A Systematic Literature Analysis on Electrolyte Filling and Wetting in Lithium-Ion Battery Production
Abstract
1. Introduction
2. Research Background
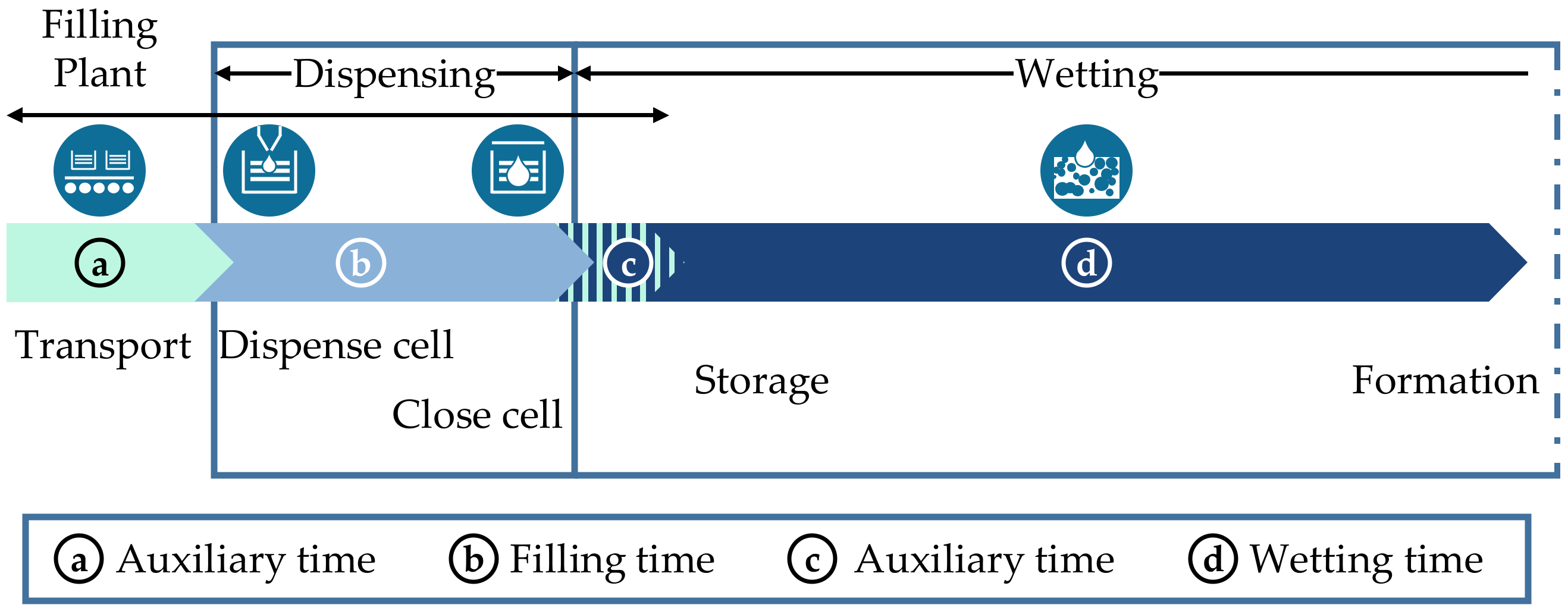
2.1. Explanation of the “Electrolyte Filling” Process
- Scaling can be direct, based on the pore volume of all cell composites (electrodes and separators) [11].
- Scaling can be indirectly influenced by the separator based on the theoretical capacity of the battery cell, which changes with the area and thickness of the electrodes and therefore correlates with the pore volume of the electrodes.
- Scaling can refer to the weight of the cell composite [12]. The latter changes proportionally to the area and thickness of the electrodes and the separator, resulting in a dispensing quantity that is proportional to the pore volume.
- Moreover, scaling can be based on the electrode surface, where electrochemical reactions between the electrolyte and the cell components occur [13]. These approaches typically rely on the assumption that all pores must be fully wetted.
2.2. Classification in the Process Chain
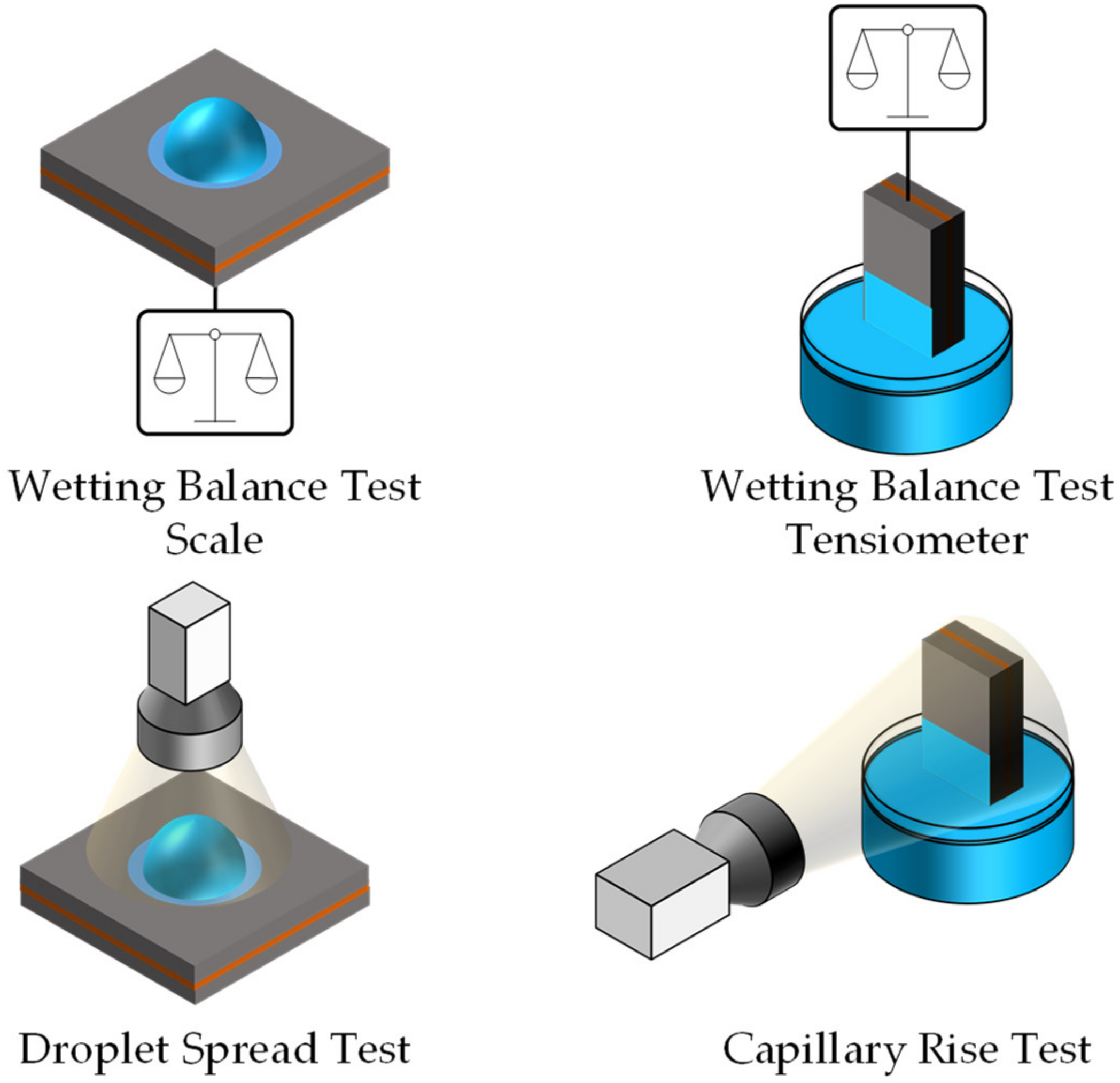
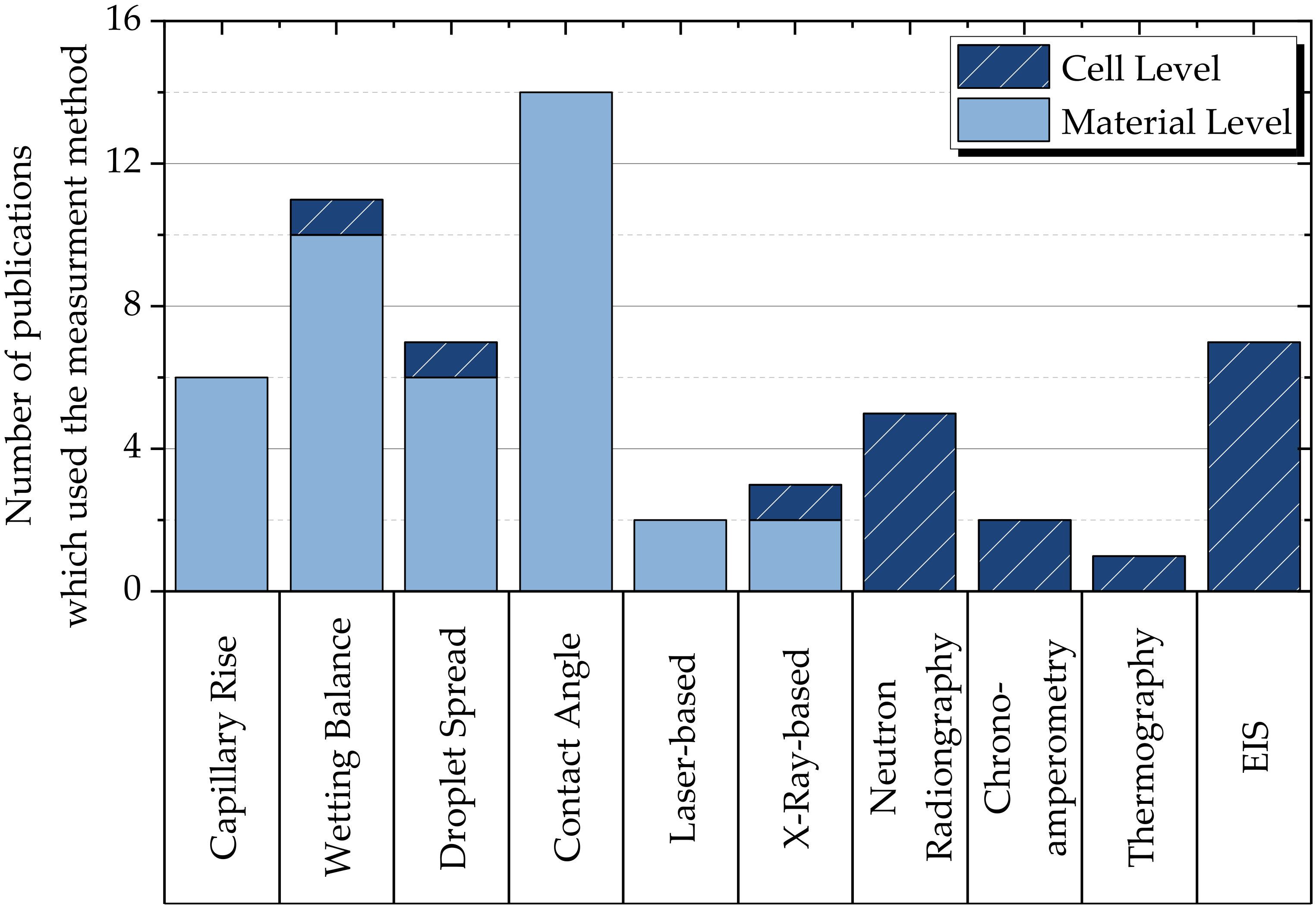
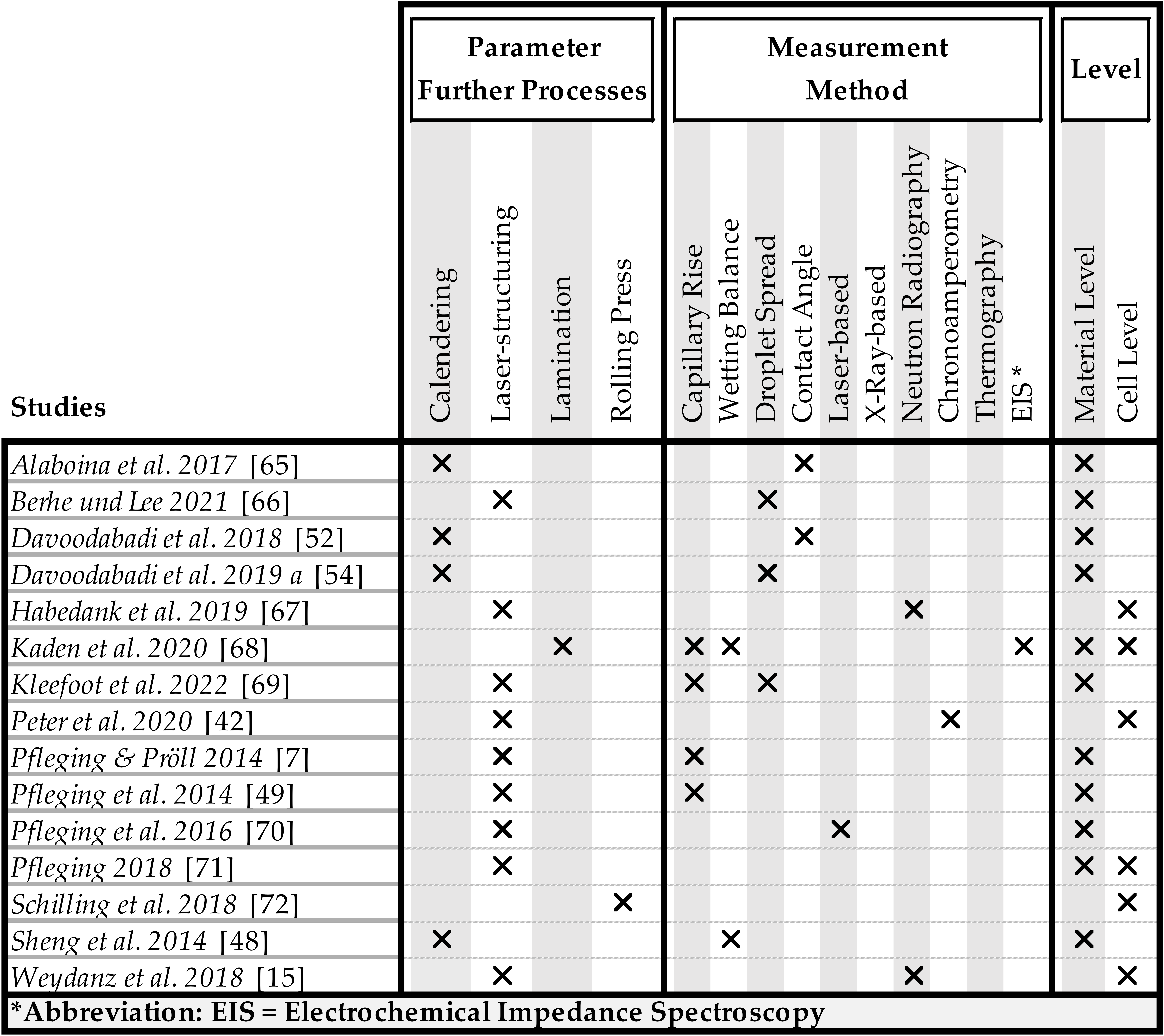
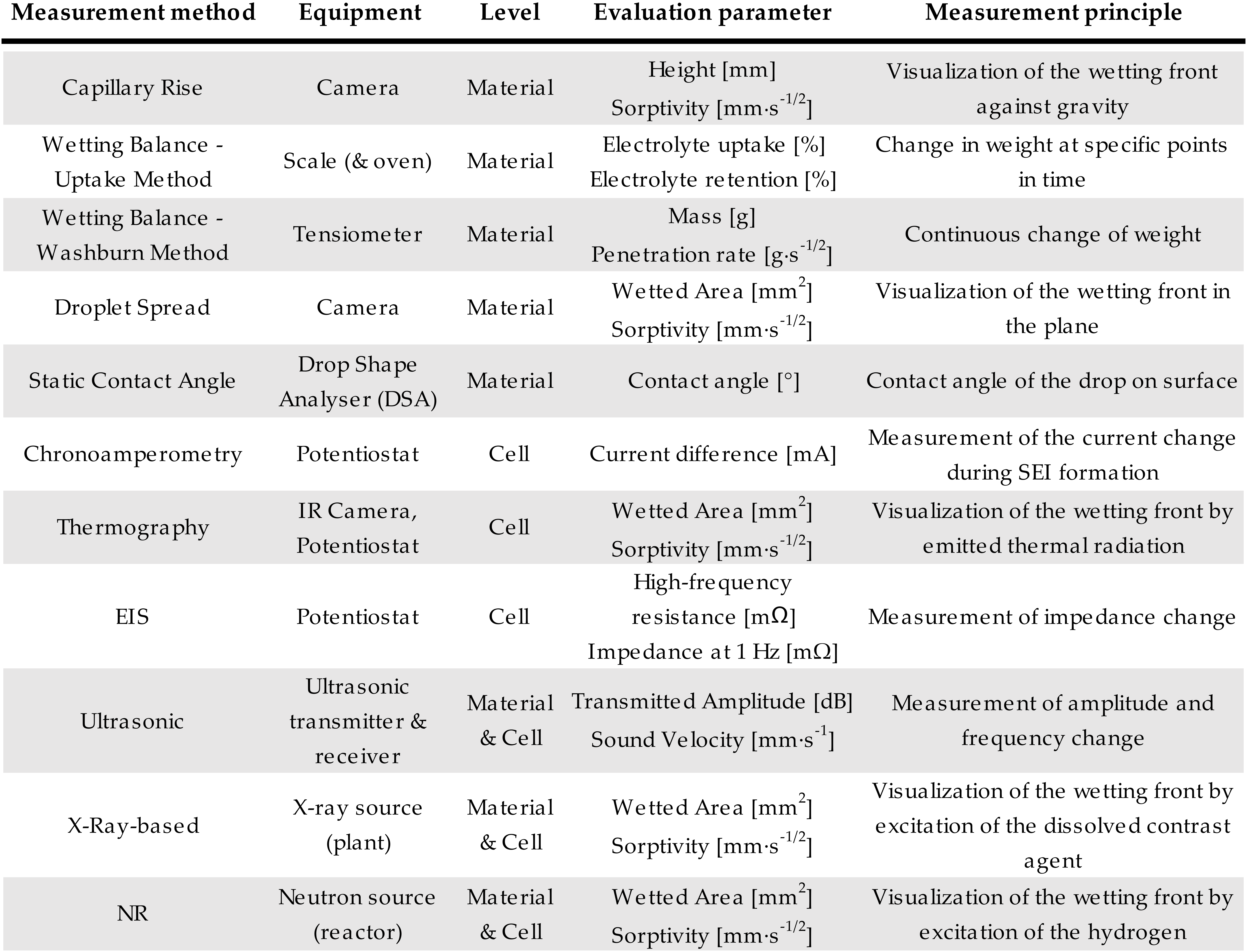
2.3. Explanation of the Common Methods for Measuring Electrolyte Filling
3. Methods
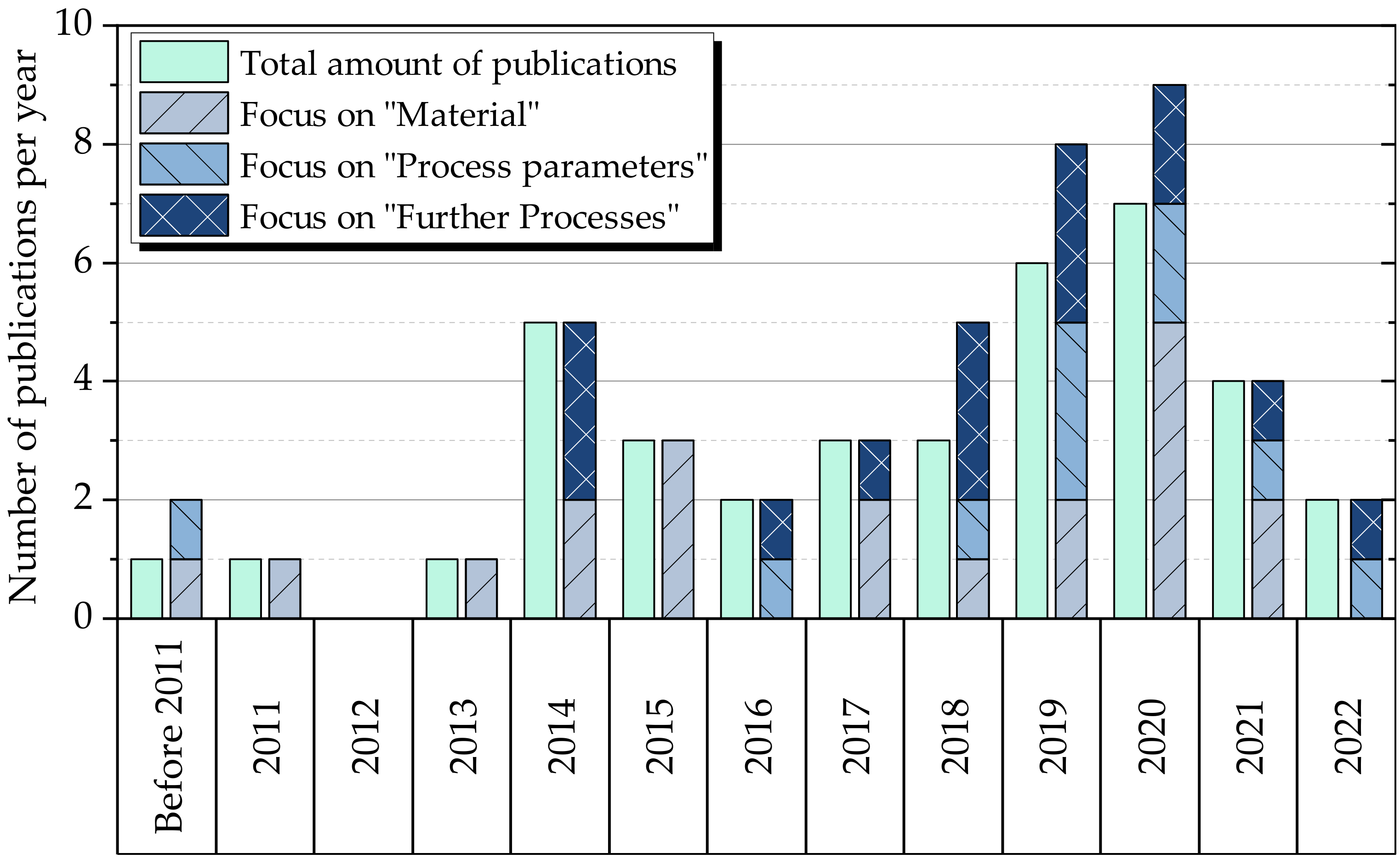
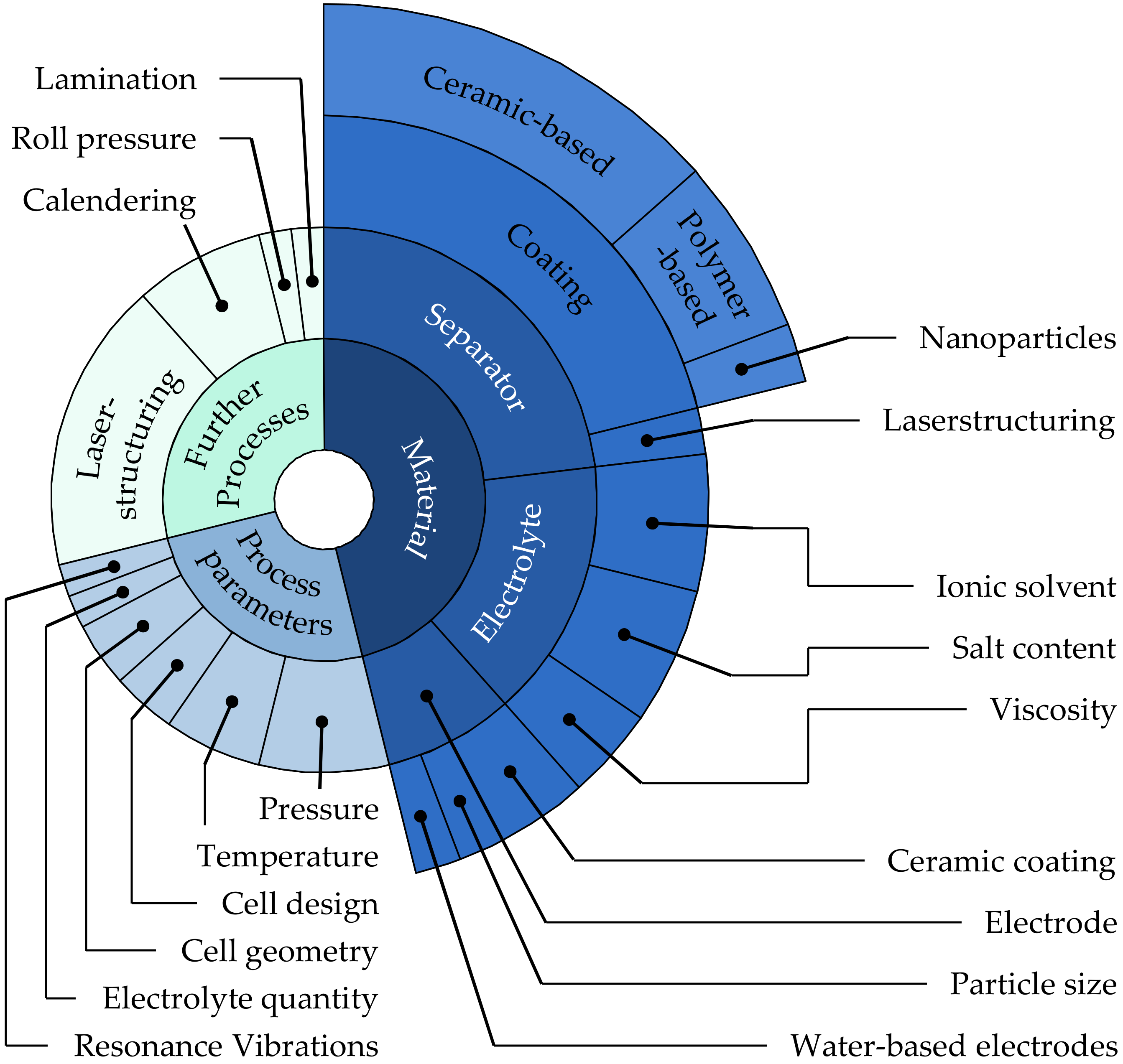
4. Results
5. Discussion
- What types of models and simulations are applied, and what computational algorithms are employed?
- At what level or scale are dispensing and wetting represented in these models?
- Are there simulations that focus on the wetting processes in the pore systems of cell composite materials, the dispensing process in the housing, or the cause-and-effect relationships in the overall process?
- What are the inputs and outputs of the respective model?
- What resources or computing power are required for a simulation?
- In what framework have these models been validated?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2022; IEA: Paris, France, 2022. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EU) 2018/858 of the European Parliament and of the Council on the Approval and Market Surveillance of Motor Vehicles and Their Trailers, and of Systems, Components and Separate Technical Units Intended for Such Vehicles, amending Regulations (EC) No 715/2007 and (EC) No 595/2009 and Repealing Directive 2007/46/EC: Regulation (EU) 2018/858; The European Parliament and the Council of the European Union: Strasbourg, France, 2018. [Google Scholar]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Daniel, C. Materials and processing for lithium-ion batteries. J. Miner. Met. Mater. Soc. 2008, 60, 43–48. [Google Scholar] [CrossRef]
- Wood, D.L.; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Pfleging, W.; Pröll, J. A new approach for rapid electrolyte wetting in tape cast electrodes for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14918–14926. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Korthauer, R. Lithium-Ion Batteries: Basics and Applications; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3662530696. [Google Scholar]
- Günter, F.J.; Burgstaller, C.; Konwitschny, F.; Reinhart, G. Influence of the Electrolyte Quantity on Lithium-Ion Cells. J. Electrochem. Soc. 2019, 166, A1709–A1714. [Google Scholar] [CrossRef]
- Hoffmann, L.; Grathwol, J.-K.; Haselrieder, W.; Leithoff, R.; Jansen, T.; Dilger, K.; Dröder, K.; Kwade, A.; Kurrat, M. Capacity Distribution of Large Lithium-Ion Battery Pouch Cells in Context with Pilot Production Processes. Energy Technol. 2020, 8, 1900196. [Google Scholar] [CrossRef]
- Kang, S.-J.; Yu, S.; Lee, C.; Yang, D.; Lee, H. Effects of electrolyte-volume-to-electrode-area ratio on redox behaviors of graphite anodes for lithium-ion batteries. Electrochim. Acta 2014, 141, 367–373. [Google Scholar] [CrossRef]
- Knoche, T.; Reinhart, G. Electrolyte Filling of Large-Scale Lithium-Ion Batteries: Challenges for Production Technology and Possible Approaches. Appl. Mech. Mater. 2015, 794, 11–18. [Google Scholar] [CrossRef]
- Weydanz, W.J.; Reisenweber, H.; Gottschalk, A.; Schulz, M.; Knoche, T.; Reinhart, G.; Masuch, M.; Franke, J.; Gilles, R. Visualization of electrolyte filling process and influence of vacuum during filling for hard case prismatic lithium ion cells by neutron imaging to optimize the production process. J. Power Sources 2018, 380, 126–134. [Google Scholar] [CrossRef]
- Günter, F.J.; Keilhofer, J.; Rauch, C.; Rössler, S.; Schulz, M.; Braunwarth, W.; Gilles, R.; Daub, R.; Reinhart, G. Influence of pressure and temperature on the electrolyte filling of lithium-ion cells: Experiment, model and method. J. Power Sources 2022, 517, 230668. [Google Scholar] [CrossRef]
- Günter, F.J.; Rössler, S.; Schulz, M.; Braunwarth, W.; Gilles, R.; Reinhart, G. Influence of the Cell Format on the Electrolyte Filling Process of Lithium-Ion Cells. Energy Technol. 2020, 8, 1801108. [Google Scholar] [CrossRef]
- Hagemeister, J.; Stock, S.; Linke, M.; Fischer, M.; Drees, R.; Kurrat, M.; Daub, R. Lean Cell Finalization in Lithium-Ion Battery Production: Determining the Required Electrolyte Wetting Degree to Begin the Formation. Energy Technol. 2022, 2200686. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Wu, M.-S.; Liao, T.-L.; Wang, Y.-Y.; Wan, C.-C. Assessment of the Wettability of Porous Electrodes for Lithium-Ion Batteries. J. Appl. Electrochem. 2004, 34, 797–805. [Google Scholar] [CrossRef]
- Hawley, W.B.; Li, J. Electrode manufacturing for lithium-ion batteries—Analysis of current and next generation processing. J. Energy Storage 2019, 25, 100862. [Google Scholar] [CrossRef]
- Kraft, L.; Habedank, J.B.; Frank, A.; Rheinfeld, A.; Jossen, A. Modeling and Simulation of Pore Morphology Modifications using Laser-Structured Graphite Anodes in Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 13506. [Google Scholar] [CrossRef]
- Yourey, W. Theoretical Impact of Manufacturing Tolerance on Lithium-Ion Electrode and Cell Physical Properties. Batteries 2020, 6, 23. [Google Scholar] [CrossRef]
- Schilling, A.; Gümbel, P.; Möller, M.; Kalkan, F.; Dietrich, F.; Dröder, K. X-ray Based Visualization of the Electrolyte Filling Process of Lithium Ion Batteries. J. Electrochem. Soc. 2019, 166, A5163–A5167. [Google Scholar] [CrossRef]
- Huttner, F.; Marth, A.; Eser, J.C.; Heckmann, T.; Mohacsi, J.; Mayer, J.K.; Scharfer, P.; Schabel, W.; Kwade, A. Design of Vacuum Post-Drying Procedures for Electrodes of Lithium-Ion Batteries. Batter. Supercaps 2021, 4, 1499–1515. [Google Scholar] [CrossRef]
- Huttner, F.; Haselrieder, W.; Kwade, A. The Influence of Different Post-Drying Procedures on Remaining Water Content and Physical and Electrochemical Properties of Lithium-Ion Batteries. Energy Technol. 2020, 8, 1900245. [Google Scholar] [CrossRef]
- Rowden, B.; Garcia-Araez, N. A review of gas evolution in lithium ion batteries. Energy Rep. 2020, 6, 10–18. [Google Scholar] [CrossRef]
- Wiemers-Meyer, S.; Jeremias, S.; Winter, M.; Nowak, S. Influence of Battery Cell Components and Water on the Thermal and Chemical Stability of LiPF6 Based Lithium Ion Battery Electrolytes. Electrochim. Acta 2016, 222, 1267–1271. [Google Scholar] [CrossRef]
- Knoche, T.; Zinth, V.; Schulz, M.; Schnell, J.; Gilles, R.; Reinhart, G. In situ visualization of the electrolyte solvent filling process by neutron radiography. J. Power Sources 2016, 331, 267–276. [Google Scholar] [CrossRef]
- Kühnel, R.-S.; Obeidi, S.; Lübke, M.; Lex-Balducci, A.; Balducci, A. Evaluation of the wetting time of porous electrodes in electrolytic solutions containing ionic liquid. J. Appl. Electrochem. 2013, 43, 697–704. [Google Scholar] [CrossRef]
- Günter, F.J.; Habedank, J.B.; Schreiner, D.; Neuwirth, T.; Gilles, R.; Reinhart, G. Introduction to Electrochemical Impedance Spectroscopy as a Measurement Method for the Wetting Degree of Lithium-Ion Cells. J. Electrochem. Soc. 2018, 165, A3249–A3256. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, B.; Pan, F.; Qiao, L.; Guo, J.; Duan, C.; Wu, W.; Chen, Z.; Su, Y. Nano-silica-decorated Poly(m-Phenylene Isophthalamide) Separator with Enhanced Mechanical and Electrolyte Wetting Properties for Lithium-Ion Batteries. Trans. Tianjin Univ. 2020, 26, 256–264. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Colloid Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Beyer, S.; Kobsch, O.; Pospiech, D.; Simon, F.; Peter, C.; Nikolowski, K.; Wolter, M.; Voit, B. Influence of surface characteristics on the penetration rate of electrolytes into model cells for lithium ion batteries. J. Adhes. Sci. Technol. 2020, 34, 849–866. [Google Scholar] [CrossRef]
- Schilling, A.; Wiemers-Meyer, S.; Winkler, V.; Nowak, S.; Hoppe, B.; Heimes, H.H.; Dröder, K.; Winter, M. Influence of Separator Material on Infiltration Rate and Wetting Behavior of Lithium-Ion Batteries. Energy Technol. 2020, 8, 1900078. [Google Scholar] [CrossRef]
- Masuch, S.; Gümbel, P.; Kaden, N.; Dröder, K. Applications and Development of X-ray Inspection Techniques in Battery Cell Production. Processes 2023, 11, 10. [Google Scholar] [CrossRef]
- Gold, L.; Herzog, T.; Schubert, F.; Heuer, H.; Giffin, G.A. Ultrasound Propagation in Lithium-Ion Battery Cell Materials: Basis for Developing Monitoring and Imaging Methods. Energy Technol. 2022, 2200861. [Google Scholar] [CrossRef]
- Deng, Z.; Huang, Z.; Shen, Y.; Huang, Y.; Ding, H.; Luscombe, A.; Johnson, M.; Harlow, J.E.; Gauthier, R.; Dahn, J.R. Ultrasonic Scanning to Observe Wetting and “Unwetting” in Li-Ion Pouch Cells. Joule 2020, 4, 2017–2029. [Google Scholar] [CrossRef]
- Peter, C.; Nikolowski, K.; Reuber, S.; Wolter, M.; Michaelis, A. Chronoamperometry as an electrochemical in situ approach to investigate the electrolyte wetting process of lithium-ion cells. J. Appl. Electrochem. 2020, 50, 295–309. [Google Scholar] [CrossRef]
- Brendel, A.B.; Trang, S.; Marrone, M.; Lichtenberg, S.; Kolbe, L.M. What to Do for a Literature Review?—A Synthesis of Literature Review Practices. In Proceedings of Americas Conference on Information Systems; Association for Information Systems: Atlanta, GA, USA, 2020; ISBN 9781733632546. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Elsevier. About Elsevier Scopus Blog. Available online: https://blog.scopus.com/about (accessed on 5 January 2023).
- Bandara, W.; Furtmueller, E.; Gorbacheva, E.; Miskon, S.; Beekhuyzen, J. Achieving Rigor in Literature Reviews: Insights from Qualitative Data Analysis and Tool-Support. Commun. Assoc. Inf. Syst. 2015, 37, 8. [Google Scholar] [CrossRef]
- Mayring, P. Qualitative Inhaltsanalyse: Grundlagen und Techniken, 13. überarbeitete Auflage; Beltz: Weinheim, Germany; Preselect.media GmbH: Grünwald, Germany, 2022; ISBN 9783407258991. [Google Scholar]
- Sheng, Y.; Fell, C.R.; Son, Y.K.; Metz, B.M.; Jiang, J.; Church, B.C. Effect of Calendering on Electrode Wettability in Lithium-Ion Batteries. Front. Energy Res. 2014, 2, 56. [Google Scholar] [CrossRef]
- Pfleging, W.; Kohler, R.; Pröll, J. Laser generated microstructures in tape cast electrodes for rapid electrolyte wetting: New technical approach for cost efficient battery manufacturing. In Laser-Based Micro- and Nanoprocessing VIII; Klotzbach, U., Washio, K., Arnold, C.B., Eds.; SPIE LASE: San Francisco, CA, USA, 2014; 89680B. [Google Scholar]
- An, M.-Y.; Kim, H.-T.; Chang, D.-R. Multilayered separator based on porous polyethylene layer, Al2O3 layer, and electro-spun PVdF nanofiber layer for lithium batteries. J. Solid State Electrochem. 2014, 18, 1807–1814. [Google Scholar] [CrossRef]
- Bolimowska, E.; Morales-Ugarte, J.E.; Rouault, H.; Santos-Peña, J.; Santini, C.; Benayad, A. Influence of the Vinylene Carbonate on the Wetting and Interface Chemical Structure of Doped Ionic Liquid Electrolyte at Porous Graphite Surface. J. Phys. Chem. C 2017, 121, 16166–16173. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Li, J.; Liang, Y.; Wang, R.; Zhou, H.; Wood, D.L.; Singler, T.J.; Jin, C. Characterization of Surface Free Energy of Composite Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A2493–A2501. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Li, J.; Liang, Y.; Wood, D.L.; Singler, T.J.; Jin, C. Analysis of electrolyte imbibition through lithium-ion battery electrodes. J. Power Sources 2019, 424, 193–203. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Li, J.; Zhou, H.; Wood, D.L.; Singler, T.J.; Jin, C. Effect of calendering and temperature on electrolyte wetting in lithium-ion battery electrodes. J. Energy Storage 2019, 26, 101034. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Jin, C.; Wood III, D.L.; Singler, T.J.; Li, J. On electrolyte wetting through lithium-ion battery separators. Extrem. Mech. Lett. 2020, 40, 100960. [Google Scholar] [CrossRef]
- Fang, J.; Kelarakis, A.; Lin, Y.-W.; Giannelis, E.; Tsai, L.-D. Nanoparticle Coated Separators for Lithium-Ion Batteries with Advanced Electrochemical Performance. ECS Meet. Abstr. 2011, MA2011-02, 1456. [Google Scholar] [CrossRef]
- Jang, D.; Suh, S.; Yoon, H.; Kim, J.; Kim, H.; Baek, J.; Kim, H.-J. Enhancing rate capability of graphite anodes for lithium-ion batteries by pore-structuring. Appl. Surf. Sci. Adv. 2021, 6, 100168. [Google Scholar] [CrossRef]
- Man, C.; Jiang, P.; Wong, K.; Zhao, Y.; Tang, C.; Fan, M.; Lau, W.; Mei, J.; Li, S.; Liu, H.; et al. Enhanced wetting properties of a polypropylene separator for a lithium-ion battery by hyperthermal hydrogen induced cross-linking of poly(ethylene oxide). J. Mater. Chem. A 2014, 2, 11980–11986. [Google Scholar] [CrossRef]
- Pröll, J.; Schmitz, B.; Niemöeller, A.; Robertz, B.; Schäfer, M.; Torge, M.; Smyrek, P.; Seifert, H.J.; Pfleging, W. Femtosecond laser patterning of lithium-ion battery separator materials: Impact on liquid electrolyte wetting and cell performance. In Laser-Based Micro- and Nanoprocessing IX; Klotzbach, U., Washio, K., Arnold, C.B., Eds.; SPIE LASE: San Francisco, CA, USA, 2015; 93511F. [Google Scholar]
- Sun, Y.; Wang, J.; Prausnitz, J.M. Interfacial properties between ionic-liquid-based electrolytes and lithium-ion-battery separator. AIChE J. 2021, 67, e17208. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, F.; Chen, C.; Shi, L.; Yuan, S.; Sun, L.; Zhu, J. Self-assembly of PEI/SiO2 on polyethylene separators for Li-ion batteries with enhanced rate capability. ACS Appl. Mater. Interfaces 2015, 7, 3314–3322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Fang, J.; Ding, L.-X.; Wang, H. A nano-silica modified polyimide nanofiber separator with enhanced thermal and wetting properties for high safety lithium-ion batteries. J. Membr. Sci. 2017, 537, 248–254. [Google Scholar] [CrossRef]
- Yoo, Y.; Kim, B.G.; Pak, K.; Han, S.J.; Song, H.-S.; Choi, J.W.; Im, S.G. Initiated Chemical Vapor Deposition (iCVD) of Highly Cross-Linked Polymer Films for Advanced Lithium-Ion Battery Separators. ACS Appl. Mater. Interfaces 2015, 7, 18849–18855. [Google Scholar] [CrossRef]
- Jin, D.; Kang, H.; Do, H.W.; Kim, G.; Kim, T.; Kim, S.; Choi, S.; Won, J.; Park, I.; Jung, K.; et al. Enhancing Li Ion Battery Performance by Mechanical Resonance. Nano Lett. 2021, 21, 5345–5352. [Google Scholar] [CrossRef]
- Alaboina, P.K.; Cho, J.-S.; Cho, S.-J. Engineering and Optimization of Silicon-Iron-Manganese Nanoalloy Electrode for Enhanced Lithium-Ion Battery. Nanomicro. Lett. 2017, 9, 41. [Google Scholar] [CrossRef]
- Berhe, M.G.; Lee, D. A Comparative Study on the Wettability of Unstructured and Structured LiFePO4 with Nanosecond Pulsed Fiber Laser. Micromachines 2021, 12, 582. [Google Scholar] [CrossRef]
- Habedank, J.B.; Günter, F.J.; Billot, N.; Gilles, R.; Neuwirth, T.; Reinhart, G.; Zaeh, M.F. Rapid electrolyte wetting of lithium-ion batteries containing laser structured electrodes: In situ visualization by neutron radiography. Int. J. Adv. Manuf. Technol. 2019, 102, 2769–2778. [Google Scholar] [CrossRef]
- Kaden, N.; Schlüter, N.; Leithoff, R.; Savas, S.; Grundmeier, S.; Dröder, K. Influence of the Lamination Process on the Wetting Behavior and the Wetting Rate of Lithium-Ion Batteries. Processes 2021, 9, 1851. [Google Scholar] [CrossRef]
- Kleefoot, M.-J.; Enderle, S.; Sandherr, J.; Bolsinger, M.; Maischik, T.; Simon, N.; Martan, J.; Ruck, S.; Knoblauch, V.; Riegel, H. Enhancement of the wettability of graphite-based lithium-ion battery anodes by selective laser surface modification using low energy nanosecond pulses. Int. J. Adv. Manuf. Technol. 2022, 118, 1987–1997. [Google Scholar] [CrossRef]
- Pfleging, W.; Zheng, Y.; Mangang, M.; Bruns, M.; Smyrek, P. Laser processes and analytics for high power 3D battery materials. In Frontiers in Ultrafast Optics: Biomedical, Scientific, and Industrial Applications XVI; Heisterkamp, A., Herman, P.R., Meunier, M., Nolte, S., Eds.; SPIE LASE: San Francisco, CA, USA, 2016; p. 974013. [Google Scholar]
- Pfleging, W. A review of laser electrode processing for development and manufacturing of lithium-ion batteries. Nanophotonics 2018, 7, 549–573. [Google Scholar] [CrossRef]
- Schilling, A. Design of an Automated System to Accelerate the Electrolyte Distribution in Lithium-Ion Batteries. Int. J. Mech. Eng. Robot. Res. 2018, 8, 162–166. [Google Scholar] [CrossRef]
- Frankenberger, M.; Mock, C.; Kaden, N.; Landwehr, I.; Veitl, J.; Ophey, J.; Schälicke, G.; Görke, M.; Holeczek, H.; Kwade, A.; et al. Improving Wetting Behavior and C-Rate Capability of Lithium-Ion Batteries by Plasma Activation. Energy Technol. 2022, 2200636. [Google Scholar] [CrossRef]
- Hagemeister, J.; Günter, F.J.; Rinner, T.; Zhu, F.; Papst, A.; Daub, R. Numerical Models of the Electrolyte Filling Process of Lithium-Ion Batteries to Accelerate and Improve the Process and Cell Design. Batteries 2022, 8, 159. [Google Scholar] [CrossRef]
- Wanner, J.; Birke, K.P. Comparison of an Experimental Electrolyte Wetting of a Lithium-Ion Battery Anode and Separator by a Lattice Boltzmann Simulation. Batteries 2022, 8, 277. [Google Scholar] [CrossRef]




 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaden, N.; Schlimbach, R.; Rohde García, Á.; Dröder, K. A Systematic Literature Analysis on Electrolyte Filling and Wetting in Lithium-Ion Battery Production. Batteries 2023, 9, 164. https://doi.org/10.3390/batteries9030164
Kaden N, Schlimbach R, Rohde García Á, Dröder K. A Systematic Literature Analysis on Electrolyte Filling and Wetting in Lithium-Ion Battery Production. Batteries. 2023; 9(3):164. https://doi.org/10.3390/batteries9030164
Chicago/Turabian StyleKaden, Nicolaj, Ricarda Schlimbach, Álvaro Rohde García, and Klaus Dröder. 2023. "A Systematic Literature Analysis on Electrolyte Filling and Wetting in Lithium-Ion Battery Production" Batteries 9, no. 3: 164. https://doi.org/10.3390/batteries9030164
APA StyleKaden, N., Schlimbach, R., Rohde García, Á., & Dröder, K. (2023). A Systematic Literature Analysis on Electrolyte Filling and Wetting in Lithium-Ion Battery Production. Batteries, 9(3), 164. https://doi.org/10.3390/batteries9030164







