LiNi0.8Fe0.1Al0.1O2 as a Cobalt-Free Cathode Material with High Capacity and High Capability for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
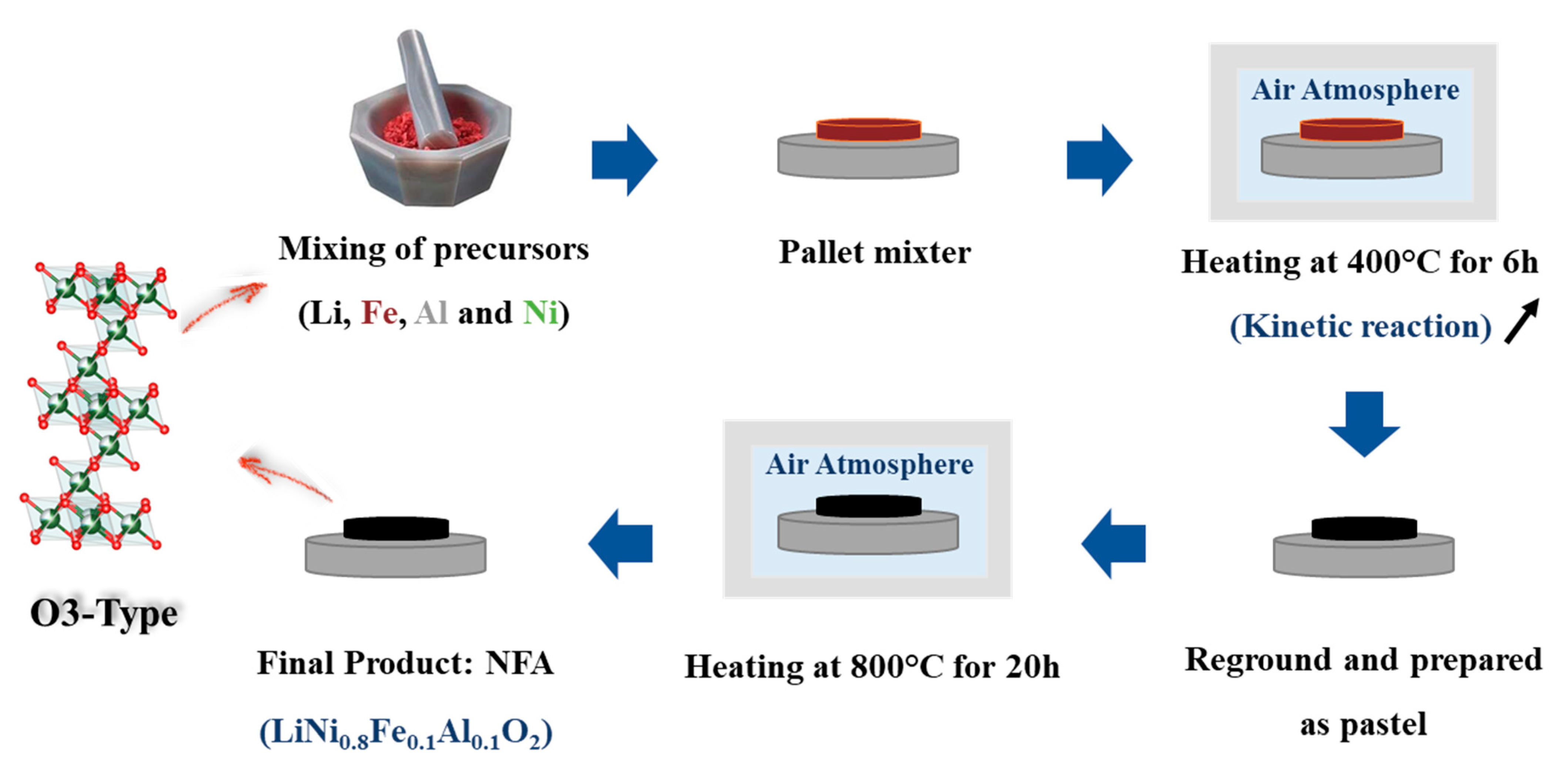
2.1. Synthesis
2.2. Structural and Morphological Observations
2.3. Electrochemical Measurements
3. Results
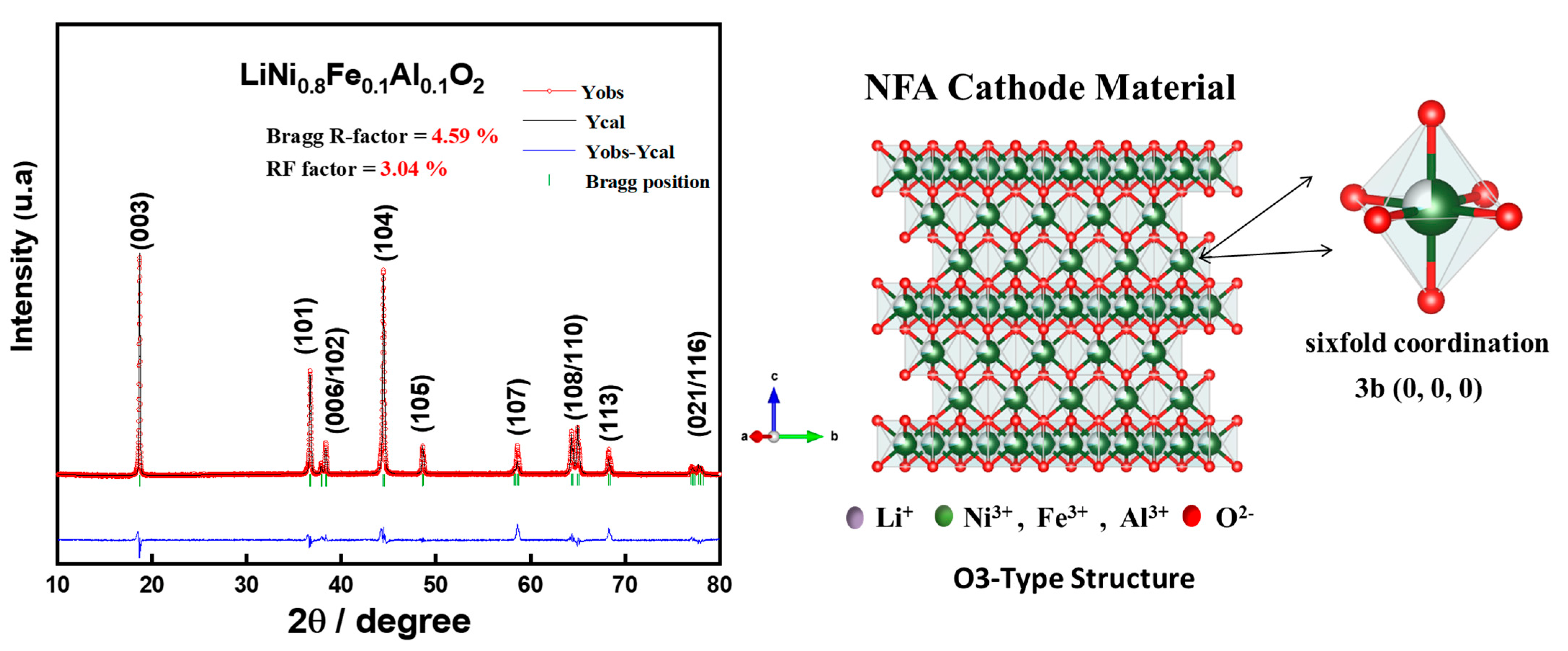
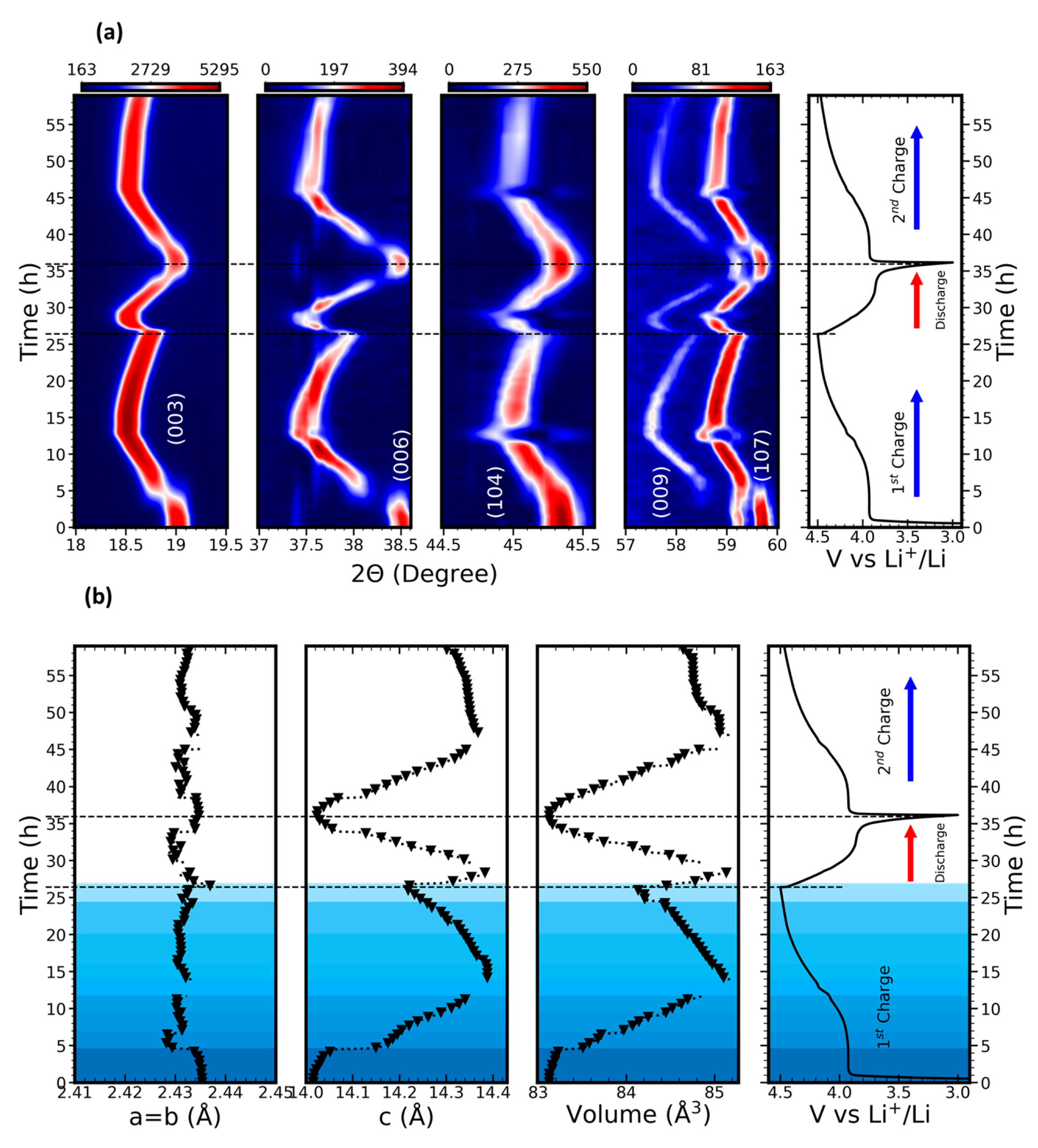
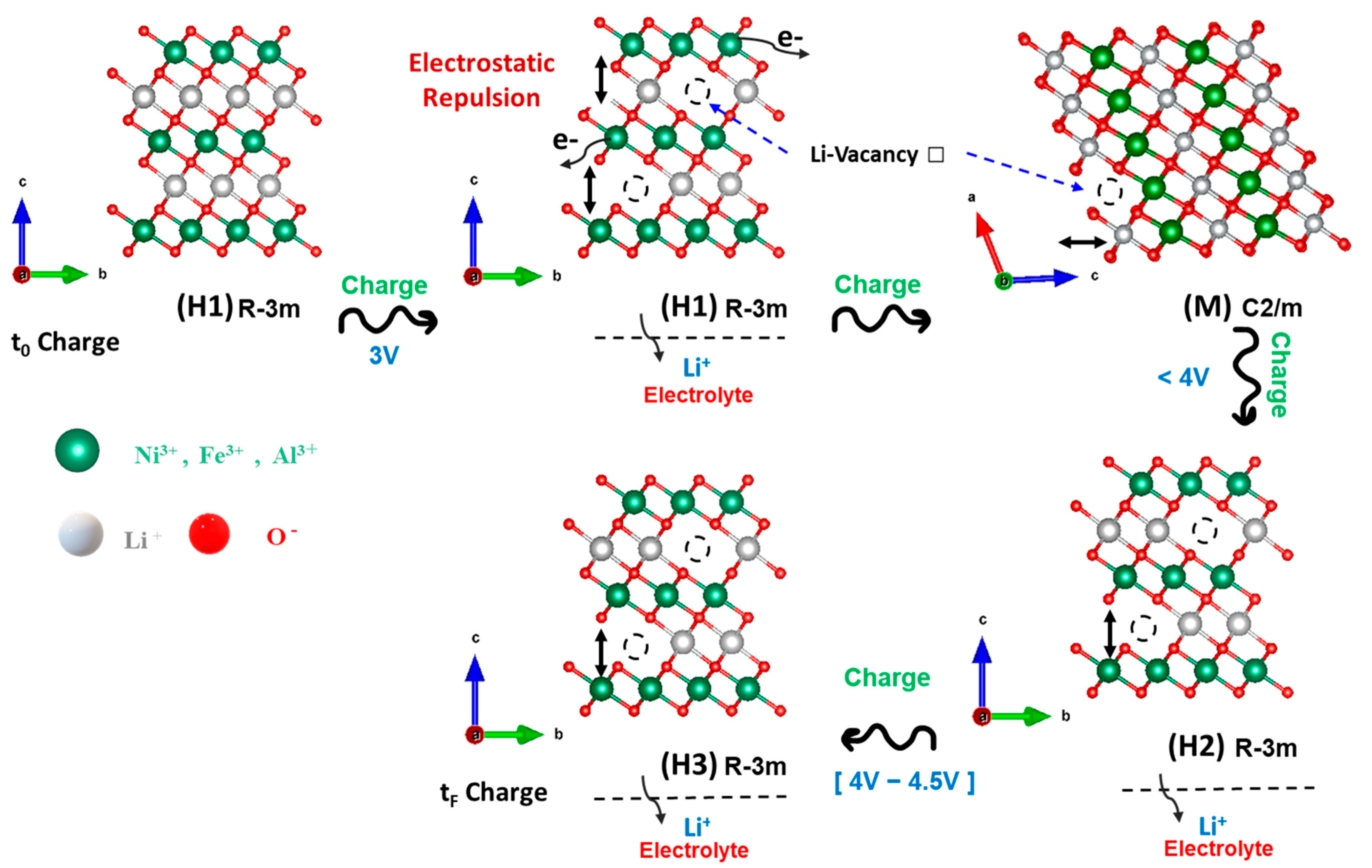
3.1. Materials Characterization
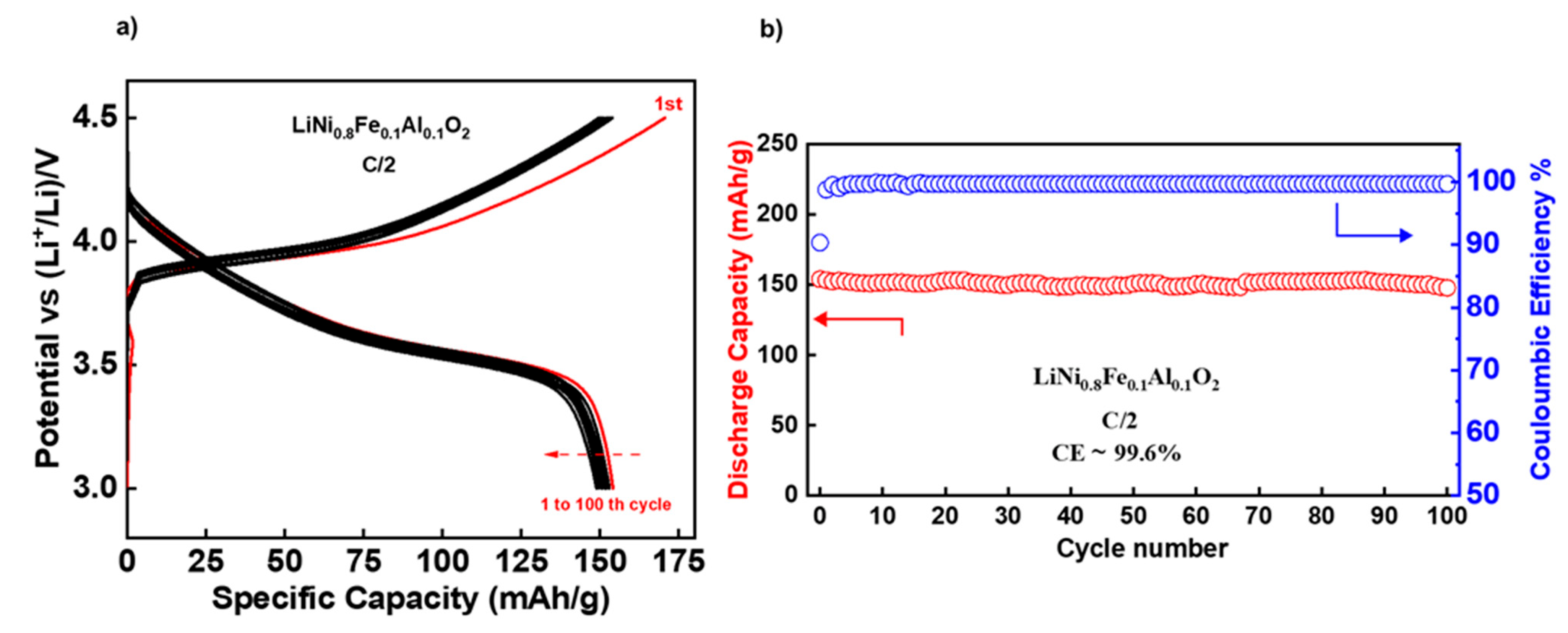
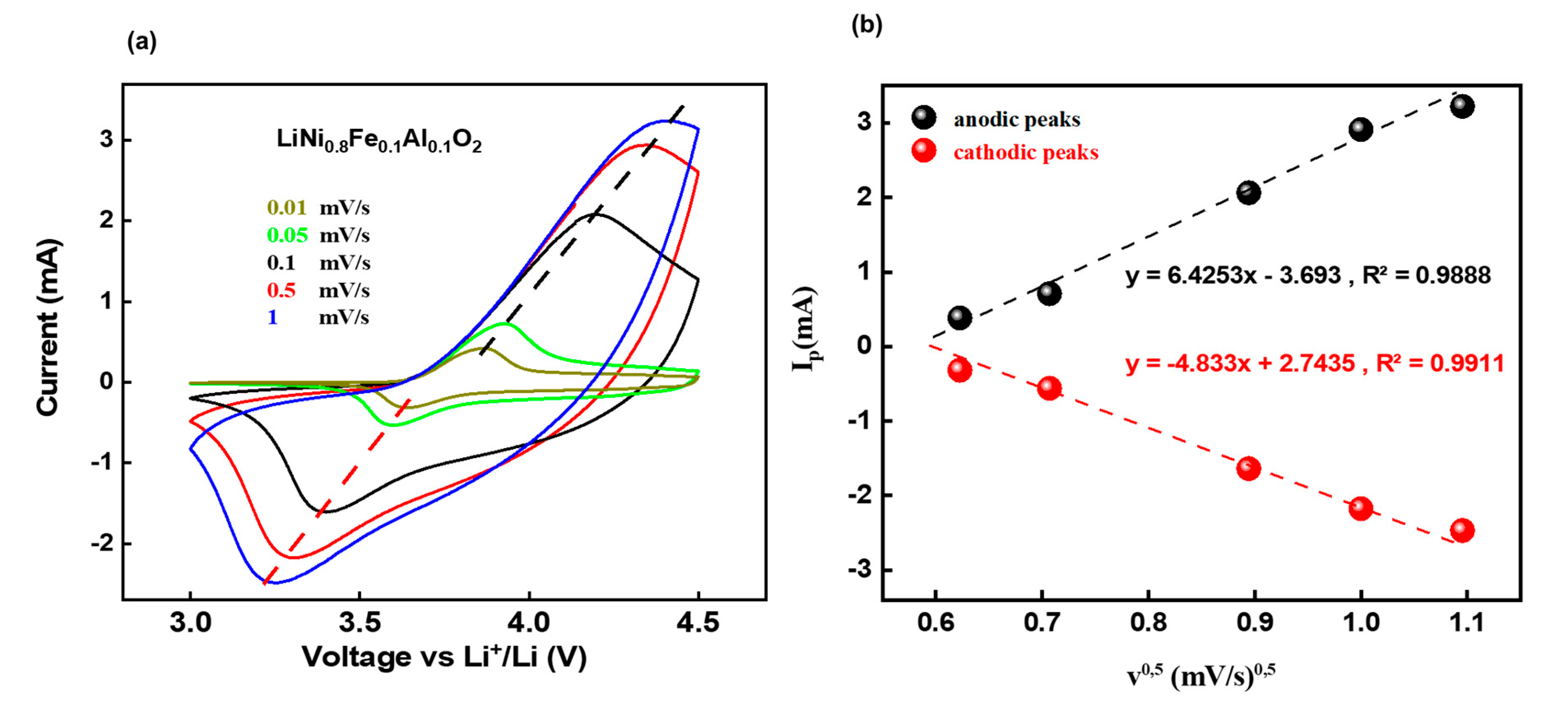
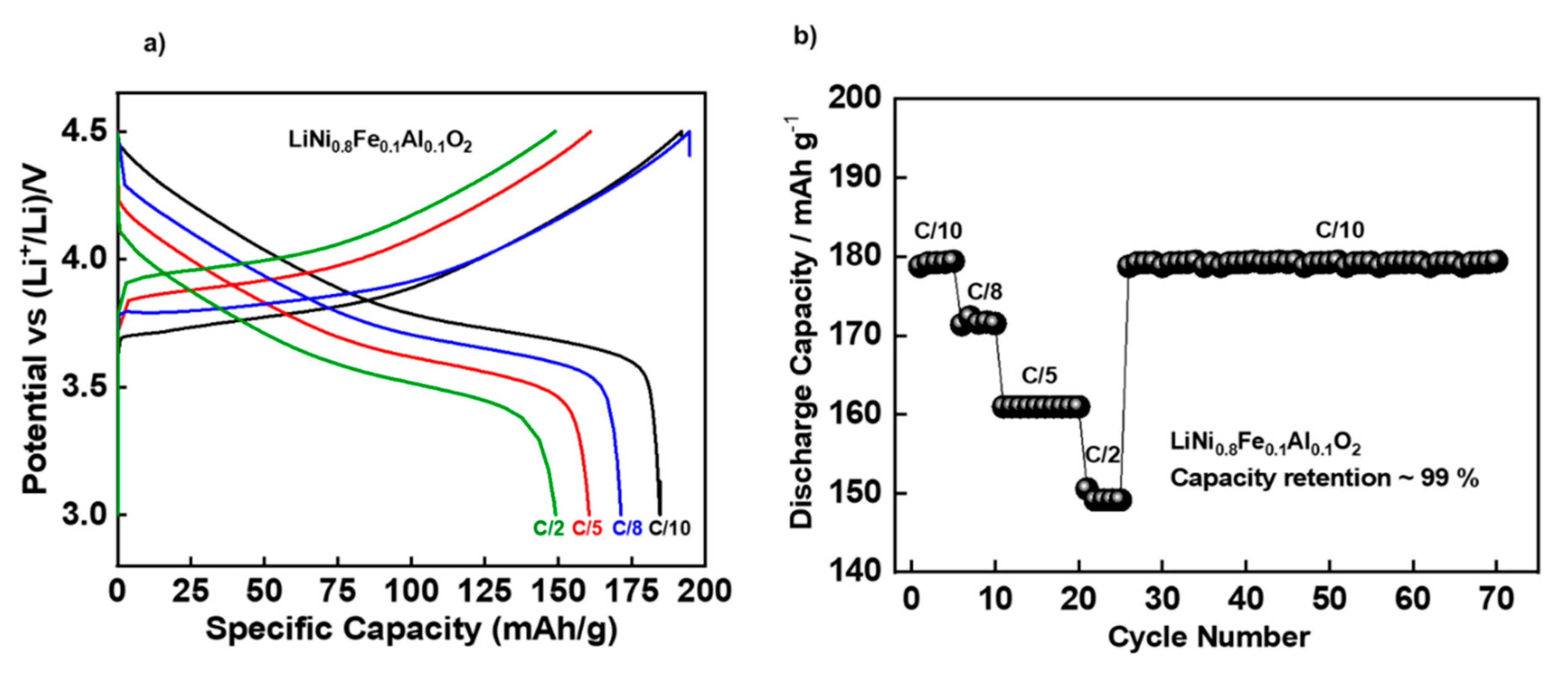
3.2. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krutilla, K.; Good, D.; Toman, M.; Arin, T. Addressing Fundamental Uncertainty in Benefit–Cost Analysis: The Case of Deep Seabed Mining. J. Benefit Cost Anal. 2021, 12, 122–151. [Google Scholar] [CrossRef]
- Zeng, A.; Chen, W.; Rasmussen, K.D.; Zhu, X.; Lundhaug, M.; Müller, D.B.; Tan, J.; Keiding, J.K.; Liu, L.; Dai, T.; et al. Battery technology and recycling alone will not save the electric mobility transition from future cobalt shortages. Nat. Commun. 2022, 13, 1341. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Bondarev, D.; Amatucci, G.G.; Yushin, G. Battery materials for low-cost electric transportation. Mater. Today 2020, 42, 57–72. [Google Scholar] [CrossRef]
- Lee, S.; Manthiram, A. Can Cobalt Be Eliminated from Lithium-Ion Batteries? ACS Energy Lett. 2022, 7, 3058–3063. [Google Scholar] [CrossRef]
- Choi, N.-S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.-K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges Facing Lithium Batteries and Electrical Double-Layer Capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Z.; Fan, M.; Yang, J.; Xiao, J.; Wang, Y. Future energy infrastructure, energy platform and energy storage. Nano Energy 2022, 104, 107915. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Myung, S.-T.; Maglia, F.; Park, K.-J.; Yoon, C.S.; Lamp, P.; Kim, S.-J.; Sun, Y.-K. Nickel-Rich Layered Cathode Materials for Automotive Lithium-Ion Batteries: Achievements and Perspectives. ACS Energy Lett. 2016, 2, 196–223. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Lee, D.-J.; Lee, Y.J.; Chen, Z.; Myung, S.-T. Cobalt-Free Nickel Rich Layered Oxide Cathodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 11434–11440. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.A. The cobalt market revisited. Miner. Econ. 2019, 33, 21–28. [Google Scholar] [CrossRef]
- Alves Dias, P.; Blagoeva, D.; Pavel, C.; Arvanitidis, N. Cobalt: Demand-supply balances in the transition to electric mobility. In EUR 29381 EN, Publications Office of the European Union; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-94311-9. [Google Scholar] [CrossRef]
- Bianchini, M.; Roca-Ayats, M.; Hartmann, P.; Brezesinski, T.; Janek, J. There and Back Again—The Journey of LiNiO2 as a Cathode Active Material. Angew. Chem. Int. Ed. 2018, 58, 10434–10458. [Google Scholar] [CrossRef]
- Deng, T.; Fan, X.; Cao, L.; Chen, J.; Hou, S.; Ji, X.; Chen, L.; Li, S.; Zhou, X.; Hu, E.; et al. Designing In-Situ-Formed Interphases Enables Highly Reversible Cobalt-Free LiNiO2 Cathode for Li-ion and Li-metal Batteries. Joule 2019, 3, 2550–2564. [Google Scholar] [CrossRef]
- Chien, P.; Wu, X.; Song, B.; Yang, Z.; Waters, C.K.; Everett, M.S.; Lin, F.; Du, Z.; Liu, J. New Insights into Structural Evolution of LiNiO2 Revealed by Operando Neutron Diffraction. Batter. Supercaps 2021, 4, 1701–1707. [Google Scholar] [CrossRef]
- Mu, L.; Lin, F. Identifying Challenges and Methods for Mitigation in No-Cobalt LiNiO2 Cathode Materials. ECS Meet. Abstr. 2020, 45, 3726. [Google Scholar] [CrossRef]
- Muralidharan, N.; Essehli, R.; Hermann, R.P.; Amin, R.; Jafta, C.; Zhang, J.; Liu, J.; Du, Z.; Meyer, H.M.; Self, E.; et al. Lithium Iron Aluminum Nickelate, LiNixFeyAlzO2 —New Sustainable Cathodes for Next-Generation Cobalt-Free Li-Ion Batteries. Adv. Mater. 2020, 32, 2002960. [Google Scholar] [CrossRef]
- Muralidharan, N.; Essehli, R.; Hermann, R.P.; Parejiya, A.; Amin, R.; Bai, Y.; Du, Z.; Belharouak, I. LiNixFeyAlzO2, a new cobalt-free layered cathode material for advanced Li-ion batteries. J. Power Sources 2020, 471, 228389. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–766. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Nagayama, M. Electrochemistry and Structural Chemistry of LiNiO2 (R3m) for 4 Volt Secondary Lithium Cells. J. Electrochem. Soc. 1993, 140, 1862–1870. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.; Mauger, A.; Qilu, A.; Gendron, F.; Julien, C. Minimization of the cation mixing in Li1+x(NMC)1−xO2 as cathode material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
- Huang, Z.; Gao, J.; He, X.; Li, J.; Jiang, C. Well-ordered spherical LiNixCo(1−2x)MnxO2 cathode materials synthesized from cobolt concentration-gradient precursors. J. Power Sources 2011, 202, 284–290. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, T.; Hu, Z.; Wei, Y.; Song, X.; Ren, Y.; Wang, W.; Rao, M.; Lin, Y.; Chen, Z.; et al. Tuning of Thermal Stability in Layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2016, 138, 13326–13334. [Google Scholar] [CrossRef] [PubMed]
- Ivanishchev, A.V.; Ushakov, A.V.; Ivanishcheva, I.A.; Churikov, A.V.; Mironov, A.V.; Fedotov, S.S.; Khasanova, N.R.; Antipov, E.V. Structural and electrochemical study of fast Li diffusion in Li3V2(PO4)3-based electrode material. Electrochim. Acta 2017, 230, 479–491. [Google Scholar] [CrossRef]
- Zhu, S. Renovation of Lithium Cobalt Oxide from Spent Lithium Ion Batteries by an Aqueous Pulsed Discharge Plasma. Int. J. Electrochem. Sci. 2016, 11, 6403–6411. [Google Scholar] [CrossRef]
- Murali, N.; Margarette, S.; Rao, V.K.; Veeraiah, V. Structural, impedance, dielectric and modulus analysis of LiNi1-xy-0.02 Mg0.02Cox ZnyO2 cathode materials for lithium-ion batteries. J. Sci. Adv. Mater. Devices 2017, 2, 233–244. [Google Scholar] [CrossRef]
- Kalyani, P.; Kalaiselvi, N. Various aspects of LiNiO2 chemistry: A review. Sci. Technol. Adv. Mater. 2005, 6, 689–703. [Google Scholar] [CrossRef]
- Weber, D.; Tripković, D.; Kretschmer, K.; Bianchini, M.; Brezesinski, T. Surface Modification Strategies for Improving the Cycling Performance of Ni-Rich Cathode Materials. Eur. J. Inorg. Chem. 2020, 2020, 3117–3130. [Google Scholar] [CrossRef]
- Jiang, X.; Sha, Y.; Cai, R.; Shao, Z. The solid-state chelation synthesis of LiNi1/3Co1/3Mn1/3O2 as a cathode material for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 10536–10544. [Google Scholar] [CrossRef]
- Hua, W.; Schwarz, B.; Azmi, R.; Müller, M.; Darma, M.S.D.; Knapp, M.; Senyshyn, A.; Heere, M.; Missyul, A.; Simonelli, L.; et al. Lithium-ion (de)intercalation mechanism in core-shell layered Li(Ni,Co,Mn)O2 cathode materials. Nano Energy 2020, 78, 105231. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhang, Y.; Dong, P.; Xia, S.; Yao, Y. A facile method for synthesis of LiNi0.8Co0.15Al0.05O2 cathode material. Solid State Ion. 2017, 307, 73–78. [Google Scholar] [CrossRef]
- Manthiram, A.; Knight, J.C.; Myung, S.-T.; Oh, S.-M.; Sun, Y.-K. Nickel-Rich and Lithium-Rich Layered Oxide Cathodes: Progress and Perspectives. Adv. Energy Mater. 2015, 6, 1501010. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, J.; Cui, S.; Song, X.; Su, Y.; Deng, W.; Wu, Z.; Wang, X.; Wang, W.; Rao, M.; et al. Kinetics Tuning of Li-Ion Diffusion in Layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2015, 137, 8364–8367. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Margolese, D.I.; Stucky, G.D. Surfactant Control of Phases in the Synthesis of Mesoporous Silica-Based Materials. Chem. Mater. 1996, 8, 1147–1160. [Google Scholar] [CrossRef]
- Bazito, F.F.C.; Torresi, R.M. Cathodes for lithium ion batteries: The benefits of using nanostructured materials. J. Braz. Chem. Soc. 2006, 17, 627–642. [Google Scholar] [CrossRef]
- Liu, T.; Yu, L.; Lu, J.; Zhou, T.; Huang, X.; Cai, Z.; Dai, A.; Gim, J.; Ren, Y.; Xiao, X.; et al. Rational design of mechanically robust Ni-rich cathode materials via concentration gradient strategy. Nat. Commun. 2021, 12, 6024. [Google Scholar] [CrossRef]
- Lin, H.; Liang, C.; Li, M.; Dai, C.; Xiong, Y. Effects of Aluminum Doping on Cobalt-Free Lithium-Iron-Nickel-Manganese-Oxygen Cathode Materials for Lithium-Ion Batteries. Energy Technol. 2017, 5, 1472–1483. [Google Scholar] [CrossRef]
- Darbar, D.; Self, E.C.; Li, L.; Wang, C.; Meyer, H.M.; Lee, C.; Croy, J.R.; Balasubramanian, M.; Muralidharan, N.; Bhattacharya, I.; et al. New synthesis strategies to improve Co-Free LiNi0.5Mn0.5O2 cathodes: Early transition metal d0 dopants and manganese pyrophosphate coating. J. Power Sources 2020, 479, 228591. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X. A novel method for preparing α-LiFeO2 nanorods for high-performance lithium-ion batteries. Ionics 2019, 26, 1057–1061. [Google Scholar] [CrossRef]
- Xu, H.; Sun, J.; Gao, L. Hydrothermal synthesis of LiMnO2 microcubes for lithium ion battery application. Ionics 2012, 19, 63–69. [Google Scholar] [CrossRef]
- Välikangas, J.; Laine, P.; Hietaniemi, M.; Hu, T.; Tynjälä, P.; Lassi, U. Precipitation and Calcination of High-Capacity LiNiO2 Cathode Material for Lithium-Ion Batteries. Appl. Sci. 2020, 10, 8988. [Google Scholar] [CrossRef]
- Shaari, H.R.; Sethuprakhash, V.; Basirun, W.J. Vanadium based lithium nickel aluminiumoxide system with good performance in lithium-ion batteries. J. Teknol. 2016, 78, 5–10. [Google Scholar] [CrossRef][Green Version]
- Ram, P.; Gören, A.; Gonçalves, R.; Choudhary, G.; Ferdov, S.; Silva, M.M.; Singhal, R.; Costa, C.M.; Sharma, R.K.; Lanceros-Méndez, S. Improved electrochemical performance of rare earth doped LiMn1.5-xNi0.5RExO4 based composite cathodes for lithium-ion batteries. Compos. Part B: Eng. 2017, 139, 55–63. [Google Scholar] [CrossRef]
- Uzun, D.; Doğrusöz, M.; Mazman, M.; Biçer, E.; Avci, E.; Şener, T.; Kaypmaz, T.C.; Demir-Cakan, R. Effect of MnO2 coating on layered Li(Li0.1Ni0.3Mn0.5Fe0.1)O2 cathode material for Li-ion batteries. Solid State Ion. 2013, 249-250, 171–176. [Google Scholar] [CrossRef]


| Cobalt-Free Based Electrode | Synthesis Method | Current Rate | Reversible Capacity (mAh g−1) | References |
|---|---|---|---|---|
| LiNi0.8Mn0.15Al0.05O2 (NMA) | coprecipitation | 0.1 C | 210 | [35] |
| LiNi0.79Mn0.2Mg0.05O2 (NMM) | coprecipitation | 0.1 C | 210 | [35] |
| LiNi0.79Mn0.2Ti0.01O2 (NMT) | coprecipitation | 0.1 C | 210 | [35] |
| Li1.1[Fe0.2Ni0.2Mn0.6]0.9O2 | sol gel | 20 mA g−1 | 175 (1st cycle) | [36] |
| LiNi0.5Mn0.5O2 | sol gel | 20 mA g−1 | 156 | [37] |
| α-LiFeO2 nanorods | hydrothermal-assisted solid-state | 0.1 C | 165.85 (1st cycle) | [38] |
| LiMnO2 microcubes | hydrothermal | 1 C | 134 | [39] |
| LiNiO2 nanoparticles | coprecipitation | 0.1 C | ~135 (after 400 cycles) | [40] |
| LiNixV1−x−y AlyO2 | carbon combustion method | - | ~80.55 (for the first 10 cycles) | [41] |
| Li Ni0.5−x Al2x Mn1.5−x O4 (0 ≤ 2x ≤ 1.0) | thermo-polymerization method | 10 C | 119 | [42] |
| Li(Li0.1Ni0.3Mn0.5Fe0.1)O2 | solid state | 0.1 C | 205 | [43] |
| LiNixFeyAlzO2 (x + y + z = 1) | sol gel | 0.1 C | 160 | [16] |
| LiNixFeyAlzO2 (x + y + z = 1) | coprecipitation | 0.2 C | 190 | [17] |
| LiNi0.8Fe0.1Al0.1O2 | solid-state | 0.1 C 0.2 C | 180 160 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmaataouy, E.; Chari, A.; El Bendali, A.; Tayoury, M.; Amine, R.; Aqil, M.; Xu, G.; Liu, T.; Alami, J.; Dahbi, M. LiNi0.8Fe0.1Al0.1O2 as a Cobalt-Free Cathode Material with High Capacity and High Capability for Lithium-Ion Batteries. Batteries 2023, 9, 23. https://doi.org/10.3390/batteries9010023
Elmaataouy E, Chari A, El Bendali A, Tayoury M, Amine R, Aqil M, Xu G, Liu T, Alami J, Dahbi M. LiNi0.8Fe0.1Al0.1O2 as a Cobalt-Free Cathode Material with High Capacity and High Capability for Lithium-Ion Batteries. Batteries. 2023; 9(1):23. https://doi.org/10.3390/batteries9010023
Chicago/Turabian StyleElmaataouy, Elhoucine, Abdelwahed Chari, Ayoub El Bendali, Marwa Tayoury, Rachid Amine, Mohamed Aqil, GuiLiang Xu, Tongchao Liu, Jones Alami, and Mouad Dahbi. 2023. "LiNi0.8Fe0.1Al0.1O2 as a Cobalt-Free Cathode Material with High Capacity and High Capability for Lithium-Ion Batteries" Batteries 9, no. 1: 23. https://doi.org/10.3390/batteries9010023
APA StyleElmaataouy, E., Chari, A., El Bendali, A., Tayoury, M., Amine, R., Aqil, M., Xu, G., Liu, T., Alami, J., & Dahbi, M. (2023). LiNi0.8Fe0.1Al0.1O2 as a Cobalt-Free Cathode Material with High Capacity and High Capability for Lithium-Ion Batteries. Batteries, 9(1), 23. https://doi.org/10.3390/batteries9010023










