.
4. Conclusions
Herein, we first introduce the synthesis methods of MXene materials, including fluorine-containing and fluorine-free etching methods as well as non-etching methods. Generally, the subsequent peeling process is closely followed, and the obtained few-layer MXene not only avoids agglomeration but also provides more active sites. The stability, mechanical and electronic properties of MXene were then analyzed. In order to make the MXene material stable, its surface-active sites should be protected as much as possible, and secondly, interlayer stacking can be avoided by expanding layer spacing, constructing three-dimensional structures, and compounding with other materials. The excellent elastic coefficient is a strong contender for flexible electrodes, and the desired electronic properties can also be achieved through the design of terminal groups. Finally, we focus on practical applications in MMIBs. Because of its large specific surface area, hydrophilicity, and high conductivity, it is used as an electrode coating and electrolyte additive, greatly enriching the diversity of MXene applications. We found that MXene has polyvalent ion storage activity, but has the characteristics of low capacity and poor cycling performance. One feasible approach is to activate MXene energy storage activity by electrochemistry or surface terminal groups, and another method is to use intercalated substances or to construct composite materials with other active materials to achieve high capacity, exceptional rate performance, and long-cycle life.
5. Outlooks
The research on MXene materials is rich and diverse, but there is still a long way to go to meet the requirements of industrial production. There are still a number of issues that deserve our attention and need to be addressed urgently.
(1) The stability of MXene material. MXene materials are very sensitive to oxygen and water. The high surface activity makes the layered structure of MXene prone to collapse, and it is important to stabilize MXene materials in practical applications.
(2) Synthesis of MXene material. So far, more than 30 kinds of MXene materials have been discovered, but there are more than 70 MAX precursors. Each MXene has its own unique etching parameters, so matching the synthesis conditions for each MXene is essential to broaden the MXene family.
(3) MXene species. The application of MXene is still only in the common Ti3C2, V2C, etc., but different components of MXene may bring unexpected results. For example, nitrogen-doped carbides and nitride MXene exhibit higher electrical conductivity, while there is little research on nitride MXene due to synthesis difficulty. Therefore, putting more types of MXene into practice is a major topic for subsequent research, in which the issue of cost should also be considered.
(4) Terminal group of MXene material. MXene material has rich surface chemistry, which can change the terminal group to change its properties, thereby broadening the application scenarios. Although MXene materials of various terminal groups such as NH, S, Cl, Se, Br, Te, etc. can be prepared by post-treatment methods, it is still very rare to apply them to practice. The general lack of attention to the terminal groups on the surface undoubtedly deprives MXene materials of a significant advantage.
(5) Composite structure. Because of its high surface activity and high electrical conductivity, MXene is often used to build composite materials with other materials. However, whether all materials can be combined and whether they can have a favorable effect on the performance after compounding should be summarized in this regard. The optimal composite ratio should also be discussed.
(6) Application in MMIBs. Because of the inherent problem of multivalent ions as carriers, there is still a huge space for exploration in the application of MXene in MMIBs. For example, the application of MXene in CIBs is still in the theoretical research stage, and experiments are urgently needed to prove it. The second adsorption layer of MIBs can exhibit dissolution/deposition behavior, and how to release the capacity of this part is also a question worth considering. The problem of actual carriers in AIBs is also worth studying. Whether the method of activating more redox activity of ZIBs can be extended to other materials or other fields will also be an interesting research topic.
(7) MXene exhibits the characteristics of pseudocapacitance in the storage of MMIBs, and although it has high conductivity, its low capacity cannot meet the needs of high-energy devices. Fortunately, composites partially compensate for these shortcomings, but they are still a long way from practical applications. Commercial LiFeO4 materials can still maintain 90% capacity after more than 6000 cycles at 1 C, while MXene is rarely achieved even based on composite materials. However, because MMIBs are different from LIBs, this comparison is not convincing. Therefore, determining the application scenarios and evaluation criteria of MMIBs is convenient for more objective evaluations. Another limitation is that the amount of material used in the lab is small and the cost is often rarely considered. In this regard, titanium-based MXene is the most attractive because precursors (i.e., Ti, Al, C) are relatively abundant and inexpensive.
To date, there have been many fantastic research works on the application of MXenes in MMIBs. It is believed that the application of MXene will be extended to the fields of supercapacitors and catalysis on the basis of these achievements.
Author Contributions
Conceptualization, X.P. and Z.C.; investigation, C.W. and Z.P.; data curation, C.W. and H.C.; writing—original draft preparation, C.W., Z.P. and H.C.; writing—review and editing, C.W., X.P. and Z.C.; supervision, X.P. and Z.C.; funding acquisition, Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (21875171 and U22A20438) and the Fundamental Research Funds for the Central Universities.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guan, P.; Zhou, L.; Yu, Z.; Sun, Y.; Liu, Y.; Wu, F.; Jiang, Y.; Chu, D. Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries. J. Energy Chem. 2020, 43, 220–235. [Google Scholar] [CrossRef]
- Hussain, I.; Lamiel, C.; Sufyan Javed, M.; Ahmad, M.; Chen, X.; Sahoo, S.; Ma, X.; Bajaber, M.A.; Zahid Ansari, M.; Zhang, K. Earth- and marine-life-resembling nanostructures for electrochemical energy storage. Chem. Eng. J. 2023, 454, 140313. [Google Scholar] [CrossRef]
- Kim, T.-H.; Park, J.-S.; Chang, S.K.; Choi, S.; Ryu, J.H.; Song, H.-K. The Current Move of Lithium Ion Batteries Towards the Next Phase. Adv. Energy Mater. 2012, 2, 860–872. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Fu, C.; Ding, Y.; Liu, G.; Cao, Y.; Chen, Z. Recent Advances in Conversion-Type Electrode Materials for Post Lithium-Ion Batteries. ACS Mater. Lett. 2021, 3, 956–977. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Huang, G.; Bayhan, Z.; Alshareef, H.N. MXenes for Rechargeable Batteries Beyond the Lithium-Ion. Adv. Mater. 2021, 33, 2004039. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Kravchyk, K.V.; Widmer, R.; Erni, R.; Dubey, R.J.C.; Krumeich, F.; Kovalenko, M.V.; Bodnarchuk, M.I. Copper sulfide nanoparticles as high-performance cathode materials for Mg-ion batteries. Sci. Rep. 2019, 9, 7988. [Google Scholar] [CrossRef]
- Dymek, C.J.; Williams, J.L.; Groeger, D.J.; Auborn, J.J. An Aluminum Acid-Base Concentration Cell Using Room Temperature Chloroaluminate Ionic Liquids. J. Electrochem. Soc. 1984, 131, 2887. [Google Scholar] [CrossRef]
- Geng, D.; Yang, H.Y. Recent Advances in Growth of Novel 2D Materials: Beyond Graphene and Transition Metal Dichalcogenides. Adv. Mater. 2018, 30, 1800865. [Google Scholar] [CrossRef]
- Li, S.-L.; Tsukagoshi, K.; Orgiu, E.; Samorì, P. Charge transport and mobility engineering in two-dimensional transition metal chalcogenide semiconductors. Chem. Soc. Rev. 2016, 45, 118–151. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.J.; Liao, C.; Hafez, A.M.; Zhu, H.L.; Wu, S.P. Two-dimensional MXenes for energy storage. Chem. Eng. J. 2018, 338, 27–45. [Google Scholar] [CrossRef]
- Mateen, A.; Ansari, M.Z.; Hussain, I.; Eldin, S.M.; Albaqami, M.D.; Bahajjaj, A.A.A.; Javed, M.S.; Peng, K.-Q. Ti2CTx–MXene aerogel based ultra–stable Zn–ion supercapacitor. Compos. Commun. 2023, 38, 101493. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Sun, H.; Zhou, J.; Yang, F.; Li, H.; Chen, H.; Chen, Y.; Liu, Z.; Qiu, Z.; et al. Progress and Perspective: MXene and MXene-Based Nanomaterials for High-Performance Energy Storage Devices. Adv. Electron. Mater. 2021, 7, 2000967. [Google Scholar] [CrossRef]
- Greaves, M.; Barg, S.; Bissett, M.A. MXene-Based Anodes for Metal-Ion Batteries. Batter. Supercaps 2020, 3, 214–235. [Google Scholar] [CrossRef]
- Cheng, L.; Li, X.; Zhang, H.; Xiang, Q. Two-Dimensional Transition Metal MXene-Based Photocatalysts for Solar Fuel Generation. J. Phys. Chem. Lett. 2019, 10, 3488–3494. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Deysher, G.; Shuck, C.E.; Hantanasirisakul, K.; Frey, N.C.; Foucher, A.C.; Maleski, K.; Sarycheva, A.; Shenoy, V.B.; Stach, E.A.; Anasori, B.; et al. Synthesis of Mo4VAlC4 MAX Phase and Two-Dimensional Mo4VC4 MXene with Five Atomic Layers of Transition Metals. ACS Nano 2020, 14, 204–217. [Google Scholar] [CrossRef]
- Tao, Q.; Dahlqvist, M.; Lu, J.; Kota, S.; Meshkian, R.; Halim, J.; Palisaitis, J.; Hultman, L.; Barsoum, M.W.; Persson, P.O.Å.; et al. Two-dimensional Mo1.33C MXene with divacancy ordering prepared from parent 3D laminate with in-plane chemical ordering. Nat. Commun. 2017, 8, 14949. [Google Scholar] [CrossRef]
- Thörnberg, J.; Halim, J.; Lu, J.; Meshkian, R.; Palisaitis, J.; Hultman, L.; Persson, P.O.Å.; Rosen, J. Synthesis of (V2/3Sc1/3)2AlC i-MAX phase and V2−xC MXene scrolls. Nanoscale 2019, 11, 14720–14726. [Google Scholar] [CrossRef]
- Zhu, J.; Ha, E.; Zhao, G.; Zhou, Y.; Huang, D.; Yue, G.; Hu, L.; Sun, N.; Wang, Y.; Lee, L.Y.S.; et al. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coord. Chem. Rev. 2017, 352, 306–327. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K.; Ricciardulli, A.G.; Zhang, P.; Liao, Z.; Lohe, M.R.; Zschech, E.; Blom, P.W.M.; Pisula, W.; Müllen, K.; et al. A Delamination Strategy for Thinly Layered Defect-Free High-Mobility Black Phosphorus Flakes. Angew. Chem. Int. Ed. 2018, 57, 4677–4681. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef]
- Cui, G.; Zheng, X.; Lv, X.; Jia, Q.; Xie, W.; Gu, G. Synthesis and microwave absorption of Ti3C2Tx MXene with diverse reactant concentration, reaction time, and reaction temperature. Ceram. Int. 2019, 45, 23600–23610. [Google Scholar] [CrossRef]
- Liu, F.F.; Zhou, A.G.; Chen, J.F.; Jin, J.; Zhou, W.J.; Wang, L.B.; Hu, Q.K. Preparation of Ti3C2 and Ti2C MXenes by fluoride salts etching and methane adsorptive properties. Appl. Surf. Sci. 2017, 416, 781–789. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, L.M.; Tang, H.; Du, F.; Guo, Y.H.; Qiu, T.; Yang, J. Synthesis of two-dimensional Ti3C2Tx MXene using HCl plus LiF etchant: Enhanced exfoliation and delamination. J. Alloys Compd. 2017, 695, 818–826. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Liu, F.F.; Zhou, J.; Wang, S.W.; Wang, B.X.; Shen, C.; Wang, L.B.; Hu, Q.K.; Huang, Q.; Zhou, A.G. Preparation of High-Purity V2C MXene and Electrochemical Properties as Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A709–A713. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Dyatkin, B.; Gogotsi, Y.; Barsoum, M.W. Kinetics of aluminum extraction from Ti3AlC2 in hydrofluoric acid. Mater. Chem. Phys. 2013, 139, 147–152. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Näslund, L.-Å.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Urbankowski, P.; Anasori, B.; Makaryan, T.; Er, D.Q.; Kota, S.; Walsh, P.L.; Zhao, M.Q.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene). Nanoscale 2016, 8, 11385–11391. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Man Hong, S.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef]
- Naguib, M.; Unocic, R.R.; Armstrong, B.L.; Nanda, J. Large-scale delamination of multi-layers transition metal carbides and carbonitrides “MXenes”. Dalton Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef]
- Mashtalir, O.; Lukatskaya, M.R.; Zhao, M.-Q.; Barsoum, M.W.; Gogotsi, Y. Amine-Assisted Delamination of Nb2C MXene for Li-Ion Energy Storage Devices. Adv. Mater. 2015, 27, 3501–3506. [Google Scholar] [CrossRef]
- Ma, R.; Sasaki, T. Two-Dimensional Oxide and Hydroxide Nanosheets: Controllable High-Quality Exfoliation, Molecular Assembly, and Exploration of Functionality. Acc. Chem. Res. 2015, 48, 136–143. [Google Scholar] [CrossRef]
- Xu, M.; Lei, S.L.; Qi, J.; Dou, Q.Y.; Liu, L.Y.; Lu, Y.L.; Huang, Q.; Shi, S.Q.; Yan, X.B. Opening Magnesium Storage Capability of Two-Dimensional MXene by Intercalation of Cationic Surfactant. ACS Nano 2018, 12, 3733–3740. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, P.P.; Wang, F.X.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X.L. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem. Int. Ed. 2018, 57, 15491–15495. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yuan, M.; Lin, L.; Yang, H.; Nan, C.; Li, H.; Sun, G.; Yang, X. Selective Lithiation–Expansion–Microexplosion Synthesis of Two-Dimensional Fluoride-Free Mxene. ACS Mater. Lett. 2019, 1, 628–632. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Li, Y.B.; Shao, H.; Lin, Z.F.; Lu, J.; Liu, L.Y.; Duployer, B.; Persson, P.O.A.; Eklund, P.; Hultman, L.; Li, M.; et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020, 19, 894–899. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.C.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Tsipas, P.; Speliotis, T.; Kordas, G.; Vavouliotis, A.; Dimoulas, A. Insight and control of the chemical vapor deposition growth parameters and morphological characteristics of graphene/Mo2C heterostructures over liquid catalyst. J. Cryst. Growth 2018, 495, 46–53. [Google Scholar] [CrossRef]
- Zhao, H.; Cai, K.; Ma, Z.; Cheng, Z.; Jia, T.; Kimura, H.; Fu, Q.; Tao, H.; Xiong, L. Synthesis of molybdenum carbide superconducting compounds by microwave-plasma chemical vapor deposition. J. Appl. Phys. 2018, 123, 053301. [Google Scholar] [CrossRef]
- Meshkian, R.; Ingason, A.S.; Dahlqvist, M.; Petruhins, A.; Arnalds, U.B.; Magnus, F.; Lu, J.; Rosen, J. Theoretical stability, thin film synthesis and transport properties of the Mon +1GaCn MAX phase. Phys. Status Solidi (RRL) Rapid Res. Lett. 2015, 9, 197–201. [Google Scholar] [CrossRef]
- Meshkian, R.; Näslund, L.-Å.; Halim, J.; Lu, J.; Barsoum, M.W.; Rosen, J. Synthesis of two-dimensional molybdenum carbide, Mo2C, from the gallium based atomic laminate Mo2Ga2C. Scr. Mater. 2015, 108, 147–150. [Google Scholar] [CrossRef]
- Verger, L.; Natu, V.; Carey, M.; Barsoum, M.W. MXenes: An Introduction of Their Synthesis, Select Properties, and Applications. Trends Chem. 2019, 1, 656–669. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.-H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Xie, Y.; Dall’Agnese, Y.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W.; Zhuang, H.L.; Kent, P.R.C. Prediction and Characterization of MXene Nanosheet Anodes for Non-Lithium-Ion Batteries. ACS Nano 2014, 8, 9606–9615. [Google Scholar] [CrossRef]
- Wang, H.-W.; Naguib, M.; Page, K.; Wesolowski, D.J.; Gogotsi, Y. Resolving the Structure of Ti3C2Tx MXenes through Multilevel Structural Modeling of the Atomic Pair Distribution Function. Chem. Mater. 2016, 28, 349–359. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Z.R.X.; Zhu, Q.Z.; Anasori, B.; Gogotsi, Y.; Xu, B. Enhanced Ionic Accessibility of Flexible MXene Electrodes Produced by Natural Sedimentation. Nano-Micro Lett. 2020, 12, 89. [Google Scholar] [CrossRef]
- Zhou, J.F.; Lin, S.; Huang, Y.N.; Tong, P.; Zhao, B.C.; Zhu, X.B.; Sun, Y.P. Synthesis and lithium ion storage performance of two-dimensional V4C3 MXene. Chem. Eng. J. 2019, 373, 203–212. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.Z.; Miao, J.W.; Zhang, P.; Wan, P.B.; He, L.Z.; Xu, B. Flexible 3D Porous MXene Foam for High-Performance Lithium-Ion Batteries. Small 2019, 15, 1904293. [Google Scholar] [CrossRef]
- Ren, C.E.; Zhao, M.-Q.; Makaryan, T.; Halim, J.; Boota, M.; Kota, S.; Anasori, B.; Barsoum, M.W.; Gogotsi, Y. Porous Two-Dimensional Transition Metal Carbide (MXene) Flakes for High-Performance Li-Ion Storage. ChemElectroChem 2016, 3, 689–693. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, C.; Zhou, G.; Pan, Z.-Z.; Ma, J.; Nishihara, H.; He, Y.-B.; Kang, F.; Lv, W.; Yang, Q.-H. Lamellar MXene Composite Aerogels with Sandwiched Carbon Nanotubes Enable Stable Lithium–Sulfur Batteries with a High Sulfur Loading. Adv. Funct. Mater. 2021, 31, 2100793. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, J.; Si, C.; Sun, Z. Flexible two-dimensional Tin+1Cn (n = 1, 2 and 3) and their functionalized MXenes predicted by density functional theories. Phys. Chem. Chem. Phys. 2015, 17, 15348–15354. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.H.; Zhang, Q.F.; Legut, D.; Si, C.; Germann, T.C.; Lookman, T.; Du, S.Y.; Francisco, J.S.; Zhang, R.F. Stabilization and strengthening effects of functional groups in two-dimensional titanium carbide. Phys. Rev. B 2016, 94, 104103. [Google Scholar] [CrossRef]
- Zha, X.-H.; Luo, K.; Li, Q.; Huang, Q.; He, J.; Wen, X.; Du, S. Role of the surface effect on the structural, electronic and mechanical properties of the carbide MXenes. Europhys. Lett. 2015, 111, 26007. [Google Scholar] [CrossRef]
- Kurtoglu, M.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W. First principles study of two-dimensional early transition metal carbides. MRS Commun. 2012, 2, 133–137. [Google Scholar] [CrossRef]
- Plummer, G.; Anasori, B.; Gogotsi, Y.; Tucker, G.J. Nanoindentation of monolayer Tin+1CnTx MXenes via atomistic simulations: The role of composition and defects on strength. Comput. Mater. Sci. 2019, 157, 168–174. [Google Scholar] [CrossRef]
- Si, C.; Jin, K.-H.; Zhou, J.; Sun, Z.; Liu, F. Large-Gap Quantum Spin Hall State in MXenes: D-Band Topological Order in a Triangular Lattice. Nano Lett. 2016, 16, 6584–6591. [Google Scholar] [CrossRef] [PubMed]
- Dillon, A.D.; Ghidiu, M.J.; Krick, A.L.; Griggs, J.; May, S.J.; Gogotsi, Y.; Barsoum, M.W.; Fafarman, A.T. Highly Conductive Optical Quality Solution-Processed Films of 2D Titanium Carbide. Adv. Funct. Mater. 2016, 26, 4162–4168. [Google Scholar] [CrossRef]
- Lai, S.; Jeon, J.; Jang, S.K.; Xu, J.; Choi, Y.J.; Park, J.-H.; Hwang, E.; Lee, S. Surface group modification and carrier transport properties of layered transition metal carbides (Ti2CTx, T: -OH, -F and -O). Nanoscale 2015, 7, 19390–19396. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and Optical Properties of 2D Transition Metal Carbides and Nitrides (MXenes). Adv. Mater. 2018, 30, 1804779. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Yunoki, S. Topological insulators in the ordered double transition metals M′2M″C2 MXenes (M′ = Mo, W; M″ = Ti, Zr, Hf). Phys. Rev. B 2016, 94, 125152. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.-Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel Electronic and Magnetic Properties of Two-Dimensional Transition Metal Carbides and Nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Enyashin, A.N.; Ivanovskii, A.L. Two-dimensional titanium carbonitrides and their hydroxylated derivatives: Structural, electronic properties and stability of MXenes Ti3C2−xNx(OH)2 from DFTB calculations. J. Solid State Chem. 2013, 207, 42–48. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of Large-Area Graphene Films for High-Performance Transparent Conductive Electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y.; Yu, X.Q.; Nam, K.W.; Yang, X.Q.; Kolesnikov, A.I.; Kent, P.R.C. Role of Surface Structure on Li-Ion Energy Storage Capacity of Two-Dimensional Transition-Metal Carbides. J. Am. Chem. Soc. 2014, 136, 6385–6394. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.A.; Shao, X.F.; Li, F.; Zhao, M.W. Anchoring effects of S-terminated Ti2C MXene for lithium-sulfur batteries: A first-principles study. Appl. Surf. Sci. 2018, 455, 522–526. [Google Scholar] [CrossRef]
- Hope, M.A.; Forse, A.C.; Griffith, K.J.; Lukatskaya, M.R.; Ghidiu, M.; Gogotsi, Y.; Grey, C.P. NMR reveals the surface functionalisation of Ti3C2 MXene. Phys. Chem. Chem. Phys. 2016, 18, 5099–5102. [Google Scholar] [CrossRef]
- Persson, I.; Näslund, L.-Å.; Halim, J.; Barsoum, M.W.; Darakchieva, V.; Palisaitis, J.; Rosen, J.; Persson, P.O.Å. On the organization and thermal behavior of functional groups on Ti3C2 MXene surfaces in vacuum. 2D Mater. 2018, 5, 015002. [Google Scholar] [CrossRef]
- Tang, J.; Peng, X.; Lin, T.; Huang, X.; Luo, B.; Wang, L. Confining ultrafine tin monophosphide in Ti3C2Tx interlayers for rapid and stable sodium ion storage. eScience 2021, 1, 203–211. [Google Scholar] [CrossRef]
- Jiang, M.; Fu, C.; Meng, P.; Ren, J.; Wang, J.; Bu, J.; Dong, A.; Zhang, J.; Xiao, W.; Sun, B. Challenges and Strategies of Low-Cost Aluminum Anodes for High-Performance Al-Based Batteries. Adv. Mater. 2022, 34, 2102026. [Google Scholar] [CrossRef]
- Li, D.; Yuan, Y.; Liu, J.; Fichtner, M.; Pan, F. A review on current anode materials for rechargeable Mg batteries. J. Magnes. Alloys 2020, 8, 963–979. [Google Scholar] [CrossRef]
- Liang, P.; Yi, J.; Liu, X.; Wu, K.; Wang, Z.; Cui, J.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Highly Reversible Zn Anode Enabled by Controllable Formation of Nucleation Sites for Zn-Based Batteries. Adv. Funct. Mater. 2020, 30, 1908528. [Google Scholar] [CrossRef]
- Chen, S.S.; Zhao, D.; Chen, L.; Liu, G.R.; Ding, Y.; Cao, Y.L.; Chen, Z.X. Emerging Intercalation Cathode Materials for Multivalent Metal-Ion Batteries: Status and Challenges. Small Struct. 2021, 2, 2100082. [Google Scholar] [CrossRef]
- Wei, C.; Tan, L.; Zhang, Y.; Wang, Z.; Feng, J.; Qian, Y. Towards better Mg metal anodes in rechargeable Mg batteries: Challenges, strategies, and perspectives. Energy Storage Mater. 2022, 52, 299–319. [Google Scholar] [CrossRef]
- Canepa, P.; Sai Gautam, G.; Hannah, D.C.; Malik, R.; Liu, M.; Gallagher, K.G.; Persson, K.A.; Ceder, G. Odyssey of Multivalent Cathode Materials: Open Questions and Future Challenges. Chem. Rev. 2017, 117, 4287–4341. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Ren, C.E.; Alhabeb, M.; Anasori, B.; Barsoum, M.W.; Gogotsi, Y. Magnesium-Ion Storage Capability of MXenes. ACS Appl. Energy Mater. 2019, 2, 1572–1578. [Google Scholar] [CrossRef]
- Gao, Q.; Come, J.; Naguib, M.; Jesse, S.; Gogotsi, Y.; Balke, N. Synergetic effects of K+ and Mg2+ ion intercalation on the electrochemical and actuation properties of the two-dimensional Ti3C2 MXene. Faraday Discuss. 2017, 199, 393–403. [Google Scholar] [CrossRef]
- Liu, F.F.; Liu, Y.C.; Zhao, X.D.; Liu, X.B.; Fan, L.Z. Pursuit of a high-capacity and long-life Mg-storage cathode by tailoring sandwich-structured MXene@carbon nanosphere composites. J. Mater. Chem. A 2019, 7, 16712–16719. [Google Scholar] [CrossRef]
- Xu, M.; Bai, N.; Li, H.-X.; Hu, C.; Qi, J.; Yan, X.-B. Synthesis of MXene-supported layered MoS2 with enhanced electrochemical performance for Mg batteries. Chin. Chem. Lett. 2018, 29, 1313–1316. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Zhang, D.; Wei, Y.; Qu, D.; Guo, Y. Study on MXene-supported Layered TiS2 as Cathode Materials for Magnesium Batteries. Int. J. Electrochem. Sci. 2019, 14, 11102–11109. [Google Scholar] [CrossRef]
- Liu, F.F.; Wang, T.T.; Liu, X.B.; Jiang, N.; Fan, L.Z. High-performance heterojunction Ti3C2/CoSe2 with both intercalation and conversion storage mechanisms for magnesium batteries. Chem. Eng. J. 2021, 426, 130747. [Google Scholar] [CrossRef]
- Zhu, J.L.; Zhang, X.; Gao, H.G.; Shao, Y.T.; Liu, Y.N.; Zhu, Y.F.; Zhang, J.G.; Li, L.Q. VS4 anchored on Ti3C2 MXene as a high-performance cathode material for magnesium ion battery. J. Power Sources 2022, 518, 230731. [Google Scholar] [CrossRef]
- Yang, C.; Yang, L.; Chai, Z.; Zheng, X.; Li, J.; Yang, Y.; Zhu, J.; Jin, X.; Li, Q.; Xu, D. Alternate-stacked Li4Ti5O12 nanosheets/d-Ti3C2 flexible film as a current collector-free, high-capacity and robust cathode for rechargeable Mg batteries. Nano Sel. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.N.; Xu, D.H.; Zhang, D.H.; Wei, Y.C.; Guo, Y.X. Study on MnO2/MXene-V2C composite as cathode for magnesium ion battery. Int. J. Electrochem. Sci. 2020, 15, 11227–11237. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, B.Q.; Hu, C.F.; Chen, H.D.; Huang, J.J.; Yan, D.; Peng, S.L. Construction Strategy of VO2@V2C 1D/2D Heterostructure and Improvement of Zinc-Ion Diffusion Ability in VO2 (B). ACS Appl. Mater. Interfaces 2022, 14, 28760–28768. [Google Scholar] [CrossRef]
- Chen, C.; Xie, X.Q.; Anasori, B.; Sarycheva, A.; Makaryan, T.; Zhao, M.Q.; Urbankowski, P.; Miao, L.; Jiang, J.J.; Gogotsi, Y. MoS2-on-MXene Heterostructures as Highly Reversible Anode Materials for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 1846–1850. [Google Scholar] [CrossRef]
- Sha, D.W.; Lu, C.J.; He, W.; Ding, J.X.; Zhang, H.; Bao, Z.H.; Cao, X.; Fan, J.C.; Dou, Y.; Pan, L.; et al. Surface Selenization Strategy for V2CTx MXene toward Superior Zn-Ion Storage. ACS Nano 2022, 16, 2711–2720. [Google Scholar] [CrossRef]
- Gao, P.; Shi, H.; Ma, T.; Liang, S.; Xia, Y.; Xu, Z.; Wang, S.; Min, C.; Liu, L. MXene/TiO2 Heterostructure-Decorated Hard Carbon with Stable Ti–O–C Bonding for Enhanced Sodium-Ion Storage. ACS Appl. Mater. Interfaces 2021, 13, 51028–51038. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, S.; Lai, Y.; Ding, M.; Cao, Y.; Chen, Z. A stable “rocking-chair” zinc-ion battery boosted by low-strain Zn3V4(PO4)6 cathode. Nano Energy 2022, 100, 107520. [Google Scholar] [CrossRef]
- Shi, M.J.; Wang, B.; Chen, C.; Lang, J.W.; Yan, C.; Yan, X.B. 3D high-density MXene@MnO2 microflowers for advanced aqueous zinc-ion batteries. J. Mater. Chem. A 2020, 8, 24635–24644. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Gogotsi, Y.; Alshareef, H.N. Atomic layer deposition of SnO2 on MXene for Li-ion battery anodes. Nano Energy 2017, 34, 249–256. [Google Scholar] [CrossRef]
- Byeon, A.; Zhao, M.Q.; Ren, C.E.; Halim, J.; Kota, S.; Urbankowski, P.; Anasori, B.; Barsoum, M.W.; Gogotsi, Y. Two-Dimensional Titanium Carbide MXene As a Cathode Material for Hybrid Magnesium/Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 4296–4300. [Google Scholar] [CrossRef]
- Liu, F.F.; Liu, Y.C.; Zhao, X.D.; Liu, K.Y.; Yin, H.Q.; Fan, L.Z. Prelithiated V2C MXene: A High-Performance Electrode for Hybrid Magnesium/Lithium-Ion Batteries by Ion Cointercalation. Small 2020, 16, 1906076. [Google Scholar] [CrossRef]
- Li, X.H.; Tang, Y.K.; Liu, L.; Zhang, Y.; Sheng, R.; NuLi, Y.N. Ti3C2 MXene with pillared structure for hybrid magnesium-lithium batteries cathode material with long cycle life and high rate capability. J. Colloid Interface Sci. 2022, 608, 2455–2462. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X. Electrochemical insertion of magnesium ions into V2O5 from aprotic electrolytes with varied water content. J. Colloid Interface Sci. 2004, 278, 160–165. [Google Scholar] [CrossRef]
- Du, A.; Zhang, Z.; Qu, H.; Cui, Z.; Qiao, L.; Wang, L.; Chai, J.; Lu, T.; Dong, S.; Dong, T.; et al. An efficient organic magnesium borate-based electrolyte with non-nucleophilic characteristics for magnesium–sulfur battery. Energy Environ. Sci. 2017, 10, 2616–2625. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, X.; Nam, K.-W.; Ling, C.; Arthur, T.S.; Song, W.; Knapp, A.M.; Ehrlich, S.N.; Yang, X.-Q.; Matsui, M. α-MnO2 as a cathode material for rechargeable Mg batteries. Electrochem. Commun. 2012, 23, 110–113. [Google Scholar] [CrossRef]
- Zhang, E.; Cao, W.; Wang, B.; Yu, X.; Wang, L.; Xu, Z.; Lu, B. A novel aluminum dual-ion battery. Energy Storage Mater. 2018, 11, 91–99. [Google Scholar] [CrossRef]
- Mohandas, K.S.; Sanil, N.; Noel, M.; Rodriguez, P. Electrochemical intercalation of aluminium chloride in graphite in the molten sodium chloroaluminate medium. Carbon 2003, 41, 927–932. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Li, J.; Sun, Z.; He, K.; Cheng, H.-M.; Li, F. The Rechargeable Aluminum Battery: Opportunities and Challenges. Angew. Chem. Int. Ed. 2019, 58, 11978–11996. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Hadjikhani, A.; Shahbazmohamadi, S.; Beidaghi, M. Two-Dimensional Vanadium Carbide (MXene) as a High-Capacity Cathode Material for Rechargeable Aluminum Batteries. ACS Nano 2017, 11, 11135–11144. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jung, S.C.; Han, Y.K. Fe2CS2 MXene: A promising electrode for Al-ion batteries. Nanoscale 2020, 12, 5324–5331. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Wang, X.X.; Zhang, W.M.; Yang, S.P. Two-dimensional Ti3C2@CTAB-Se (MXene) composite cathode material for high-performance rechargeable aluminum batteries. Chem. Eng. J. 2020, 398, 125679. [Google Scholar] [CrossRef]
- Huo, X.G.; Wang, X.X.; Li, Z.Y.; Liu, J.; Li, J.L. Two-dimensional composite of D-Ti3C2Tx@S@TiO2 (MXene) as the cathode material for aluminum-ion batteries. Nanoscale 2020, 12, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Ponrouch, A.; Araujo, R.B.; Barde, F.; Johansson, P.; Palacín, M.R. Multivalent Batteries—Prospects for High Energy Density: Ca Batteries. Front. Chem. 2019, 7, 79. [Google Scholar] [CrossRef]
- Aurbach, D.; Skaletsky, R.; Gofer, Y. The Electrochemical Behavior of Calcium Electrodes in a Few Organic Electrolytes. J. Electrochem. Soc. 1991, 138, 3536. [Google Scholar] [CrossRef]
- Ponrouch, A.; Frontera, C.; Bardé, F.; Palacín, M.R. Towards a calcium-based rechargeable battery. Nat. Mater. 2016, 15, 169–172. [Google Scholar] [CrossRef]
- Dinda, P.P.; Meena, S. A V3C2 MXene/graphene heterostructure as a sustainable electrode material for metal ion batteries. J. Phys. Condens. Matter 2021, 33, 175001. [Google Scholar] [CrossRef]
- Demiroglu, I.; Peeters, F.M.; Gulseren, O.; Cakir, D.; Sevik, C. Alkali Metal Intercalation in MXene/Graphene Heterostructures: A New Platform for Ion Battery Applications. J. Phys. Chem. Lett. 2019, 10, 727–734. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Ma, Y.H.; Zhang, Q.F.; Huang, R.; Gao, B.L.; Li, Z.W.; Li, G.N.; Liang, F. First-principles investigation of V2CSe2 MXene as a potential anode material for non-lithium metal ion batteries. Curr. Appl. Phys. 2022, 41, 7–13. [Google Scholar] [CrossRef]
- Ma, L.; Zhi, C. Zn electrode/electrolyte interfaces of Zn batteries: A mini review. Electrochem. Commun. 2021, 122, 106898. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Xie, X.; Lu, B.; He, Z.; Liang, S.; Zhou, J. Anode Materials for Aqueous Zinc Ion Batteries: Mechanisms, Properties, and Perspectives. ACS Nano 2020, 14, 16321–16347. [Google Scholar] [CrossRef] [PubMed]
- Yufit, V.; Tariq, F.; Eastwood, D.S.; Biton, M.; Wu, B.; Lee, P.D.; Brandon, N.P. Operando Visualization and Multi-scale Tomography Studies of Dendrite Formation and Dissolution in Zinc Batteries. Joule 2019, 3, 485–502. [Google Scholar] [CrossRef]
- Li, H.F.; Ma, L.T.; Han, C.P.; Wang, Z.F.; Liu, Z.X.; Tang, Z.J.; Zhi, C.Y. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.L.; Wei, C.L.; Xi, B.J.; Xiong, S.L.L.; Feng, J.K.; Qian, Y.T. Flexible and Free-Standing Ti3C2Tx MXene@Zn Paper for Dendrite-Free Aqueous Zinc Metal Batteries and Nonaqueous Lithium Metal Batteries. ACS Nano 2019, 13, 11676–11685. [Google Scholar] [CrossRef]
- Zhou, J.H.; Xie, M.; Wu, F.; Mei, Y.; Hao, Y.T.; Li, L.; Chen, R.J. Encapsulation of Metallic Zn in a Hybrid MXene/Graphene Aerogel as a Stable Zn Anode for Foldable Zn-Ion Batteries. Adv. Mater. 2022, 34, 2106897. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Y.; Cao, B.; Zhu, Q.; Xu, B. Recent Progress of MXenes in Aqueous Zinc-Ion Batteries. Acta Phys. Chim. Sin. 2023, 39, 2210027. [Google Scholar] [CrossRef]
- Li, X.L.; Li, Q.; Hou, Y.; Yang, Q.; Chen, Z.; Huang, Z.D.; Liang, G.J.; Zhao, Y.W.; Ma, L.T.; Li, M.A.; et al. Toward a Practical Zn Powder Anode: Ti3C2Tx MXene as a Lattice-Match Electrons/Ions Redistributor. ACS Nano 2021, 15, 14631–14642. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Zhang, N.N.; Huang, S.; Yuan, Z.S.; Zhu, J.C.; Zhao, Z.F.; Niu, Z.Q. Direct Self-Assembly of MXene on Zn Anodes for Dendrite-Free Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 2861–2865. [Google Scholar] [CrossRef]
- Li, X.L.; Li, M.A.; Luo, K.; Hou, Y.; Li, P.; Yang, Q.; Huang, Z.D.; Liang, G.J.; Chen, Z.; Du, S.Y.; et al. Lattice Matching and Halogen Regulation for Synergistically Induced Uniform Zinc Electrodeposition by Halogenated Ti3C2 MXenes. ACS Nano 2022, 16, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Shen, Y.; Min, J.; Pang, J.; Zheng, Y.; Gu, T.; Wang, G.; Chen, L. MOF-derived Zn/Co co-doped MnO/C microspheres as cathode and Ti3C2@Zn as anode for aqueous zinc-ion full battery. Chem. Eng. J. 2023, 454, 140394. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Liu, C.; Xiong, S.; Feng, J.; Qian, Y. Rational Design of Sulfur-Doped Three-Dimensional Ti3C2Tx MXene/ZnS Heterostructure as Multifunctional Protective Layer for Dendrite-Free Zinc-Ion Batteries. ACS Nano 2021, 15, 15259–15273. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wei, C.; Zhang, Y.; An, Y.; Xiong, S.; Feng, J. Long-life and dendrite-free zinc metal anode enabled by a flexible, green and self-assembled zincophilic biomass engineered MXene based interface. Chem. Eng. J. 2022, 431, 134277. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Liu, C.; Xiong, S.; Feng, J.; Qian, Y. Reversible zinc-based anodes enabled by zincophilic antimony engineered MXene for stable and dendrite-free aqueous zinc batteries. Energy Storage Mater. 2021, 41, 343–353. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.M.; Jiang, Y.P.; Li, X.L.; Liu, Y.; Xiao, H.H.; Ma, Y.; Huang, Y.Y.; Yuan, G.H. Tailoring Ultrahigh Energy Density and Stable Dendrite-Free Flexible Anode with Ti3C2Tx MXene Nanosheets and Hydrated Ammonium Vanadate Nanobelts for Aqueous Rocking-Chair Zinc Ion Batteries. Adv. Funct. Mater. 2021, 31, 2103210. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, S.; Yu, Q.; Wang, Q.; Wang, M.; Ni, T.; Ruan, L.; Zeng, W. A flexible, heat-resistant and self-healable “rocking-chair” zinc ion microbattery based on MXene-TiS2 (de)intercalation anode. J. Power Sources 2021, 504, 230076. [Google Scholar] [CrossRef]
- Sun, C.; Wu, C.; Gu, X.; Wang, C.; Wang, Q. Interface Engineering via Ti3C2Tx MXene Electrolyte Additive toward Dendrite-Free Zinc Deposition. Nano-Micro Lett. 2021, 13, 89. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Wang, D.; Yang, Q.; Ma, L.; Huang, Z.; Liang, G.; Chen, A.; Guo, Y.; Dong, B.; et al. Grafted MXene/polymer electrolyte for high performance solid zinc batteries with enhanced shelf life at low/high temperatures. Energy Environ. Sci. 2021, 14, 3492–3501. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Zeng, W.; Wang, M.; Ruan, L.; Ma, Y. A New Free-Standing Aqueous Zinc-Ion Capacitor Based on MnO2–CNTs Cathode and MXene Anode. Nano-Micro Lett. 2019, 11, 70. [Google Scholar] [CrossRef]
- Shi, J.; Wang, S.; Wang, Q.; Chen, X.; Du, X.; Wang, M.; Zhao, Y.; Dong, C.; Ruan, L.; Zeng, W. A new flexible zinc-ion capacitor based on δ-MnO2@Carbon cloth battery-type cathode and MXene@Cotton cloth capacitor-type anode. J. Power Sources 2020, 446, 227345. [Google Scholar] [CrossRef]
- Huang, L.; Lin, Y.; Zeng, W.; Xu, C.; Chen, Z.; Wang, Q.; Zhou, H.; Yu, Q.; Zhao, B.; Ruan, L.; et al. Highly Transparent and Flexible Zn-Ti3C2Tx MXene Hybrid Capacitors. Langmuir 2022, 38, 5968–5976. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ao, H.; Qi, K.; Zhang, X.; Liu, M.; Zhou, T.; Wang, S.; Xia, G.; Zhu, Y. A High-rate, Long Life, and Anti-self-discharge Aqueous N-doped Ti3C2/Zn Hybrid Capacitor. Mater. Today Energy 2021, 19, 100598. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.L.; Guo, X.H.; Ruan, L.M.; Wei, N.; Ma, Y.; Li, J.Y.; Wang, M.; Li, W.Q.; Zeng, W. MXene-Reduced Graphene Oxide Aerogel for Aqueous Zinc-Ion Hybrid Supercapacitor with Ultralong Cycle Life. Adv. Electron. Mater. 2019, 5, 1900537. [Google Scholar] [CrossRef]
- Wang, C.D.; Wei, S.Q.; Chen, S.M.; Cao, D.F.; Song, L. Delaminating Vanadium Carbides for Zinc-Ion Storage: Hydrate Precipitation and H+/Zn2+ Co-Action Mechanism. Small Methods 2019, 3, 1900495. [Google Scholar] [CrossRef]
- Maughan, P.A.; Tapia-Ruiz, N.; Bimbo, N. In-situ pillared MXene as a viable zinc-ion hybrid capacitor. Electrochim. Acta 2020, 341, 136061. [Google Scholar] [CrossRef]
- Fan, Z.; Jin, J.; Li, C.; Cai, J.; Wei, C.; Shao, Y.; Zou, G.; Sun, J. 3D-Printed Zn-Ion Hybrid Capacitor Enabled by Universal Divalent Cation-Gelated Additive-Free Ti3C2 MXene Ink. ACS Nano 2021, 15, 3098–3107. [Google Scholar] [CrossRef]
- Li, Z.; Guo, D.; Wang, D.; Sun, M.; Sun, H. Exploration of Metal/Ti3C2 MXene-derived composites as anode for high-performance zinc-ion supercapacitor. J. Power Sources 2021, 506, 230197. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, Z.; Li, X.; Liu, Z.; Li, H.; Liang, G.; Wang, D.; Huang, Q.; Zhang, S.; Chen, S.; et al. A Wholly Degradable, Rechargeable Zn–Ti3C2 MXene Capacitor with Superior Anti-Self-Discharge Function. ACS Nano 2019, 13, 8275–8283. [Google Scholar] [CrossRef]
- Luo, S.J.; Xie, L.Y.; Han, F.; Wei, W.; Huang, Y.; Zhang, H.; Zhu, M.S.; Schmidt, O.G.; Wang, L. Nanoscale Parallel Circuitry Based on Interpenetrating Conductive Assembly for Flexible and High-Power Zinc Ion Battery. Adv. Funct. Mater. 2019, 29, 1901336. [Google Scholar] [CrossRef]
- Shi, M.; Wang, B.; Shen, Y.; Jiang, J.; Zhu, W.; Su, Y.; Narayanasamy, M.; Angaiah, S.; Yan, C.; Peng, Q. 3D assembly of MXene-stabilized spinel ZnMn2O4 for highly durable aqueous zinc-ion batteries. Chem. Eng. J. 2020, 399, 125627. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Z.; Wang, W.; Li, H.; Dong, J.; Gao, S.; Xu, D.; Li, L.; Shen, J.; Ye, M. Superior-Performance Aqueous Zinc-Ion Batteries Based on the In Situ Growth of MnO2 Nanosheets on V2CTX MXene. ACS Nano 2021, 15, 2971–2983. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, Y.; Gong, Z.; Lu, T.; Pan, L. Three-dimensional hydrated vanadium pentoxide/MXene composite for high-rate zinc-ion batteries. J. Colloid Interface Sci. 2021, 593, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, J.; Liang, S. Guest pre-intercalation strategy to boost the electrochemical performance of aqueous zinc-ion battery cathodes. Acta Phys. Chim. Sin. 2021, 37, 2005020. [Google Scholar] [CrossRef]
- Liu, C.; Xu, W.; Mei, C.; Li, M.-C.; Xu, X.; Wu, Q. Highly stable H2V3O8/Mxene cathode for Zn-ion batteries with superior rate performance and long lifespan. Chem. Eng. J. 2021, 405, 126737. [Google Scholar] [CrossRef]
- Shi, Z.; Ru, Q.; Pan, Z.; Zheng, M.; Chi-Chun Ling, F.; Wei, L. Flexible Free-Standing VO2/MXene Conductive Films as Cathodes for Quasi-Solid-State Zinc-Ion Batteries. ChemElectroChem 2021, 8, 1091–1097. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, L.; Cao, B.; Du, H.; Lu, H.; Ma, Y.; Wang, H.; Guo, H.; Huang, Q.; Xu, B.; et al. Van der Waals Interaction-Driven Self-Assembly of V2O5 Nanoplates and MXene for High-Performing Zinc-Ion Batteries by Suppressing Vanadium Dissolution. ACS Nano 2022, 16, 14539–14548. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, Q.; Li, H.; Xu, H.; Chai, Z.; Chen, K.; Liu, Z.; Tang, Z.; Ma, L.; et al. Phase Transition Induced Unusual Electrochemical Performance of V2CTX MXene for Aqueous Zinc Hybrid-Ion Battery. ACS Nano 2020, 14, 541–551. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Hu, Z.; Peng, J.; Lai, W.; Wu, D.; Zuo, S.; Zhang, J.; Chen, B.; Dai, Z.; et al. In-Situ Electrochemically Activated Surface Vanadium Valence in V2C MXene to Achieve High Capacity and Superior Rate Performance for Zn-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2008033. [Google Scholar] [CrossRef]
- Narayanasamy, M.; Kirubasankar, B.; Shi, M.; Velayutham, S.; Wang, B.; Angaiah, S.; Yan, C. Morphology restrained growth of V2O5 by the oxidation of V-MXenes as a fast diffusion controlled cathode material for aqueous zinc ion batteries. Chem. Commun. 2020, 56, 6412–6415. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, H.; Wei, C.; Tao, Y.; Li, Y.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Micron-Sized Nanoporous Vanadium Pentoxide Arrays for High-Performance Gel Zinc-Ion Batteries and Potassium Batteries. Chem. Mater. 2020, 32, 4054–4064. [Google Scholar] [CrossRef]
- Venkatkarthick, R.; Rodthongkum, N.; Zhang, X.; Wang, S.; Pattananuwat, P.; Zhao, Y.; Liu, R.; Qin, J. Vanadium-Based Oxide on Two-Dimensional Vanadium Carbide MXene (V2Ox@V2CTx) as Cathode for Rechargeable Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 4677–4689. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, W.; Cao, Z.; Gao, S.; Chee, M.O.L.; Zhang, X.; Dong, P.; Ajayan, P.M.; Ye, M.; Shen, J. Zn2+-Intercalated V2O5·nH2O derived from V2CTx MXene for hyper-stable zinc-ion storage. J. Mater. Chem. A 2021, 9, 17994–18005. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Z.; Li, X.-L.; Pei, L.; Jones, J.; Zhou, Y.-N.; Dong, P.; Wang, L.; Ye, M.; Shen, J. Ion-intercalation regulation of MXene-derived hydrated vanadates for high-rate and long-life Zn-Ion batteries. Energy Storage Mater. 2022, 45, 568–577. [Google Scholar] [CrossRef]
- Dong, C.; Xu, F.; Chen, L.; Chen, Z.; Cao, Y. Design Strategies for High-Voltage Aqueous Batteries. Small Struct. 2021, 2, 2100001. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, Q.; Liang, G.; Huang, Z.; Ma, L.; Wang, D.; Mo, F.; Dong, B.; Huang, Q.; et al. In Situ Electrochemical Synthesis of MXenes without Acid/Alkali Usage in/for an Aqueous Zinc Ion Battery. Adv. Energy Mater. 2020, 10, 2001791. [Google Scholar] [CrossRef]
- Long, F.; Zhang, Q.; Shi, J.; Wen, L.; Wu, Y.; Ren, Z.; Liu, Z.; Hou, Y.; Mao, K.; Niu, K.; et al. Ultrastable and ultrafast 3D charge–discharge network of robust chemically coupled 1 T-MoS2/Ti3C2 MXene heterostructure for aqueous Zn-ion batteries. Chem. Eng. J. 2022, 455, 140539. [Google Scholar] [CrossRef]
- Shi, J.; Hou, Y.; Liu, Z.; Zheng, Y.; Wen, L.; Su, J.; Li, L.; Liu, N.; Zhang, Z.; Gao, Y. The high-performance MoO3−x/MXene cathodes for zinc-ion batteries based on oxygen vacancies and electrolyte engineering. Nano Energy 2022, 91, 106651. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Li, J.; Yuan, Z.; Li, D.; Wang, L.; Han, W. Self-assembled Cobalt-doped NiMn-layered double hydroxide (LDH)/V2CTx MXene hybrids for advanced aqueous electrochemical energy storage properties. Chem. Eng. J. 2022, 430, 132992. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Z.; Zhang, W.; Jiang, Y.; Peng, J.; Wu, D.; Chen, B.; Wei, W.; Chen, X.; Liu, Z.; et al. Sulfonic-Group-Grafted Ti3C2Tx MXene: A Silver Bullet to Settle the Instability of Polyaniline toward High-Performance Zn-Ion Batteries. ACS Nano 2021, 15, 9065–9075. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Hou, Y.; Zhang, Z.; Lu, Y.; Huang, Z.; Liang, G.; Li, M.; Yang, Q.; Ma, J.; et al. Intrinsic voltage plateau of a Nb2CTx MXene cathode in an aqueous electrolyte induced by high-voltage scanning. Joule 2021, 5, 2993–3005. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Qin, G.; Luo, K.; Lu, J.; Li, Y.; Liang, G.; Huang, Z.; Zhou, J.; Hultman, L.; et al. Halogenated Ti3C2 MXenes with Electrochemically Active Terminals for High-Performance Zinc Ion Batteries. ACS Nano 2021, 15, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).