How the Sodium Cations in Anode Affect the Performance of a Lithium-ion Battery
Abstract
:1. Introduction
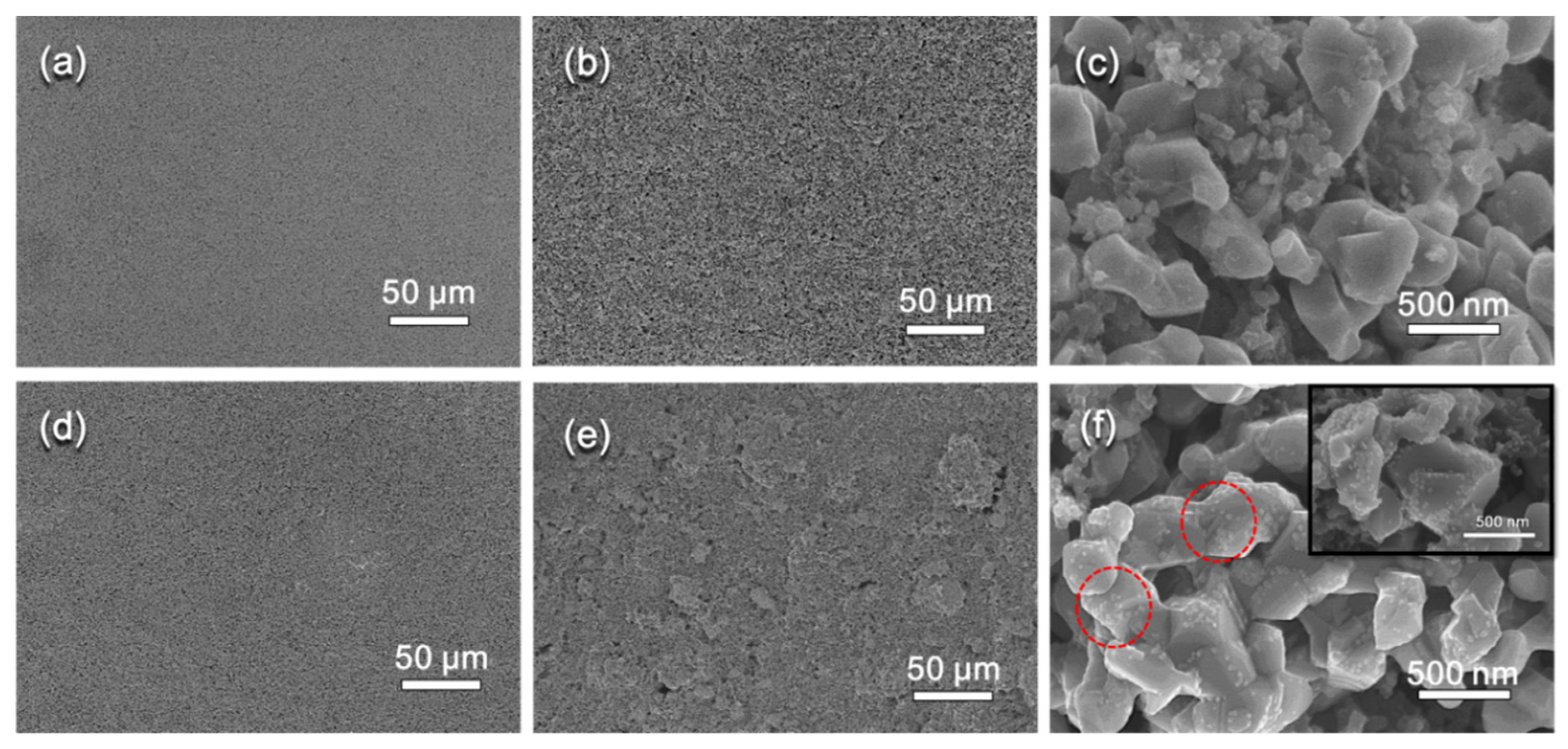
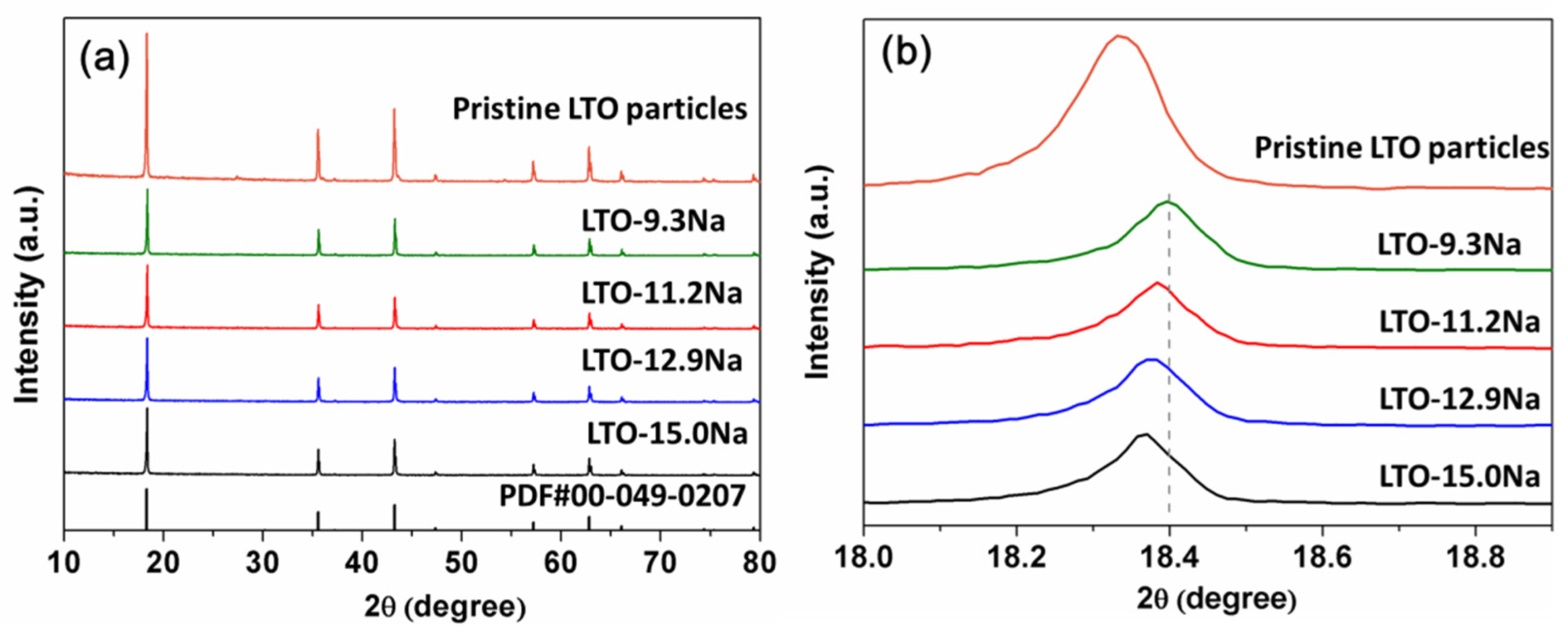
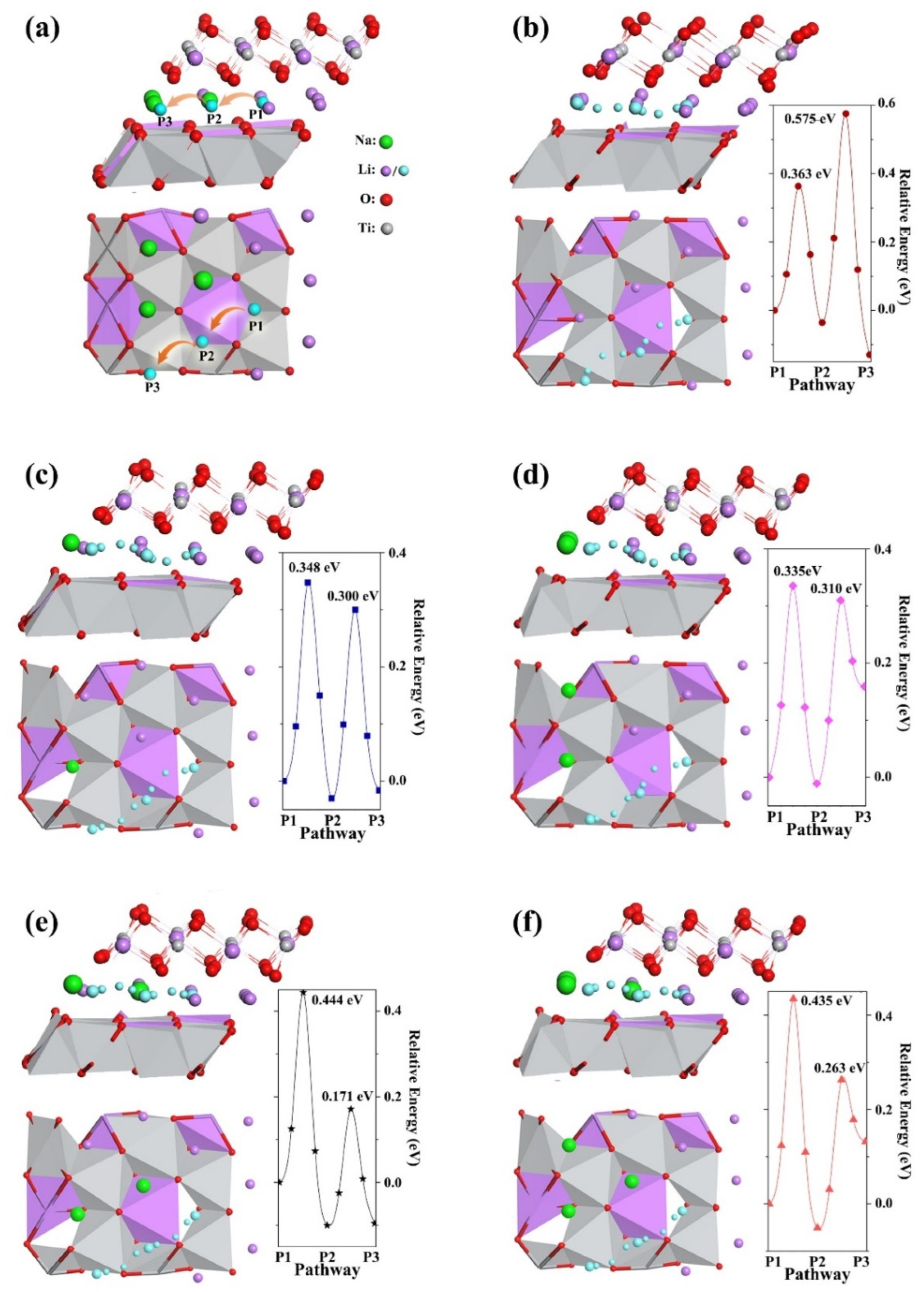
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of CMC-Na Binders
3.3. Preparation of LTO Electrodes
3.4. Characterization
3.5. Computational and Model Details
3.6. Electrochemical Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Ding, Y.L.; Cano, Z.P.; Yu, A.P.; Lu, J.; Chen, Z.W. Automotive Li-ion batteries: Current status and future perspectives. Electro. Ener. Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; VonJouanne, A.; Yokochi, A. Current Li-ion battery technologies in electric vehicles and opportunities for advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef] [Green Version]
- Fan, E.S.; Li, L.; Wang, Z.P.; Lin, J.; Huang, Y.X.; Yao, Y.; Chen, R.J.; Wu, F. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Tian, Y.S.; Zeng, G.B.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.Y.; Koettgen, J.; Sun, Y.Z.; Ouyang, B.; Chen, T.N.; et al. Promises and challenges of next-generation "Beyond Li-ion" batteries for electric vehicles and grid decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef] [PubMed]
- Logan, E.R.; Dahn, J.R. Electrolyte design for fast-charging Li-ion batteries. Trends Chem. 2020, 2, 354–366. [Google Scholar] [CrossRef]
- Wang, X.X.; Ding, Y.L.; Deng, Y.P.; Chen, Z.W. Ni-rich/Co-poor layered cathode for automotive Li-ion batteries: Promises and challenges. Adv. Energy Mater. 2020, 10, 1903864. [Google Scholar] [CrossRef]
- Yi, T.F.; Wei, T.T.; Li, Y.; He, Y.B.; Wang, Z.B. Efforts on enhancing the Li-ion diffusion coefficient and electronic conductivity of titanate-based anode materials for advanced Li-ion batteries. Energy Stor. Mater. 2020, 26, 165–197. [Google Scholar] [CrossRef]
- Li, P.; Hwang, J.Y.; Sun, Y.K. Nano/microstructured silicon-graphite composite anode for high-energy-density Li-ion battery. ACS Nano. 2019, 13, 2624–2633. [Google Scholar] [CrossRef]
- Saal, A.; Hagemann, T.; Schubert, U.S. Polymers for battery applications-active materials, membranes, and binders. Adv. Energy Mater. 2021, 11, 2001984. [Google Scholar] [CrossRef]
- Zha, G.J.; Hu, W.; Agarwal, S.; Ouyang, C.Y.; Hu, N.G.; Hou, H.Q. High performance layered LiNi0.8Co0.07Fe0.03Mn0.1O2 cathode materials for Li-ion battery. Chem. Eng. J. 2021, 409, 128343. [Google Scholar] [CrossRef]
- Banerjee, A.; Ziv, B.; Luski, S.; Aurbach, D.; Halalay, I.C. Increasing the durability of Li-ion batteries by means of manganese ion trapping materials with nitrogen functionalities. J. Power Sources 2017, 341, 457–465. [Google Scholar] [CrossRef]
- Evertz, M.; Horsthemke, F.; Kasnatscheew, J.; Börner, M.; Winter, M.; Nowak, S. Unraveling transition metal dissolution of Li1.04Ni1/3Co1/3Mn1/3O2 (NCM 111) in lithium ion full cells by using the total reflection X-ray fluorescence technique. J. Power Sources 2016, 329, 364–371. [Google Scholar] [CrossRef]
- Husmann, S.; Zarbin, A.J.G. Cation effect on the structure and properties of hexacyanometallates-based nanocomposites: Improving cathode performance in aqueous metal-ions batteries. Electrochim. Acta. 2018, 283, 1339–1350. [Google Scholar] [CrossRef]
- Yoon, Y.; Shin, D.; Kim, D.; Lee, K.; Kim, S. Effects of sodium content of titanate nanotubes on lithium battery performance. J. Nanosci. Nanotechnol. 2010, 10, 6206–6210. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhan, C.; He, K.; Chen, H.; Yao, W.; Sharifi-Asl, S.; Song, B.; Yang, Z.; Nie, A.; Luo, X.Y.; et al. The influence of large cations on the electrochemical properties of tunnel-structured metal oxides. Nat. Commun. 2016, 7, 13374. [Google Scholar] [CrossRef]
- Xu, C.Y.; Li, J.L.; Sun, J.; Zhang, W.Z.; Ji, B.M. Li-rich layered oxide single crystal with Na doping as a high-performance cathode for Li ion batteries. J. Alloys Compd. 2021, 895, 162613. [Google Scholar] [CrossRef]
- Guo, L.F.; Xie, Y.L. Na-doped LiNi1/3Co1/3Mn1/3O2 with enhanced rate performance as a cathode for Li-ion batteries. Ionics 2022, 28, 2117–2123. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Hu, W.; Zhou, Q.H.; Li, H.L. Conflicting roles of Na-doped layered cathode material LiCoO2 for Li-ion batteries. J. Solid State Electr. 2021, 25, 2565–2569. [Google Scholar] [CrossRef]
- Shahjalal, M.; Roy, P.K.; Shams, T.; Fly, A.; Chowdhury, J.I.; Ahmed, M.R.; Liu, K. A review on second-life of Li-ion batteries: Prospects, challenges, and issues. Energy 2022, 241, 122881. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.Y.; Yu, G.H. Material and structural design of novel binder systems for high-energy, high-power lithium-ion batteries. Acc. Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.M.; Yue, F.S.; Li, S.C.; Zhang, Y.; Tian, Z.R.; Xu, Q.; Xin, S.; Guo, Y.G. Advances of polymer binders for silicon-based anodes in high energy density lithium-ion batteries. InfoMat 2021, 3, 460–501. [Google Scholar] [CrossRef]
- Wennig, S.; Langklotz, U.; Prinz, G.M.; Schmidt, A.; Oberschachtsiek, B.; Lorke, A. The influence of different pre-treatments of current collectors and variation of the binders on the performance of Li4Ti5O12 anodes for lithium ion batteries. J. Appl. Electrochem. 2015, 45, 1043–1055. [Google Scholar] [CrossRef]
- Chou, S.L.; Gao, X.W.; Wang, J.Z.; Wexler, D.; Wang, Z.X.; Chen, L.Q.; Liu, H.K. Tin/polypyrrole composite anode using sodium carboxymethyl cellulose binder for lithium-ion batteries. Dalton Trans. 2011, 40, 12801–12807. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Maken, S.; Lee, S.; Chung, E.; Park, J.; Min, B. Characteristics of water-soluble fiber manufactured from carboxymethyl cellulose synthesis. Korean J. Chem. Eng. 2007, 24, 288–293. [Google Scholar] [CrossRef]
- Racz, I.; Borsa, J. Swelling of carboxymethylated cellulose fibres. Cellulose 1997, 4, 293–303. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Yin, Y.; Luo, X.; Xu, B. In-situ self-assembly synthesis of low-cost, long-life, shape-controllable spherical Li4Ti5O12 anode material for Li-ion batteries. J. Alloy. Compds. 2022, 904, 164026. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. EIS Study on the formation of solid electrolyte interface in Li-ion battery. Electrochim. Acta 2006, 51, 1636–1640. [Google Scholar] [CrossRef]
- Uhlemann, M.; Madian, M.; Leones, R.; Oswald, S.; Maletti, S.; Eychmüller, A.; Mikhailova, D. In-depth study of Li4Ti5O12 performing beyond conventional operating conditions. ACS Appl. Mater. Interfaces 2020, 12, 37227–37238. [Google Scholar] [CrossRef]
- Yuan, T.; Cai, R.; Ran, R.; Zhou, Y.K.; Shao, Z.P. A mechanism study of synthesis of Li4Ti5O12 from TiO2 anatase. J. Alloy. Compd. 2012, 505, 367. [Google Scholar] [CrossRef]
- Liu, W.; Shao, D.; Luo, G.; Gao, Q.; Yan, G.; He, J.; Chen, D.; Yu, X.; Fang, Y. Mesoporous spinel Li4Ti5O12 nanoparticles for high rate lithium-ion battery anodes. Electrochim. Acta 2014, 133, 578–582. [Google Scholar] [CrossRef]
- Zhang, W.; Seo, D.H.; Chen, T.; Wu, L.J.; Topsakal, M.; Zhu, Y.M.; Lu, D.Y.; Ceder, G.; Wang, F. Kinetic pathways of ionic transport in fast-charging lithium titanate. Science 2020, 367, 1030. [Google Scholar] [CrossRef]
- Mai, Y.J.; Shi, S.J.; Zhang, D.; Lu, Y.; Gu, C.D.; Tu, J.P. NiO–graphene hybrid as an anode material for lithium ion batteries. J. Power Sources 2012, 204, 155–161. [Google Scholar] [CrossRef]
- Dreyer, W.; Jamnik, J.; Guhlke, C.; Huth, R.; Moskon, J.; Gaberscek, M. The thermodynamic origin of hysteresis in insertion batteries. Nat. Mater. 2010, 9, 448–453. [Google Scholar]
- Lee, K.C.; Chang-Jian, C.W.; Ho, B.C.; Ding, Y.R.; Huang, J.H.; Hsiao, Y.S. Conductive PProDOT-Me2–capped Li4Ti5O12 microspheres with an optimized Ti3+/Ti4+ ratio for enhanced and rapid lithium-ion storage. Ceram. Int. 2019, 45, 15252–15261. [Google Scholar] [CrossRef]
- Hu, Y.S.; Cakan, R.D.; Titirici, M.M.; Müller, J.O.; Schlögl, R.; Antonietti, M.; Maier, J. Superior storage performance of a Si@SiOx/C nanocomposite as anode material for lithium-ion batteries. Angew. Chem. Int. Ed. 2008, 47, 1645–1649. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Hao, F.; Fang, D. The effects of elastic stiffening on the evolution of the stress field within a spherical electrode particle of lithium-ion batteries. Int. J. Appl. Mech. 2013, 5, 1350040. [Google Scholar] [CrossRef]
- Christensen, J.; John Newman, J. A mathematical model of stress generation and fracture in lithium manganese oxide. J. Electrochem. Soc. 2006, 153, A1019–A1030. [Google Scholar] [CrossRef]
- Zhou, W. Effects of external mechanical loading on stress generation during lithiation in Li-ion battery electrodes. Electrochim. Acta. 2015, 185, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Henkelman, G.; Uberuaga, B.P.; J´onsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Kick, M.; Scheurer, C.; Oberhofer, H. Formation and stability of small polarons at the lithium-terminated Li4Ti5O12 (LTO) (111) surface. J. Chem. Phys. 2020, 153, 144701. [Google Scholar] [CrossRef] [PubMed]
- Tada, K.; Kitta, M.; Ozaki, H.; Tanaka, S. A comparative study of Na3LiTi5O12 and Li4Ti5O12: Geometric andelectronic structures obtained by density functional theory calculations. Chem. Phys. Lett. 2019, 731, 136598. [Google Scholar] [CrossRef]
- Shenouda, A.Y.; Murali, K.R. Electrochemical properties of doped lithium titanate compounds and their performance in lithium rechargeable batteries. J. Power Sources 2008, 176, 332–339. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Meng, Q.; Lei, S.; Song, F.; Ma, J. Synergy of a hierarchical porous morphology and anionic defects of nanosized Li4Ti5O12 toward a high-rate and large-capacity lithium-ion battery. J. Energy. Chem. 2021, 54, 699–711. [Google Scholar] [CrossRef]
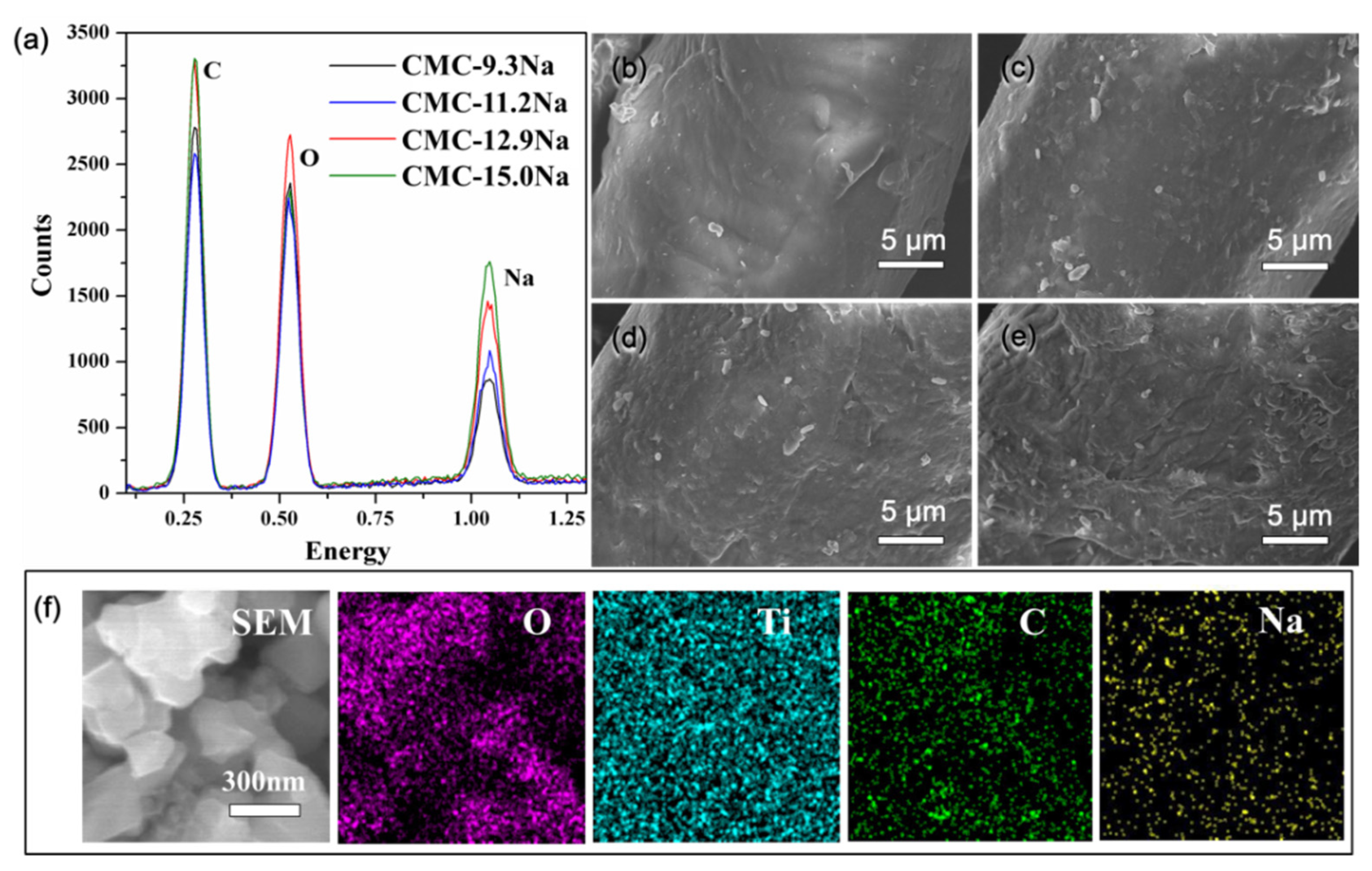
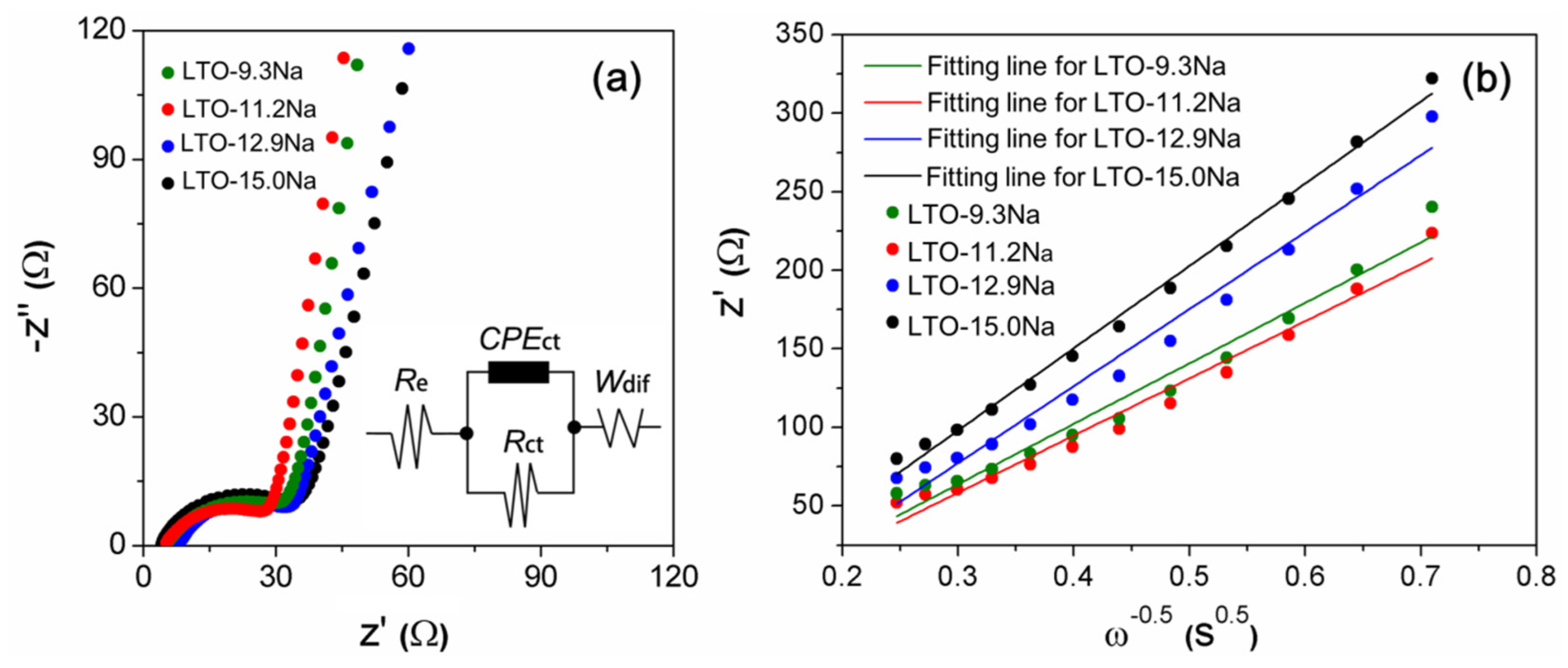
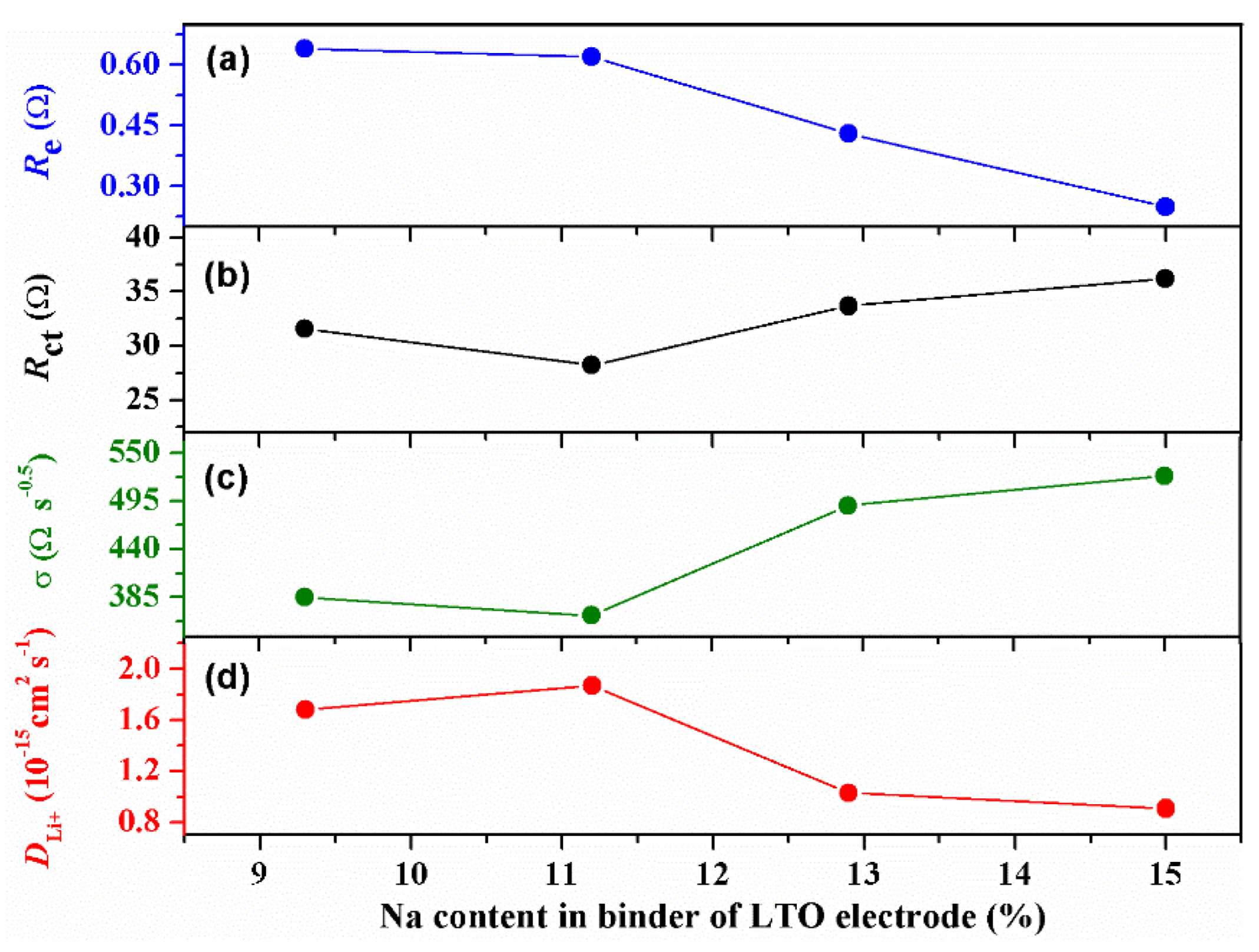

| Electrodes | Sodium Content in Binder (%) | Re (Ω) | Rct (Ω) | σ (Ωs−1/2) | DLi+ (cm2 s−1) |
|---|---|---|---|---|---|
| LTO-9.3Na | 9.3 | 0.64 | 31.57 | 384.8 | 1.68 × 10−15 |
| LTO-11.2Na | 11.2 | 0.62 | 28.22 | 364.0 | 1.87 × 10−15 |
| LTO-12.9Na | 12.9 | 0.43 | 33.70 | 490.1 | 1.03 × 10−15 |
| LTO-15.0Na | 15.0 | 0.25 | 36.21 | 523.9 | 9.06 × 10−16 |
| Electrodes | Initial Discharge Capacity (mAh/g) | Initial Charge Capacity (mAh/g) | CE (%) |
|---|---|---|---|
| LTO-9.3Na | 170.3 | 148.9 | 87.4 |
| LTO-11.2Na | 169.6 | 153.8 | 90.6 |
| LTO-12.9Na | 167.7 | 140.2 | 83.6 |
| LTO-15.0Na | 167.8 | 135.6 | 80.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, D.; Rao, D.; Wu, A.; Luo, X. How the Sodium Cations in Anode Affect the Performance of a Lithium-ion Battery. Batteries 2022, 8, 78. https://doi.org/10.3390/batteries8080078
Shao D, Rao D, Wu A, Luo X. How the Sodium Cations in Anode Affect the Performance of a Lithium-ion Battery. Batteries. 2022; 8(8):78. https://doi.org/10.3390/batteries8080078
Chicago/Turabian StyleShao, Dan, Dewei Rao, Aihua Wu, and Xiangyi Luo. 2022. "How the Sodium Cations in Anode Affect the Performance of a Lithium-ion Battery" Batteries 8, no. 8: 78. https://doi.org/10.3390/batteries8080078
APA StyleShao, D., Rao, D., Wu, A., & Luo, X. (2022). How the Sodium Cations in Anode Affect the Performance of a Lithium-ion Battery. Batteries, 8(8), 78. https://doi.org/10.3390/batteries8080078






