Influence of the Crusher Settings and a Thermal Pre-Treatment on the Properties of the Fine Fraction (Black Mass) from Mechanical Lithium-Ion Battery Recycling
Abstract
:1. Introduction
2. Black Mass Properties and Their Relevance
2.1. Black Mass Composition
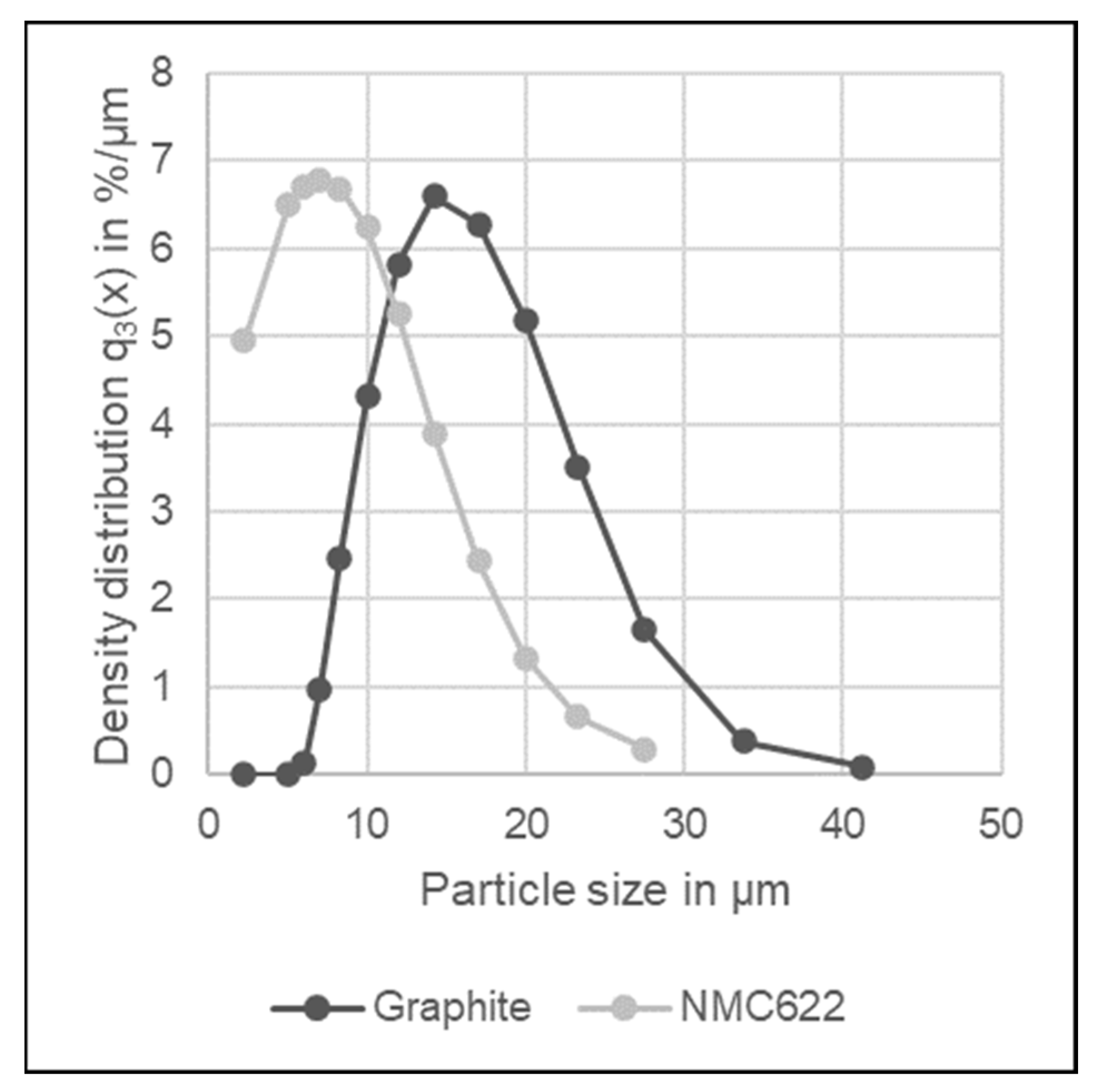
2.2. Particle Size Distribution and Flotation
2.3. Influence of Metal Impurities on the Hydrometallurgical Treatment
2.4. Effects of Metal Impurities in the Cell Resynthesis
3. Materials, Methods and Calculations
3.1. Materials
3.2. Methods
3.3. Calculations
4. Results
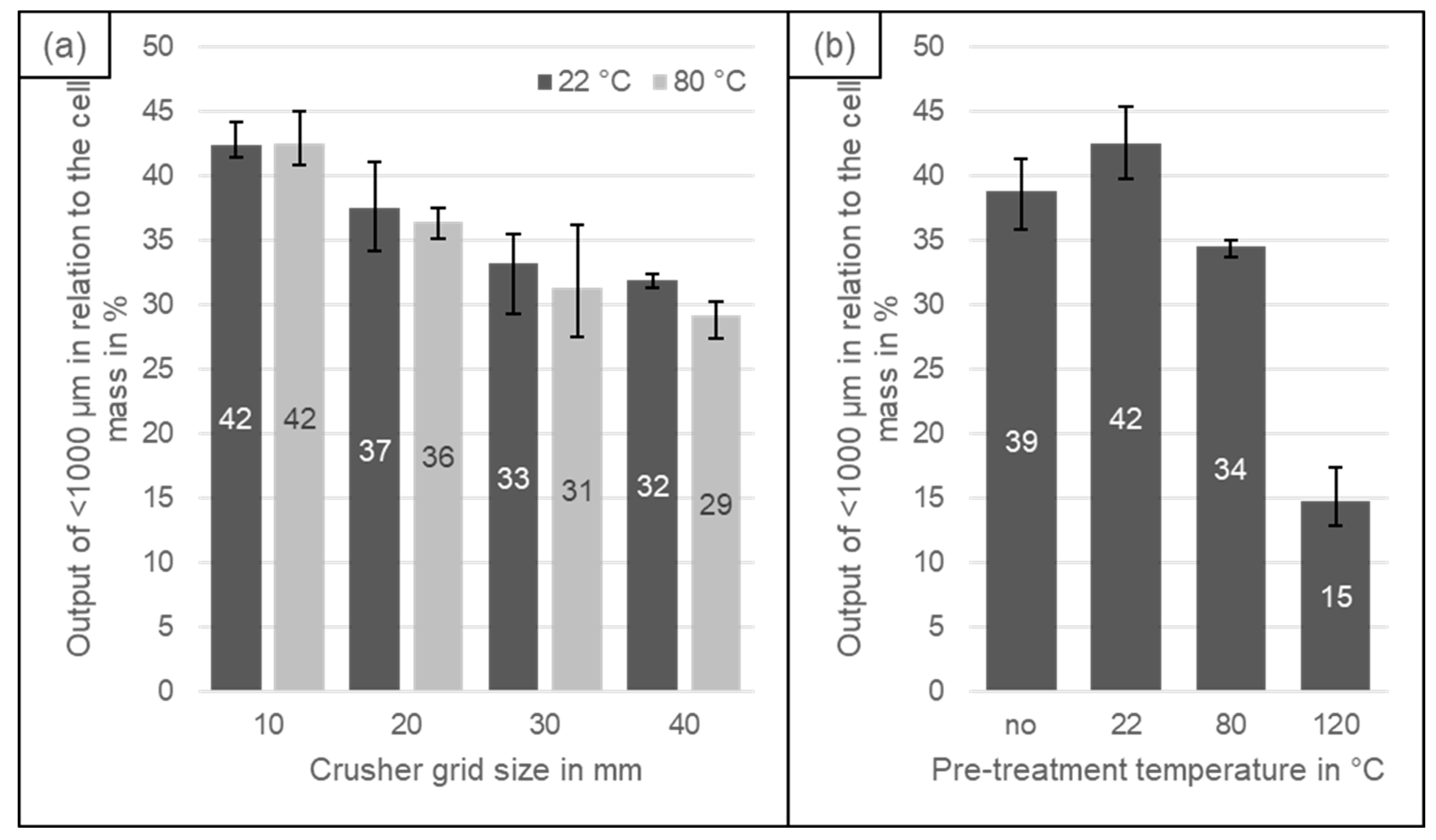
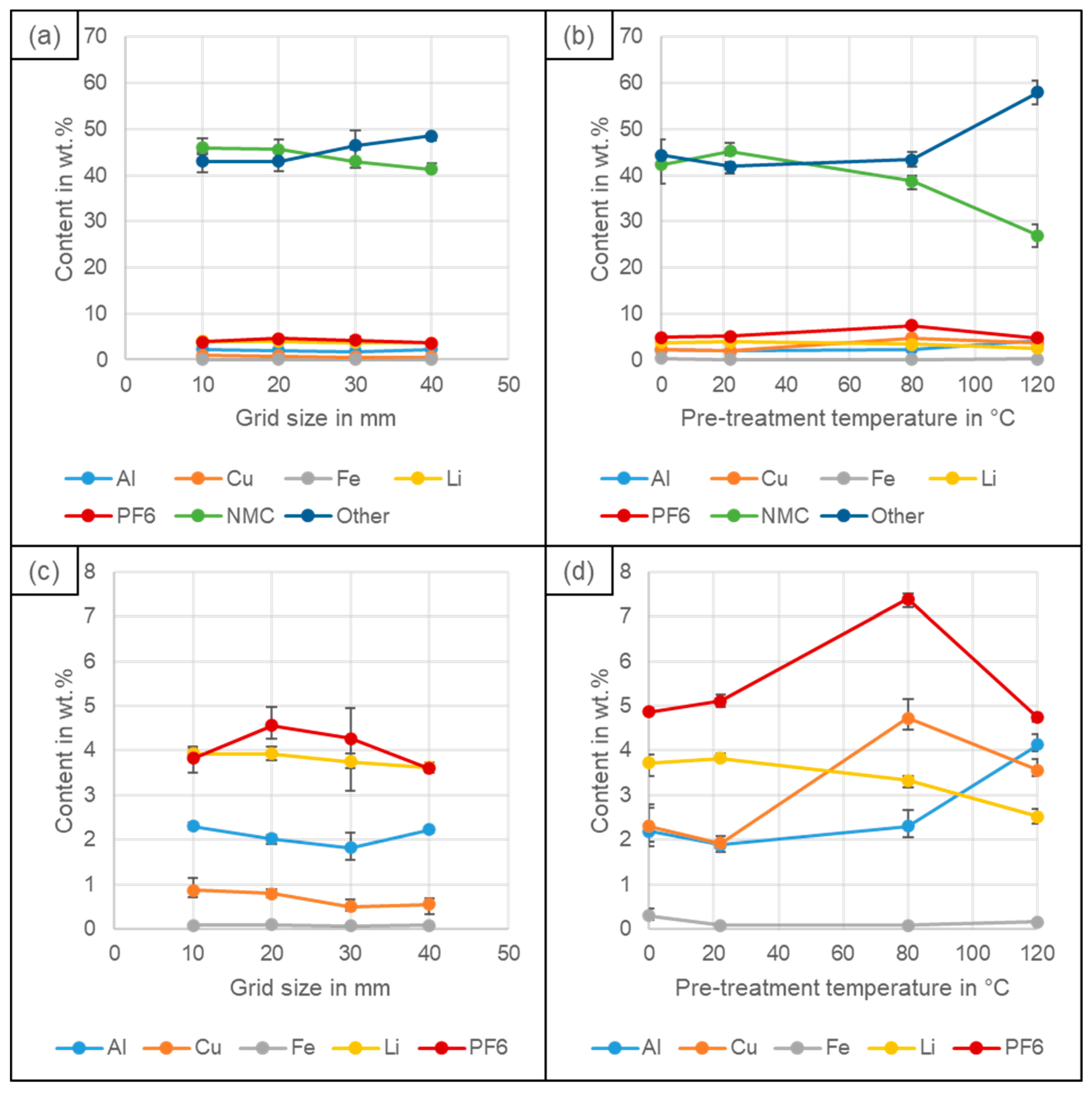
4.1. Black Mass Yield
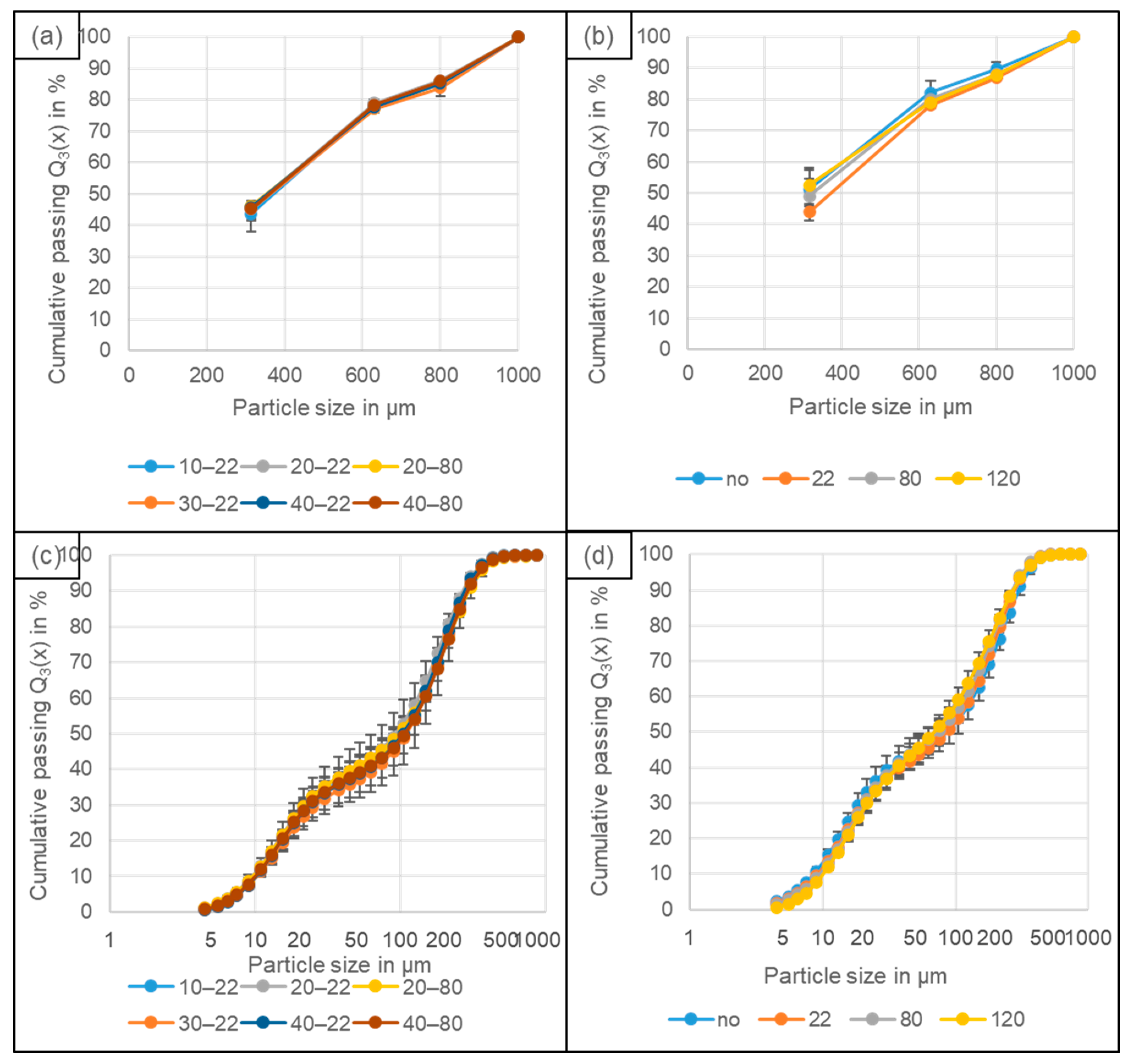
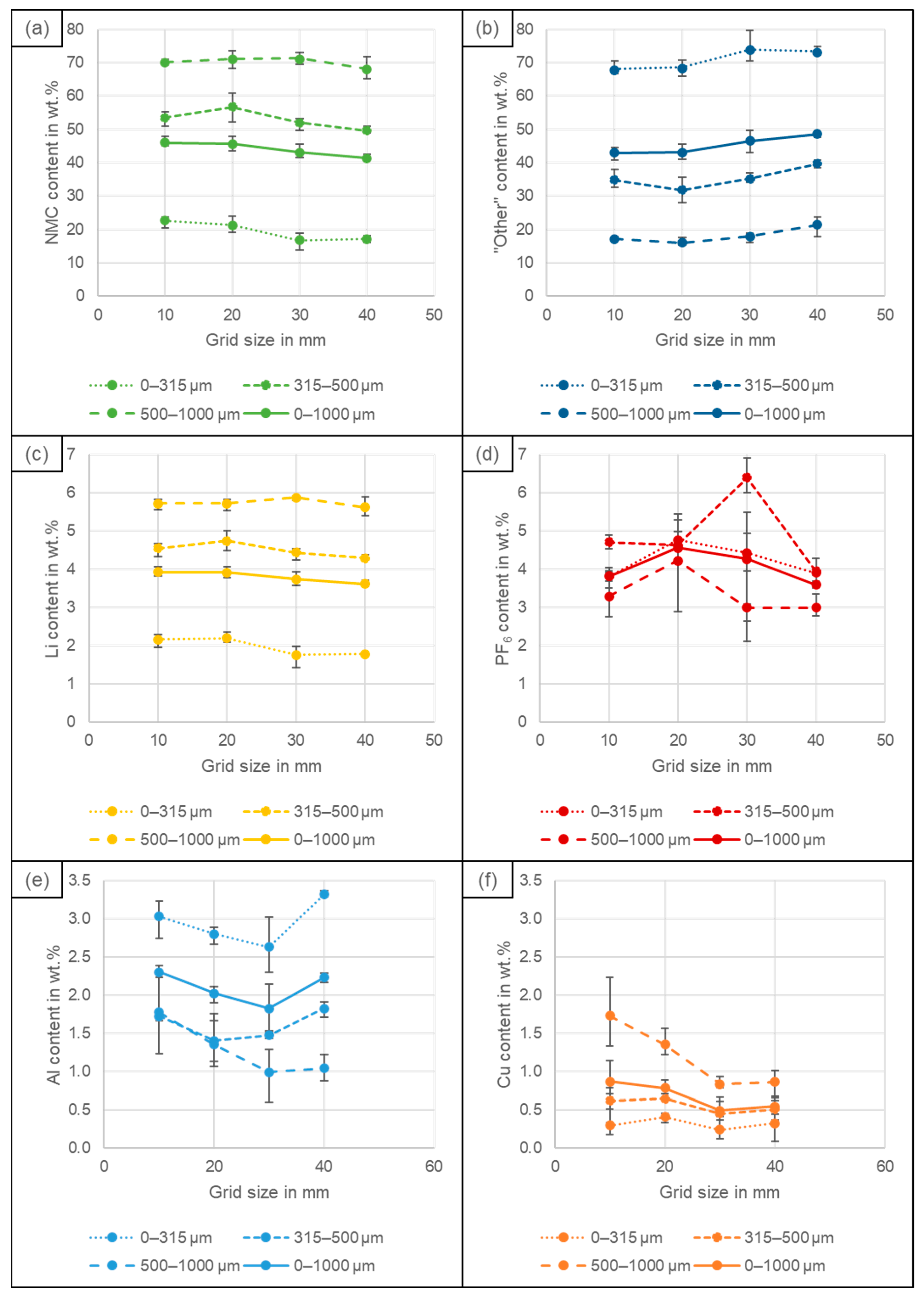
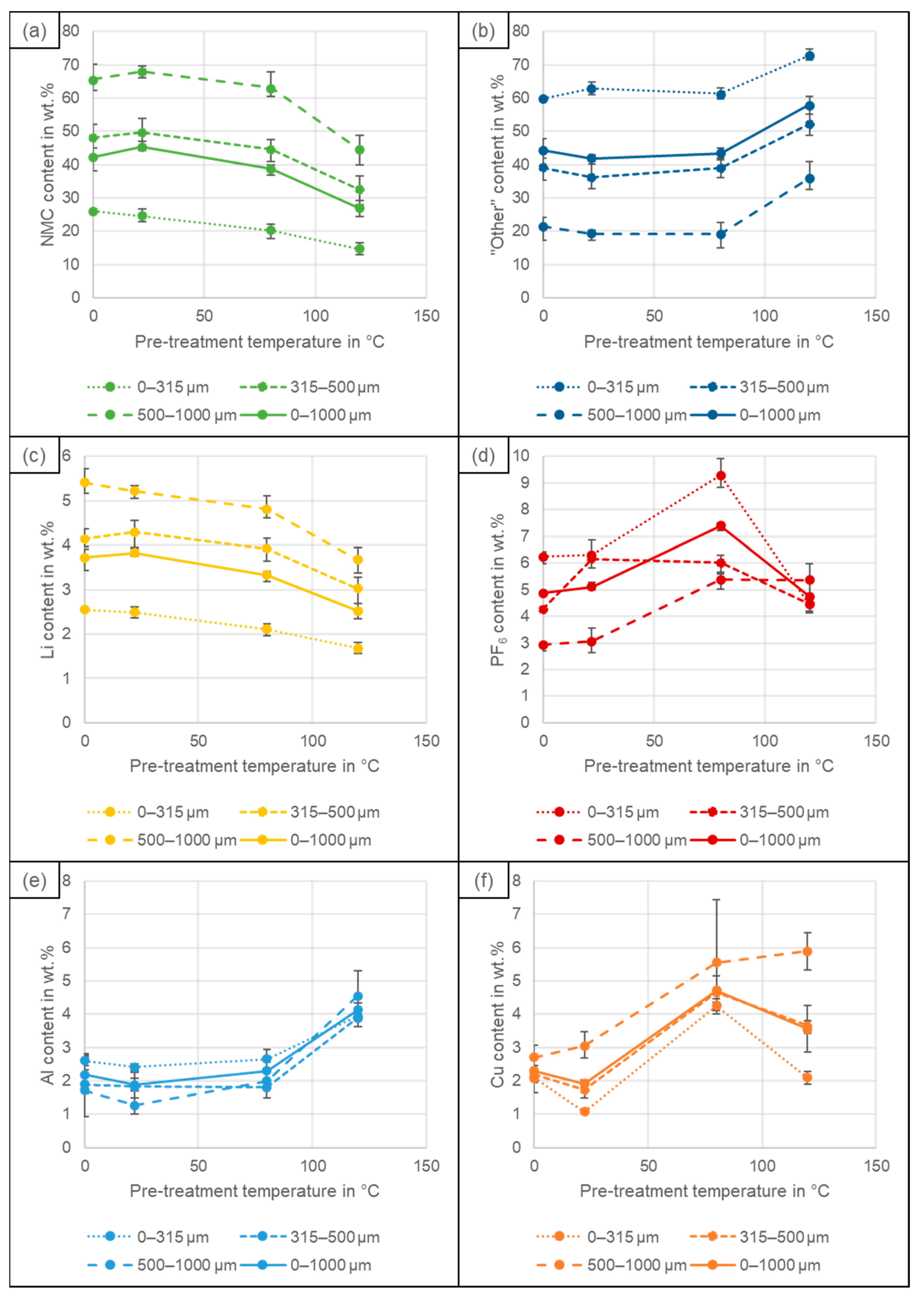
4.2. Particle Size Distribution
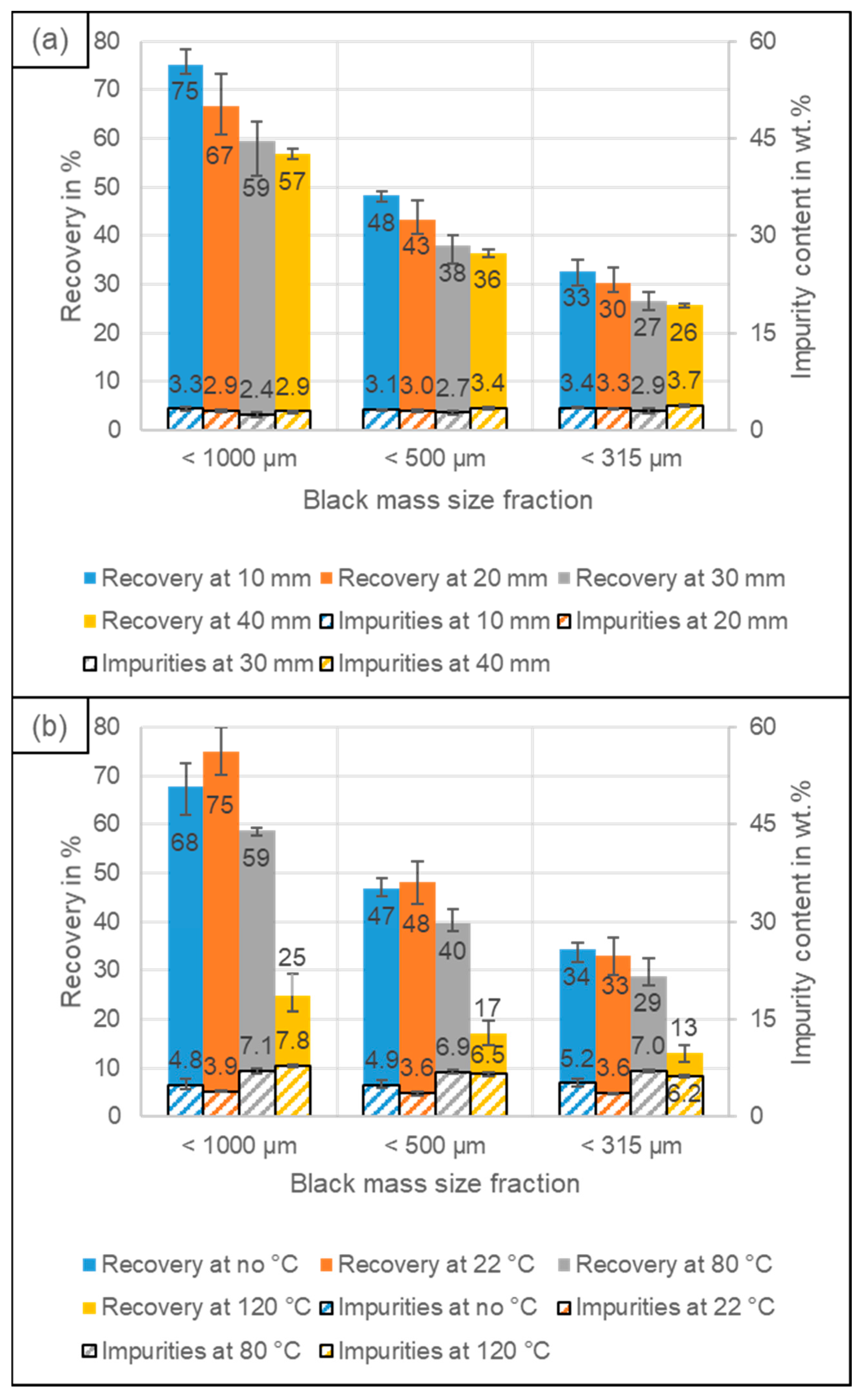
4.3. Recovery of Coating and Conductive Salt vs. Al, Cu and Fe Impurities
4.4. Elemental Composition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michaelis, S.; Rahimzei, E.; Kampker, A.; Heimer, H.; Offermanns, C.; Locke, M.; Löbberding, H.; Wennemar, S.; Thielmann, A.; Hettesheimer, T.; et al. Roadmap Batterie-Produktionsmittel 2030; VDMA: Frankfurt am Main, Germany, 2020. [Google Scholar]
- Neef, C.; Schmaltz, T.; Thielmann, A. Recycling von Lithium-Ionen Batterien: Chancen und Herausforderungen für den Maschinen- und Anlagenbau; Fraunhofer-Institut für System- und Innovationsforschung ISI: Karlsruhe, Germany, 2021. [Google Scholar]
- Council of the European Union. Proposal for a Regulation of the European Parliament and of the Council Concerning Batteries and Waste Batteries, Repealing Directive 2006/66/EC and Amending Regulation (EU) No 2019/1020—Letter to the Chair of the European Parliament Committee on the Environment; Public Health and Food Safety (ENVI): Brussels, Belgium, 2023. [Google Scholar]
- Graf, C. Cathode materials for lithium-ion batteries. In Lithium-Ion Batteries: Basics and Applications; Korthauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 29–41. [Google Scholar]
- Doose, S.; Mayer, J.K.; Michalowski, P.; Kwade, A. Challenges in Ecofriendly Battery Recycling and Closed Material Cycles: A Perspective on Future Lithium Battery Generations. Metals 2021, 11, 291. [Google Scholar] [CrossRef]
- Woehrle, T. Lithium-ion cell. In Lithium-Ion Batteries: Basics and Applications; Korthauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 101–112. [Google Scholar]
- Hauck, D.; Kurrat, M. Overdischarging Lithium-Ion Batteries. In Recycling of Lithium-Ion Batteries: The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 53–81. [Google Scholar]
- Wuschke, L. Mechanische Aufbereitung von Lithium-Ionen-Batteriezellen; TU Bergakademie Freiberg: Freiberg, Germany, 2018. [Google Scholar]
- Werner, D.M.; Mütze, T.; Peuker, U.A. Influence of Pretreatment Strategy on the Crushing of Spent Lithium-Ion Batteries. Metals 2022, 12, 1839. [Google Scholar] [CrossRef]
- Lyon, T.; Mütze, T.; Peuker, U.A. Decoating of Electrode Foils from EOL Lithium-Ion Batteries by Electrohydraulic Fragmentation. Metals 2022, 12, 209. [Google Scholar] [CrossRef]
- Krüger, S.; Hanisch, C.; Kwade, A.; Winter, M.; Nowak, S. Effect of Impurities Caused by a Recycling Process on the Electrochemical Performance of Li[Ni0.33Co0.33Mn0.33]O2. J. Electroanal. Chem. 2014, 726, 91–96. [Google Scholar] [CrossRef]
- Windisch-Kern, S.; Gerold, E.; Nigl, T.; Jandric, A.; Altendorfer, M.; Rutrecht, B.; Scherhaufer, S.; Raupenstrauch, H.; Pomberger, R.; Antrekowitsch, H.; et al. Recycling chains for lithium-ion batteries: A critical examination of current challenges, opportunities and process dependencies. Waste Manag. 2022, 138, 125–139. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Sygusch, J.; Rudolph, M.; Serna-Guerrero, R. A contribution to understanding the flotation behavior of lithium metal oxides and spheroidized graphite for lithium-ion battery recycling. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127111. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Hayagan, N.; Bachmann, K.; Ferreira, A.; Werner, D.; Horn, D.; Peuker, U.A.; Serna-Guerrero, R.; Rudolph, M. Lithium-Ion Battery Recycling─Influence of Recycling Processes on Component Liberation and Flotation Separation Efficiency. ACS ES&T Eng. 2022, 2, 2130–2141. [Google Scholar] [CrossRef]
- Salces, A.M.; Bremerstein, I.; Rudolph, M.; Vanderbruggen, A. Joint recovery of graphite and lithium metal oxides from spent lithium-ion batteries using froth flotation and investigation on process water re-use. Miner. Eng. 2022, 184, 107670. [Google Scholar] [CrossRef]
- Zhan, R.; Oldenburg, Z.; Pan, L. Recovery of active cathode materials from lithium-ion batteries using froth flotation. Sustain. Mater. Technol. 2018, 17, e00062. [Google Scholar] [CrossRef]
- Ruismäki, R.; Rinne, T.; Dancak, A.; Taskinen, P.; Serna-Guerrero, R.; Jokilaakso, A. Integrating Flotation and Pyrometallurgy for Recovering Graphite and Valuable Metals from Battery Scrap. Metals 2020, 10, 680. [Google Scholar] [CrossRef]
- Porvali, A.; Aaltonen, M.; Ojanen, S.; Velazquez-Martinez, O.; Eronen, E.; Liu, F.; Wilson, B.P.; Serna-Guerrero, R.; Lundström, M. Mechanical and hydrometallurgical processes in HCl media for the recycling of valuable metals from Li-ion battery waste. Resour. Conserv. Recycl. 2019, 142, 257–266. [Google Scholar] [CrossRef]
- Peng, C.; Hamuyuni, J.; Wilson, B.P.; Lundström, M. Selective reductive leaching of cobalt and lithium from industrially crushed waste Li-ion batteries in sulfuric acid system. Waste Manag. 2018, 76, 582–590. [Google Scholar] [CrossRef]
- Vassura, I.; Morselli, L.; Bernadi, E.; Passarini, F. Chemical characterisation of spent rechargeable batteries. Waste Manag. 2009, 29, 2332–2335. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gaustad, G.; Babbitt, C.W. Targeting high value metals in lithium-ion battery recycling via shredding and size-based separation. Waste Manag. 2016, 51, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Widijatmoko, S.D.; Fu, G.; Wang, Z.; Hall, P. Selective liberation in dry milled spent lithium-ion batteries. Sustain. Mater. Technol. 2019, 23, e00134. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Wang, F.; Ge, L.; Zhu, X.; Li, H. Chemical and process mineralogical characterizations of spent lithium-ion batteries: An approach by multi-analytical techniques. Waste Manag. 2014, 34, 1051–1058. [Google Scholar] [CrossRef]
- Schubert, H. Aufbereitung Fester Stoffe Band II: Sortierprozesse; Deutscher Verlag für Grundstoffindustrie: Stuttgart, Germany, 1996; Volume 4. [Google Scholar]
- Yu, J.; He, Y.; Ge, Z.; Li, H.; Xie, W.; Wang, S. A promising physical method for recovery of LiCoO2 and graphite from spent lithium-ion batteries: Grinding flotation. Sep. Purif. Technol. 2018, 190, 45–52. [Google Scholar] [CrossRef]
- Chernyaev, A.; Partinen, J.; Klemettinen, L.; Wilson, B.P.; Jokilaakso, A.; Lundström, M. The efficiency of scrap Cu and Al current collector materials as reductants in LIB waste leaching. Hydrometallurgy 2021, 203, 105608. [Google Scholar] [CrossRef]
- Sa, Q.; Heelan, J.A.; Lu, Y.; Apelian, D.; Wang, Y. Copper Impurity Effects on LiNi1/3Mn1/3Co1/3O2 Cathode Material. ACS Appl. Mater. Interfaces 2015, 7, 20585–20590. [Google Scholar] [CrossRef]
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of iron, aluminium, manganese and copper from leach solutions of lithium-ion battery waste using ion exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Chen, L.; Tang, X.; Zhang, Y.; Li, L.; Zeng, Z.; Zhang, Y. Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 2011, 108, 80–86. [Google Scholar] [CrossRef]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef]
- Peng, C.; Lahtinen, K.; Medina, E.; Kauranen, P.; Karppinen, M.; Kallio, T.; Wilson, B.P.; Lundström, M. Role of impurity copper in Li-ion battery recycling to LiCoO2 cathode materials. J. Power Sources 2020, 450, 227630. [Google Scholar] [CrossRef]
- Ardia, P.; Stallone, S.; Cericola, D. A quantification method for Fe based particle contaminants in high purity materials for lithium-ion batteries. Talanta 2021, 224, 121827. [Google Scholar] [CrossRef]
- Nasser, O.A.; Petranikova, M. Review of Achieved Purities after Li-ion Batteries Hydrometallurgical Treatment and Impurities Effects on the Cathode Performance. Batteries 2021, 7, 60. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, Y.; Yao, Z.; Vanaphuti, P.; Ma, X.; Bong, S.; Chen, M.; Liu, Y.; Cheng, F.; Yang, Z.; et al. Systematic Study of Al Impurity for NCM622 Cathode Materials. ACS Sustain. Chem. Eng. 2020, 8, 9875–9884. [Google Scholar] [CrossRef]
- Zhang, R.; Meng, Z.; Ma, X.; Chen, M.; Chen, B.; Zheng, Y.; Yao, Z.; Vanaphuti, P.; Bong, S.; Yang, Z.; et al. Understanding fundamental effects of Cu impurity in different forms for recovered LiNi0.6Co0.2Mn0.2O2 cathode materials. Nano Energy 2020, 78, 105214. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.; Ku, H.; Jo, M.; Kim, S.; Song, J.; Yu, J.; Kwon, K. The effect of Fe as an impurity element for sustainable resynthesis of Li[Ni1/3Co1/3Mn1/3]O2 cathode material from spent lithium-ion batteries. Electrochim. Acta 2018, 296, 814–822. [Google Scholar] [CrossRef]
- Wilke, C.; Kaas, A.; Werner, D.; Peuker, U.A. Supplementary information to the publication “Influence of the crusher settings and a thermal pre-treatment on the properties of the fine fraction (black mass) from mechanical Lithium-Ion Battery recycling”. OpARA 2023. [Google Scholar] [CrossRef]
- Kaas, A.; Wilke, C.; Vanderbruggen, A.; Peuker, U.A. Influence of different discharge levels on the mechanical recycling efficiency of lithium-ion batteries. Waste Manag. 2023, 172, 1–10. [Google Scholar] [CrossRef]
- Wurm, C.; Öttinger, O.; Wittkämper, S.; Zauter, R.; Vuorilehto, K. Anode materials for lithium-ion batteries. In Lithium-Ion Batteries: Basics and Applications; Korthauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 43–58. [Google Scholar]
- Rothermel, S.; Evertz, M.; Kasnatscheew, J.; Qi, X.; Grützke, M.; Winter, M.; Nowak, S. Graphite Recycling from Spent Lithium-Ion Batteries. ChemSusChem 2016, 9, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal Decomposition of LiPF6-Based Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A2327–A2334. [Google Scholar] [CrossRef]
- Friesen, A.; Hildebrand, S.; Horsthemke, F.; Börner, M.; Klöpsch, R.; Niehoff, P.; Schappacher, F.M.; Winter, M. Al2O3 coating on anode surface in lithium ion batteries: Impact on low temperature cycling and safety behavior. J. Power Sources 2017, 363, 70–77. [Google Scholar] [CrossRef]


| Black Mass Size (Fraction) in µm | |||||||
|---|---|---|---|---|---|---|---|
| 0–38/ 0–63 | 0–200/ 0–250 | 0–1000 | 0–2000 | 200–400/ 250–500 | 400–1250/ 500–1000 | 1000–2000/ 1000–3000 | |
| Constituent | Content in % | ||||||
| Positive electrode coating | 36.5 5 ≈40 7 | 31.4–34.3 6 ≈38 2 ≈40–45 1 | 34.1 2 37.9 4 ≈48 7 | 28.1 3 | ≈40 1 ≈40 2 6.0 6 | 4.3 6 ≈35 1 ≈30 2 ≈60 7 | 4.2 6 33.5 4 |
| Li | ≈4 2 | 3.2 4 3.6 2 | 3.7 3 | ≈4 2 | ≈4 2 | 2.7 4 | |
| P | 0.5–0.7 6 | 0.5 6 | 0.4 6 | 0.4 6 | |||
| Graphite | ≈60 7 | ≈50 7 | ≈35 7 | ||||
| Al | 1.6 5 | <1 1 <1 2 2–2.4 6 | 0.5 4 2.2 2 | 2.8 3 | ≈2 1 ≈2 2 10.8 6 | ≈7 2 ≈11 1 22.3 6 | 2.6 4 29.8 6 |
| Cu | 0.8 5 | <1 1 <1 2 0.6–1.4 6 | 1.9 4 3.0 2 | 6.2 3 | ≈2 1 ≈3 2 50.2 6 | ≈9 1 ≈11 2 48.3 6 | 12 4 30.6 6 |
| Fe | 0.1 6 <1 1 <1 2 | 0.6 4 0.7 2 | 0.7 3 | 0.3 6 <1 1 <1 2 | 0.3 6 <1 1 ≈1 2 | 0.1 4 0.3 6 | |
| Black Mass Size Fraction in µm | ||||
|---|---|---|---|---|
| 0–315 | 315–500 | 500–1000 | ||
| Mass Share in % | ||||
| Grid size in mm | 10 | 43.6 | 20.5 | 35.9 |
| 20 | 45.6 | 19.6 | 34.9 | |
| 30 | 45.2 | 18.8 | 36.0 | |
| 40 | 45.7 | 18.8 | 35.5 | |
| Pre-treatment temperature in °C | no | 51.2 | 18.2 | 30.7 |
| 22 | 44.0 | 20.1 | 36.0 | |
| 80 | 49.1 | 18.1 | 32.8 | |
| 120 | 52.5 | 15.5 | 32.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilke, C.; Werner, D.M.; Kaas, A.; Peuker, U.A. Influence of the Crusher Settings and a Thermal Pre-Treatment on the Properties of the Fine Fraction (Black Mass) from Mechanical Lithium-Ion Battery Recycling. Batteries 2023, 9, 514. https://doi.org/10.3390/batteries9100514
Wilke C, Werner DM, Kaas A, Peuker UA. Influence of the Crusher Settings and a Thermal Pre-Treatment on the Properties of the Fine Fraction (Black Mass) from Mechanical Lithium-Ion Battery Recycling. Batteries. 2023; 9(10):514. https://doi.org/10.3390/batteries9100514
Chicago/Turabian StyleWilke, Christian, Denis Manuel Werner, Alexandra Kaas, and Urs Alexander Peuker. 2023. "Influence of the Crusher Settings and a Thermal Pre-Treatment on the Properties of the Fine Fraction (Black Mass) from Mechanical Lithium-Ion Battery Recycling" Batteries 9, no. 10: 514. https://doi.org/10.3390/batteries9100514
APA StyleWilke, C., Werner, D. M., Kaas, A., & Peuker, U. A. (2023). Influence of the Crusher Settings and a Thermal Pre-Treatment on the Properties of the Fine Fraction (Black Mass) from Mechanical Lithium-Ion Battery Recycling. Batteries, 9(10), 514. https://doi.org/10.3390/batteries9100514








