Ion Transport Regulated Lithium Metal Batteries Achieved by Electrospun ZIF/PAN Composite Separator with Suitable Electrolyte Wettability
Abstract
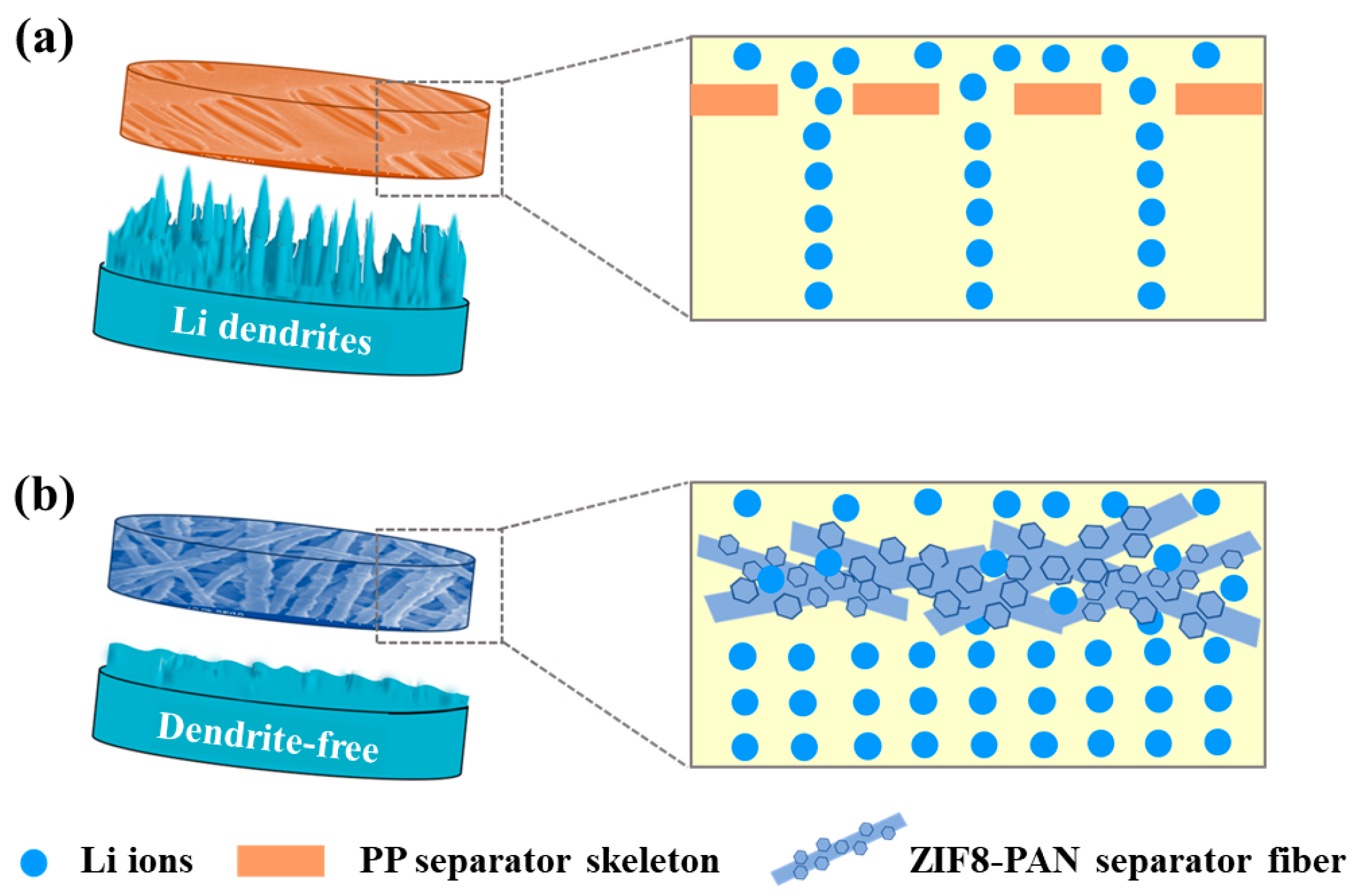
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZIF-8 and Preparation of ZIF8-PAN Solutions
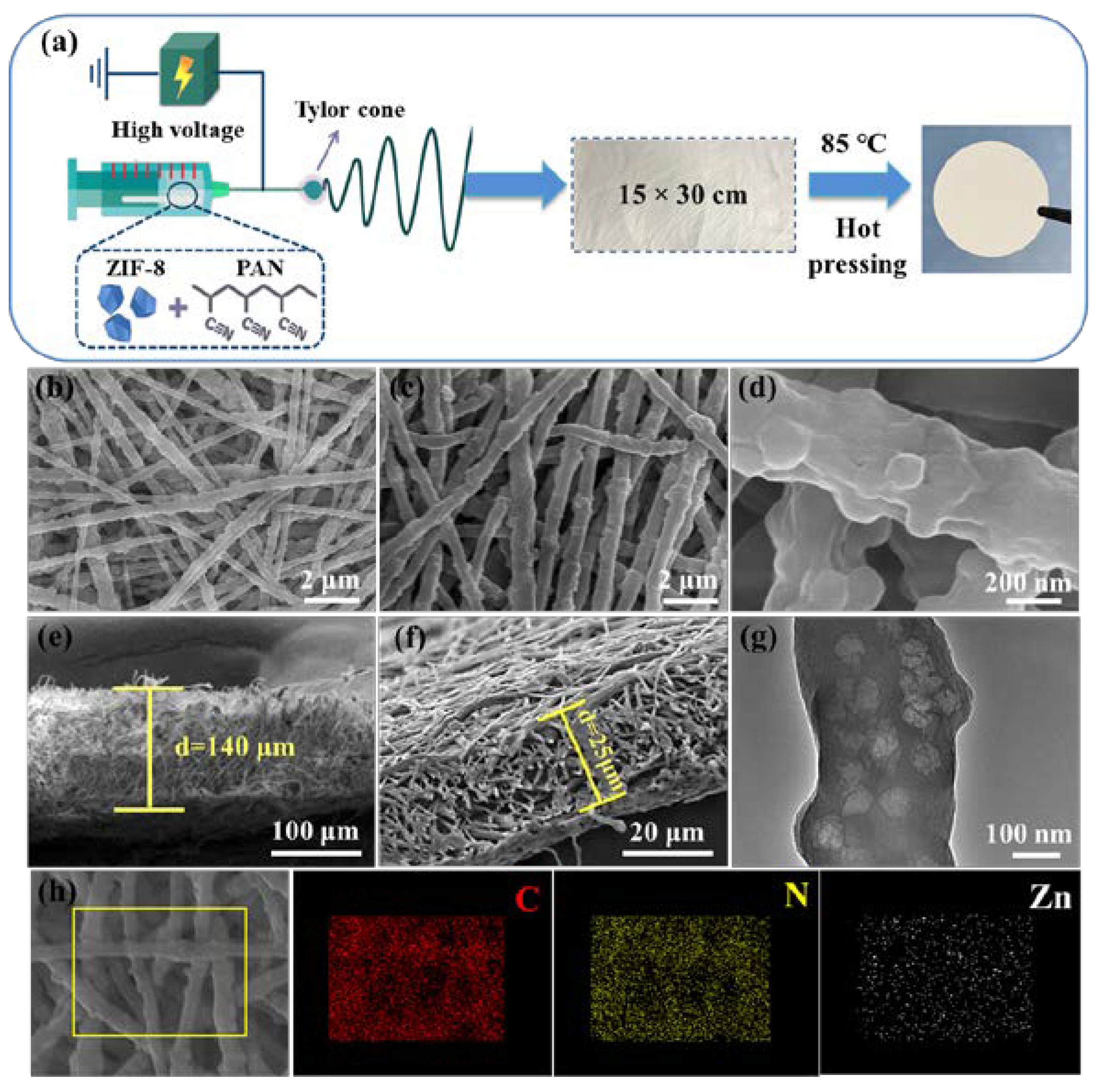
2.3. Fabrication of ZIF8-PAN Composite Separator
2.4. Physical Characterization
2.5. Electrochemical Measurements
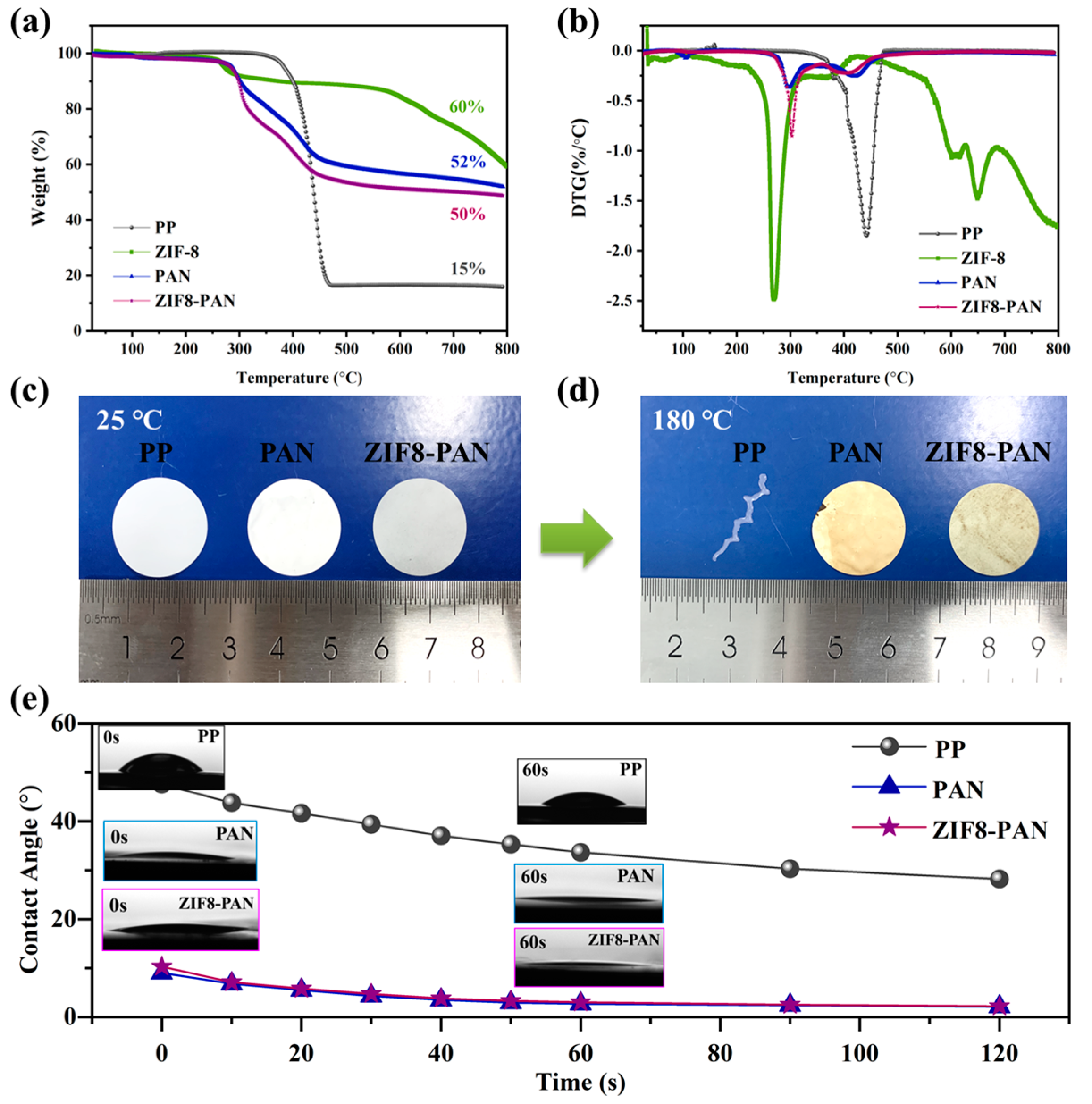
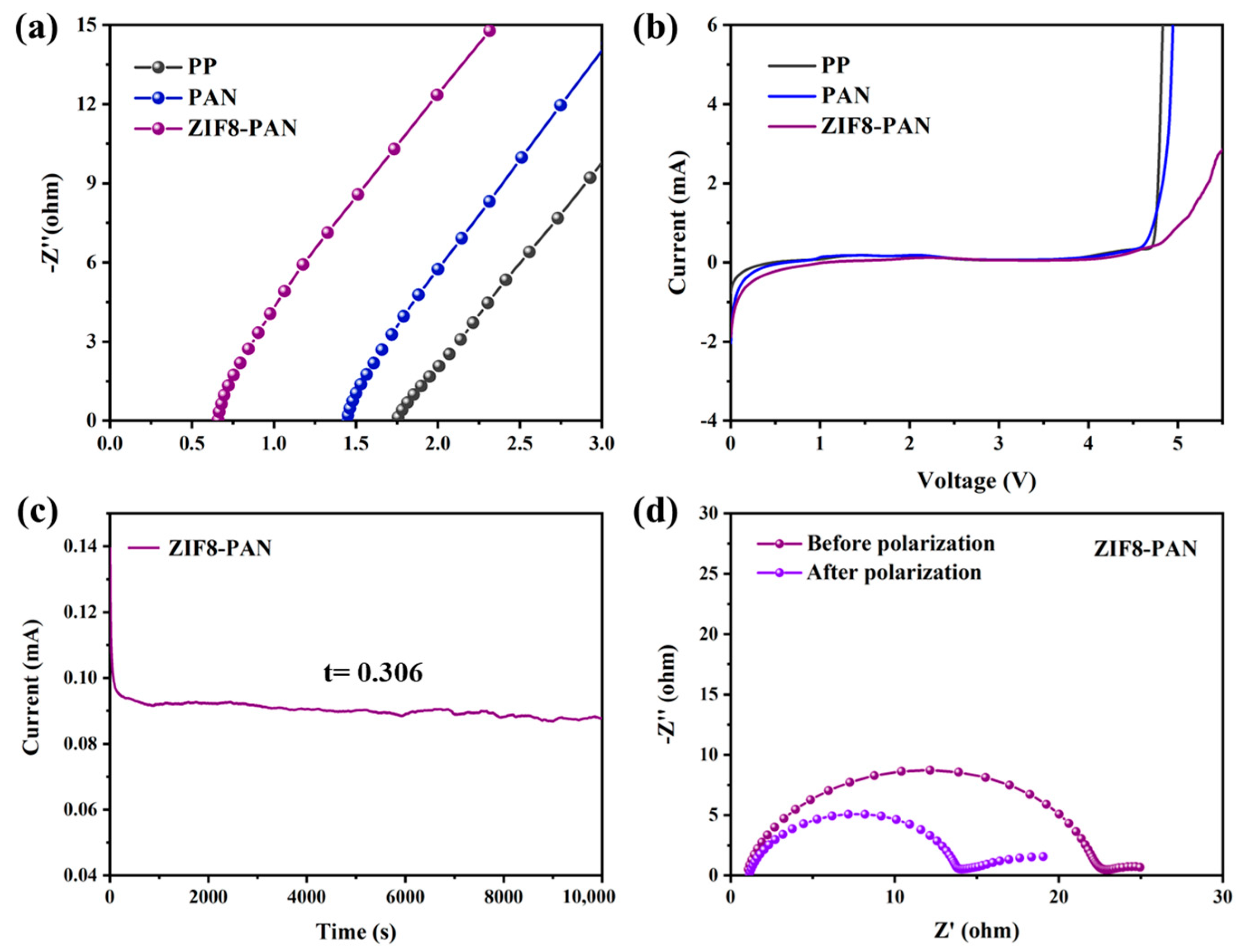
3. Results and Discussion
3.1. Morphology and Structure of ZIF8-PAN Composite Separator
3.2. Physical Properties of Separators
3.3. Electrochemical Stability and Ionic Conductivity of ZIF8-PAN Separator
3.4. The Lithium-Ion Transference Number of Separators
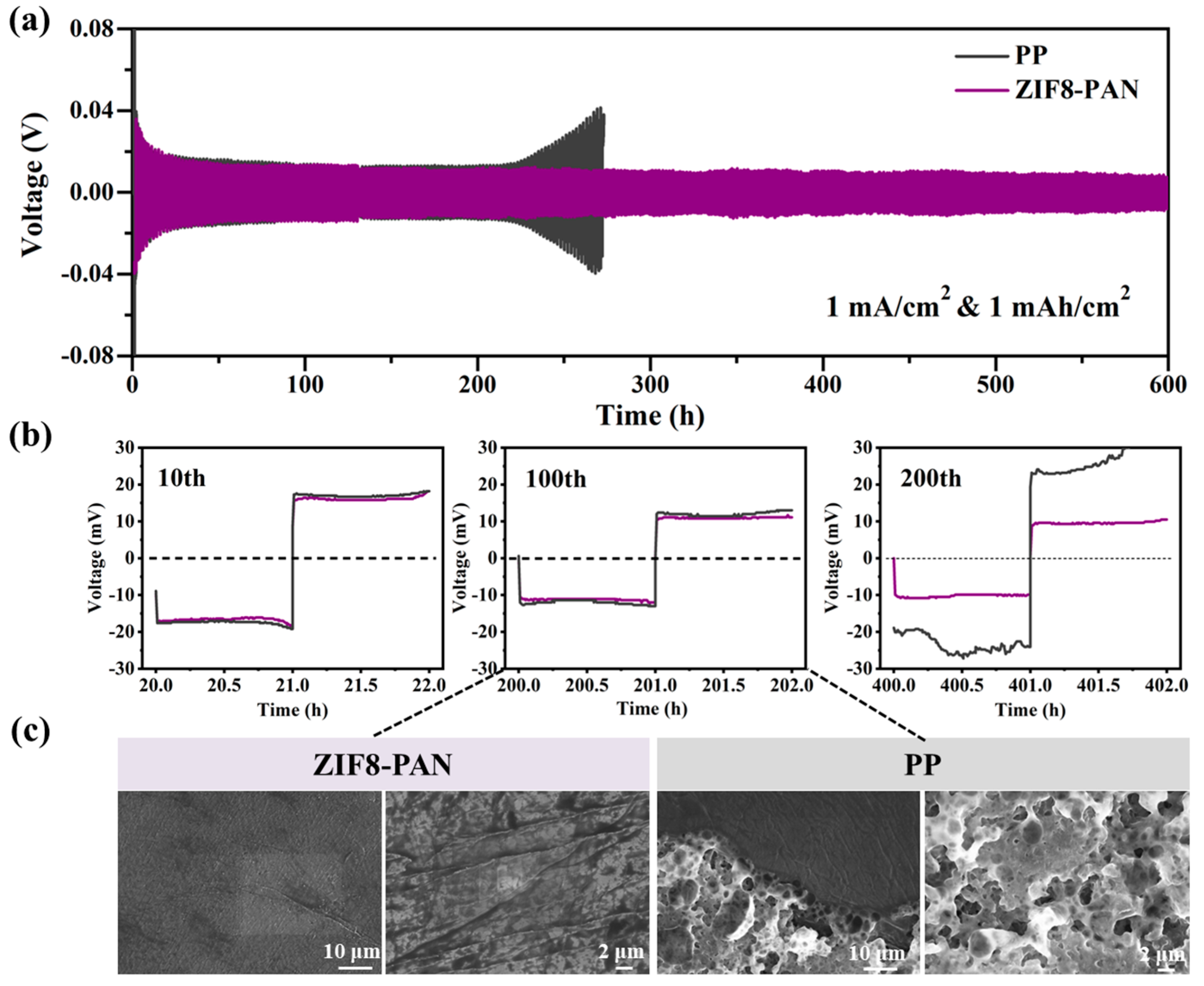
3.5. Stability of ZIF8-PAN Separator with Li Anode
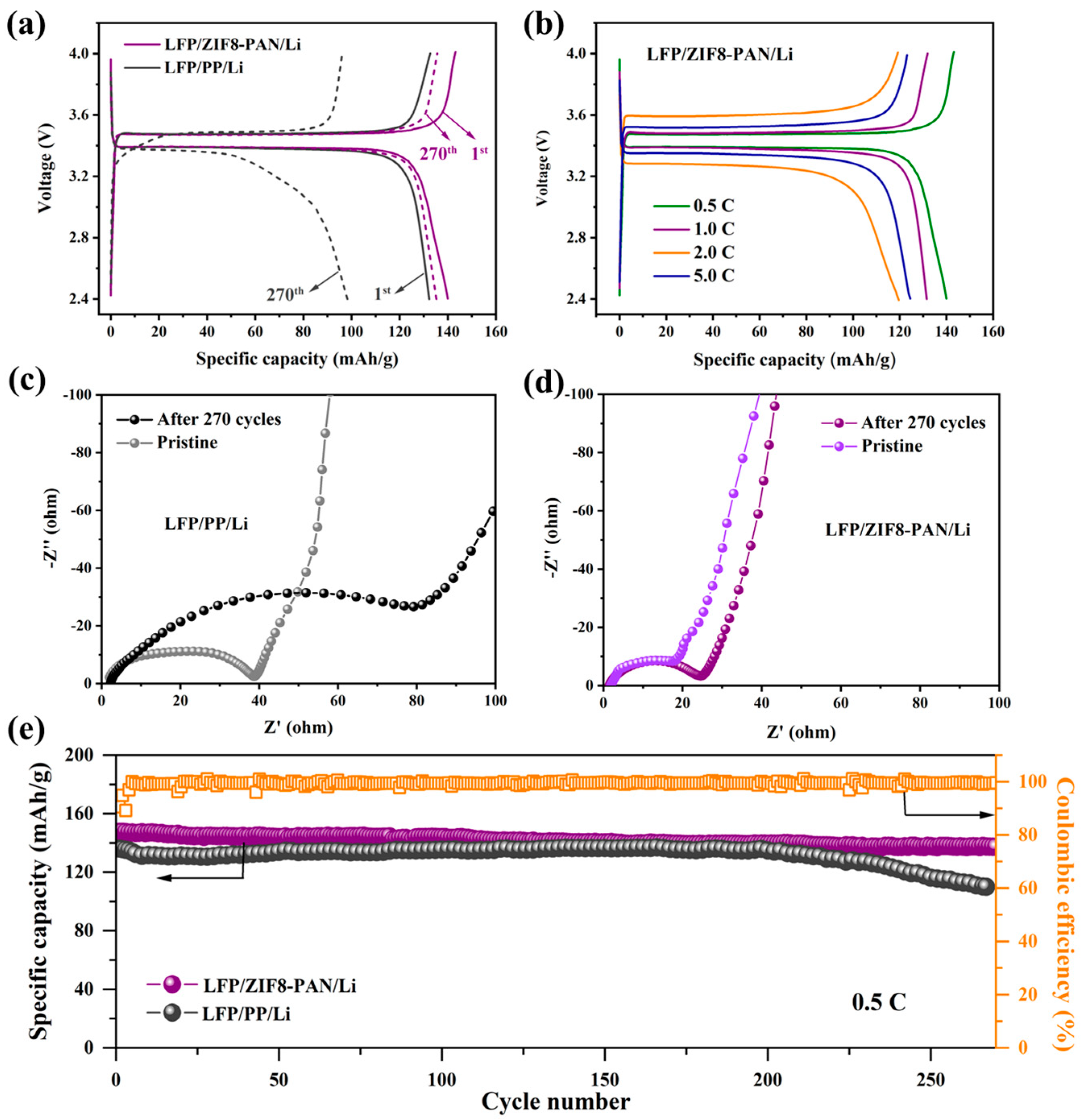
3.6. Cycling Performance of LFP/Separator/Li Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Wang, H.; Hu, C.; Jin, Y.; Liu, J.; Yan, H. Recent progresses in the suppression method based on the growth mechanism of lithium dendrite. J. Energy Chem. 2018, 27, 513–527. [Google Scholar] [CrossRef]
- Wang, P.; Qu, W.; Song, W.L.; Chen, H.; Chen, R.; Fang, D. Electro-chemo-mechanical issues at the interfaces in solid-state lithium metal batteries. Adv. Funct. Mater. 2019, 29, 1900950. [Google Scholar] [CrossRef]
- Xia, S.; Wu, X.; Zhang, Z.; Cui, Y.; Liu, W. Practical challenges and future perspectives of all-solid-state lithium-metal batteries. Chem 2019, 5, 753–785. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.N.; Han, D.; Zhou, J.; Zhou, D.; Tang, W.; Goodenough, J.B. Thermodynamic understanding of Li-dendrite formation. Joule 2020, 4, 1864–1879. [Google Scholar] [CrossRef]
- Guo, Q.; Deng, W.; Xia, S.J.; Zhang, Z.B.; Zhao, F.; Hu, B.J.; Zhang, S.S.; Zhou, X.F.; Chen, G.Z.; Liu, Z.P. Nano-channel-based physical and chemical synergic regulation for dendrite-free lithium plating. Nano Res. 2021, 14, 3585–3597. [Google Scholar] [CrossRef]
- Li, Y.N.; Wang, C.Y.; Gao, R.M.; Cao, F.F.; Ye, H. Recent smart lithium anode configurations for high-energy lithium metal batteries. Energy Storage Mater. 2021, 38, 262–275. [Google Scholar] [CrossRef]
- Yang, C.P.; Yin, Y.X.; Zhang, S.F.; Li, N.W.; Guo, Y.G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jin, H.J.; Yun, Y.S. Advances in the design of 3D-structured electrode materials for lithium-metal anodes. Adv. Mater. 2020, 32, 2002193. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, S.; Shi, C.; Liang, G.; Lu, Z.; Fu, R.; Wu, D. Two-dimensional molecular brush-functionalized porous bilayer composite separators toward ultrastable high-current density lithium metal anodes. Nat. Commun. 2019, 10, 1363. [Google Scholar] [CrossRef]
- Lu, Q.; He, Y.; Yu, Q.; Li, B.; Kaneti, Y.V.; Yao, Y.; Kang, F.; Yang, Q.H. Dendrite-free, high-rate, long-life lithium metal batteries with a 3D cross-linked network polymer electrolyte. Adv. Mater. 2017, 29, 1604460. [Google Scholar] [CrossRef]
- Huang, J.D.; Liu, J.D.; He, J.; Wu, M.G.; Qi, S.H.; Wang, H.P.; Li, F.; Ma, J.M. Optimizing electrode/electrolyte interphases and Li-ion flux/solvation for lithium-metal batteries with qua-functional heptafluorobutyric anhydride. Angew. Chem. Int. Ed. 2021, 60, 20717–20722. [Google Scholar] [CrossRef]
- Han, Y.; Liu, B.; Xiao, Z.; Zhang, W.; Wang, X.; Pan, G.; Xia, Y.; Xia, X.; Tu, J. Interface issues of lithium metal anode for high-energy batteries: Challenges, strategies, and perspectives. InfoMat 2021, 3, 155–174. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhang, Z.; Chen, X.; Lie, W.; He, Y.B.; Zhou, Z.; Xia, G.; Guo, Z. Building artificial solid-electrolyte interphase with uniform intermolecular ionic bonds toward dendrite-free lithium metal anodes. Adv. Funct. Mater. 2020, 30, 2002414. [Google Scholar] [CrossRef]
- Yang, H.; Guo, C.; Naveed, A.; Lei, J.; Yang, J.; Nuli, Y.; Wang, J. Recent progress and perspective on lithium metal anode protection. Energy Storage Mater. 2018, 14, 199–221. [Google Scholar] [CrossRef]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for lithium-ion batteries: A review on the production processes and recent developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, N.; Kang, W.; Li, Z.; Wang, G.; Cheng, B. Highly multiscale structural Poly (vinylidene fluoridehexafluoropropylene)/poly-m-phenyleneisophthalamide separator with enhanced interface compatibility and uniform lithium-ion flux distribution for dendrite-proof lithium-metal batteries. Energy Storage Mater. 2020, 26, 334–348. [Google Scholar] [CrossRef]
- Lu, X.; Bertei, A.; Finegan, D.P.; Tan, C.; Daemi, S.R.; Weaving, J.S.; O’Regan, K.B.; Heenan, T.M.M.; Hinds, G.; Emma Kendrick, E.; et al. 3D microstructure design of lithium-ion battery electrodes assisted by X-ray nano-computed tomography and modelling. Nat. Commun. 2020, 11, 2079. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, L.; Liu, D.; Liao, M.; Chen, J.; Yuan, K.; Xiang, J.; Li, Z.; Huang, Y. Elevated Lithium Ion Regulation by a “Natural Silk” Modified Separator for High-Performance Lithium Metal Anode. Adv. Funct. Mater. 2021, 31, 2100537. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Yang, C.; Jia, Z.; Guan, Z.; Wang, L. Polyvinylidene fluoride membrane by novel electrospinning system for separator of Li-ion batteries. J. Power Sources 2009, 189, 716–720. [Google Scholar] [CrossRef]
- Zeng, F.; Xu, R.; Ye, L.; Xiong, B.; Kang, J.; Xiang, M.; Li, L.; Sheng, X.; Hao, Z. Effects of heat setting on the morphology and performance of polypropylene separator for lithium ion batteries. Ind. Eng. Chem. Res. 2019, 58, 2217–2224. [Google Scholar] [CrossRef]
- Ryu, J.; Han, D.Y.; Hong, D.; Park, S. A polymeric separator membrane with chemoresistance and high Li-ion flux for high-energy-density lithium metal batteries. Energy Storage Mater. 2022, 45, 941–951. [Google Scholar] [CrossRef]
- Shen, L.; Wu, H.B.; Liu, F.; Zhang, C.; Ma, S.; Le, Z.; Lu, Y. Anchoring anions with metal-organic framework-functionalized separators for advanced lithium batteries. Nanoscale Horiz. 2019, 4, 705–711. [Google Scholar] [CrossRef]
- Luo, J.; Li, Y.; Zhang, H.; Wang, A.; Lo, W.S.; Dong, Q.; Wong, N.; Povinelli, C.; Shao, Y.; Chereddy, S.; et al. A metal-organic framework thin film for selective Mg2+ transport. Angew. Chem. Int. Ed. 2019, 58, 15313–15317. [Google Scholar] [CrossRef]
- Zhuang, T.Z.; Huang, J.Q.; Peng, H.J.; He, L.Y.; Cheng, X.B.; Chen, C.M.; Zhang, Q. Rational integration of polypropylene/graphene oxide/nafion as ternary-layered separator to retard the shuttle of polysulfides for lithium-sulfur batteries. Small 2016, 12, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Hu, X.; Dai, C.; Yi, T. Crystal structures of electrospun PVDF membranes and its separator application for rechargeable lithium metal cells. Mater. Sci. Eng. B 2006, 131, 100–105. [Google Scholar] [CrossRef]
- Hu, M.; Ma, Q.; Yuan, Y.; Pan, Y.; Chen, M.; Zhang, Y.; Long, D. Grafting polyethyleneimine on electrospun nanofiber separator to stabilize lithium metal anode for lithium sulfur batteries. Chem. Eng. J. 2020, 388, 124258. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, W.; Zhu, H.; Li, B.G.; Lu, Y.; Zhu, S. Fabrication of metal-organic framework-based nanofibrous separator via one-pot electrospinning strategy. Nano Res. 2021, 14, 1465–1470. [Google Scholar] [CrossRef]
- Ryou, M.H.; Lee, Y.M.; Park, J.K.; Choi, J.W. Mussel-inspired polydopamine-treated polyethylene separators for high-power Li-ion batteries. Adv. Mater. 2011, 23, 3066–3070. [Google Scholar] [CrossRef]
- Qin, J.; Shi, H.; Huang, K.; Lu, P.; Wen, P.; Xing, F.; Yang, B.; Ye, M.; Yu, Y.; Wu, Z.S. Achieving stable Na metal cycling via polydopamine/multilayer graphene coating of a polypropylene separator. Nat. Commun. 2021, 12, 5786. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, D.H.; Joo, S.H.; Choi, B.; Cha, A.; Kim, K.M.; Kwon, T.H.; Kwak, S.K.; Kang, S.J.; Jin, J. Hierarchical chitin fibers with aligned nanofibrillar architectures: A nonwoven-mat separator for lithium metal batteries. ACS Nano 2017, 11, 6114–6121. [Google Scholar] [CrossRef]
- Cheng, D.; Yang, X.; He, Z.; Ni, Y. Potential of cellulose-based materials for lithium-ion batteries (LIB) separator membranes. J. Bioresour. Bioprod. 2016, 1, 18–21. [Google Scholar]
- Baghali, M.; Jayathilaka, W.A.D.M.; Ramakrishna, S. The role of electrospun nanomaterials in the future of energy and environment. Materials 2021, 14, 558. [Google Scholar] [CrossRef]
- Wang, G.; He, P.; Fan, L.Z. Asymmetric Polymer Electrolyte Constructed by Metal–Organic Framework for Solid-State, Dendrite-Free Lithium Metal Battery. Adv. Func. Mater. 2021, 31, 2007198. [Google Scholar] [CrossRef]
- Hao, Z.; Wu, Y.; Zhao, Q.; Tang, J.; Zhang, Q.; Ke, X.; Liu, J.; Jin, Y.; Wang, H. Functional Separators Regulating Ion Transport Enabled by Metal-Organic Frameworks for Dendrite-Free Lithium Metal Anodes. Adv. Funct. Mater. 2021, 31, 2102938. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, W.; Muhammad, I.P.; Chen, X.; Zeng, Y.; Wang, B.; Zhang, S. Coaxially electrospun PAN/HCNFs@ PVDF/UiO-66 composite separator with high strength and thermal stability for lithium-ion battery. Micropor. Mesopor. Mater. 2021, 311, 110724. [Google Scholar] [CrossRef]
- Cho, T.H.; Tanaka, M.; Onishi, H.; Kondo, Y.; Nakamura, T.; Yamazaki, H.; Tanase, S.; Sakai, T. Battery performances and thermal stability of polyacrylonitrile nano-fiber-based nonwoven separators for Li-ion battery. J. Power Sources 2008, 181, 155–160. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Yang, W.; Ren, Q.; Li, F.; Huang, Z. Lithium ion battery separator with high performance and high safety enabled by tri-layered SiO2@ PI/m-PE/SiO2@ PI nanofiber composite membrane. J. Power Sources 2018, 396, 265–275. [Google Scholar] [CrossRef]
- Liu, X.; Song, K.; Lu, C.; Huang, Y.; Duan, X.; Li, S.; Ding, Y. Electrospun PU@ GO separators for advanced lithium ion batteries. J. Membr. Sci. 2018, 555, 1–6. [Google Scholar] [CrossRef]
- Liang, Y.; Cheng, S.; Zhao, J.; Zhang, C.; Sun, S.; Zhou, N.; Qiu, Y.; Zhang, X. Heat treatment of electrospun Polyvinylidene fluoride fibrous membrane separators for rechargeable lithium-ion batteries. J. Power Sources 2013, 240, 204–211. [Google Scholar] [CrossRef]
- Gong, W.Z.; Gu, J.F.; Ruan, S.L.; Shen, C.Y. A high-strength electrospun PPESK fibrous membrane for lithium-ion battery separator. Polym. Bull. 2019, 76, 5451–5462. [Google Scholar] [CrossRef]
- Huang, D.; Liang, C.; Chen, L.; Tang, M.; Zheng, Z.; Wang, Z. MOF composite fibrous separators for high-rate lithium-ion batteries. J. Mater. Sci. 2021, 56, 5868–5877. [Google Scholar] [CrossRef]
- He, Y.; Chang, Z.; Wu, S.; Qiao, Y.; Bai, S.; Jiang, K.; He, P.; Zhou, H. Simultaneously Inhibiting Lithium Dendrites Growth and Polysulfides Shuttle by a Flexible MOF-Based Membrane in Li-S Batteries. Adv. Energy. Mater. 2018, 8, 1802130. [Google Scholar] [CrossRef]
- Yang, L.Y.; Cao, J.H.; Cai, B.R.; Liang, T.; Wu, D.Y. Electrospun MOF/PAN composite separator with superior electrochemical performances for high energy density lithium batteries. Electrochim. Acta 2021, 382, 138346. [Google Scholar] [CrossRef]
- Hao, Z.; Zhao, Q.; Tang, J.; Zhang, Q.; Liu, J.; Jin, Y.; Wang, H. Functional separators towards the suppression of lithium dendrites for rechargeable high-energy batteries. Mater. Horiz. 2021, 8, 12–32. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Hu, X.; Zhang, Y.; He, T.; Zhou, J.; Qiao, J. Ion Transport Regulated Lithium Metal Batteries Achieved by Electrospun ZIF/PAN Composite Separator with Suitable Electrolyte Wettability. Batteries 2023, 9, 166. https://doi.org/10.3390/batteries9030166
Liu T, Hu X, Zhang Y, He T, Zhou J, Qiao J. Ion Transport Regulated Lithium Metal Batteries Achieved by Electrospun ZIF/PAN Composite Separator with Suitable Electrolyte Wettability. Batteries. 2023; 9(3):166. https://doi.org/10.3390/batteries9030166
Chicago/Turabian StyleLiu, Ting, Xuemei Hu, Yadong Zhang, Ting He, Jianping Zhou, and Junqiang Qiao. 2023. "Ion Transport Regulated Lithium Metal Batteries Achieved by Electrospun ZIF/PAN Composite Separator with Suitable Electrolyte Wettability" Batteries 9, no. 3: 166. https://doi.org/10.3390/batteries9030166
APA StyleLiu, T., Hu, X., Zhang, Y., He, T., Zhou, J., & Qiao, J. (2023). Ion Transport Regulated Lithium Metal Batteries Achieved by Electrospun ZIF/PAN Composite Separator with Suitable Electrolyte Wettability. Batteries, 9(3), 166. https://doi.org/10.3390/batteries9030166






