Comparison of Cooling Performance in a Cylindrical Battery with Single-Phase Direct Contact Cooling under Various Operating Conditions
Abstract
1. Introduction
2. Numerical Methodology
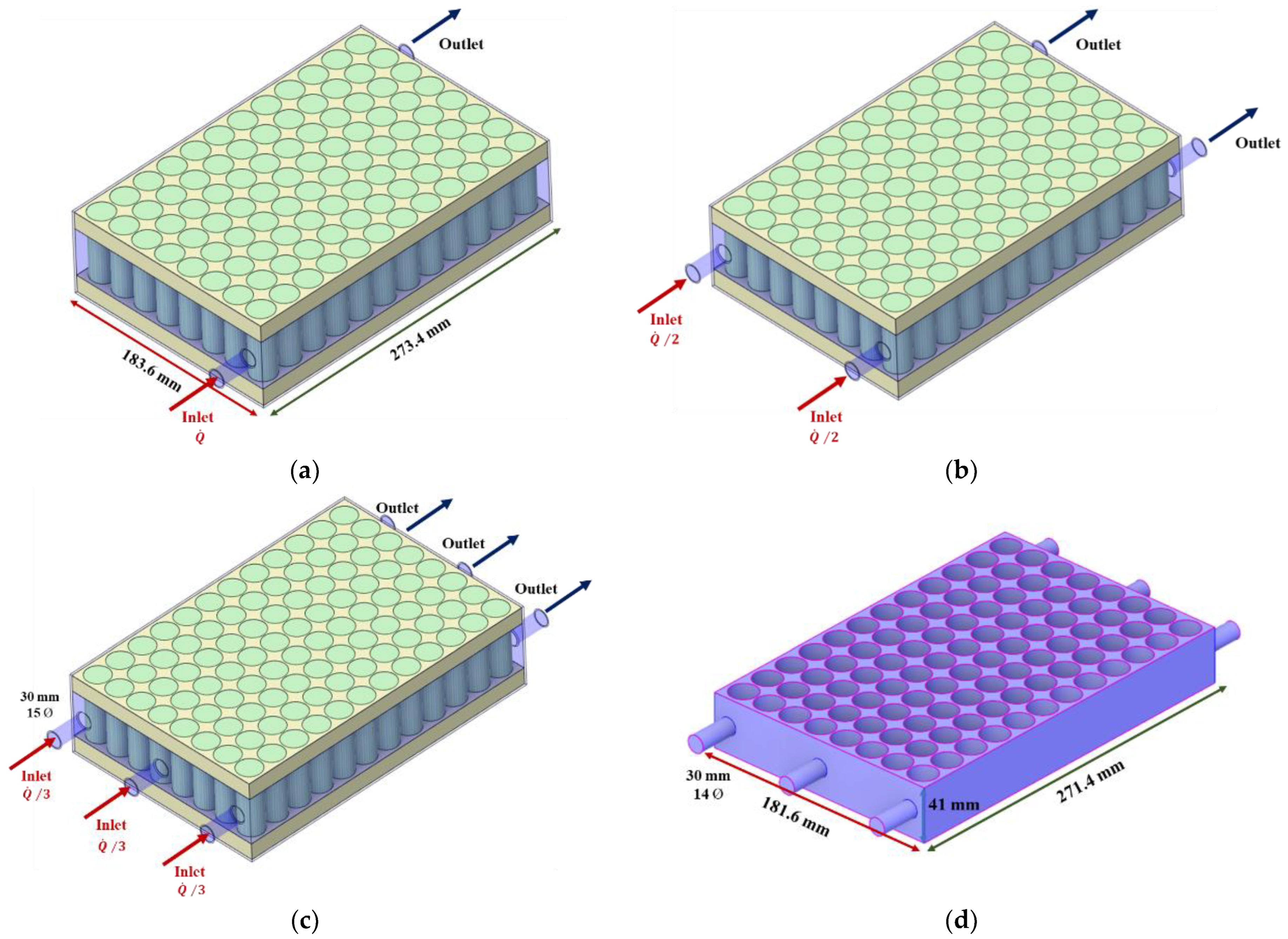
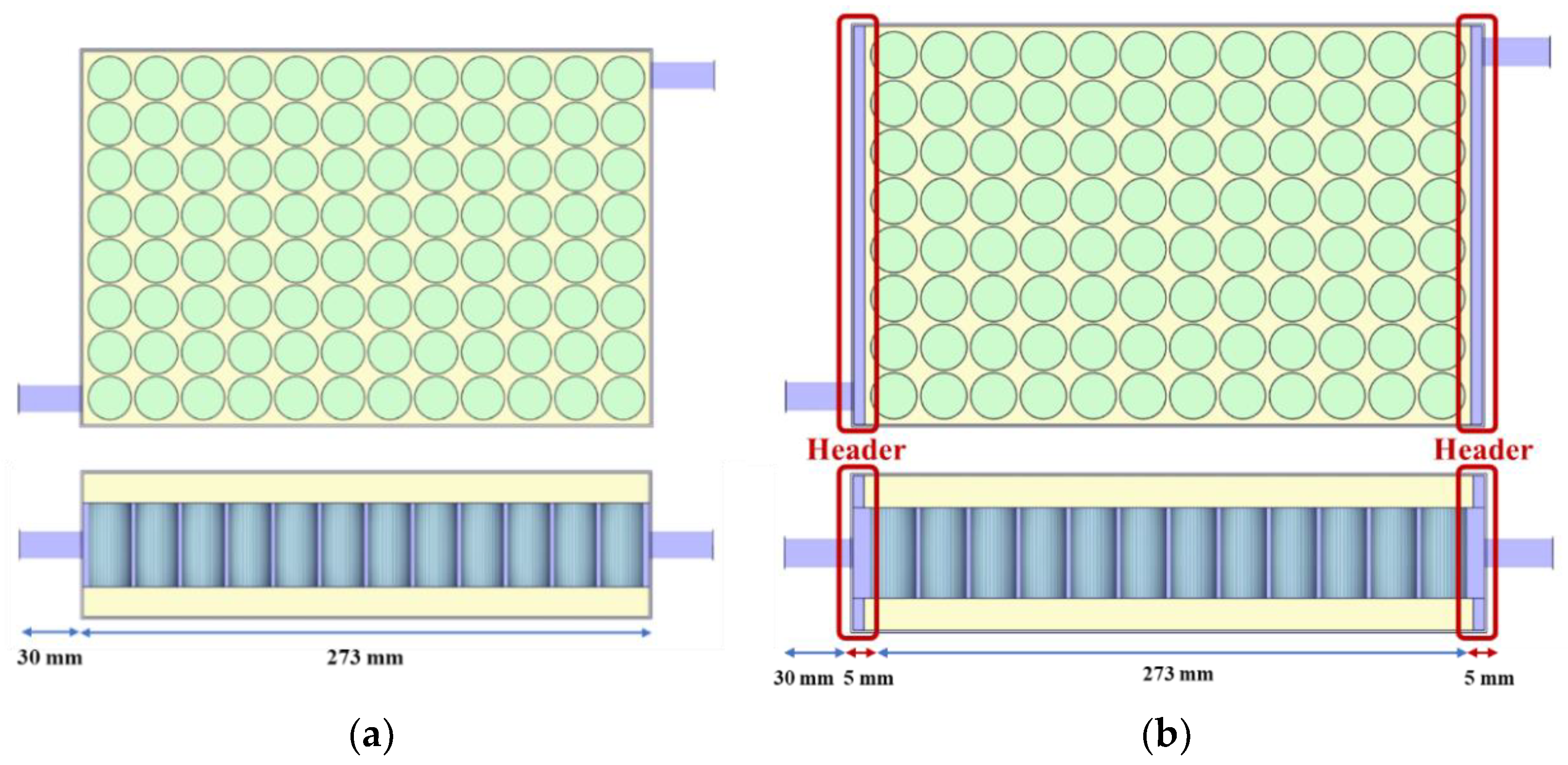
2.1. Battery and Cooling System Modeling
2.2. Governing Equation and Boundary Conditions
- (1)
- There is no phase change in the interpretation of this analysis.
- (2)
- It is assumed that the working fluid is filled with the cooling model, and there is no air.
- (3)
- Thermal resistance and radiation are not considered.
- (4)
- The working fluid is incompressible.
2.3. Grid Independence Test
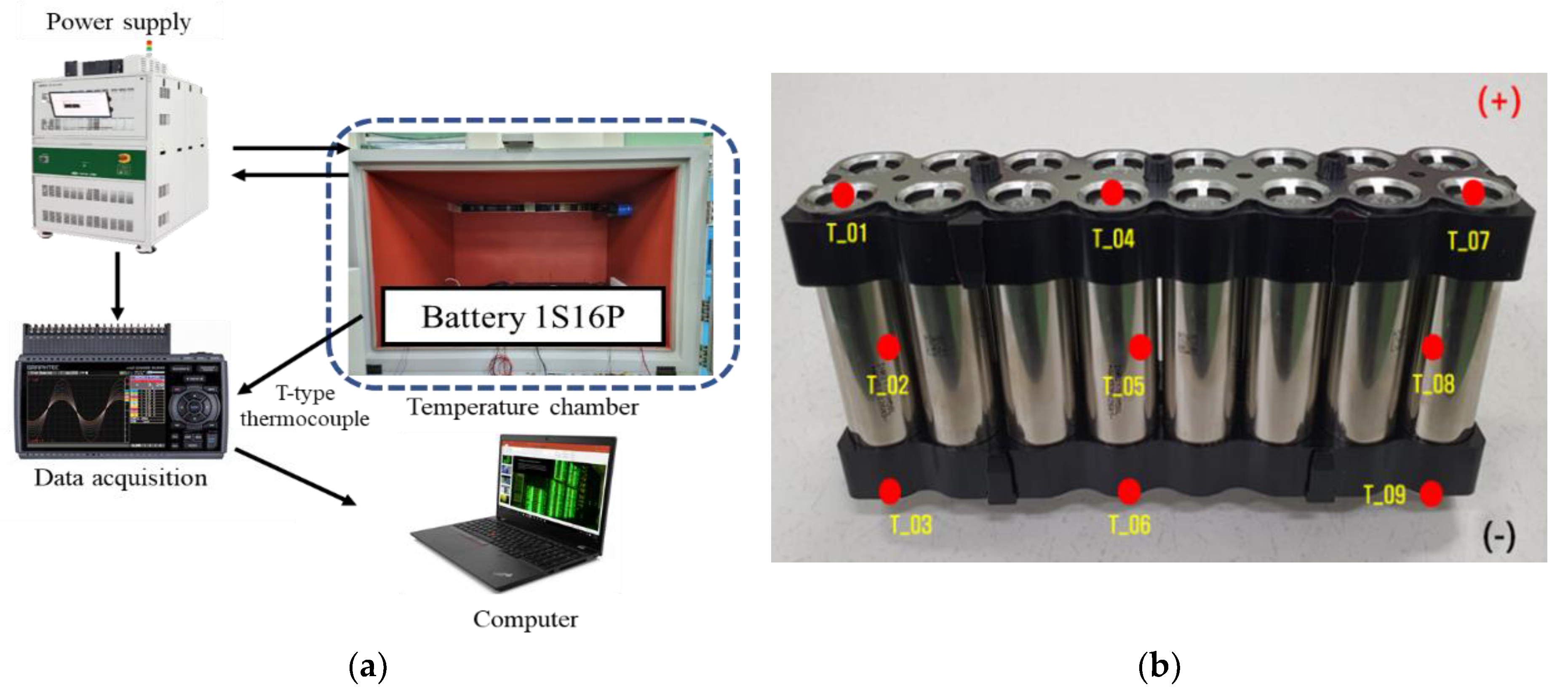
2.4. Verification Experiment Setup and Method
3. Results and Discussion
3.1. Verification of Battery Analytical Model
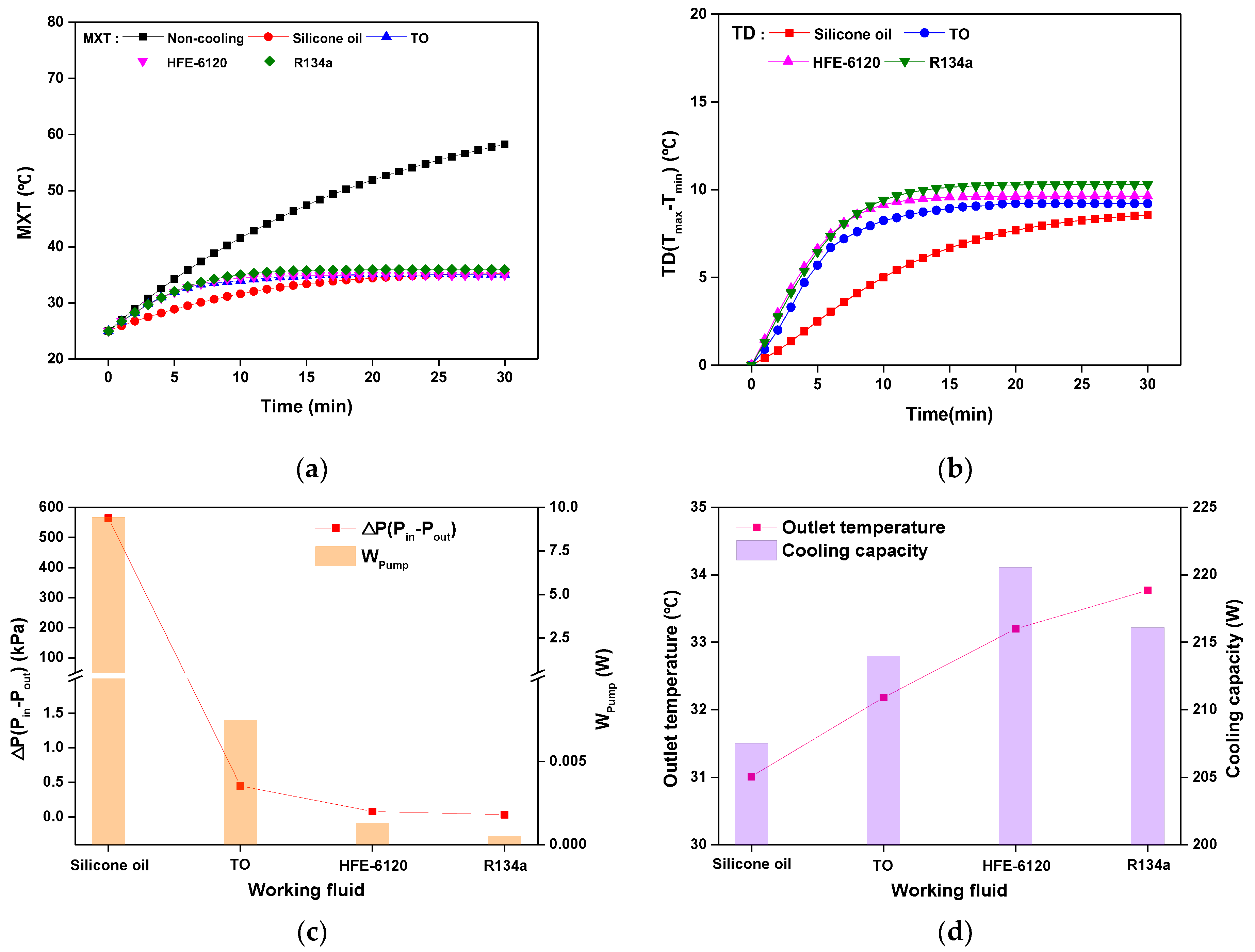
3.2. Cooling Performance according to the Working Fluid
3.3. Cooling Performance according to the Number of Port and Header Width Using R134a
3.4. Comparison of Cooling Performance according to the Flow Rate of R134a under Standard and Summer Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| BTMS | Battery thermal management system | U | Voltage (V) |
| c | Specific heat capacity (J/kg·°C) | u | Velocity, (m/s) |
| D | Diameter (m) | W | Power |
| EES | Engineering equation solver | 1S16P | 1 serial × 16 parallel |
| g | Gravitational acceleration (m/s2) | ||
| H | Height (m) | Greek symbols | |
| HVAC | Heating, ventilation, and air conditioning | ρ | Density (kg/m3) |
| I | Current (A) | λ | Thermal conductivity (W/m·°C) |
| L | Length, (m) | ||
| MXT | Maximum temperature of the battery (°C) | Subscript | |
| Mass flow rate (kg/s) | avg | average | |
| P | Pressure (Pa) | ba | Battery |
| PCM | Phase change material | in | inlet |
| Flow rate (m3/s) | ir | irreversible heat | |
| Q | Heat generation rate (W) | max | Maximum |
| Volumetric heat generation rate (W/m3) | min | Minimum | |
| R | Equivalent internal resistance, Ω | pump | Pump |
| T | Temperature (°C) | re | reversible heat |
| TD | Temperature difference between batteries (°C) | OCV | Open circuit voltage |
| TO | Transformer oil | wf | Working fluid |
| t | Time (s) | ||
References
- Chen, S.; Bao, N.; Garg, A.; Peng, X.; Gao, L. A Fast Charging–Cooling Coupled Scheduling Method for a Liquid Cooling-Based Thermal Management System for Lithium-Ion Batteries. Engineering 2021, 7, 1165–1176. [Google Scholar] [CrossRef]
- Amjad, S.; Neelakrishnan, S.; Rudramoorthy, R. Review of design considerations and technological challenges for successful development and deployment of plug-in hybrid electric vehicles. Renew. Sustain. Energy Rev. 2010, 14, 1104–1110. [Google Scholar] [CrossRef]
- Roe, C.; Feng, X.; White, G.; Li, R.; Wang, H.; Rui, X.; Li, C.; Zhang, F.; Null, V.; Parkes, M.; et al. Immersion cooling for lithium-ion batteries—A review. J. Power Sources 2022, 525, 231094. [Google Scholar] [CrossRef]
- Chitta, S.D.; Akkaldevi, C.; Jaidi, J.; Panchal, S.; Fowler, M.; Fraser, R. Comparison of lumped and 1D electrochemical models for prismatic 20Ah LiFePO4 battery sandwiched between minichannel cold-plates. Appl. Therm. Eng. 2021, 199, 117586. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, D.; Jiang, L.; Zhang, G.; Wu, H.; Day, R.; Jiang, W. Advanced thermal management system driven by phase change materials for power lithium-ion batteries: A review. Renew. Sustain. Energy Rev. 2022, 159, 112207. [Google Scholar] [CrossRef]
- Gan, Y.; He, L.; Liang, J.; Tan, M.; Xiong, T.; Li, Y. A numerical study on the performance of a thermal management system for a battery pack with cylindrical cells based on heat pipes. Appl. Therm. Eng. 2020, 179, 115740. [Google Scholar] [CrossRef]
- Ramadass, P.; Haran, B.; White, R.; Popov, B.N. Capacity fade of Sony 18650 cells cycled at elevated temperatures: Part II. Capacity fade analysis. J. Power Sources 2002, 112, 614–620. [Google Scholar] [CrossRef]
- Huang, P.; Yao, C.; Mao, B.; Wang, Q.; Sun, J.; Bai, Z. The critical characteristics and transition process of lithium-ion battery thermal runaway. Energy 2020, 213, 119082. [Google Scholar] [CrossRef]
- Wang, S.; Takyi-Aninakwa, P.; Jin, S.; Yu, C.; Fernandez, C.; Stroe, D.I. An improved feedforward-long short-term memory modeling method for the whole-life-cycle state of charge prediction of lithium-ion batteries considering current-voltage-temperature variation. Energy 2022, 254, 124224. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Yuan, Q.; Wang, T.; Ji, H.; Papović, S.; Raleva, K.; Pan, F.; Yang, T.; Li, J. Effects of Minor Mechanical Deformation on the Lifetime and Performance of Commercial 21700 lithium-Ion Battery. J. Electrochem. Soc. 2022, 169, 060544. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, H.; Jin, Y. Experimental and Numerical Study on Mechanical Deformation Characteristics of Lithium Iron Phosphate Pouch Battery Modules under Overcharge Conditions. Energy Fuels 2021, 35, 15172–15184. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Kalogiannis, T.; Jaguemont, J.; Jin, L.; Behi, H.; Karimi, D.; Beheshti, H.; Van Mierlo, J.; Berecibar, M. A comparative study between air cooling and liquid cooling thermal management systems for a high-energy lithium-ion battery module. Appl. Therm. Eng. 2021, 198, 117503. [Google Scholar] [CrossRef]
- Egab, K.; Oudah, S.K. Thermal management analysis of li-ion battery-based on cooling system using dimples with air fins and perforated fins. Int. J. Therm. Sci. 2022, 171, 107200. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Xiang, G.; Li, H. Optimization of liquid cooling and heat dissipation system of lithium-ion battery packs of automobile. Case Stud. Therm. Eng. 2021, 26, 101012. [Google Scholar] [CrossRef]
- Wang, N.; Li, C.; Li, W.; Chen, X.; Li, Y.; Qi, D. Heat dissipation optimization for a serpentine liquid cooling battery thermal management system: An application of surrogate assisted approach. J. Energy Storage 2021, 40, 102771. [Google Scholar] [CrossRef]
- Liu, H.; Ahmad, S.; Shi, Y.; Zhao, J. A parametric study of a hybrid battery thermal management system that couples PCM/copper foam composite with helical liquid channel cooling. Energy 2021, 231, 120869. [Google Scholar] [CrossRef]
- Xin, Q.; Xiao, J.; Yang, T.; Zhang, H.; Long, X. Thermal management of lithium-ion batteries under high ambient temperature and rapid discharging using composite PCM and liquid cooling. Appl. Therm. Eng. 2022, 210, 118230. [Google Scholar] [CrossRef]
- Jang, D.S.; Yun, S.; Hong, S.H.; Cho, W.; Kim, Y. Performance characteristics of a novel heat pipe-assisted liquid cooling system for the thermal management of lithium-ion batteries. Energy Convers. Manag. 2022, 251, 115001. [Google Scholar] [CrossRef]
- E, J.; Yi, F.; Li, W.; Zhang, B.; Zuo, H.; Wei, K.; Chen, J.; Zhu, H.; Zhu, H.; Deng, Y. Effect analysis on heat dissipation performance enhancement of a lithium-ion-battery pack with heat pipe for central and southern regions in China. Energy 2021, 226, 120336. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Negnevitsky, M.; Zhang, H. A review of air-cooling battery thermal management systems for electric and hybrid electric vehicles. J. Power Sources 2021, 501, 230001. [Google Scholar] [CrossRef]
- Jouhara, H.; Delpech, B.; Bennett, R.; Chauhan, A.; Khordehgah, N.; Serey, N.; Lester, S.P. Heat pipe based battery thermal management: Evaluating the potential of two novel battery pack integrations. Int. J. Thermofluids 2021, 12, 100115. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Zhang, Y.; Lv, S.; Ni, H.; Deng, Y.; Yuan, Y. A Review of the Power Battery Thermal Management System with Different Cooling, Heating and Coupling System. Energies 2022, 15, 1963. [Google Scholar] [CrossRef]
- Shen, M.; Gao, Q. Structure design and effect analysis on refrigerant cooling enhancement of battery thermal management system for electric vehicles. J. Energy Storage 2020, 32, 101940. [Google Scholar] [CrossRef]
- Hong, S.H.; Jang, D.S.; Park, S.; Yun, S.; Kim, Y. Thermal performance of direct two-phase refrigerant cooling for lithium-ion batteries in electric vehicles. Appl. Therm. Eng. 2020, 173, 115213. [Google Scholar] [CrossRef]
- Tan, X.; Lyu, P.; Fan, Y.; Rao, J.; Ouyang, K. Numerical investigation of the direct liquid cooling of a fast-charging lithium-ion battery pack in hydrofluoroether. Appl. Therm. Eng. 2021, 196, 117279. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; He, S.; Zhang, G.; Liu, X.; Yang, X. A simple cooling structure with precisely-tailored liquid cooling plate for thermal management of large battery module. Appl. Therm. Eng. 2022, 212, 118575. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, G.; Zhu, J.; Feng, X.; Wei, X.; Ouyang, M.; Dai, H. Multi-objective optimization design and experimental investigation for a parallel liquid cooling-based Lithium-ion battery module under fast charging. Appl. Therm. Eng. 2022, 211, 118503. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Liu, F.; Liu, Y.; Wang, F.; Yang, N.; Lu, D.; Jia, Y. Performance optimization of Tesla valve-type channel for cooling lithium-ion batteries. Appl. Therm. Eng. 2022, 212, 118583. [Google Scholar] [CrossRef]
- Wu, S.; Lao, L.; Wu, L.; Liu, L.; Lin, C.; Zhang, Q. Effect analysis on integration efficiency and safety performance of a battery thermal management system based on direct contact liquid cooling. Appl. Therm. Eng. 2022, 201, 117788. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.; Xie, Q. Feasibility study of a novel oil-immersed battery cooling system: Experiments and theoretical analysis. Appl. Therm. Eng. 2022, 208, 118251. [Google Scholar] [CrossRef]
- Al-Zareer, M.; Dincer, I.; Rosen, M.A. Heat and mass transfer modeling and assessment of a new battery cooling system. Int. J. Heat Mass Transf. 2018, 126, 765–778. [Google Scholar] [CrossRef]
- Fluent, I. Fluent 2021 user’s guide. Fluent Solut. 2021. [Google Scholar]
- Sheng, L.; Zhang, Z.; Su, L.; Zhang, H.; Zhang, H.; Li, K.; Fang, Y.; Ye, W. A calibration calorimetry method to investigate the thermal characteristics of a cylindrical lithium-ion battery. Int. J. Therm. Sci. 2021, 165, 106891. [Google Scholar] [CrossRef]
- Yataganbaba, A.; Kilicarslan, A.; Kurtbaş, İ. Exergy analysis of R1234yf and R1234ze as R134a replacements in a two evaporator vapour compression refrigeration system. Int. J. Refrig. 2015, 60, 26–37. [Google Scholar] [CrossRef]
- A/C Pro Certified Auto Air Conditioner R-134a Refrigerant, 12 oz, 301CA. Available online: Walmart.com (accessed on 17 August 2022).
- Silicone oil Dow Corning Corporation 200 fluid, 60,000cSt 25 °C 63148-62-9. Available online: Sigmaaldrich.com (accessed on 20 September 2022).
- Sigma Aldrich Fine Chemicals Biosciences Novec 7000 Engineered Fluid, 375-03-1. Fisher Scientific: Waltham, MA, USA. Available online: Fishersci.com (accessed on 17 August 2022).
- HD TO4 NO.10 18L|Transmission Oil. Sinopec: Sydney, NSW, Australia. Available online: Sinopeconline.com (accessed on 20 September 2022).
- Sheng, L.; Zhang, H.; Zhang, H.; Su, L.; Zhang, Z. Lightweight liquid cooling based thermal management to a prismatic hard-cased lithium-ion battery. Int. J. Heat Mass Transf. 2021, 170, 120998. [Google Scholar] [CrossRef]
- Lai, Y.; Wu, W.; Chen, K.; Wang, S.; Xin, C. A compact and lightweight liquid-cooled thermal management solution for cylindrical lithium-ion power battery pack. Int. J. Heat Mass Transf. 2019, 144, 118581. [Google Scholar] [CrossRef]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A general energy balance for battery systems. J. Electrochem. Soc. 1985, 132, 5–12. [Google Scholar] [CrossRef]
- Ghahfarokhi, P.S.; Kallaste, A.; Vaimann, T.; Rassolkin, A.; Belahcen, A. Determination of natural convection heat transfer coefficient over the fin side of a coil system. Int. J. Heat Mass Transf. 2018, 126, 677–682. [Google Scholar] [CrossRef]
- Shin, Y.; Im, G.; Yu, K.; Cho, H. Experimental study on the change in driver’s physiological signals in automobile HVAC system under Full load condition. Appl. Therm. Eng. 2017, 112, 1213–1222. [Google Scholar] [CrossRef]
- Dong, J.; Lan, H.; Liu, Y.; Wang, X.; Yu, C. Indoor environment of nearly zero energy residential buildings with conventional air conditioning in hot-summer and cold-winter zone. Energy Built Environ. 2022, 3, 129–138. [Google Scholar] [CrossRef]
- Huang, Z.; Li, G.; Tian, S.; Song, X.; Sheng, M.; Shah, S. Operation Parameters Calculation. Abras. Water Jet Perforation Multi-Stage Fract. 2018, 191–203. [Google Scholar] [CrossRef]






| Parameters | Battery | Battery Holder | Battery Cooling Block |
|---|---|---|---|
| Density (kg m−3) | 2525.5 | 1200 | 2719 |
| Specific heat (J kg−1 °C−1) | 1040.5 + 2.29 × T | 1250 | 871 |
| Thermal conductivity (W m−1 °C−1) | 23.6 | 0.2 | 202.4 |
| Parameters | Silicone Oil | TO | HFE-6120 | R134a |
|---|---|---|---|---|
| Density (kg m−3) | 965 | 820 | 1600 | (−1.20 × 10−2) × T2 − 3.28 × T + 1297.94 |
| Specific heat (J kg−1 °C−1) | 2490 | 2530 | 1170 | (3.62 × 10−2) × T2 + 2.52 × T + 1336.14 |
| Thermal conductivity (W m−1 °C−1) | 0.16 | 0.3875 | 0.23 | (−1.42 × 10−6) × T2 − (4.29 × 10−4) × T + 0.10 |
| Viscosity (Pa s) | 50 | 0.0082 | 0.0019 | (1.83 × 10−8) × T2 + (−3.40 × 10−6) × T + (2.69 × 10−4) |
| Cost ($ kg−1) | 245 | 81.68 | 217 | 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Ham, J.; Shin, D.; Cho, H. Comparison of Cooling Performance in a Cylindrical Battery with Single-Phase Direct Contact Cooling under Various Operating Conditions. Batteries 2022, 8, 195. https://doi.org/10.3390/batteries8100195
Kim M, Ham J, Shin D, Cho H. Comparison of Cooling Performance in a Cylindrical Battery with Single-Phase Direct Contact Cooling under Various Operating Conditions. Batteries. 2022; 8(10):195. https://doi.org/10.3390/batteries8100195
Chicago/Turabian StyleKim, Minjun, Jeonggyun Ham, Donghyeon Shin, and Honghyun Cho. 2022. "Comparison of Cooling Performance in a Cylindrical Battery with Single-Phase Direct Contact Cooling under Various Operating Conditions" Batteries 8, no. 10: 195. https://doi.org/10.3390/batteries8100195
APA StyleKim, M., Ham, J., Shin, D., & Cho, H. (2022). Comparison of Cooling Performance in a Cylindrical Battery with Single-Phase Direct Contact Cooling under Various Operating Conditions. Batteries, 8(10), 195. https://doi.org/10.3390/batteries8100195








