Electrochemical Impedance Spectroscopy as an Analytical Tool for the Prediction of the Dynamic Charge Acceptance of Lead-Acid Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Laboratory Test Cells
2.2. Charge Acceptance Tests
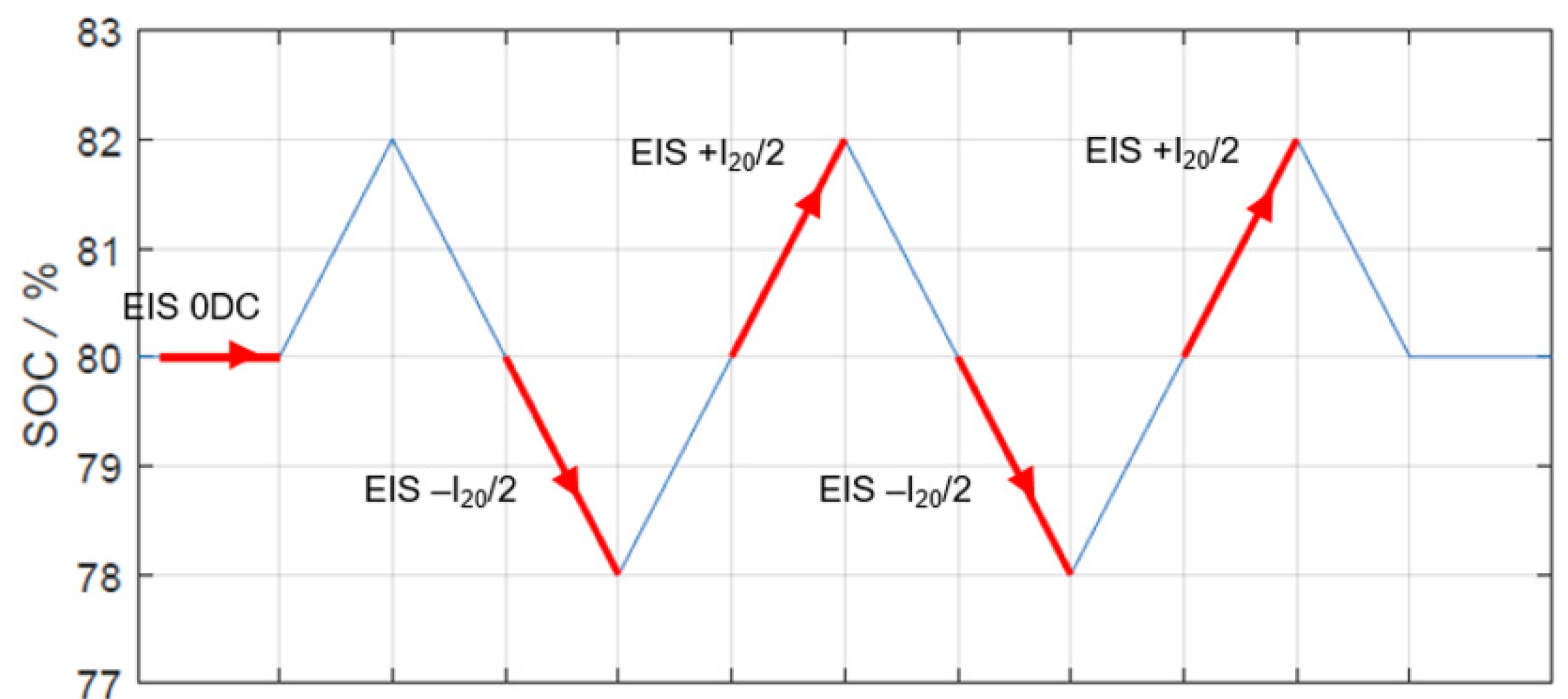
2.3. Electrochemical Impedance Spectroscopy
3. Results
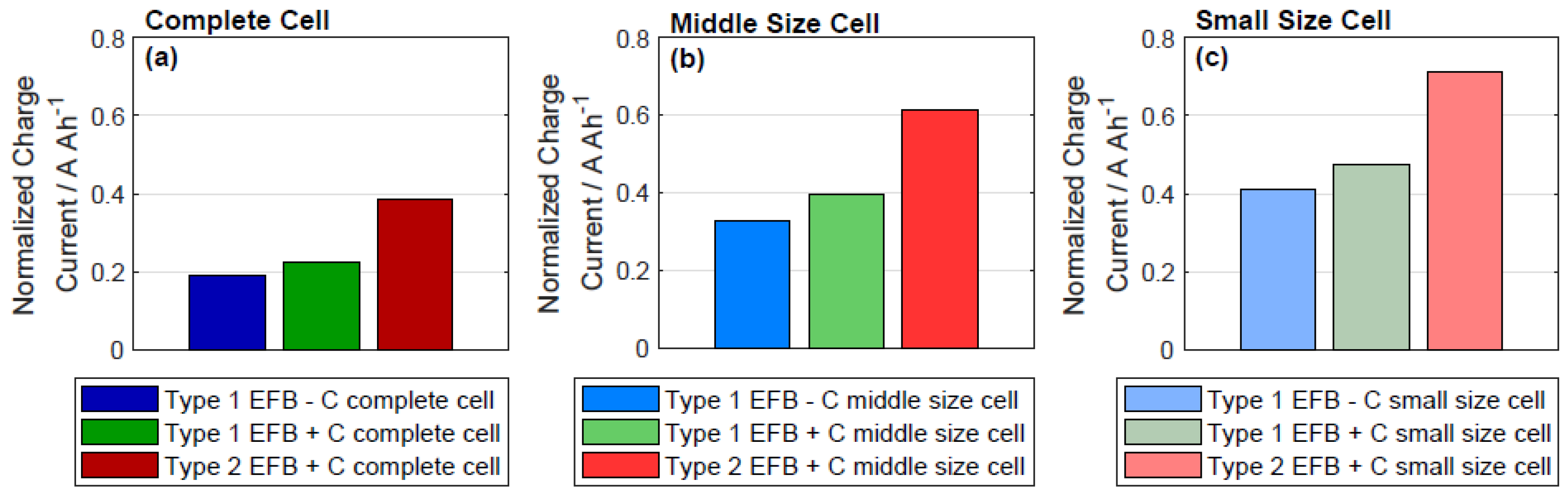
3.1. Dynamic Charge Acceptance
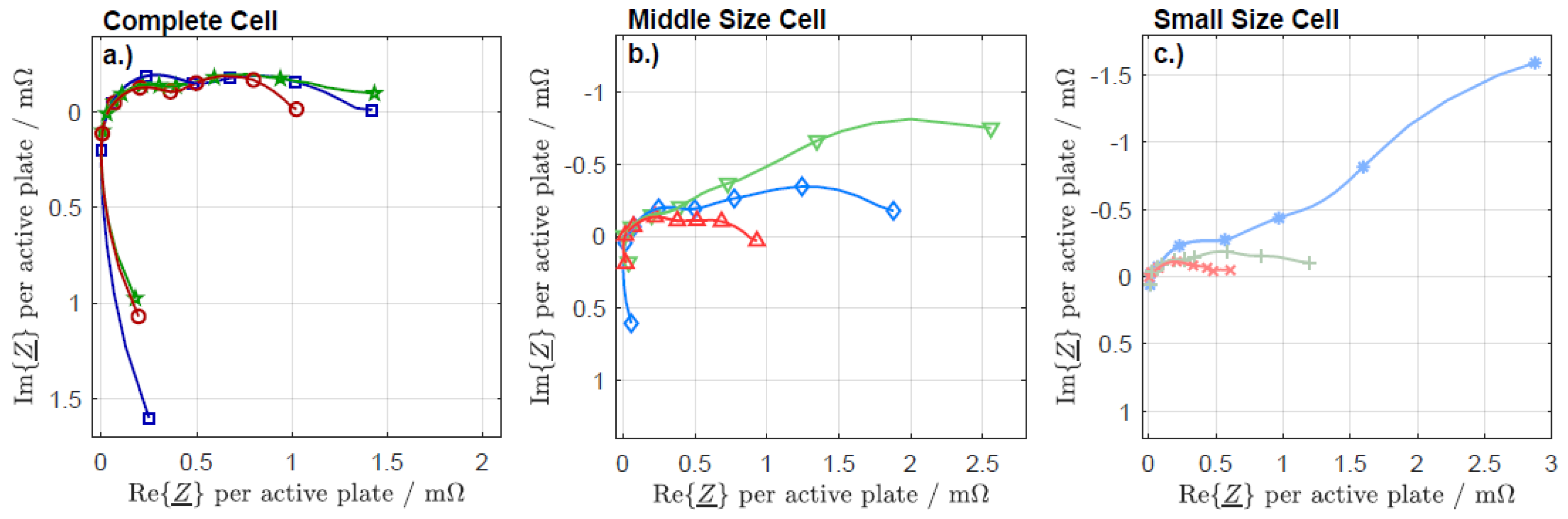
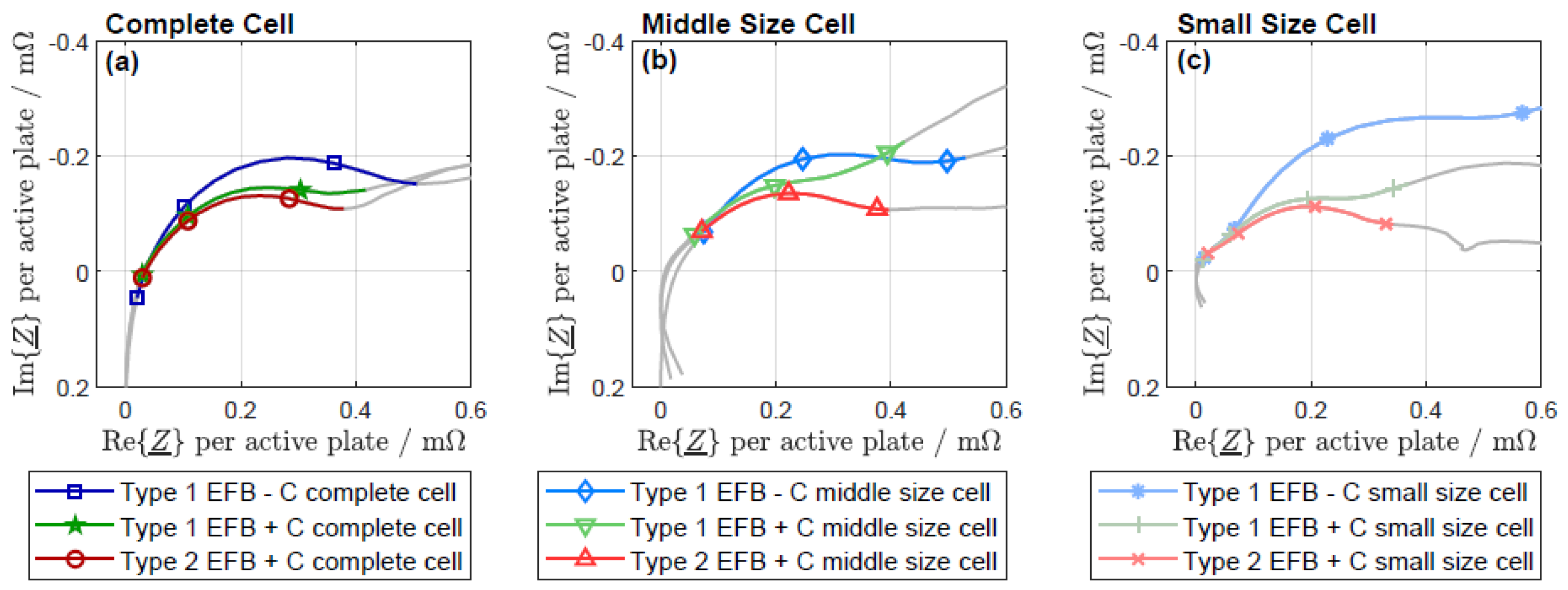
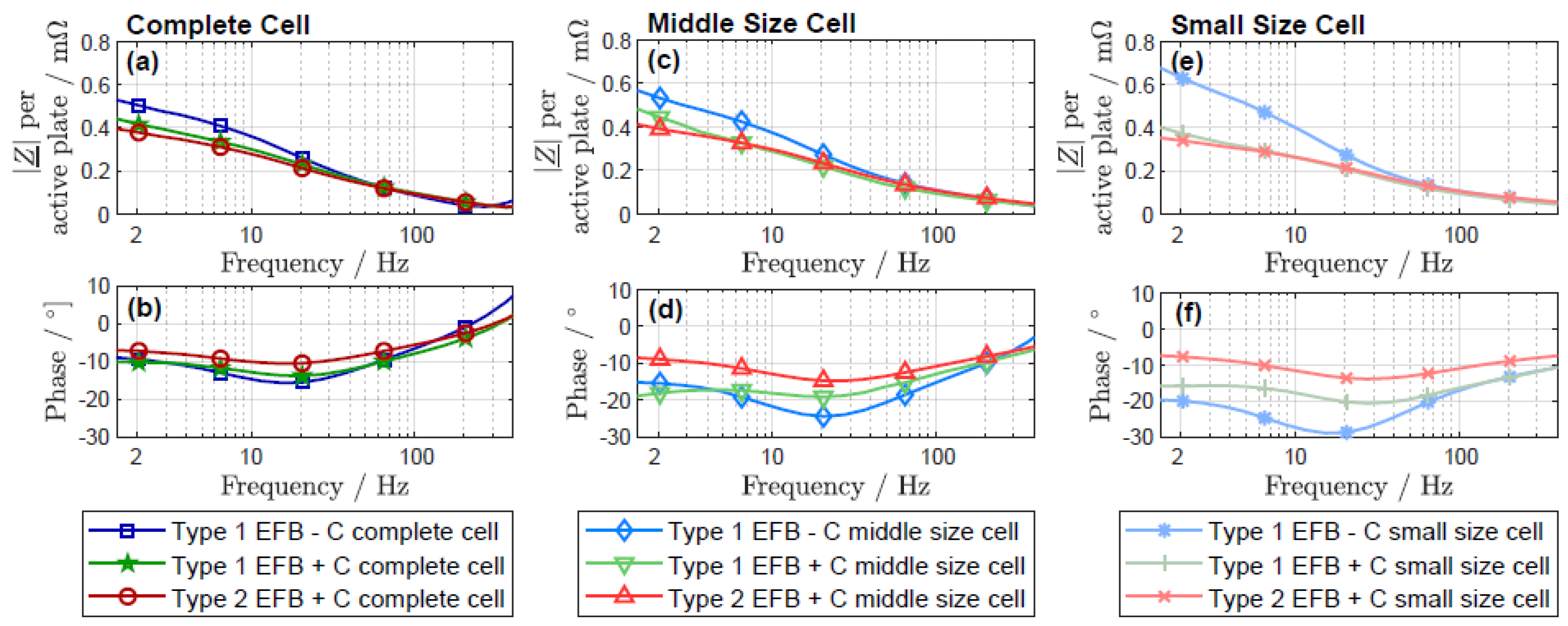
3.2. Electrochemical Impedance Spectroscopy
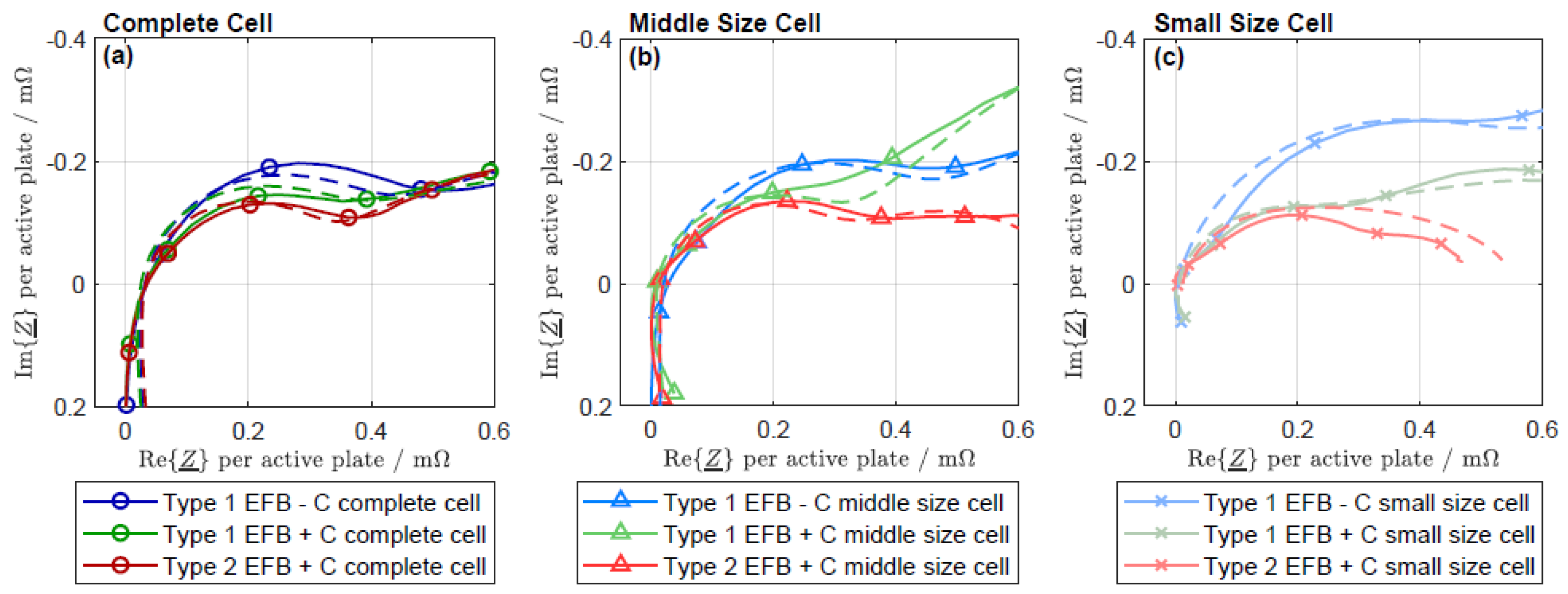
3.3. Kramers-Kronig
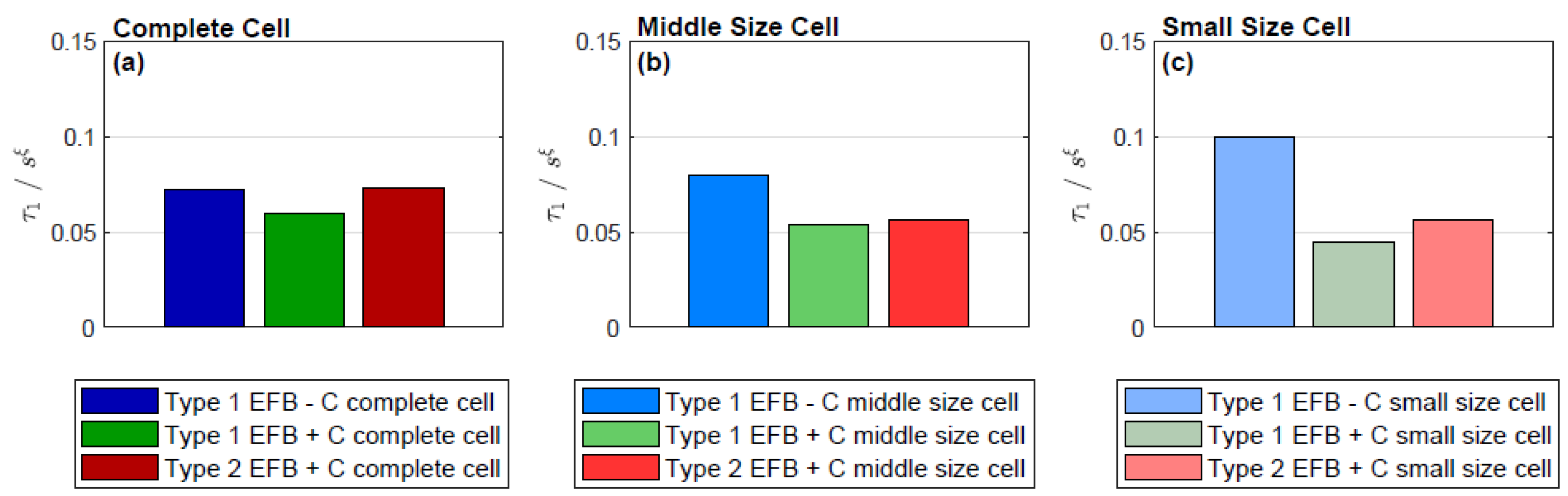
3.4. Distribution of Relaxation Times
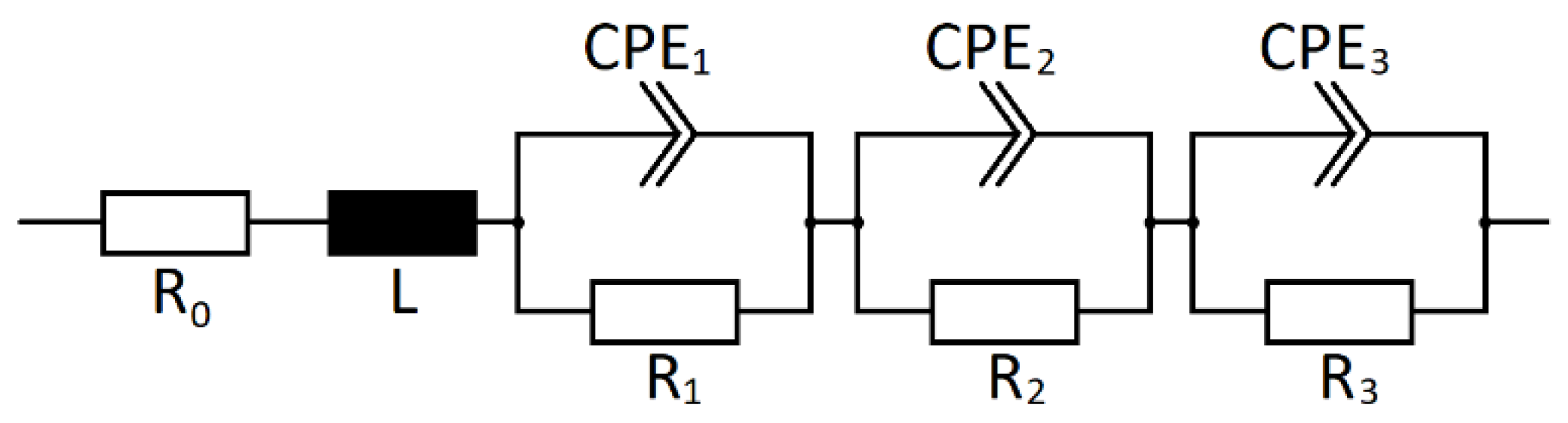
3.5. Fitting with an Equivalent Circuit Model
3.6. Parameterization of the Equivalent Circuit Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy-Theory, Experiment and Application, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2005; ISBN 978-0-471-64749-2. [Google Scholar]
- Willihnganz, E. The Voltage of the Sponge Lead Plate During Discharge of the Storage Battery. Trans. Electrochem. Soc. 1941, 79, 243–251. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Wang, F.-C.; Chang, H.-P.; Ma, Y.-W.; Weng, B.-J. Low power proton exchange membrane fuel cell system identification and adaptive control. J. Power Source 2007, 164, 761–771. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, H.; Zhu, J. Modelling and control of hybrid UPS system with backup PEM fuel cell/battery. Int. J. Electr. Power Energy Syst. 2012, 43, 1322–1331. [Google Scholar] [CrossRef]
- Křivík, P.; Vaculík, S.; Bača, P.; Kazelle, J. Determination of state of charge of lead-acid battery by EIS. J. Energy Storage 2019, 21, 581–585. [Google Scholar] [CrossRef]
- Křivík, P.; Bača, P.; Kazelle, J. Effect of ageing on the impedance of the lead-acid battery. J. Energy Storage 2021, 36, 102382. [Google Scholar] [CrossRef]
- Meissner, E.; Richter, G. The challenge to the automotive battery industry: The battery has to become an increasingly integrated component within the vehicle electric power system. J. Power Source 2005, 144, 438–460. [Google Scholar] [CrossRef]
- Budde-Meiwes, H.; Schulte, D.; Kowal, J.; Sauer, D.U.; Hecke, R.; Karden, E. DCA of lead acid batteries comparison of methods for conditioning and testing. J. Power Source 2012, 207, 30–36. [Google Scholar] [CrossRef]
- Pavlov, D.; Nikolov, P.; Rogachev, T. Influence of expander components on the processes at the negative plates of lead-acid cells on high-rate partial-state-of-charge cycling. Part II. Effects of carbon additives on the processes of charge and discharge of negative plates. J. Power Source 2010, 195, 4444–4457. [Google Scholar] [CrossRef]
- Pavlov, D.; Nikolov, P.; Rogachev, T. Influence of carbons on the structure of the negative active material of lead acid batteries and on battery performance. J. Power Source 2011, 196, 5155–5167. [Google Scholar] [CrossRef]
- Bozkaya, B.; Bauknecht, S.; Settelein, J.; Kowal, J.; Karden, E.; Giffin, G.A. Comparison of Dynamic Charge Acceptance Tests on Lead–Acid Cells for Carbon Additive Screening. Energy Technol. 2022, 10, 2101051. [Google Scholar] [CrossRef]
- Karden, E.; Jöris, F.; Budde-Meiwes, H.; Sauer, D.U. Test Methods for Dynamic Charge Acceptance (DCA) of Microhybrid Starter Batteries. In Proceedings of the 13ELBC European Lead Battery Conference, Paris, France, 25–28 September 2012. [Google Scholar]
- Blanke, H.; Bohlen, O.; Buller, S.; De Doncker, R.W.; Fricke, B.; Hammouche, A.; Linzen, D.; Thele, M.; Sauer, D.U. Impedance measurements on lead-acid batteries for state-of-charge, state-of-health and cranking capability prognosis in electric and hybrid electric vehicles. J. Power Source 2005, 144, 418–425. [Google Scholar] [CrossRef]
- Majchrzycki, W.; Jankowska, E.; Baraniak, M.; Handzlik, P.; Samborski, R. Electrochemical Impedance Spectroscopy and Determination of the Internal Resistance as a Way to Estimate Lead-Acid Batteries Condition. Batteries 2018, 4, 70. [Google Scholar] [CrossRef] [Green Version]
- Bauknecht, S.; Kowal, J.; Bozkaya, B.; Settelein, J.; Karden, E. The effects of cell configuration and scaling factors on constant current discharge and dynamic charge acceptance in lead-acid batteries. J. Energy Storage 2022, 45, 103667. [Google Scholar] [CrossRef]
- Bauknecht, S.; Kowal, J.; Bozkaya, B.; Settelein, J.; Karden, E. Jochen Settelein, Eckhard Karden, The Influence of Cell Size on Dynamic Charge Acceptance Tests in Laboratory Lead-Acid Cells. Energy Technol. 2022, 10, 2101053. [Google Scholar] [CrossRef]
- Karden, E.; Buller, S.; De Doncker, R.W. A method for measurement and interpretation of impedance spectra for industrial batteries. J. Power Source 2000, 85, 72–78. [Google Scholar] [CrossRef]
- Pavlov, D.; Petkova, G.; Rogachev, T. Influence of H2SO4 concentration on the performance of lead-acid battery negative plates. J. Power Source 2008, 175, 586–594. [Google Scholar] [CrossRef]
- Craig, D.; Vinal, G. Solubility of Lead Sulfate in Solutions of Sulfuric Acid, Determined by Dithizone with A Photronic Cell. J. Res. Natnl. Bur. Stand. Technol. 1939, 22, 55. [Google Scholar] [CrossRef]
- Schaeck, S.; Stoermer, A.; Albers, J.; Weirather-Koestner, D.; Kabza, H. Lead-acid batteries in micro-hybrid applications. Part II. Test proposal. J. Power Source 2011, 196, 1555–1560. [Google Scholar] [CrossRef]
- Doraswamy, S.; Srinivas, K.; Murthy, K.; Jagadish, M.; Rao, V.V. Effect of milled carbon as negative electrode additive for lead acid energy storage device. Mater. Proc. 2021, 38, 3131–3135. [Google Scholar] [CrossRef]
- Blecua, M.; Romero, A.; Ocon, P.; Fatas, E.; Valenciano, J.; Trinidad, F. Improvement of the lead acid battery performance by the addition of graphitized carbon nanofibers together with a mix of organic expanders in the negative active material. J. Energy Storage 2019, 23, 106–115. [Google Scholar] [CrossRef]
- Kramers, H.A. Die Dispersion und Absorption von Röntgenstrahlen. Phys. Z 1929, 30, 522–523. [Google Scholar]
- Kronig, R.D.L. On the theory of dispersion of X-rays. J. Opt. Soc. Am. 1926, 12, 547–557. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Linear Kronig-Kramers Transform Test for Immittance Data Validation. J. Electrochem. Soc. 1995, 142, 1885–1894. [Google Scholar] [CrossRef]
- Hahn, M.; Schindler, S.; Triebs, L.-C.; Danzer, M.A. Optimized Process Parameters for a Reproducible Distribution of Relaxation Times Analysis of Electrochemical Systems. Batteries 2019, 5, 43. [Google Scholar] [CrossRef] [Green Version]
- Danzer, M.A. Generalized Distribution of Relaxation Times Analysis for the Characterization of Impedance Spectra. MDTI Batter. 2019, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Tikhonov, A.N. Numerical Methods for the Solution of Ill-Posed Problems; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Randles, J.E.B. Kinetics of rapid electrode reactions. Diss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Thele, M. A Contribution to the Modelling of the Charge Acceptance of Lead-Acid Batteries-Using Frequency and Time Domain Based Concepts. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2007. [Google Scholar]
- Barsoukov, E.; Kim, J.H.; Yoon, C.O.; Lee, H. Universal battery parameterization to yield a non-linear equivalent circuit valid for battery simulation at arbitrary load. J. Power Source 1999, 83, 61–70. [Google Scholar] [CrossRef]
- Takehara, Z.-I. Dissolution and precipitation reaction of lead sulfate in positive and negative electrodes in lead acid battery. J. Power Source 2000, 85, 29–37. [Google Scholar] [CrossRef]
- Soria, M.; Trinidad, F.; Lacadena, J.; Sánchez, A.; Valenciano, J. Advanced valve-regulated lead-acid batteries for hybrid vehicle applications. J. Power Source 2007, 168, 12–21. [Google Scholar] [CrossRef]
- Lam, L.; Louey, R. Development of ultra-battery for hybrid-electric vehicle applications. J. Power Source 2006, 158, 1140–1148. [Google Scholar] [CrossRef]
- Yahchouchi, N. Mesure de l’impédance d’un Accumulateur Tubulaire au Plomb: Application à la Détermination de la Capacité Électrique. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 1981. [Google Scholar]
- Huet, F. A review of impedance measurements for determination of the state-of-charge or state-of-health of secondary batteries. J. Power Source 1998, 70, 59–69. [Google Scholar] [CrossRef]
- Kirchev, A.; Mattera, F.; Lemaire, E.; Dong, K. Studies of the pulse charge of lead-acid batteries for photovoltaic applications: Part IV. Pulse charge of the negative plate. J. Power Source 2009, 191, 82–90. [Google Scholar] [CrossRef]
- D’Alkaine, C.; Mengarda, P.; Impinnisi, P. Discharge mechanisms and electrochemical impedance spectroscopy measurements of single negative and positive lead-acid battery plates. J. Power Source 2009, 191, 28–35. [Google Scholar] [CrossRef]
- Alao, O.; Barendse, P. Characterization of Lithium NCM and Sealed Lead Acid Batteries using Electrochemical Impedance Spectroscopy. In Proceedings of the IEEE PES/IAS PowerAfrica, Cape Town, South Africa, 28–29 June 2018; pp. 166–171. [Google Scholar]
- Kowal, J.; Budde-Meiwes, H.; Sauer, D.U. Interpretation of processes at positive and negative electrode by measurement and simulation of impedance spectra. Part I: Inductive semicircles. J. Power Source 2012, 207, 10–18. [Google Scholar] [CrossRef]
- Tenno, A.; Tenno, R.; Suntio, T. A method for Battery Impedance Analysis. J. Electrochem. Soc. 2004, 151, 806–824. [Google Scholar] [CrossRef]
- Schlesinger, M. Modern Aspects of Electrochemistry; Springer: New York, NY, USA, 2009; Volume 43, ISBN 978-0-387-49580-4. [Google Scholar]
- Lindbergh, G. Experimental determination of the effective electrolyte conductivity in porous lead electrodes in the lead-acid battery. Electrochim. Acta 1997, 42, 1239–1246. [Google Scholar] [CrossRef]
- Niya, S.M.R.; Hejabi, M.; Gobal, F. Estimation of the kinetic parameters of processes at the negative plate of lead-acid batteries by impedance studies. J. Power Source 2010, 195, 5789–5793. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Doan, V.-T.; Lee, G.-H.; Kim, H.-W.; Choi, W.; Kim, D.-W. Development of an Intelligent Charger with a Battery Diagnosis Function Using Online Impedance Spectroscopy. J. Power Electron. 2016, 16, 1981–1989. [Google Scholar] [CrossRef] [Green Version]
- Calborean, A.; Murariu, T.; Morari, C. Determination of current homogeneity on the electrodes of lead-acid batteries through electrochemical impedance spectroscopy. Electrochim. Acta 2019, 320, 134636. [Google Scholar] [CrossRef]
- Kwiecien, M.; Huck, M.; Badeda, J.; Zorer, C.; Komut, K.; Yu, Q.; Sauer, D.U. Variation of Impedance in Lead-Acid Batteries in the Presence of Acid Stratification. Appl. Sci. 2018, 8, 1018. [Google Scholar] [CrossRef] [Green Version]
- Hampson, N.; Lakeman, J. The oxidation of porous lead electrodes in Sulphuric acid solution. J. Power Source 1979, 4, 21–32. [Google Scholar] [CrossRef]
- Hampson, N.A.; Lazarides, C. The anodic oxidation of lead in H2SO4-based solutions to form soluble species. Surf. Technol. 1981, 14, 301–308. [Google Scholar] [CrossRef]
- Huck, M. Modelling the Transient Behaviour of Lead-Acid Batteries: Electrochemical Impedance of Adsorbed Species. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2020. [Google Scholar]


| Cell Size | Plate Count Type 1/Type 2 | Nominal Capacity Cn Type 1/Type 2 |
|---|---|---|
| Complete cell | 8P8N/8P9N | 70 Ah |
| Middle size cells | 3P2N | 17.50 Ah/18.70 Ah |
| Small size cells | 2P1N | 8.75 Ah/9.30 Ah |
| Parameter | Value |
|---|---|
| IDC | I20/2 |
| IAC,max | 0.5 A |
| fmin | 10 mHz |
| fmax | 6.5 kHz |
| T | 25 °C |
| SoC | 80% |
| ΔSoC | 2% |
| Original Plate Count | Type 1: 8P8N | Type 2: 8P9N |
|---|---|---|
| Complete cell | 15/16 | 16/18 |
| Middle size cells | 4/16 | 4/18 |
| Small size cells | 2/16 | 2/18 |
| Type 1 EFB − C | Type 1 EFB + C | Type 2 EFB + C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time Constant | Complete Cell | Middle Size Cell | Small Size Cell | Complete Cell | Middle Size Cell | Small Size Cell | Complete Cell | Middle Size Cell | Small Size Cell |
| τ0/sξ | 0.003 | - | - | 0.002 | - | - | 0.003 | - | - |
| τ1/sξ | 0.072 | 0.08 | 0.10 | 0.06 | 0.054 | 0.044 | 0.073 | 0.056 | 0.056 |
| τ2/sξ | 2.359 | 2.32 | 1.094 | 1.094 | 2.816 | 0.568 | 2.984 | 1.436 | 0.451 |
| τ3/sξ | 13.495 | 11.19 | 5.838 | 7.415 | 15.9 | 3.629 | 19.025 | 19.025 | 2.283 |
| Parameter | Lower Limit | Starting Value | Upper Limit |
|---|---|---|---|
| R0/Ω | R0 | R0 | R0 + 0.05 |
| L/μH | 0 | 200 | 10,000 |
| λL | 0 | 0.4 | 1 |
| R1/Ω | 0 | 0.3 | 1 |
| τ1/sξ | τ1 | τ1 | τ1 |
| ξ1 | 0.849 | 0.849 | 0.849 |
| R2/Ω | 0 | 0.4 | 1 |
| τ2/sξ | τ2 | τ2 | τ2 |
| ξ2 | 0.664 | 0.664 | 0.664 |
| R3/Ω | 0 | 0.5 | 2 |
| τ3/sξ | τ3 | τ3 | τ3 |
| ξ3 | 0.75 | 0.75 | 0.75 |
| Type 1 EFB − C | Type 1 EFB + C | Type 2 EFB + C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time Constant | Complete Cell | Middle Size Cell | Small Size Cell | Complete Cell | Middle Size Cell | Small Size Cell | Complete Cell | Middle Size Cell | Small Size Cell |
| R0/Ω | 0 | 0.0062 | 0 | 0 | 0 | 0 | 0.0119 | 0.0121 | 0 |
| L/μH | 420 | 108 | 11.8 | 249 | 46.8 | 13.1 | 257 | 277 | 2500 |
| λL | 0.94 | 0.98 | 0.97 | 0.94 | 0.94 | 0.95 | 0.95 | 1 | 0.18 |
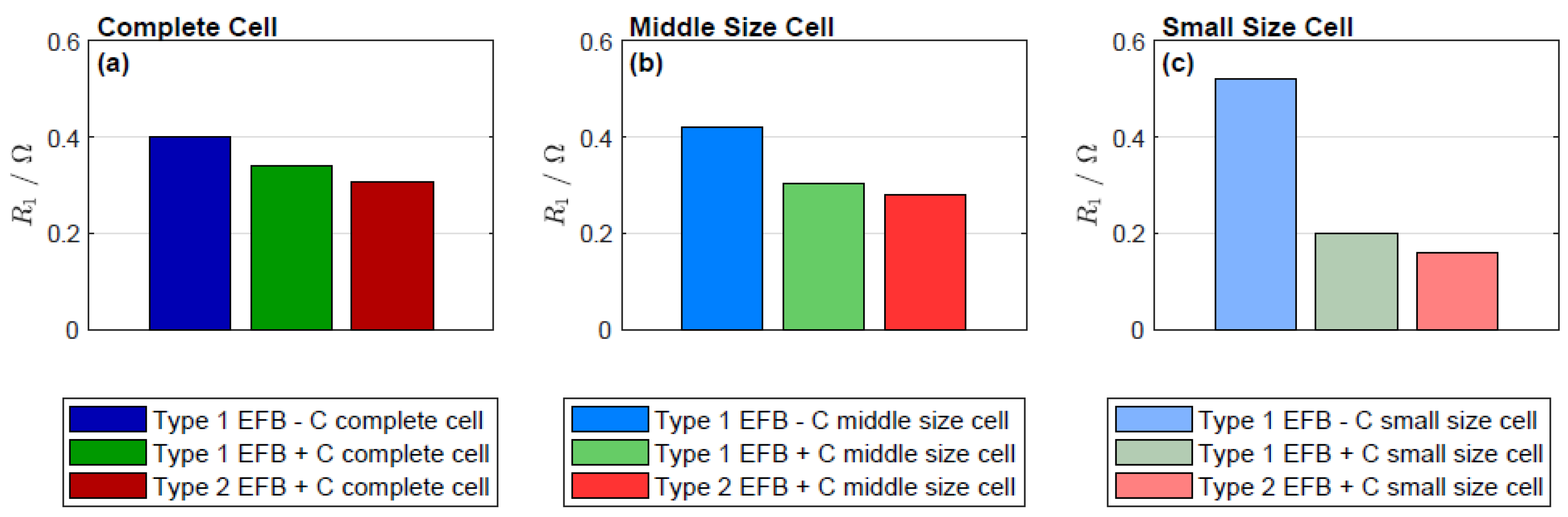
| R1/Ω | 0.4 | 0.42 | 0.52 | 0.34 | 0.303 | 0.2 | 0.309 | 0.28 | 0.16 |
| τ1/sξ | 0.072 | 0.08 | 0.10 | 0.06 | 0.054 | 0.044 | 0.073 | 0.056 | 0.056 |
| ξ1 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| R2/Ω | 0.534 | 0.533 | 0.3 | 0.3 | 0.6 | 0.3 | 0.384 | 0.3 | 0.3 |
| τ2/sξ | 2.359 | 2.32 | 1.094 | 1.094 | 2.816 | 0.568 | 2.984 | 1.436 | 0.451 |
| ξ2 | 0.664 | 0.664 | 0.664 | 0.664 | 0.664 | 0.664 | 0.664 | 0.664 | 0.664 |
| R3/Ω | 0.218 | 0.62 | 1.16 | 0.41 | 1.452 | 0.366 | 0.37 | 0.101 | 0.1 |
| τ3/sξ | 13.495 | 11.19 | 5.838 | 7.415 | 15.9 | 3.629 | 19.025 | 19.025 | 2.283 |
| ξ3 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| error | 0.042 | 0.029 | 0.066 | 0.051 | 0.047 | 0.034 | 0.036 | 0.023 | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauknecht, S.; Kowal, J.; Bozkaya, B.; Settelein, J.; Karden, E. Electrochemical Impedance Spectroscopy as an Analytical Tool for the Prediction of the Dynamic Charge Acceptance of Lead-Acid Batteries. Batteries 2022, 8, 66. https://doi.org/10.3390/batteries8070066
Bauknecht S, Kowal J, Bozkaya B, Settelein J, Karden E. Electrochemical Impedance Spectroscopy as an Analytical Tool for the Prediction of the Dynamic Charge Acceptance of Lead-Acid Batteries. Batteries. 2022; 8(7):66. https://doi.org/10.3390/batteries8070066
Chicago/Turabian StyleBauknecht, Sophia, Julia Kowal, Begüm Bozkaya, Jochen Settelein, and Eckhard Karden. 2022. "Electrochemical Impedance Spectroscopy as an Analytical Tool for the Prediction of the Dynamic Charge Acceptance of Lead-Acid Batteries" Batteries 8, no. 7: 66. https://doi.org/10.3390/batteries8070066
APA StyleBauknecht, S., Kowal, J., Bozkaya, B., Settelein, J., & Karden, E. (2022). Electrochemical Impedance Spectroscopy as an Analytical Tool for the Prediction of the Dynamic Charge Acceptance of Lead-Acid Batteries. Batteries, 8(7), 66. https://doi.org/10.3390/batteries8070066







