Organic and Inorganic Hybrid Composite Phase Change Material for Inhibiting the Thermal Runaway of Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experimental Setup
2.3. Experimental Conditions
3. Results and Discussions
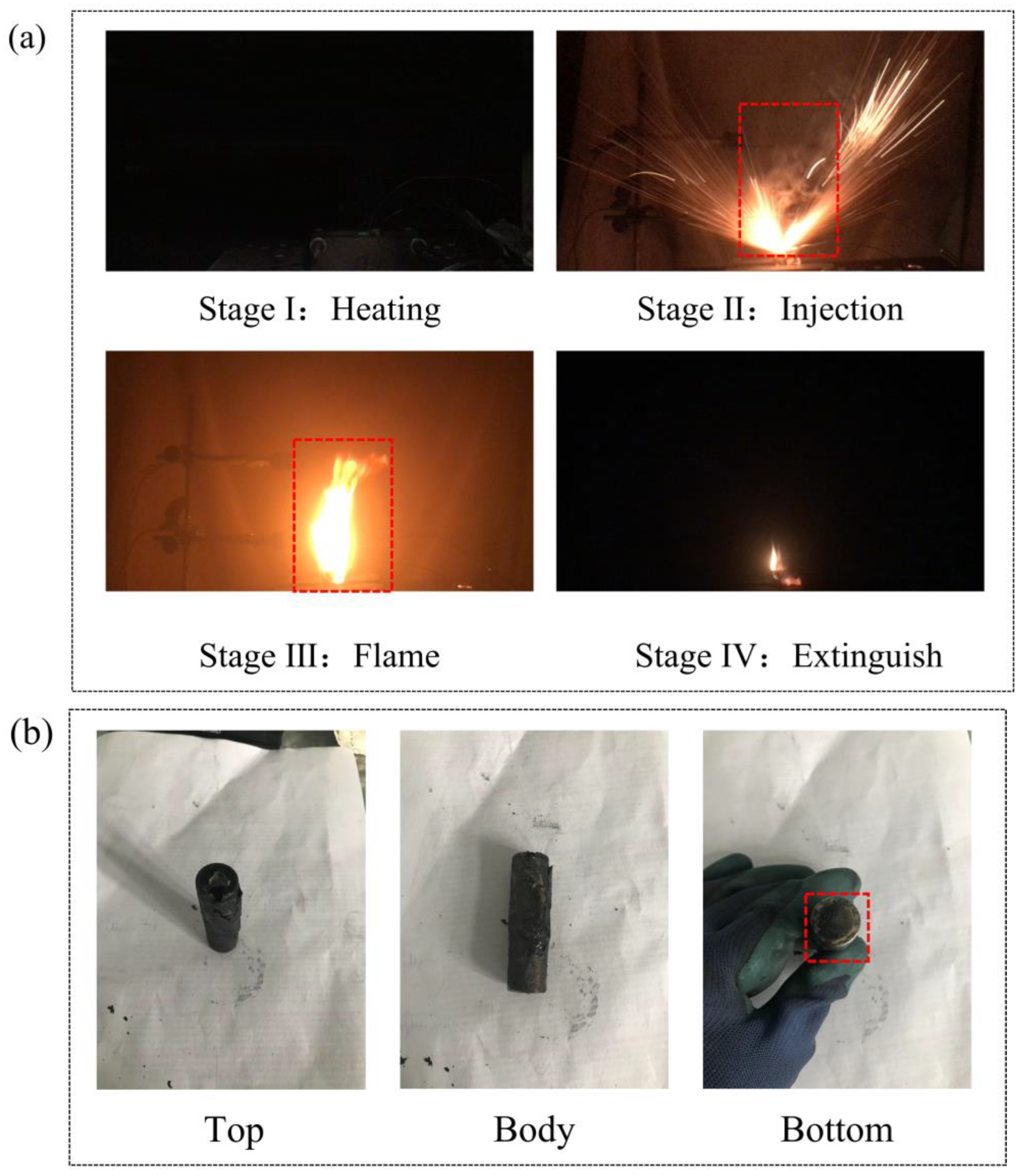
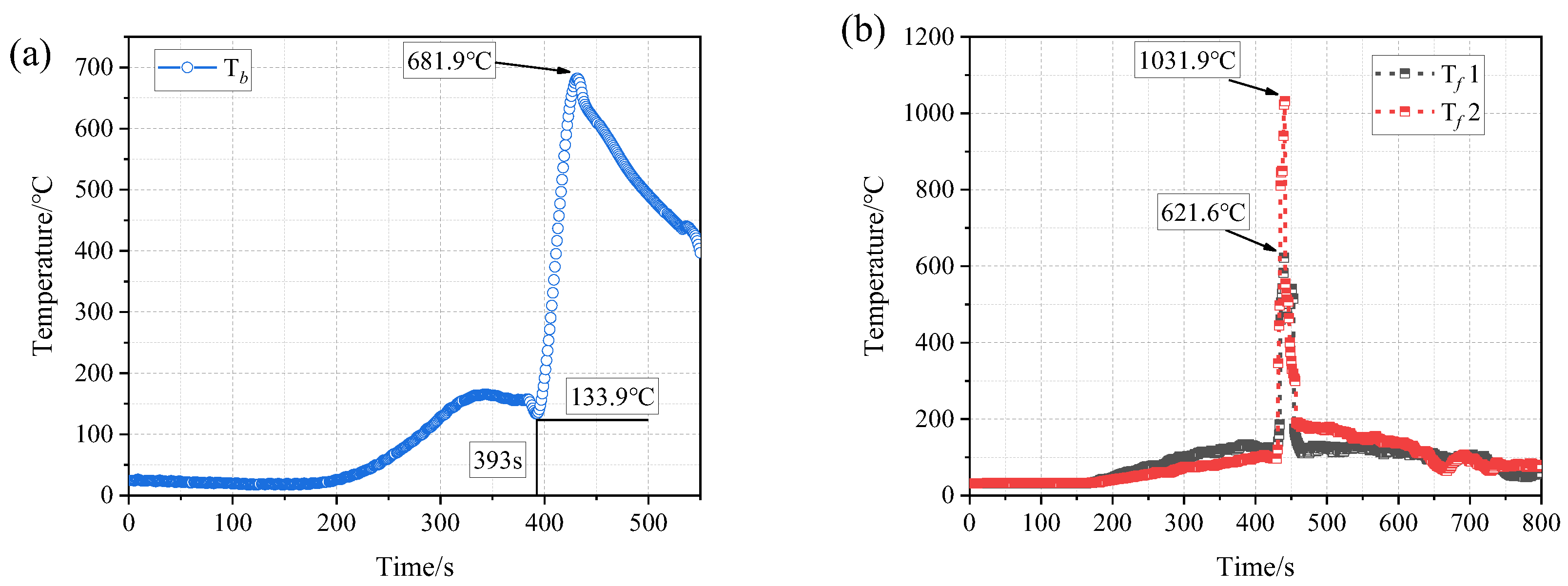
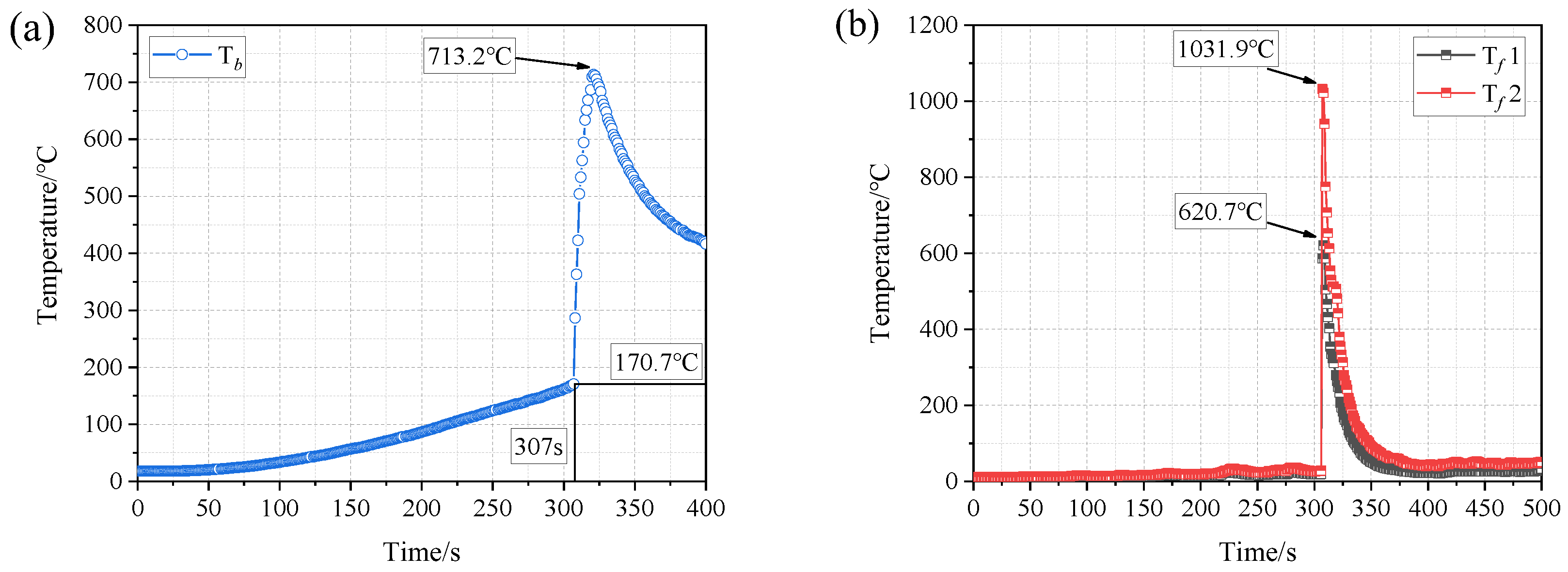
3.1. Thermal Runaway Behaviors of a Single Battery
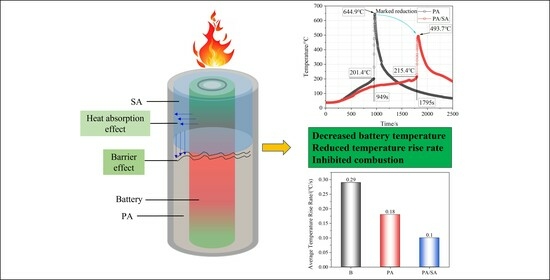
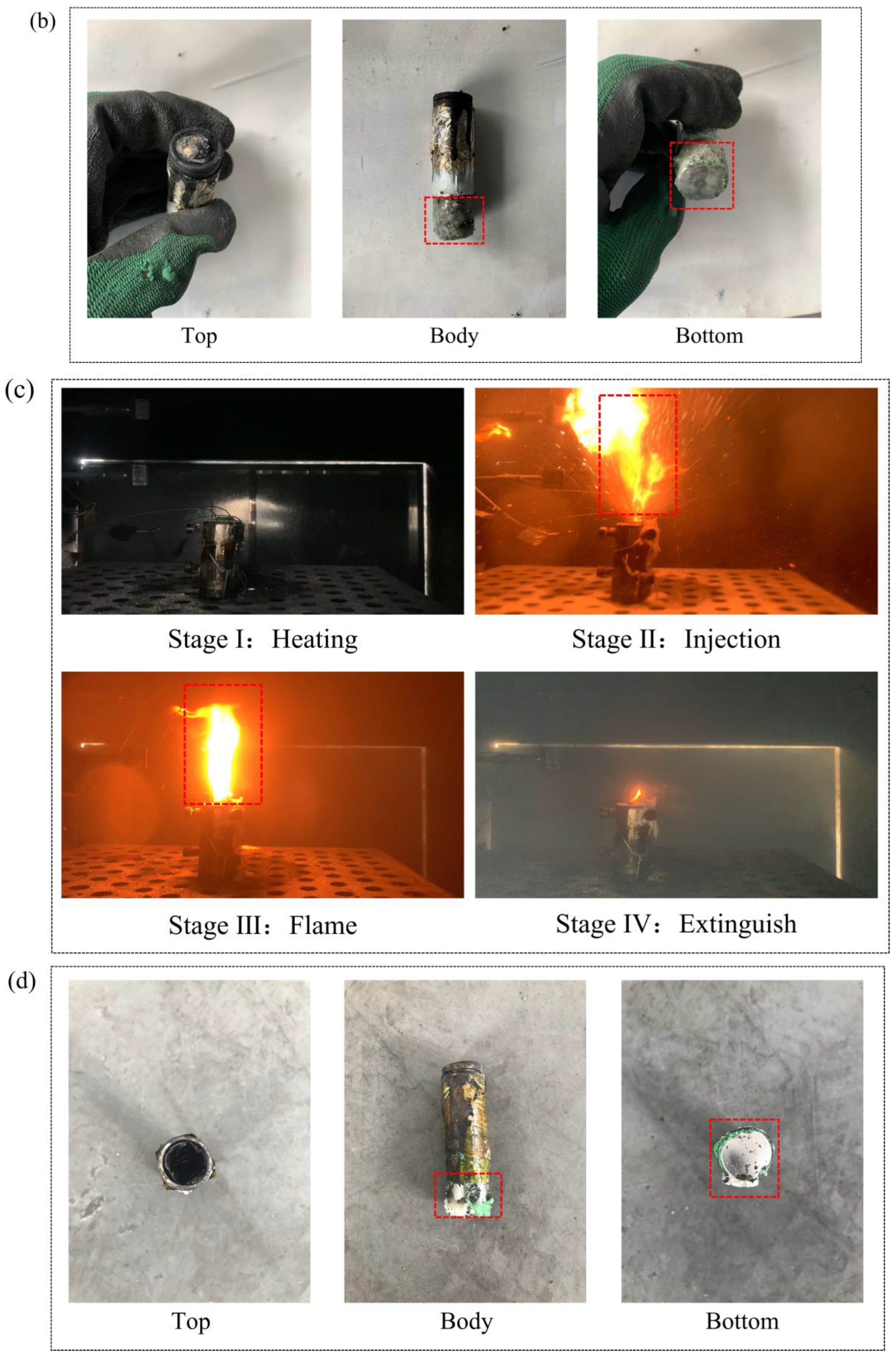
3.2. Effect of the PCMs on Thermal Runaway
3.3. Influence of Heating Power
4. Conclusions
- (1)
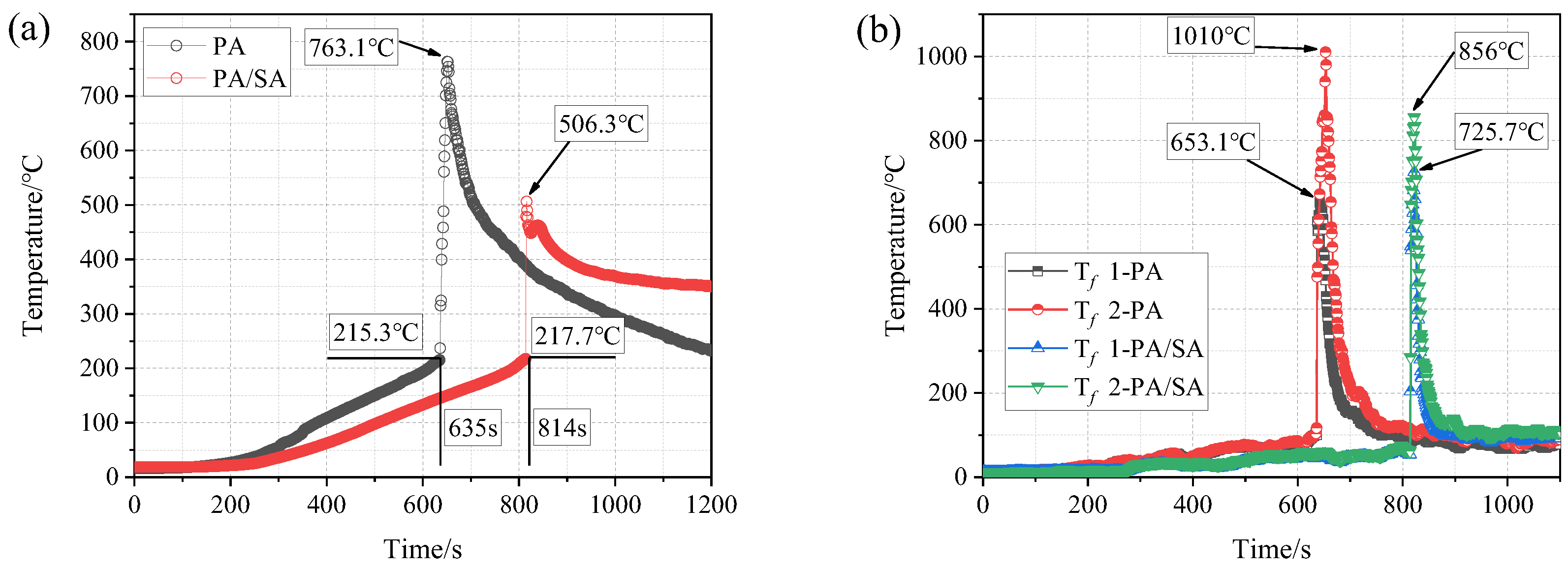
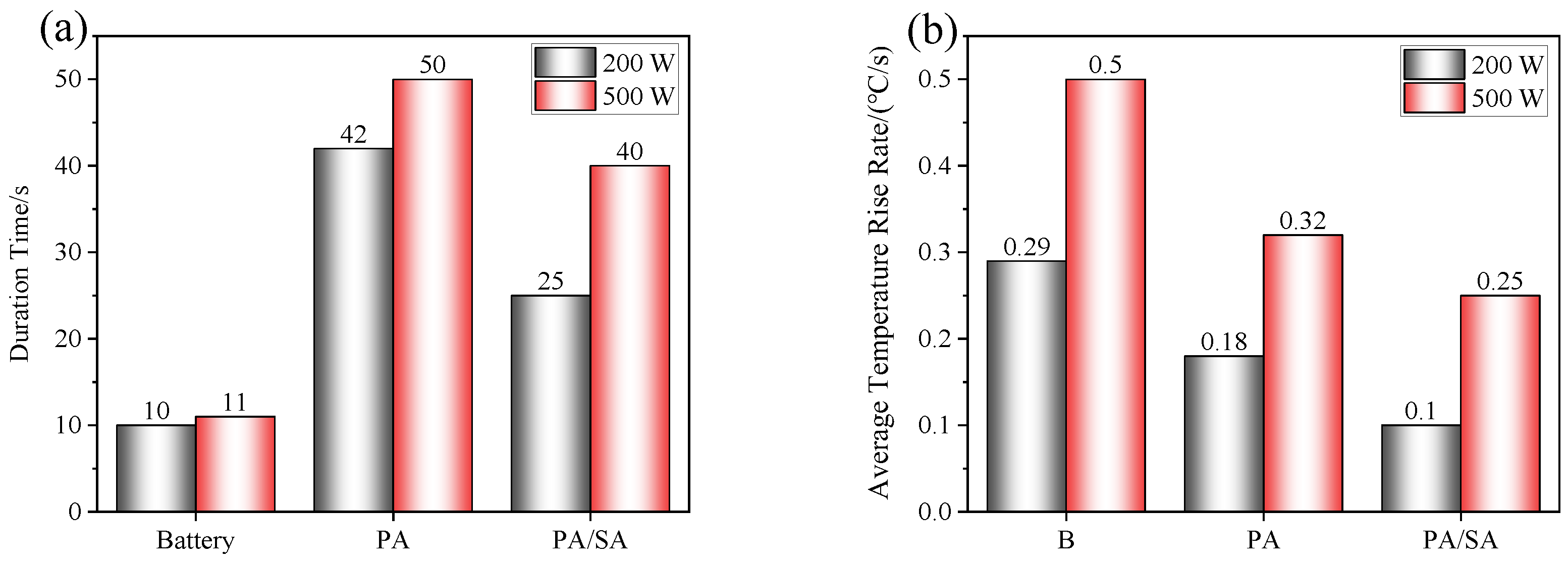
- The thermal runaway of the battery was accompanied by violent combustion behaviors and a high temperature. The heat absorption of PA delayed the thermal runaway by 33.5%, but its flammable characteristic led to more violent combustion and a longer combustion duration. The tcom of Group II (PA) was 42 s, while that of Group I (single battery) was only 10 s.
- (2)
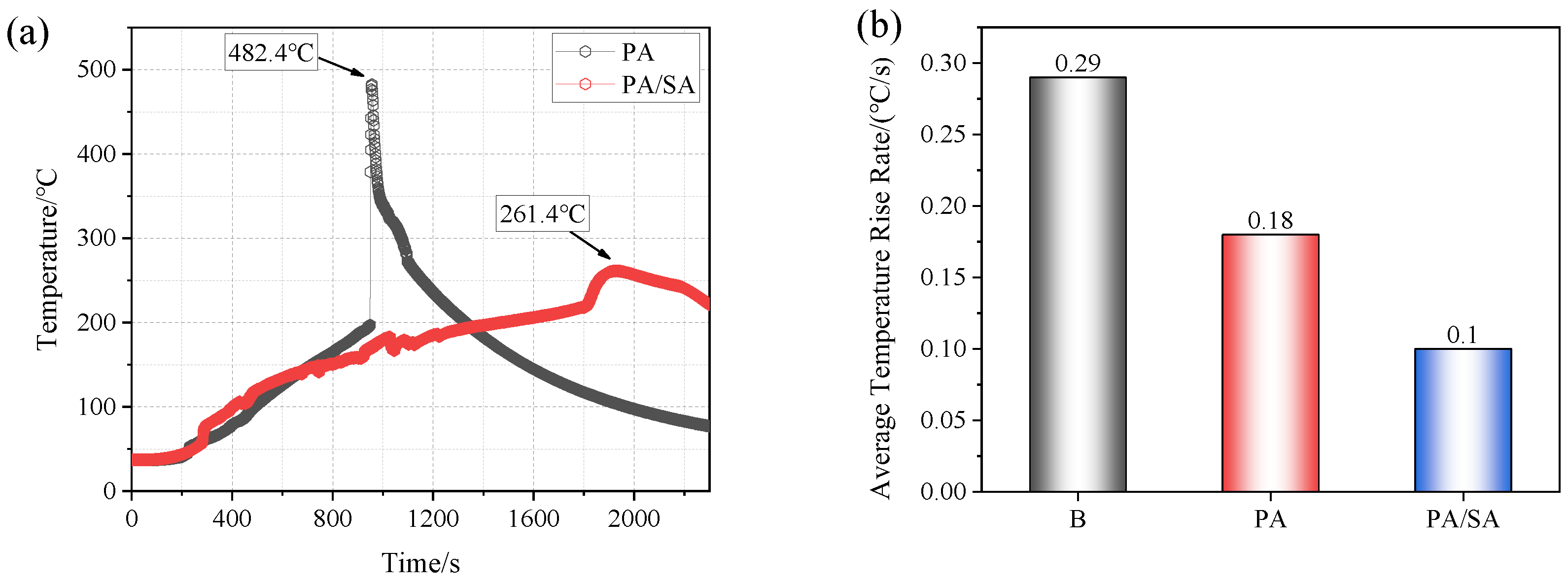
- The heat absorption of SA reduced the combustion of PA, and Group III (PA/SA) had a 40.5% reduction in tcom than that of Group II (PA). The rise in battery temperature was significantly slowed down by PA/SA, and its Trate-ave was decreased by 87.3% and 44.4% in comparison to PA and a single battery, respectively. The temperature of the battery flame was essentially unaffected by either PCM.
- (3)
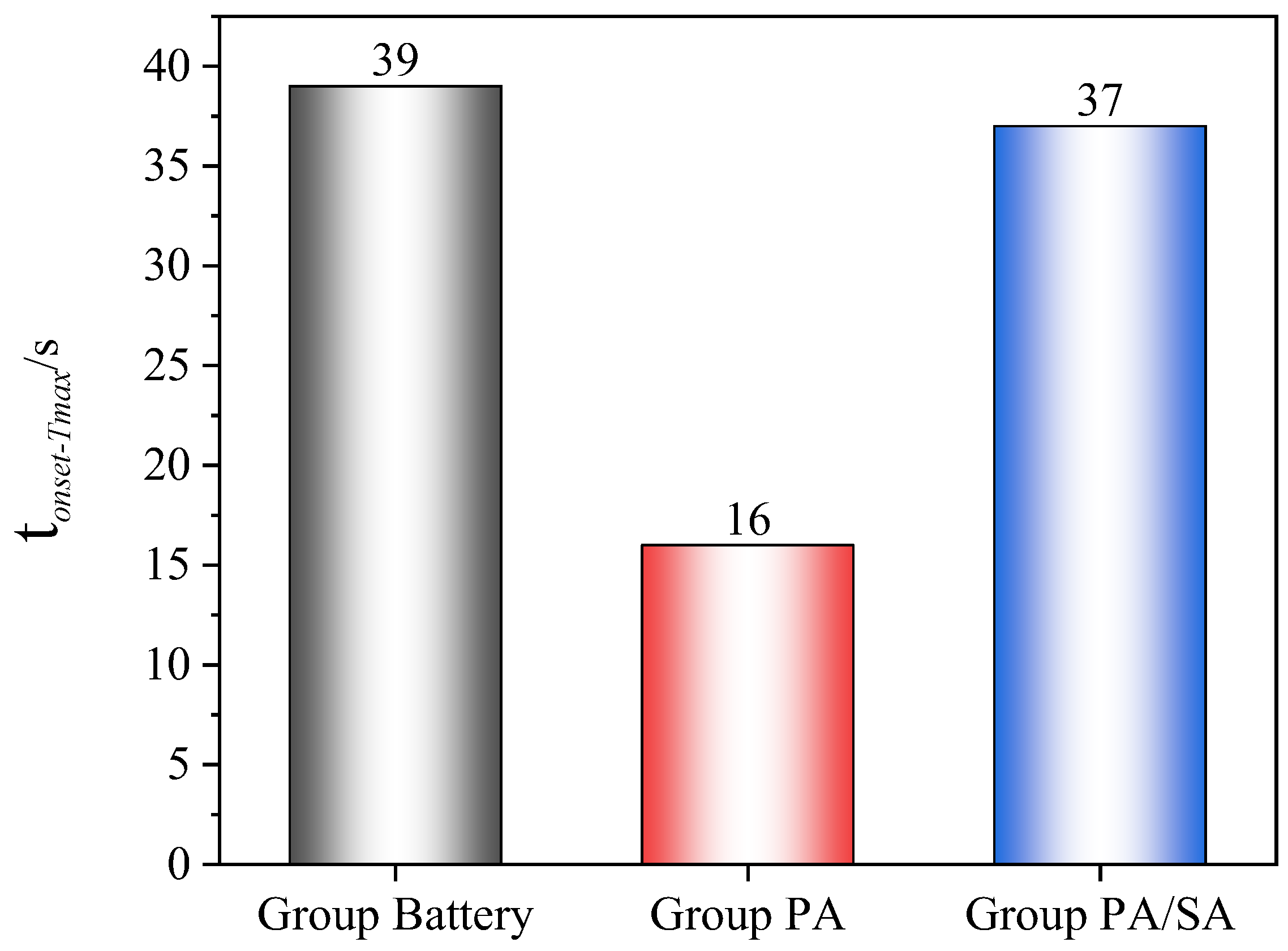
- Although the battery and flame temperatures of the two PCM groups were essentially unaffected by the increasing heating power, the thermal runaway mitigation effect was significantly reduced, while the thermal runaway onset time was advanced by at least 33.1%. The heating power had a pronounced impact on SA/PA, with tcom and Trate-ave increasing by 37.5% and 60%, respectively. The fact that the tone-max for Group III (PA/SA) changed from 37 to 2 s further illustrates the tremendous impact of the heating power on the suppression effect of PA/SA on a battery’ temperature rise.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adnan, M. The Future of Energy Storage: Advancements and Roadmaps for Lithium-Ion Batteries. Int. J. Mol. Sci. 2023, 24, 7457. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Ouyang, D.; Weng, J.; Chen, M.; Wang, J. Impact of high-temperature environment on the optimal cycle rate of lithium-ion battery. J. Energy Storage 2020, 28, 101242. [Google Scholar] [CrossRef]
- Leng, F.; Tan, C.M.; Pecht, M. Effect of Temperature on the Aging rate of Li Ion Battery Operating above Room Temperature. Sci. Rep. 2015, 5, 12967. [Google Scholar] [CrossRef]
- Feng, X.; Sun, J.; Ouyang, M.; He, X.; Lu, L.; Han, X.; Fang, M.; Peng, H. Characterization of large format lithium ion battery exposed to extremely high temperature. J. Power Sources 2014, 272, 457–467. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Ping, P.; Peng, R.; Kong, D.; Chen, G.; Wen, J. Investigation on thermal management performance of PCM-fin structure for Li-ion battery module in high-temperature environment. Energy Convers. Manag. 2018, 176, 131–146. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Huang, S.; Jiao, Y.; Chen, M. Recent Progress and Prospects in Liquid Cooling Thermal Management System for Lithium-Ion Batteries. Batteries 2023, 9, 400. [Google Scholar] [CrossRef]
- Abbas, S.; Ramadan, Z.; Park, C.W. Thermal performance analysis of compact-type simulative battery module with paraffin as phase-change material and flat plate heat pipe. Int. J. Heat Mass Transf. 2021, 173, 121269. [Google Scholar] [CrossRef]
- An, Z.; Luo, Y.; Zhang, C.; Li, D. Performance of chocolate bar-shaped modular thermal management system combined metal lattice liquid-cooling plate with paraffin in high-rate discharge. J. Energy Storage 2022, 56, 106017. [Google Scholar] [CrossRef]
- Hussain, A.; Abidi, I.H.; Tso, C.Y.; Chan, K.C.; Luo, Z.; Chao, C.Y.H. Thermal management of lithium ion batteries using graphene coated nickel foam saturated with phase change materials. Int. J. Therm. Sci. 2018, 124, 23–35. [Google Scholar] [CrossRef]
- Wang, Z.; Du, C.; Qi, R.; Wang, Y. Experimental study on thermal management of lithium-ion battery with graphite powder based composite phase change materials covering the whole climatic range. Appl. Therm. Eng. 2022, 216, 119072. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, B.; Ding, J.; Ding, Y. Experimental and numerical investigation of a hybrid battery thermal management system based on copper foam-paraffin composite phase change material and liquid cooling. Appl. Therm. Eng. 2023, 218, 119312. [Google Scholar] [CrossRef]
- Greco, A.; Jiang, X.; Cao, D. An investigation of lithium-ion battery thermal management using paraffin/porous-graphite-matrix composite. J. Power Sources 2015, 278, 50–68. [Google Scholar] [CrossRef]
- Kang, W.; Zhao, Y.; Jia, X.; Hao, L.; Dang, L.; Wei, H. Paraffin/SiC as a Novel Composite Phase-Change Material for a Lithium-Ion Battery Thermal Management System. Trans. Tianjin Univ. 2021, 27, 55–63. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Li, C.; Wang, J.; Zhang, J.; Cheng, Y.; Mei, W.; Cheng, S.; Qin, P.; Duan, Q.; et al. Numerical optimization for a phase change material based lithium-ion battery thermal management system. Appl. Therm. Eng. 2023, 222, 119839. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, S.; Zhao, L.; Weng, J.; Ouyang, D.; Chen, Q.; Kong, Q.; Wang, J. Preparation of thermally conductive composite phase change materials and its application in lithium-ion batteries thermal management. J. Energy Storage 2022, 52, 104857. [Google Scholar] [CrossRef]
- Chen, H.; Abidi, A.; Hussein, A.K.; Younis, O.; Degani, M.; Heidarshenas, B. Investigation of the use of extended surfaces in paraffin wax phase change material in thermal management of a cylindrical lithium-ion battery: Applicable in the aerospace industry. J. Energy Storage 2022, 45, 103685. [Google Scholar] [CrossRef]
- Mei, J.; Shi, G.; Liu, H.; Wang, Z.; Chen, M. Investigation on the optimization strategy of phase change material thermal management system for lithium-ion battery. J. Energy Storage 2022, 55, 105365. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bai, J.; Gao, T.; Mao, N. Heat generation and thermal runaway mechanisms induced by overcharging of aged lithium-ion battery. Appl. Therm. Eng. 2022, 212, 118565. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Z.; Sun, J.; Wang, Q. Heat generation and thermal runaway of lithium-ion battery induced by slight overcharging cycling. J. Power Sources 2022, 526, 231136. [Google Scholar] [CrossRef]
- Mao, N.; Zhang, T.; Wang, Z.; Gadkari, S.; Wang, J.; He, T.; Gao, T.; Cai, Q. Revealing the thermal stability and component heat contribution ratio of overcharged lithium-ion batteries during thermal runaway. Energy 2023, 263, 125786. [Google Scholar] [CrossRef]
- Hu, J.; Liu, T.; Wang, X.; Wang, Z.; Wu, L. Investigation on thermal runaway of 18,650 lithium ion battery under thermal abuse coupled with charging. J. Energy Storage 2022, 51, 104482. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, X.; Meng, N.; Yu, Z.; Yang, H. Study of thermal runaway and the combustion behavior of lithium-ion batteries overcharged with high current rates. Thermochim. Acta 2022, 715, 179276. [Google Scholar] [CrossRef]
- García, A.; Zhao, P.; Monsalve-Serrano, J.; Villalta, D.; Martinez-Boggio, S. Optical diagnostics of the venting spray and combustion behaviour during Li-ion battery thermal runaway induced by ramp heating. Appl. Therm. Eng. 2023, 218, 119308. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Planteu, R.; Krohn, P.; Rasch, B.; Brunnsteiner, B.; Thaler, A.; Hacker, V. Thermal runaway of large automotive Li-ion batteries. RSC Adv. 2018, 8, 40172–40186. [Google Scholar] [CrossRef]
- He, C.X.; Yue, Q.L.; Chen, Q.; Zhao, T.S. Modeling thermal runaway of lithium-ion batteries with a venting process. Appl. Energy 2022, 327, 120110. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Zhang, Y.; Ouyang, M. Flammability characteristics of the battery vent gas: A case of NCA and LFP lithium-ion batteries during external heating abuse. J. Energy Storage 2019, 24, 100775. [Google Scholar] [CrossRef]
- Huang, Z.; Shen, T.; Jin, K.; Sun, J.; Wang, Q. Heating power effect on the thermal runaway characteristics of large-format lithium ion battery with Li(Ni1/3Co1/3Mn1/3)O2 as cathode. Energy 2022, 239, 121885. [Google Scholar] [CrossRef]
- Md Said, M.S.; Mohd Tohir, M.Z. Visual and thermal imaging of lithium-ion battery thermal runaway induced by mechanical impact. J. Loss Prev. Process Ind. 2022, 79, 104854. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, L.; Zhang, Z.; Li, X.; Ma, R.; Ren, Y.; Wu, W. Non-uniform phase change material strategy for directional mitigation of battery thermal runaway propagation. Renew. Energy 2022, 200, 1338–1351. [Google Scholar] [CrossRef]
- Dai, X.; Kong, D.; Du, J.; Zhang, Y.; Ping, P. Investigation on effect of phase change material on the thermal runaway of lithium-ion battery and exploration of flame retardancy improvement. Process Saf. Environ. Prot. 2022, 159, 232–242. [Google Scholar] [CrossRef]
- Weng, J.; Ouyang, D.; Yang, X.; Chen, M.; Zhang, G.; Wang, J. Alleviation of thermal runaway propagation in thermal management modules using aerogel felt coupled with flame-retarded phase change material. Energy Convers. Manag. 2019, 200, 112071. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Zhang, G.; Weng, J.; Wang, Y.; Deng, J. Innovative thermal management and thermal runaway suppression for battery module with flame retardant flexible composite phase change material. J. Clean. Prod. 2022, 330, 129718. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J. Investigation of external heating-induced failure propagation behaviors in large-size cell modules with different phase change materials. Energy 2020, 204, 117946. [Google Scholar] [CrossRef]
- Mei, J.; Shi, G.; Liu, H.; Wang, Z.; Chen, M. Experimental study on the effect of passive retardation method for thermal runaway mitigation of lithium-ion battery. Appl. Therm. Eng. 2023, 230, 120861. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, K.; Peng, H.; Ling, X. Preparation and thermal properties of sodium acetate trihydrate as a novel phase change material for energy storage. Energy 2019, 167, 269–274. [Google Scholar] [CrossRef]
- Hu, P.; Lu, D.-J.; Fan, X.-Y.; Zhou, X.; Chen, Z.-S. Phase change performance of sodium acetate trihydrate with AlN nanoparticles and CMC. Sol. Energy Mater. Sol. Cells 2011, 95, 2645–2649. [Google Scholar] [CrossRef]




| Name | Molecular Formula | ρ/(g/cm3) | Melting Point/°C | Boiling Point/°C | Specific Heat Capacity (J/(kg·°C)) |
|---|---|---|---|---|---|
| Paraffin | CnH2n+2 | 0.88 | 45–48 | 322 | 2140 |
| SA | CH3COONa·3H2O | 1.45 | 58 | 400 | 1970 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, J.; Shi, G.; Liu, H.; Wang, Z. Organic and Inorganic Hybrid Composite Phase Change Material for Inhibiting the Thermal Runaway of Lithium-Ion Batteries. Batteries 2023, 9, 513. https://doi.org/10.3390/batteries9100513
Mei J, Shi G, Liu H, Wang Z. Organic and Inorganic Hybrid Composite Phase Change Material for Inhibiting the Thermal Runaway of Lithium-Ion Batteries. Batteries. 2023; 9(10):513. https://doi.org/10.3390/batteries9100513
Chicago/Turabian StyleMei, Jie, Guoqing Shi, He Liu, and Zhi Wang. 2023. "Organic and Inorganic Hybrid Composite Phase Change Material for Inhibiting the Thermal Runaway of Lithium-Ion Batteries" Batteries 9, no. 10: 513. https://doi.org/10.3390/batteries9100513
APA StyleMei, J., Shi, G., Liu, H., & Wang, Z. (2023). Organic and Inorganic Hybrid Composite Phase Change Material for Inhibiting the Thermal Runaway of Lithium-Ion Batteries. Batteries, 9(10), 513. https://doi.org/10.3390/batteries9100513