Electrochemical Performance and Stress Distribution of Sb/Sb2O3 Nanoparticles as Anode Materials for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
3. Calculations Stress Analysis during Sodiation Process
4. Results and Discussion
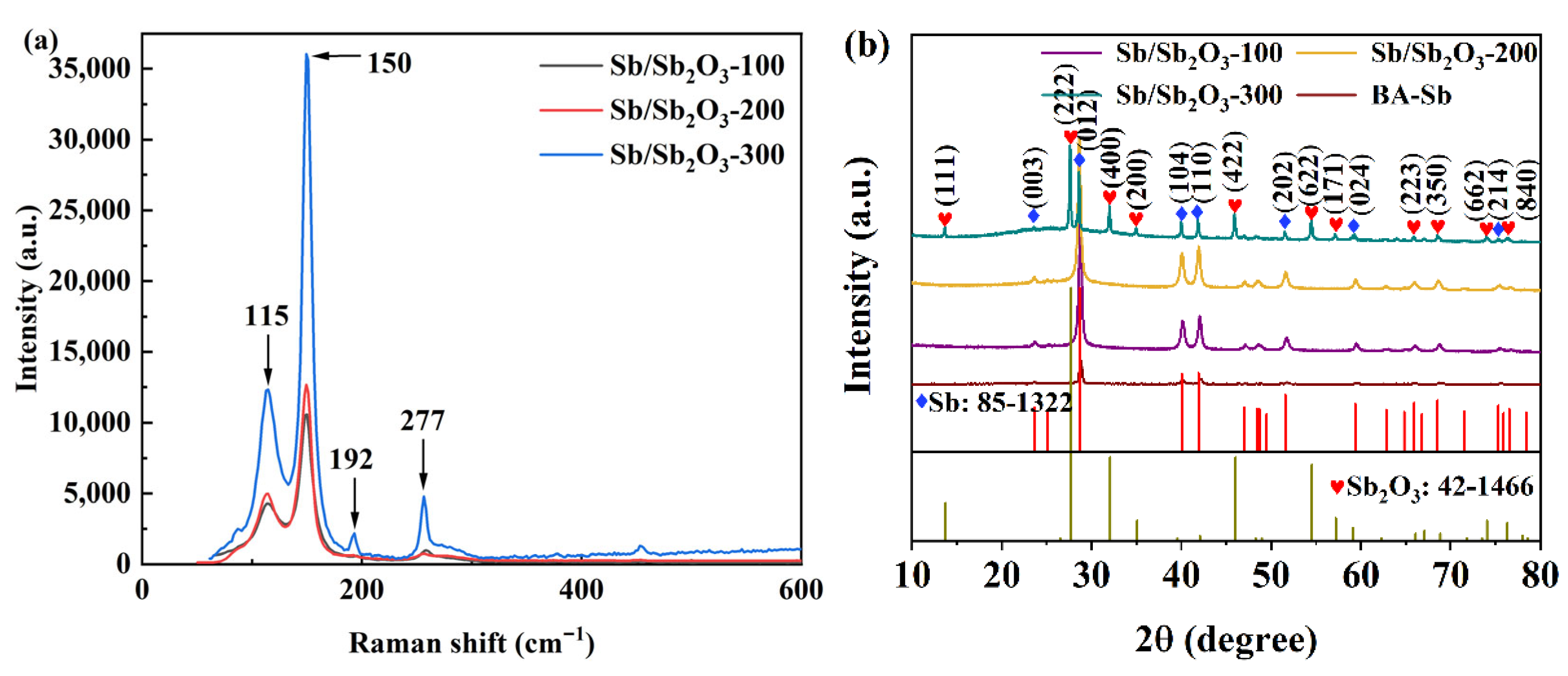
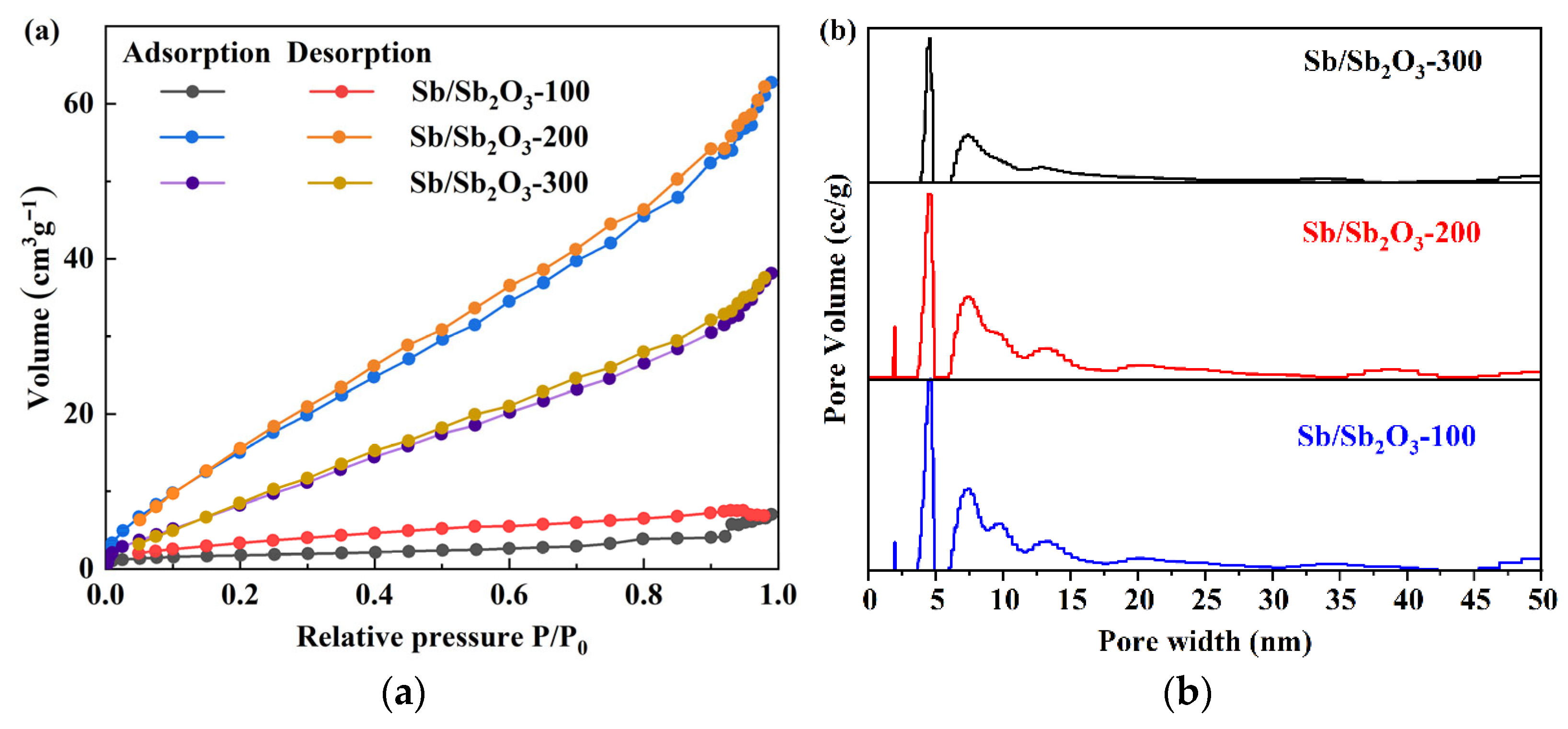
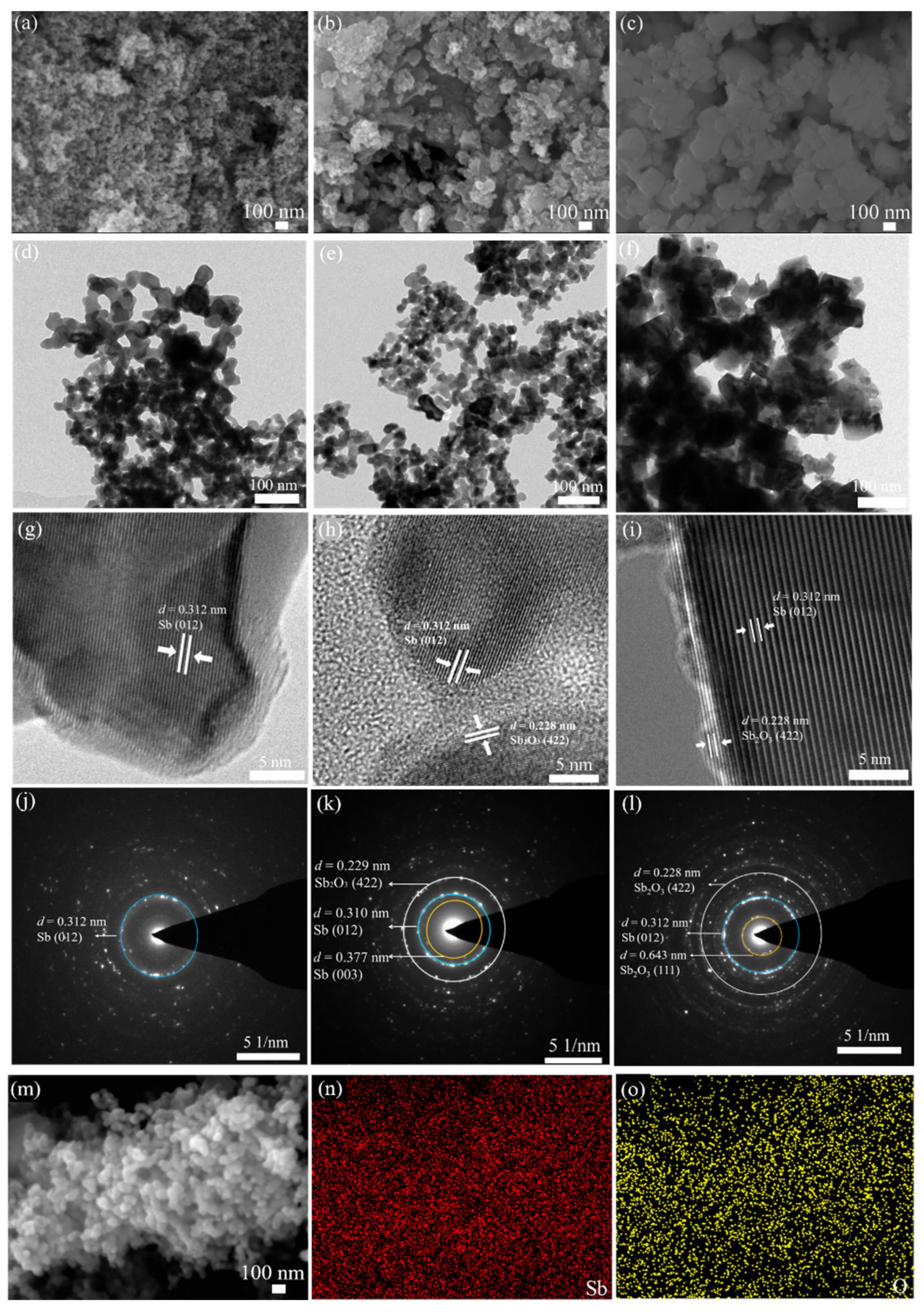
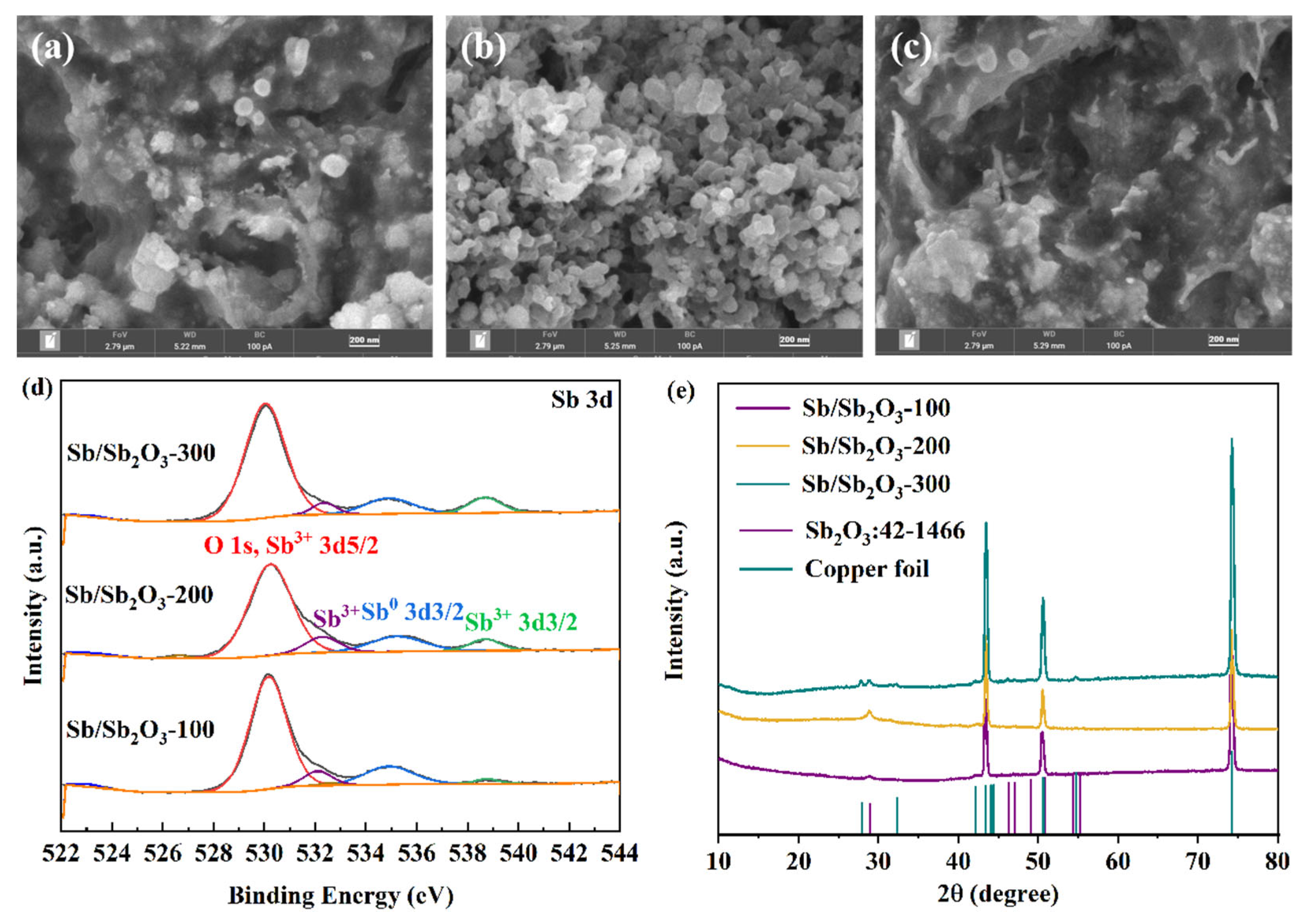
4.1. Morphology, Structures, and Electrochemical Analysis
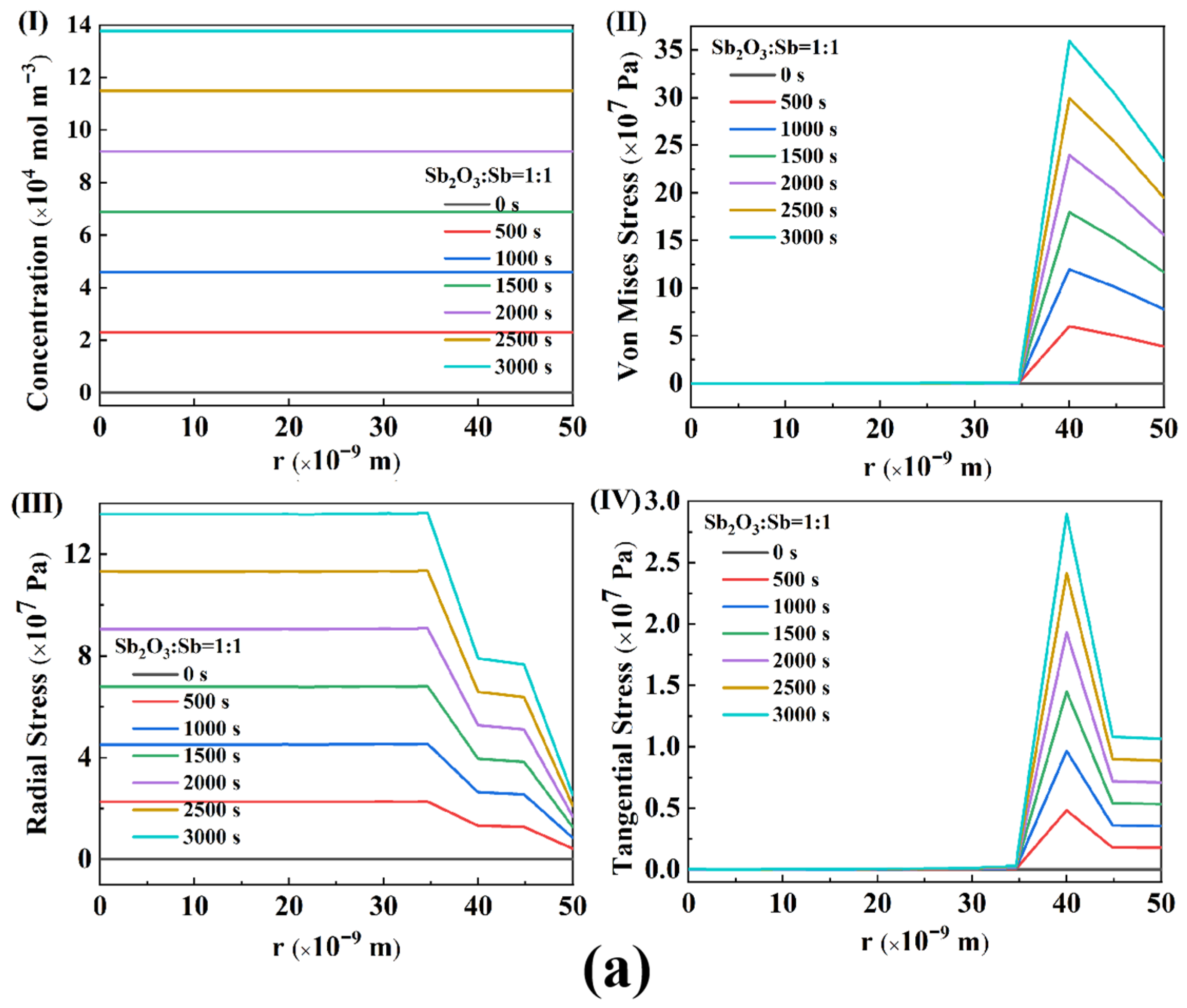
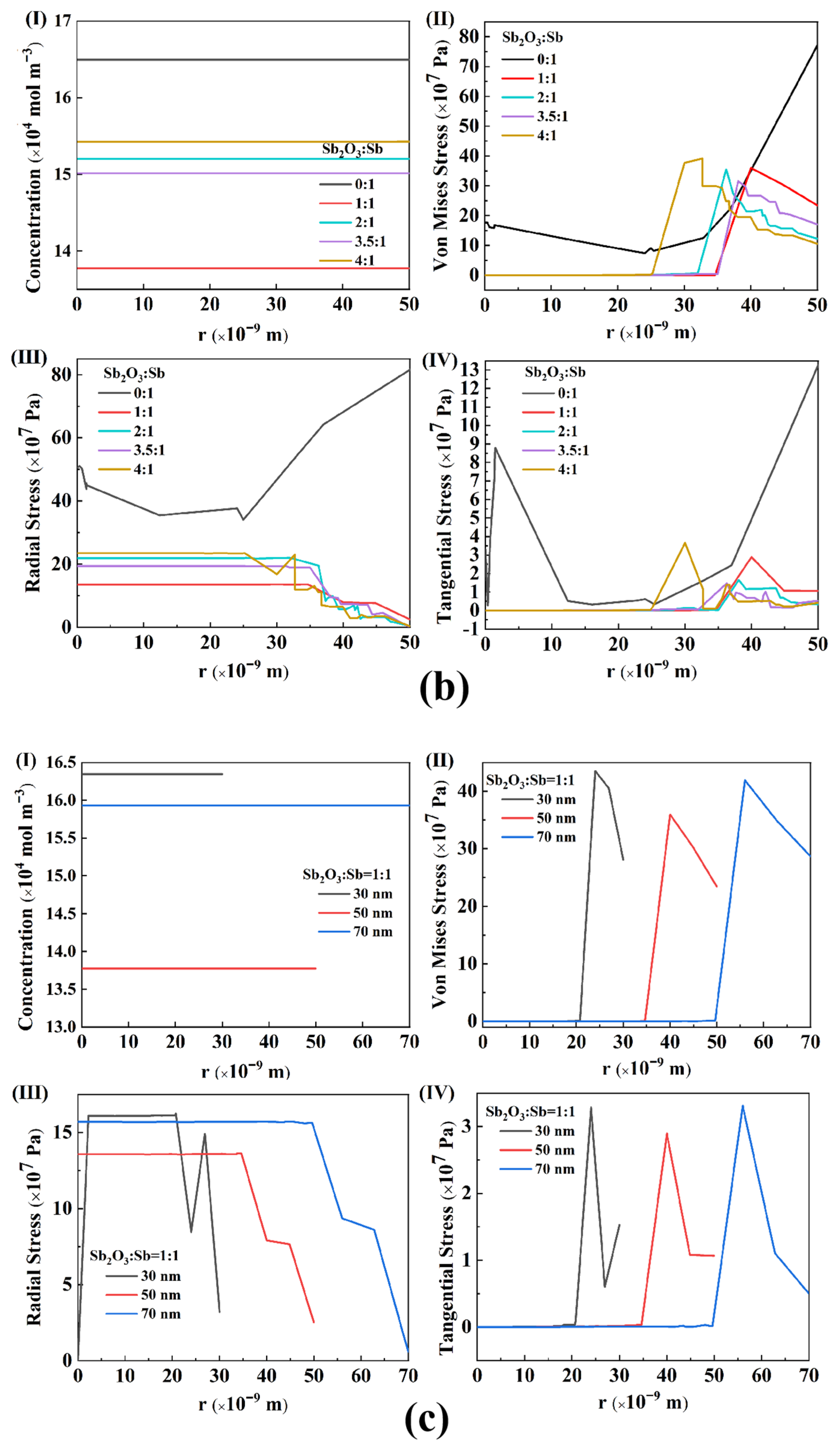
4.2. Stress Analysis
5. Conclusions
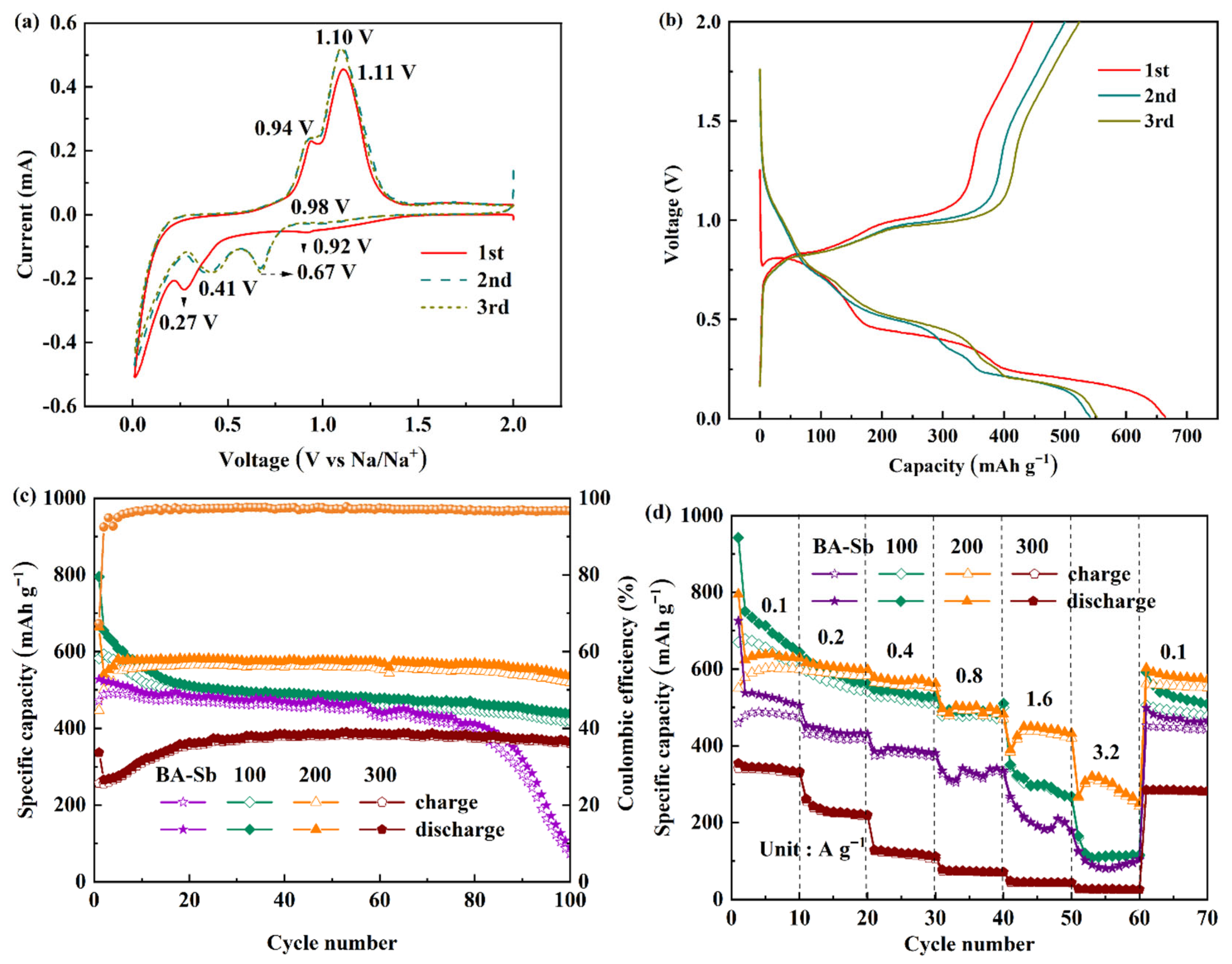
- The half SIBs with the optimized Sb/Sb2O3-200 nanoparticles exhibit superior reversible performance, maintaining up to six times more capacity (540 mAh g−1) than the pure Sb (90 mAh g−1) after 100 cycles at 0.1 A g−1, and a superior rate performance with 95.7% retention after cycling at the varied current densities. One reason for this is that the oxidation temperature affects the content of Sb2O3 in Sb/Sb2O3 nanoparticle and the particle size of Sb/Sb2O3, and Sb/Sb2O3-200 is at exactly the optimum composition ratio of Sb2O3:Sb in Sb/Sb2O3 and the particle size to ensure both a high capacity for Na+ and small strain-induced stress during sodiation/desodiation, which is supported by the diffusion-stress coupled results.
- The results of the diffusion-stress coupled model indicate that increasing the composition ratio of Sb2O3:Sb in Sb/Sb2O3 will lead to a decrease of Mises equivalent stress, radial stress, and tangential stress in the ratio range 1:1–3.5:1 and cause a stress increase in the ratio range 3.5:1–4:1. The Mises equivalent stress, radial stress, and tangential stress show a decrease with the particle radius in the range 30–50 nm of Sb/Sb2O3 nanoparticles, and an increase with a particle radius in the range 50–70 nm.
- The superior performance of Sb/Sb2O3-200 is also related to the formation of cycling-induced coral-like structure Sb particles, which can promote Na+ diffusion, relieve cycling-induced volume changes, and provide exceptional Na+ storage. This morphology transformation is associated with the state of stress of active materials.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, L.; Rao, M.; Aloni, S.; Wang, L.; Cairns, E.J.; Zhang, Y. Porous carbon nanofiber–sulfur composite electrodes for lithium/sulfur cells. Energy Environ. Sci. 2011, 4, 5053–5059. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kim, H.-J. Preparation and electrochemical performance of NiCo2O4@NiCo2O4 composite nanoplates for high performance supercapacitor applications. New J. Chem. 2018, 42, 19971–19978. [Google Scholar] [CrossRef]
- Wang, N.; Bai, Z.; Qian, Y.; Yang, J. One-Dimensional Yolk–Shell Sb@Ti–O–P Nanostructures as a High-Capacity and High-Rate Anode Material for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2016, 9, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Nam, D.-H.; Hong, K.-S.; Lim, S.-J.; Kim, T.-H.; Kwon, H.-S. Electrochemical Properties of Electrodeposited Sn Anodes for Na-Ion Batteries. J. Phys. Chem. C 2014, 118, 20086–20093. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. The Mechanisms of Lithium and Sodium Insertion in Carbon Materials. J. Electrochem. Soc. 2001, 148, A803–A811. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Kohandehghan, A.; Cui, K.; Xu, Z.; Zahiri, B.; Tan, X.; Lotfabad, E.M.; Olsen, B.C.; et al. Carbon Nanosheet Frameworks Derived from Peat Moss as High Performance Sodium Ion Battery Anodes. ACS Nano 2013, 7, 11004–11015. [Google Scholar] [CrossRef]
- Jache, M.S.B.; Adelhelm, P. Use of Graphite as a Highly Reversible Electrode with Superior Cycle Life for Sodium-Ion Batteries by Making Use of Co-Intercalation Phenomena. Angew. Chem. Int. Ed. 2014, 53, 10169–10173. [Google Scholar] [CrossRef]
- Bodenes, L.; Darwiche, A.; Monconduit, L.; Martinez, H. The Solid Electrolyte Interphase a key parameter of the high performance of Sb in sodium-ion batteries: Comparative X-ray Photoelectron Spectroscopy study of Sb/Na-ion and Sb/Li-ion batteries. J. Power Sources 2015, 273, 14–24. [Google Scholar] [CrossRef]
- Zhao, Y.; Manthiram, A. High-Capacity, High-Rate Bi–Sb Alloy Anodes for Lithium-Ion and Sodium-Ion Batteries. Chem. Mater. 2015, 27, 3096–3101. [Google Scholar] [CrossRef]
- Tao, T.; Chen, Y. Direct synthesis of rutile TiO2 nanorods with improved electrochemical lithium ion storage properties. Mater. Lett. 2013, 98, 112–115. [Google Scholar] [CrossRef]
- Hariharan, S.; Saravanan, K.; Balaya, P. α-MoO3: A high performance anode material for sodium-ion batteries. Electrochem. Commun. 2013, 31, 5–9. [Google Scholar] [CrossRef]
- Moniruzzaman; Kumar, Y.A.; Pallavolu, M.R.; Arbi, H.M.; Alzahmi, S.; Obaidat, I.M. Two-Dimensional Core-Shell Structure of Cobalt-Doped@MnO2 Nanosheets Grown on Nickel Foam as a Binder-Free Battery-Type Electrode for Supercapacitor Application. Nanomaterials 2022, 12, 3187. [Google Scholar] [CrossRef]
- Alcantara, R.; Jiménez-Mateos, J.M.; Lavela, P.; Tirado, J.L. Carbon black: A promising electrode material for sodium-ion batteries. Electrochem. Commun. 2001, 3, 639–642. [Google Scholar] [CrossRef]
- Qian, J.; Wu, X.; Cao, Y.; Ai, X.; Yang, H. High Capacity and Rate Capability of Amorphous Phosphorus for Sodium Ion Batteries. Angew. Chem. Int. Ed. 2013, 52, 4633–4636. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Wang, L.L.; Zhu, Z.Q.; Hu, Z. All organic sodium-ion batteries with Na4C8H2O6. Angew. Chem. Int. Ed. 2014, 53, 5892–5896. [Google Scholar] [CrossRef]
- Hou, H.; Jing, M.; Yang, Y.; Zhu, Y.; Fang, L.; Song, W.; Pan, C.; Yang, X.; Ji, X. Sodium/Lithium Storage Behavior of Antimony Hollow Nanospheres for Rechargeable Batteries. ACS Appl. Mater. Interfaces 2014, 6, 16189–16196. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.; Wu, L.; Cao, Y.; Ai, X.; Yang, H. High capacity Na-storage and superior cyclability of nanocomposite Sb/C anode for Na-ion batteries. Chem. Commun. 2012, 48, 7070–7072. [Google Scholar] [CrossRef]
- Nie, A.; Gan, L.-Y.; Cheng, Y.; Tao, X.; Yuan, Y.; Sharifi-Asl, S.; He, K.; Asayesh-Ardakani, H.; Vasiraju, V.; Lu, J.; et al. Ultrafast and Highly Reversible Sodium Storage in Zinc-Antimony Intermetallic Nanomaterials. Adv. Funct. Mater. 2015, 26, 543–552. [Google Scholar] [CrossRef]
- Baggetto, L.; Marszewski, M.; Górka, J.; Jaroniec, M.; Veith, G. AlSb thin films as negative electrodes for Li-ion and Na-ion batteries. J. Power Sources 2013, 243, 699–705. [Google Scholar] [CrossRef]
- Li, N.; Liao, S.; Sun, Y.; Song, H.W.; Wang, C.X. Uniformly dispersed self-assembled growth of Sb2O3/Sb@graphene nanocomposites on a 3D carbon sheet network for high Na-storage capacity and excellent stability. J. Mater. Chem. A 2015, 3, 5820–5828. [Google Scholar] [CrossRef]
- Sun, Q.; Ren, Q.-Q.; Li, H.; Fu, Z.-W. High capacity Sb2O4 thin film electrodes for rechargeable sodium battery. Electrochem. Commun. 2011, 13, 1462–1464. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, G.; Lin, Y.; Wang, Y.; Ou, X.; Zheng, F.; Yang, C.; Wang, J.-H.; Liu, M. Enhancing Sodium Ion Battery Performance by Strongly Binding Nanostructured Sb2S3 on Sulfur-Doped Graphene Sheets. ACS Nano 2016, 10, 10953–10959. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yan, P.; Luo, L.; Qi, X.; Rong, X.; Zheng, J.; Xiao, B.; Feng, S.; Wang, C.; Hu, Y.-S.; et al. Yolk-shell structured Sb@C anodes for high energy Na-ion batteries. Nano Energy 2017, 40, 504–511. [Google Scholar] [CrossRef]
- Hou, H.; Jing, M.; Yang, Y.; Zhang, Y.; Zhu, Y.; Song, W.; Yang, X.; Ji, X. Sb porous hollow microspheres as advanced anode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 2971–2977. [Google Scholar] [CrossRef]
- Liang, L.; Xu, Y.; Wang, C.; Wen, L.; Fang, Y.; Mi, Y.; Zhou, M.; Zhao, H.; Lei, Y. Large-scale highly ordered Sb nanorod array anodes with high capacity and rate capability for sodium-ion batteries. Energy Environ. Sci. 2015, 8, 2954–2962. [Google Scholar] [CrossRef]
- Jaramillo-Quintero, O.; Benítez-Cruz, M.; García-Ocampo, J.; Cano, A.; Rincón, M. Enhanced performance of S-doped Sb/Sb2O3/CNT/GNR nanocomposite as anode material in lithium-ion batteries. J. Alloy. Compd. 2019, 807, 151647. [Google Scholar] [CrossRef]
- Ye, J.J.; Xia, G.; Zheng, Z.Q.; Hu, C. Facile controlled synthesis of coral-like nanostructured Sb2O3@Sb anode materials for high performance sodium-ion batteries. Int. J. Hydrogen Energy 2020, 45, 9969–9978. [Google Scholar] [CrossRef]
- Hong, K.-S.; Nam, D.-H.; Lim, S.-J.; Sohn, D.; Kim, T.-H.; Kwon, H. Electrochemically Synthesized Sb/Sb2O3 Composites as High-Capacity Anode Materials Utilizing a Reversible Conversion Reaction for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 17264–17271. [Google Scholar] [CrossRef]
- Nam, D.-H.; Hong, K.-S.; Lim, S.-J.; Kim, M.-J.; Kwon, H.-S. High-Performance Sb/Sb2O3Anode Materials Using a Polypyrrole Nanowire Network for Na-Ion Batteries. Small 2015, 11, 2885–2892. [Google Scholar] [CrossRef]
- Pan, J.; Wang, N.; Zhou, Y.; Yang, X.; Zhou, W.; Qian, Y.; Yang, J. Simple synthesis of a porous Sb/Sb2O3 nanocomposite for a high-capacity anode material in Na-ion batteries. Nano Res. 2017, 10, 1794–1803. [Google Scholar] [CrossRef]
- Baggetto, L.; Ganesh, P.; Sun, C.-N.; Meisner, R.A.; Zawodzinski, T.A.; Veith, G.M. Intrinsic thermodynamic and kinetic properties of Sb electrodes for Li-ion and Na-ion batteries: Experiment and theory. J. Mater. Chem. A 2013, 1, 7985–7994. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Woodford, W.; Chiang, Y.-M.; Carter, W.C. “Electrochemical Shock” of Intercalation Electrodes: A Fracture Mechanics Analysis. J. Electrochem. Soc. 2010, 157, A1052–A1059. [Google Scholar] [CrossRef]
- Zhang, X.; Shyy, W.; Sastry, A.M. Numerical Simulation of Intercalation-Induced Stress in Li-Ion Battery Electrode Particles. J. Electrochem. Soc. 2007, 154, A910–A916. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, J.; Chen, B.; Liu, Z.; Liu, T. Dislocation effect on diffusion-induced stress for lithiation in hollow spherical electrode. J. Solid State Electrochem. 2016, 20, 37–46. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Verbrugge, M.W. The influence of surface mechanics on diffusion induced stresses within spherical nanoparticles. J. Appl. Phys. 2008, 104, 083521. [Google Scholar] [CrossRef]
- Meng, W.; Guo, M.; Liu, X.; Chen, J.; Bai, Z.; Wang, Z. Spherical nano Sb@HCMs as high-rate and superior cycle performance anode material for sodium-ion batteries. J. Alloy. Compd. 2019, 795, 141–150. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.J. Wearable super-high specific performance supercapacitors using a honeycomb with folded silk-like composite of NiCo2O4 nanoplates decorated with NiMoO4 honeycombs on nickel foam. Dalton Trans. 2018, 47, 15545–15554. [Google Scholar] [CrossRef]
- Ye, Y.; Shi, Y.; Cai, N.; Lee, J.; He, X. Electro-thermal modeling and experimental validation for lithium ion battery. J. Power Sources 2012, 199, 227–238. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kumar, K.D.; Kim, H.-J. Reagents assisted ZnCo2O4 nanomaterial for supercapacitor application. Electrochim. Acta 2019, 330, 135261. [Google Scholar] [CrossRef]
- Yang, C.; Li, W.; Yang, Z.; Gu, L.; Yu, Y. Nanoconfined antimony in sulfur and nitrogen co-doped three-dimensionally (3D) interconnected macroporous carbon for high-performance sodium-ion batteries. Nano Energy 2015, 18, 12–19. [Google Scholar] [CrossRef]
- Hui, J.; Burgess, M.; Zhang, J.; Rodríguez-López, J. Layer Number Dependence of Li+ Intercalation on Few-Layer Graphene and Electrochemical Imaging of Its Solid–Electrolyte Interphase Evolution. ACS Nano 2016, 10, 4248–4257. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, M.; Pol, V.G.; Evans, S.F.; Jackson, K.; Jafta, C.J.; Bridges, C.A.; Dai, S.; Levine, A.M.; Lee, R.J.; Paranthaman, M.P. Encapsulated Sb and Sb2O3 particles in waste-tire derived carbon as stable composite anodes for sodium-ion batteries. Sustain. Energy Fuels 2020, 4, 3613–3622. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Salunkhe, T.T.; Vo, T.N.; Choi, H.W.; Lee, Y.-C.; Choi, J.-S.; Hur, J.; Kim, I.T. Tailored synthesis of antimony-based alloy/oxides nanosheets for high-performance sodium-ion battery anodes. J. Power Sources 2019, 414, 470–478. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Q.; Wei, Q.; Sun, R.; Liu, X.; An, Q.; Mai, L. NiSe2 Nanooctahedra as an Anode Material for High-Rate and Long-Life Sodium-Ion Battery. ACS Appl. Mater. Interfaces 2017, 9, 311–316. [Google Scholar] [CrossRef]
- Huang, Z.; Hou, H.; Zou, G.; Chen, J.; Zhang, Y.; Liao, H.; Li, S.; Ji, X. 3D Porous Carbon Encapsulated SnO2 Nanocomposite for Ultrastable Sodium Ion Batteries. Electrochim. Acta 2016, 214, 156–164. [Google Scholar] [CrossRef]



| Electrode Materials | Key Improvements | Electrolyte/ Concentration | Electrode Size (Mass Loading of Active Material) | Cycling Performance (mAh g−1) | Capacity Retention Ratio | Ref. |
|---|---|---|---|---|---|---|
| Sb/Sb2O3 nanoparticles | Sb alloys & Sb oxides & special morphology nanostructure | 1 M NaClO4 + PC a + 3%FEC b | Copper foil diameters of 12 mm (1.0–1.2 mg) | 540 at 100 mA g−1 after 100 cycles | 98.0% | This work |
| Sb/Sb2O3-polypyrone electrode | 3D porous Sb/Sb2O3 & fabricated with a polypyrrole nanowire network | 1 M NaClO4 +2% FEC b | A sheet of nodular Cu (0.67 mg cm−2) | 512.01 at 66 mA g−1 after 100 cycles | 99% | [30] |
| Sb2O3/Sb@ graphene-CSN electrode | Sb2O3/Sb Nanoparticles & graphene shell nanostructure & anchored on carbon sheet networks | 1 M NaClO4 + EC c + DMC d | Copper foil with 0.97 mg cm−2 (1.5 mg) | 525.4 at 100 mA g−1 after 100 cycles | 98% | [21] |
| Sb and Sb2O3 particles in waste-tire derived carbon | A conductive network of waste tire derived carbon & Sb composites | 1 M NaPF6 + EC c + PC a | Cu current collector with the size of 14 mm diameter (3.6 mg) | 207 at 37 mA g−1 after 100 cycles | 88% | [44] |
| Antimony-based alloy/oxides nanosheets | Sb-based alloy/oxides & nanosheet structure | 1 M NaClO4 + PC a + EC c + 2% FEC b | Cu foil (1.4–1.7 mg cm−2) | 311 at 100 mA g−1 after 120 cycles | 89% | [45] |
| Porous Sb/Sb2O3 nanocomposite | Porous nano-structure & Sb/Sb2O3 composites | 1.0 M NaClO4+EC c + DEC e+10% FEC b | Copper foil diameters of 12 mm (0.9–1.2 mg cm−2) | 481 at 660 mA g−1 after 180 cycles | ~92.8% | [31] |
| Coral-like nanostructured Sb2O3@Sb | Mild oxidization in air & etching of element & coral-like nanostructure | 1 M NaPF6 + EC c + DEC d + 5% FEC b | Cu foil (0.7–0.9 mg cm−2) | 574.8 at 100 mA g−1 after 150 cycles | 99.0% | [28] |
| Sb/Sb2O3 composites | The electrodeposition of Sb & the chemical deposition of Sb2O3 & morula-like Sb/Sb2O3 particles | 1 M NaClO4 + PC a + 0.5% FEC b | The nodule-type Cu foil (0.86 mg cm−2, thickness of 1.34 μm) | 615 at 66 mA g−1 after 100 cycles | 97.56% | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhao, S.; Meng, W.; Guo, M.; Wang, G.; Guo, C.; Bai, Z.; Li, Z.; Ye, J.; Song, H.; et al. Electrochemical Performance and Stress Distribution of Sb/Sb2O3 Nanoparticles as Anode Materials for Sodium-Ion Batteries. Batteries 2023, 9, 98. https://doi.org/10.3390/batteries9020098
Chen J, Zhao S, Meng W, Guo M, Wang G, Guo C, Bai Z, Li Z, Ye J, Song H, et al. Electrochemical Performance and Stress Distribution of Sb/Sb2O3 Nanoparticles as Anode Materials for Sodium-Ion Batteries. Batteries. 2023; 9(2):98. https://doi.org/10.3390/batteries9020098
Chicago/Turabian StyleChen, Jiajun, Songnan Zhao, Weijia Meng, Meiqing Guo, Genwei Wang, Chunli Guo, Zhongchao Bai, Zhiqiang Li, Jiaye Ye, Hui Song, and et al. 2023. "Electrochemical Performance and Stress Distribution of Sb/Sb2O3 Nanoparticles as Anode Materials for Sodium-Ion Batteries" Batteries 9, no. 2: 98. https://doi.org/10.3390/batteries9020098
APA StyleChen, J., Zhao, S., Meng, W., Guo, M., Wang, G., Guo, C., Bai, Z., Li, Z., Ye, J., Song, H., & Wang, X. (2023). Electrochemical Performance and Stress Distribution of Sb/Sb2O3 Nanoparticles as Anode Materials for Sodium-Ion Batteries. Batteries, 9(2), 98. https://doi.org/10.3390/batteries9020098








