Electrolyte Optimization to Improve the High-Voltage Operation of Single-Crystal LiNi0.83Co0.11Mn0.06O2 in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.3. Electrochemical Measurement
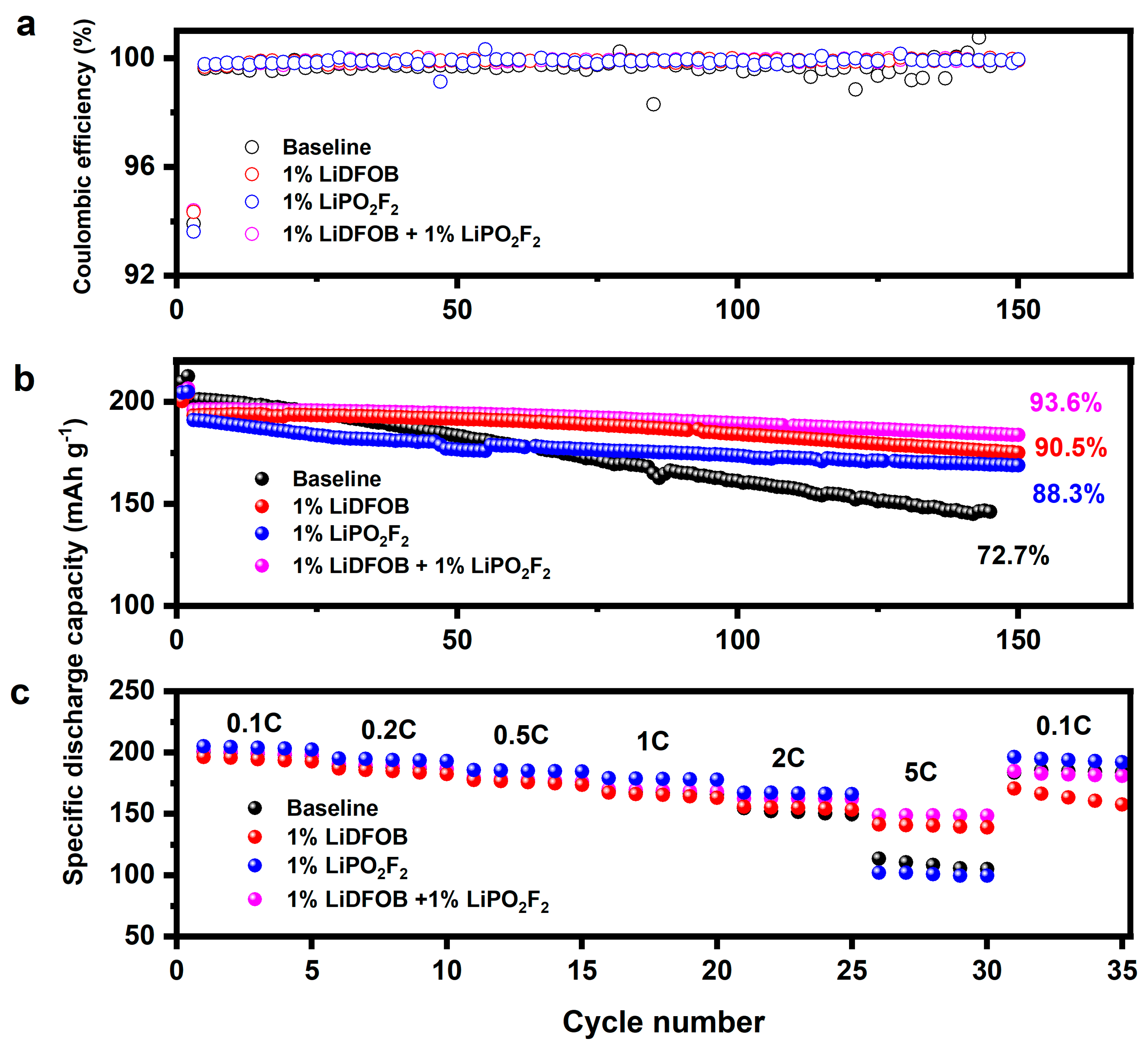
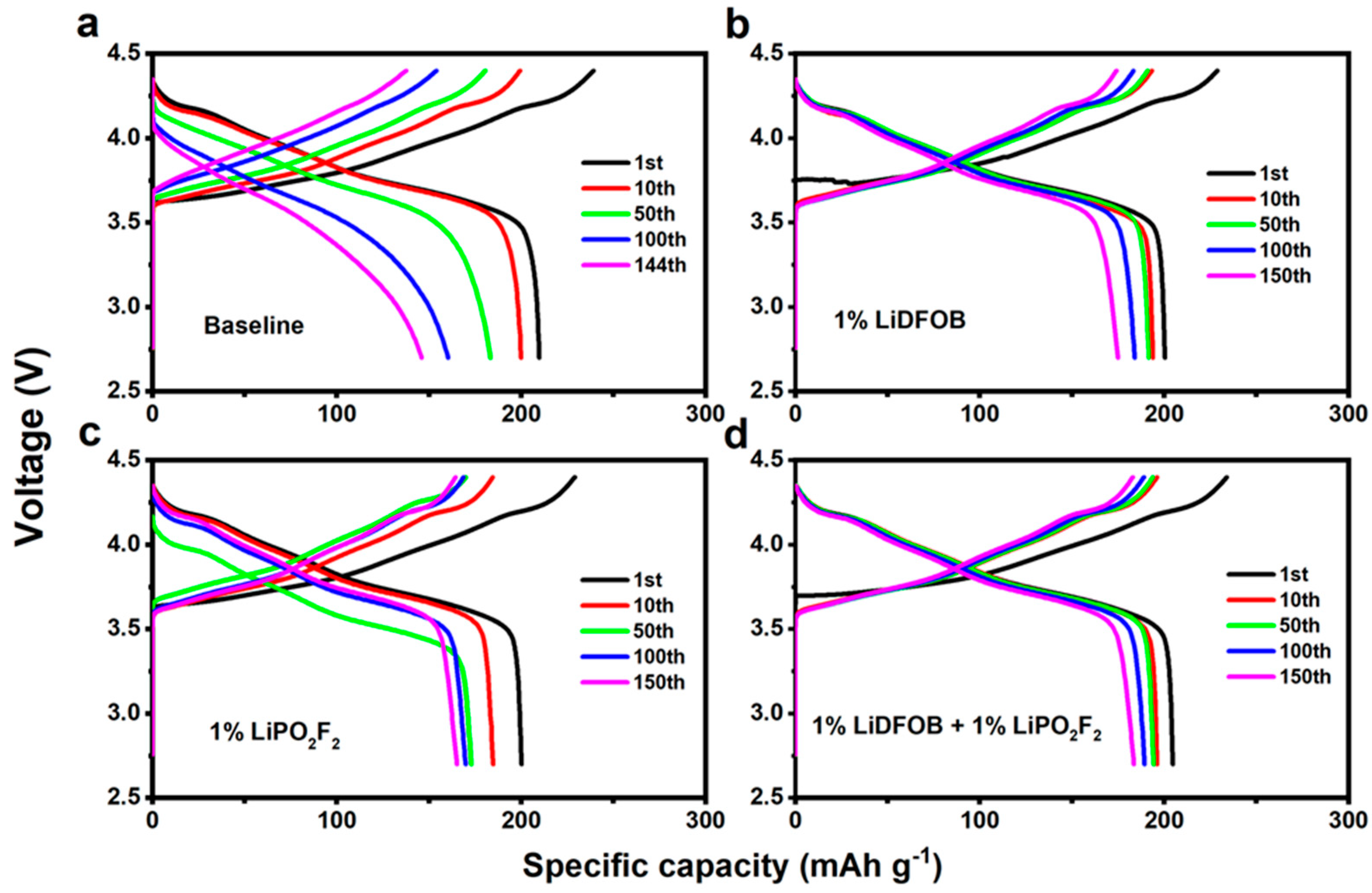
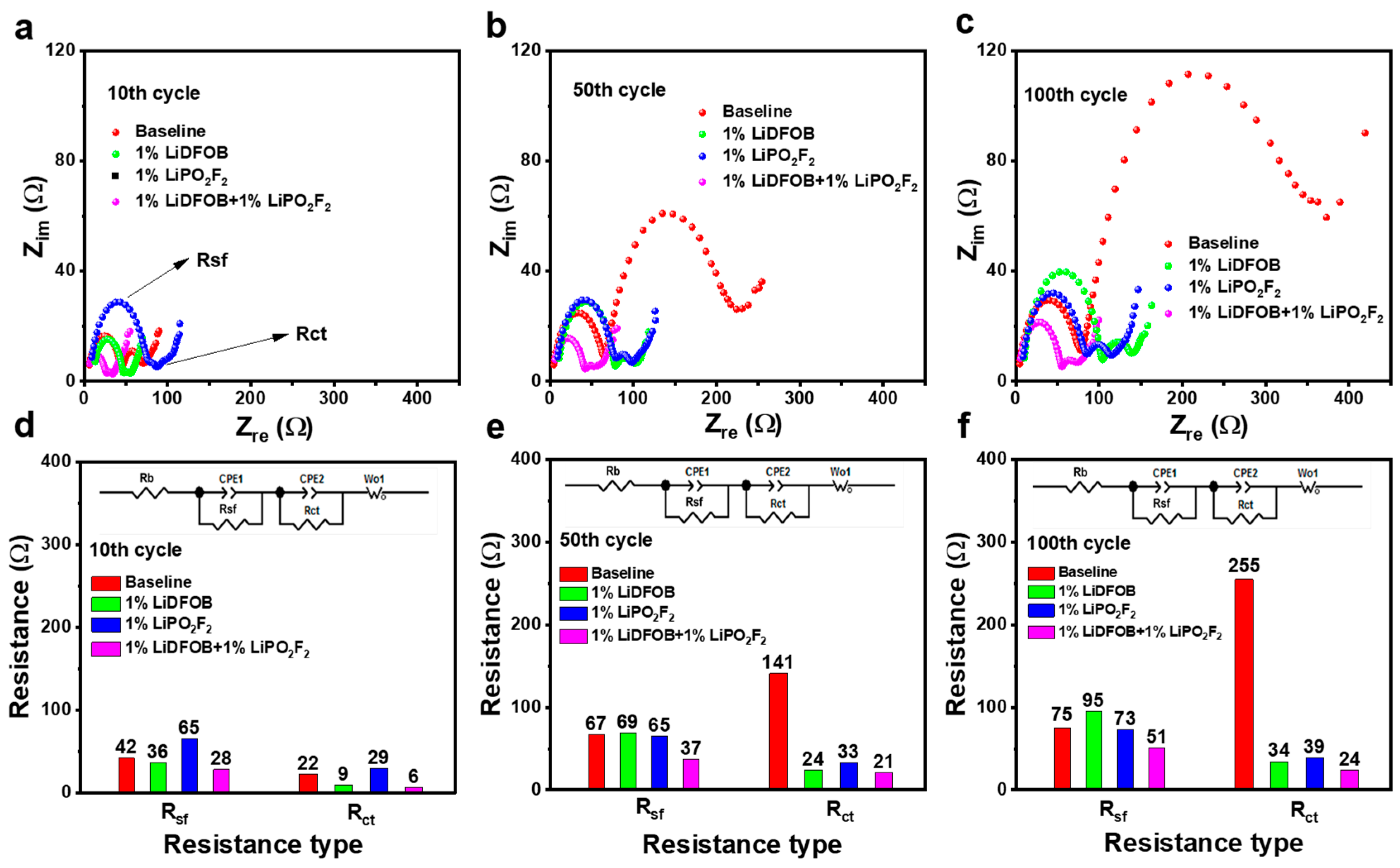
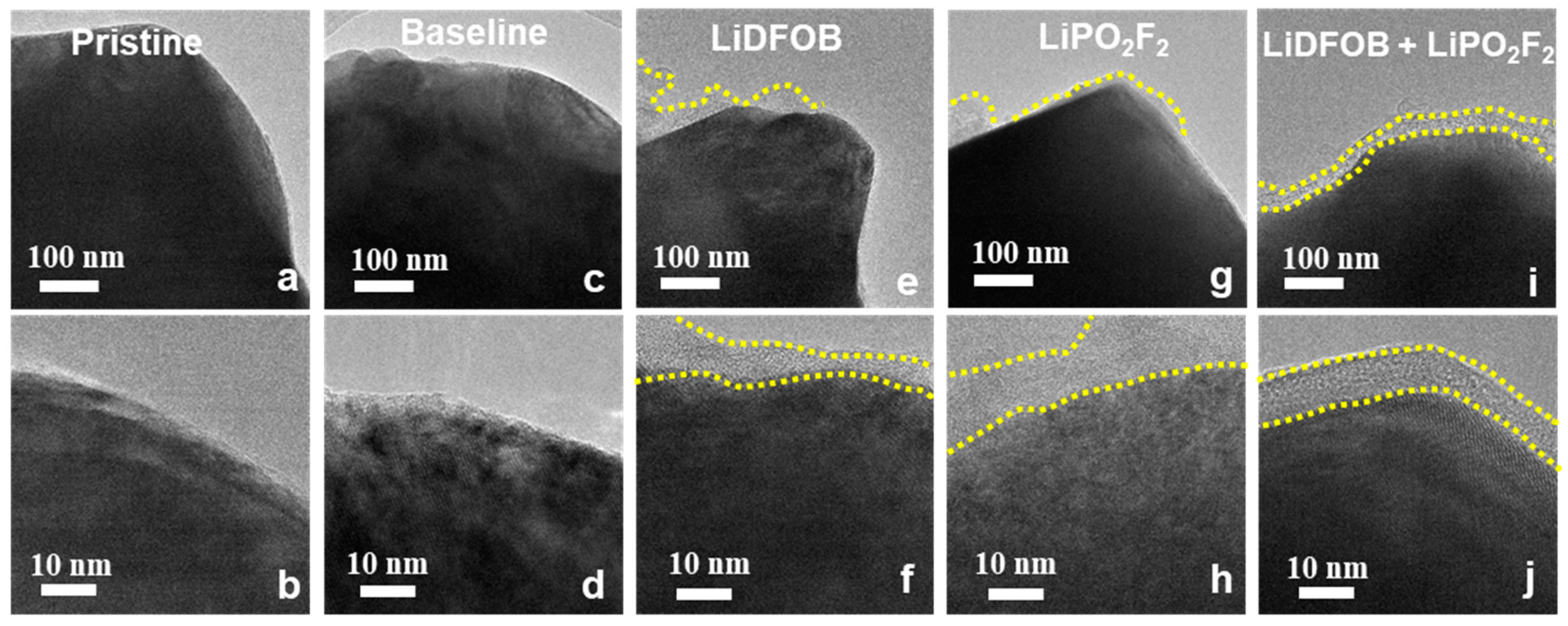
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Friedrich, F.; Strehle, B.; Freiberg, A.T.S.; Kleiner, K.; Day, S.J.; Erk, C.; Piana, M.; Gasteiger, H.A. Editors’ Choice—Capacity Fading Mechanisms of NCM-811 Cathodes in Lithium-Ion Batteries Studied by X-ray Diffraction and Other Diagnostics. J. Electrochem. Soc. 2019, 166, A3760–A3774. [Google Scholar] [CrossRef]
- Wu, Q.; Mao, S.; Wang, Z.; Tong, Y.; Lu, Y. Improving LiNixCoyMn1−x−yO2 Cathode Electrolyte Interface under High Voltage in Lithium Ion Batteries. Nano Select 2020, 1, 111–134. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, Y.; Li, L.; Zhang, R.; Xu, J.; Cheng, Z.; Xie, S.; Wang, H.; Rao, Q.; He, Y.; et al. Single-Crystal Nickel-Rich Layered-Oxide Battery Cathode Materials: Synthesis, Electrochemistry, and Intra-Granular Fracture. Energy Storage Mater. 2020, 27, 140–149. [Google Scholar] [CrossRef]
- Fan, X.; Hu, G.; Zhang, B.; Ou, X.; Zhang, J.; Zhao, W.; Jia, H.; Zou, L.; Li, P.; Yang, Y. Crack-Free Single-Crystalline Ni-Rich Layered NCM Cathode Enable Superior Cycling Performance of Lithium-Ion Batteries. Nano Energy 2020, 70, 104450. [Google Scholar] [CrossRef]
- Xu, C.; Märker, K.; Lee, J.; Mahadevegowda, A.; Reeves, P.J.; Day, S.J.; Groh, M.F.; Emge, S.P.; Ducati, C.; Layla Mehdi, B.; et al. Bulk Fatigue Induced by Surface Reconstruction in Layered Ni-Rich Cathodes for Li-Ion Batteries. Nat. Mater. 2021, 20, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sharifi-Asl, S.; Garcia, J.C.; Iddir, H.H.; Croy, J.R.; Shahbazian-Yassar, R.; Chen, G. Atomic-Level Understanding of Surface Reconstruction Based on Li[NixMnyCo1−x−y]O2 Single-Crystal Studies. ACS Appl. Energy Mater. 2020, 3, 4799–4811. [Google Scholar] [CrossRef]
- Wan, H.; Liu, Z.; Liu, G.; Yi, S.; Yan, P.; Deng, H.; Hu, W.; Gao, F. Unraveling TM Migration Mechanisms in LiNi1/3 Mn1/3Co1/3O2 by Modeling and Experimental Studies. Nano Lett. 2021, 21, 6875–6881. [Google Scholar] [CrossRef]
- Wang, H.; Yang, G.; Lai, F.; Chu, Y.; Zhang, X.; Ma, Z.; Li, Q. Ultrathin Al2O3 Layer Modified LiNi0.6Co0.2Mn0.2O2 with Al-Doping for High Performance Lithium Ion Batteries. Ionics 2020, 26, 2147–2156. [Google Scholar] [CrossRef]
- David, L.; Dahlberg, K.; Mohanty, D.; Ruther, R.E.; Huq, A.; Chi, M.; An, S.J.; Mao, C.; King, D.M.; Stevenson, L.; et al. Unveiling the Role of Al2O3 in Preventing Surface Reconstruction During High-Voltage Cycling of Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 1308–1313. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Wang, L.; Wang, K.; Wu, X.; Zhou, P.; Miao, Z.; Zhou, J.; Zhao, Y.; Zhuo, S. Stabilizing the High-Voltage Cycle Performance of LiNi0.8Co0.1Mn0.1O2 Cathode Material by Mg Doping. J. Power Sources 2019, 438, 227017. [Google Scholar] [CrossRef]
- Yue, P.; Wang, Z.; Guo, H.; Xiong, X.; Li, X. A Low Temperature Fluorine Substitution on the Electrochemical Performance of Layered LiNi0.8Co0.1Mn0.1O2-zFz Cathode Materials. Electrochim. Acta 2013, 92, 1–8. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, K.; Dubey, R.; Ren, F.; Brack, E.; Becker, M.; Grissa, R.; Seidl, L.; Pagani, F.; Egorov, K.; et al. Extending the High-Voltage Operation of Graphite/NCM811 Cells by Constructing a Robust Electrode/Electrolyte Interphase Layer. Mater. Today Energy 2023, 34, 101301. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, G.; Lin, M.; Zhao, W.; Li, D.; Guan, X.; Ji, Y.; Ortiz, G.F.; Yang, Y. Toward a Stable Solid-Electrolyte-Interfaces on Nickel-Rich Cathodes: LiPO2F2 Salt-Type Additive and Its Working Mechanism for LiNi0.5Mn0.25Co0.25O2 Cathodes. J. Power Sources 2018, 380, 149–157. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Guan, X.; Guo, R.; Che, Y.; Lan, J.; Xing, L.; Xu, M.; Fan, W.; Li, W. Efficiently Suppressing Oxygen Evolution in High Voltage Graphite/NCM Pouch Cell with Tributyl Borate as Electrolyte Additive. Electrochim. Acta 2020, 354, 136722. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, F.; Huang, Z.; Yi, M.; Fan, X.; Bai, M.; Hong, B.; Zhang, Z.; Li, J.; Lai, Y. Improving Interfacial Stability of Ultrahigh-Voltage Lithium Metal Batteries with Single-Crystal Ni-Rich Cathode via a Multifunctional Additive Strategy. J. Colloid Interface Sci. 2022, 608, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Beltrop, K.; Klein, S.; Nölle, R.; Wilken, A.; Lee, J.J.; Köster, T.K.-J.; Reiter, J.; Tao, L.; Liang, C.; Winter, M.; et al. Triphenylphosphine Oxide as Highly Effective Electrolyte Additive for Graphite/NMC811 Lithium Ion Cells. Chem. Mater. 2018, 30, 2726–2741. [Google Scholar] [CrossRef]
- Su, C.-C.; He, M.; Peebles, C.; Zeng, L.; Tornheim, A.; Liao, C.; Zhang, L.; Wang, J.; Wang, Y.; Zhang, Z. Functionality Selection Principle for High Voltage Lithium-Ion Battery Electrolyte Additives. ACS Appl. Mater. Interfaces 2017, 9, 30686–30695. [Google Scholar] [CrossRef]
- Dong, Q.; Guo, F.; Cheng, Z.; Mao, Y.; Huang, R.; Li, F.; Dong, H.; Zhang, Q.; Li, W.; Chen, H.; et al. Insights into the Dual Role of Lithium Difluoro(Oxalato)Borate Additive in Improving the Electrochemical Performance of NMC811||Graphite Cells. ACS Appl. Energy Mater. 2020, 3, 695–704. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Zhu, Y.; Marin, T.W.; Abraham, D.P. Mechanistic Insight into the Protective Action of Bis(Oxalato)Borate and Difluoro(Oxalate)Borate Anions in Li-Ion Batteries. J. Phys. Chem. C 2013, 117, 23750–23756. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, B.; Jiao, F.; Wu, K. The Roles and Working Mechanism of Salt-Type Additives on the Performance of High-Voltage Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 16298–16307. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, W.; Zhang, Q.; Yang, G.; Zheng, J.; Tang, W.; Xu, Q.; Lai, C.; Yang, J.; Peng, C. Armoring LiNi1/3Co 1/3Mn1/3O2 Cathode with Reliable Fluorinated Organic–Inorganic Hybrid Interphase Layer toward Durable High Rate Battery. Adv. Funct. Mater. 2020, 30, 2000396. [Google Scholar] [CrossRef]
- Ma, L.; Ellis, L.; Glazier, S.L.; Ma, X.; Dahn, J.R. Combinations of LiPO2F2 and Other Electrolyte Additives in Li[Ni0.5 Mn0.3 Co0.2] O2/Graphite Pouch Cells. J. Electrochem. Soc. 2018, 165, A1718–A1724. [Google Scholar] [CrossRef]
- Gao, H.; Maglia, F.; Lamp, P.; Amine, K.; Chen, Z. Mechanistic Study of Electrolyte Additives to Stabilize High-Voltage Cathode–Electrolyte Interface in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 44542–44549. [Google Scholar] [CrossRef]
- Li, X.; Zheng, J.; Ren, X.; Engelhard, M.H.; Zhao, W.; Li, Q.; Zhang, J.; Xu, W. Dendrite-Free and Performance-Enhanced Lithium Metal Batteries through Optimizing Solvent Compositions and Adding Combinational Additives. Adv. Energy Mater. 2018, 8, 1703022. [Google Scholar] [CrossRef]
- Li, X.; Zheng, J.; Engelhard, M.H.; Mei, D.; Li, Q.; Jiao, S.; Liu, N.; Zhao, W.; Zhang, J.-G.; Xu, W. Effects of Imide–Orthoborate Dual-Salt Mixtures in Organic Carbonate Electrolytes on the Stability of Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2018, 10, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zou, L.; Zheng, J.; Jia, H.; Song, J.; Engelhard, M.H.; Wang, C.; Xu, W.; Yang, Y.; Zhang, J. Simultaneous Stabilization of LiNi 0.76 Mn 0.14 Co 0.10 O 2 Cathode and Lithium Metal Anode by Lithium Bis(Oxalato)Borate as Additive. ChemSusChem 2018, 11, 2211–2220. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, J.; Zou, L.; Jia, H.; Liu, B.; Wang, H.; Engelhard, M.H.; Wang, C.; Xu, W.; Yang, Y.; et al. High Voltage Operation of Ni-Rich NMC Cathodes Enabled by Stable Electrode/Electrolyte Interphases. Adv. Energy Mater. 2018, 8, 1800297. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Huang, W.; Wang, H.; Zhou, W.; Li, Y.; Li, Y.; Xu, J.; Huang, W.; Chiu, W.; et al. Cathode-Electrolyte Interphase in Lithium Batteries Revealed by Cryogenic Electron Microscopy. Matter 2021, 4, 302–312. [Google Scholar] [CrossRef]


| a (Å) | b (Å) | c (Å) | c/a | Vol(Å3) | Ni in Li Layer (%) | |
|---|---|---|---|---|---|---|
| SC-NCM83 | 2.875 (3) | 2.875 (3) | 14.187 (3) | 4.934 (1) | 101.580 | 2.42 |
| Coulombic Efficiency (%) | ||||
|---|---|---|---|---|
| Electrolyte | Baseline | 1% LiDFOB + 1% LiPO2F2 | 1% LiDFOB | 1% LiPO2F2 |
| 1st cycle | 87.7 | 87.5 | 87.6 | 87.4 |
| Cycle | 10th | 50th | 100th | |||
|---|---|---|---|---|---|---|
| Impedance | Rsf (Ω) | Rct (Ω) | Rsf (Ω) | Rct (Ω) | Rsf (Ω) | Rct (Ω) |
| Baseline | 42 | 22 | 67 | 141 | 75 | 255 |
| LiDFOB | 36 | 9 | 69 | 24 | 95 | 34 |
| LiPO2F2 | 65 | 29 | 65 | 33 | 73 | 39 |
| LiDFOB + LiPO2F2 | 28 | 6 | 37 | 21 | 51 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Si, M.; Wang, K.; Brack, E.; Zhang, Z.; Fan, X.; Battaglia, C. Electrolyte Optimization to Improve the High-Voltage Operation of Single-Crystal LiNi0.83Co0.11Mn0.06O2 in Lithium-Ion Batteries. Batteries 2023, 9, 528. https://doi.org/10.3390/batteries9110528
Zhao W, Si M, Wang K, Brack E, Zhang Z, Fan X, Battaglia C. Electrolyte Optimization to Improve the High-Voltage Operation of Single-Crystal LiNi0.83Co0.11Mn0.06O2 in Lithium-Ion Batteries. Batteries. 2023; 9(11):528. https://doi.org/10.3390/batteries9110528
Chicago/Turabian StyleZhao, Wengao, Mayan Si, Kuan Wang, Enzo Brack, Ziyan Zhang, Xinming Fan, and Corsin Battaglia. 2023. "Electrolyte Optimization to Improve the High-Voltage Operation of Single-Crystal LiNi0.83Co0.11Mn0.06O2 in Lithium-Ion Batteries" Batteries 9, no. 11: 528. https://doi.org/10.3390/batteries9110528
APA StyleZhao, W., Si, M., Wang, K., Brack, E., Zhang, Z., Fan, X., & Battaglia, C. (2023). Electrolyte Optimization to Improve the High-Voltage Operation of Single-Crystal LiNi0.83Co0.11Mn0.06O2 in Lithium-Ion Batteries. Batteries, 9(11), 528. https://doi.org/10.3390/batteries9110528








