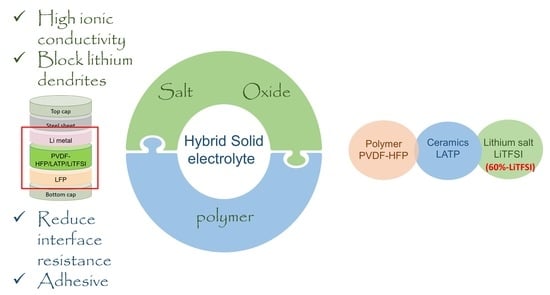
Effect of Lithium Salt Concentration on Materials Characteristics and Electrochemical Performance of Hybrid Inorganic/Polymer Solid Electrolyte for Solid-State Lithium-Ion Batteries
Abstract
1. Introduction
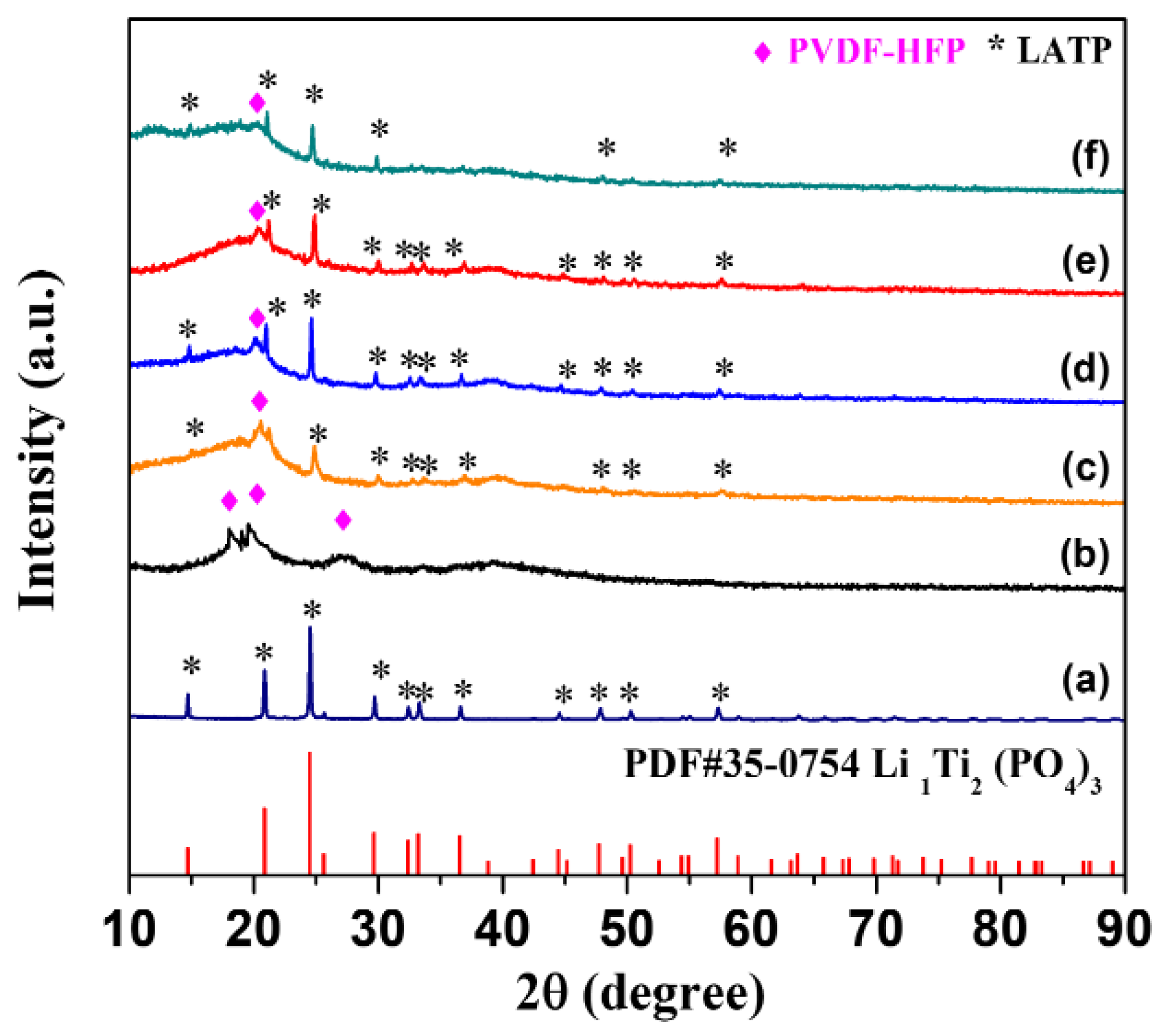
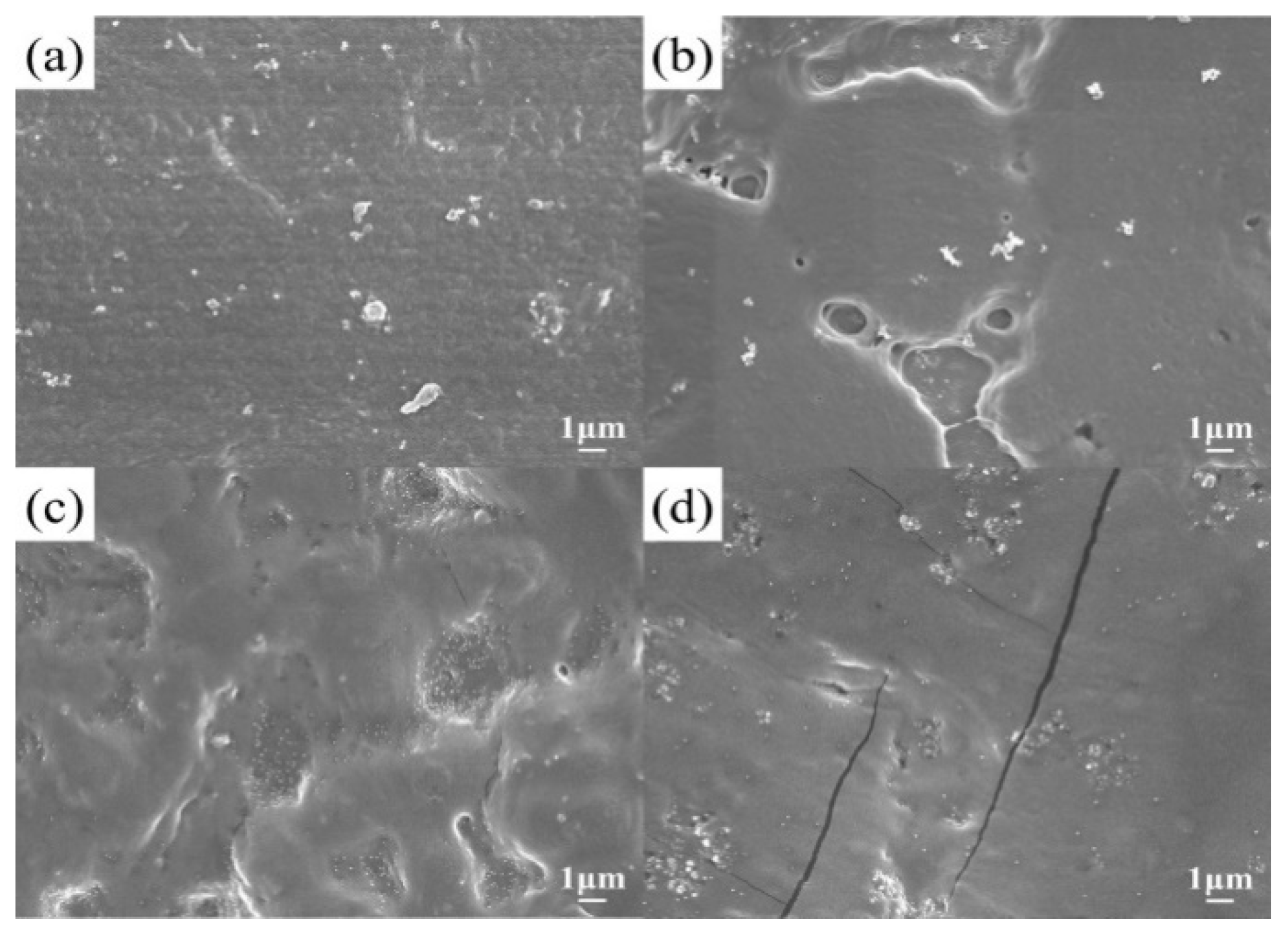
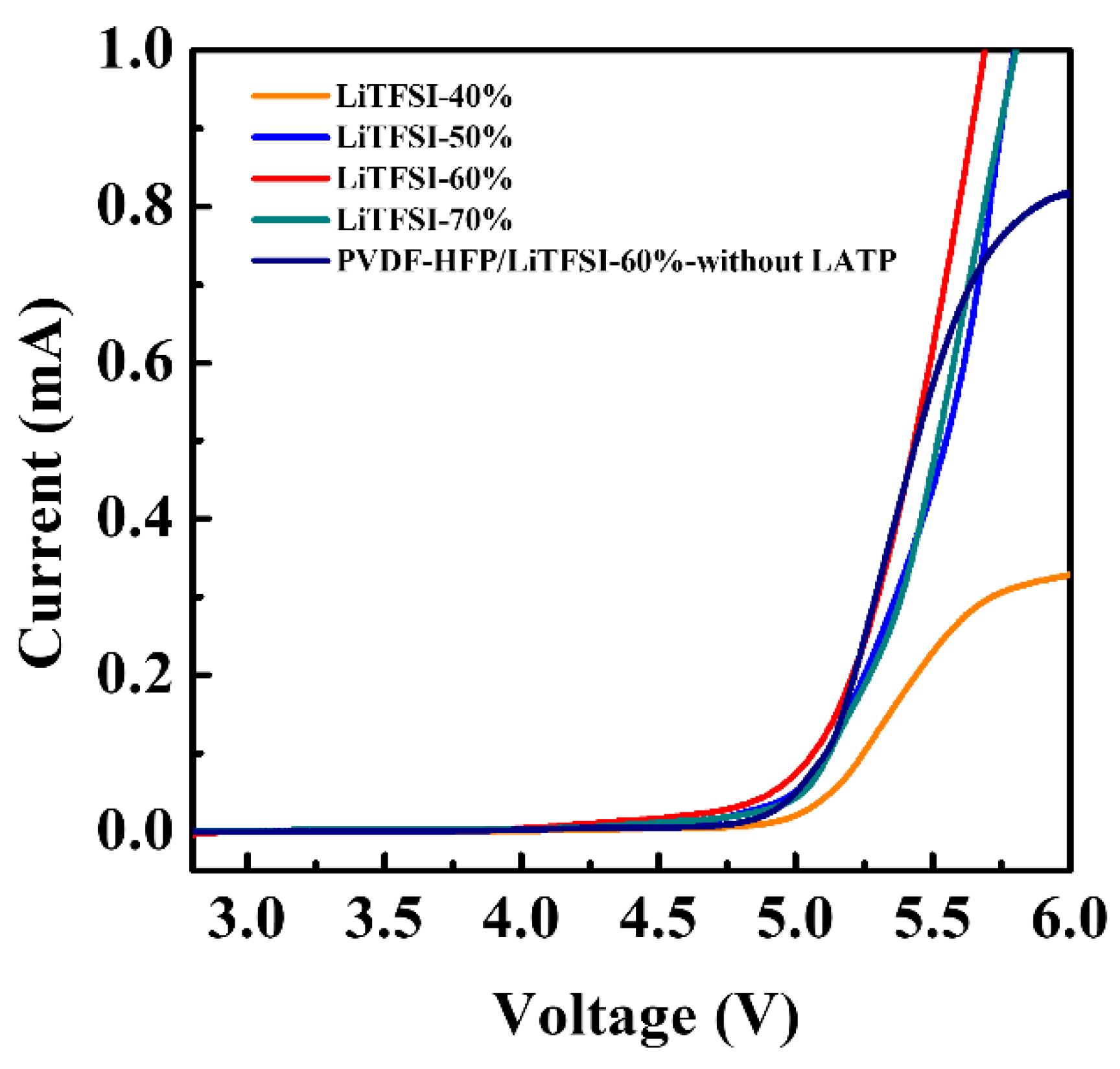
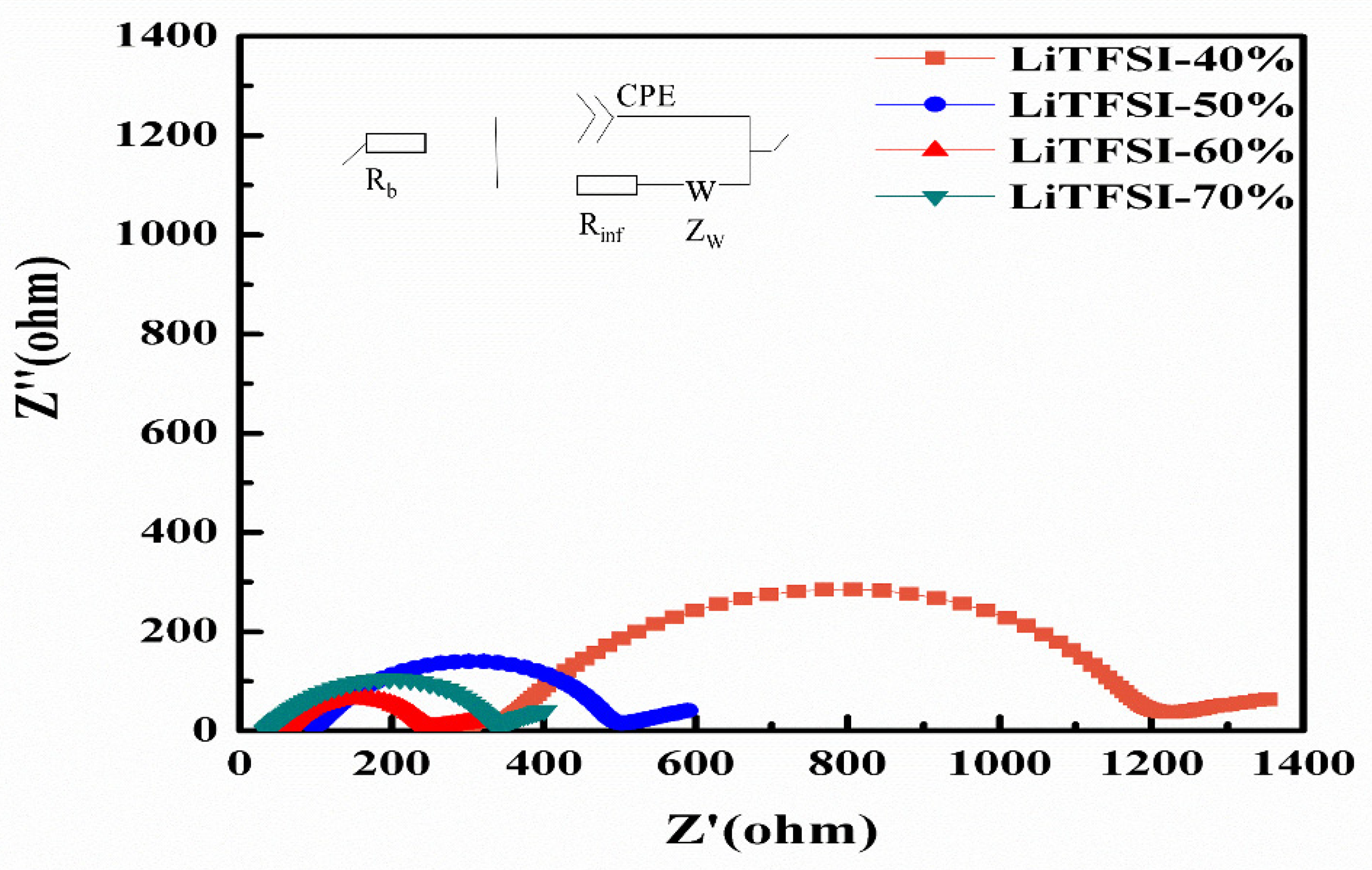
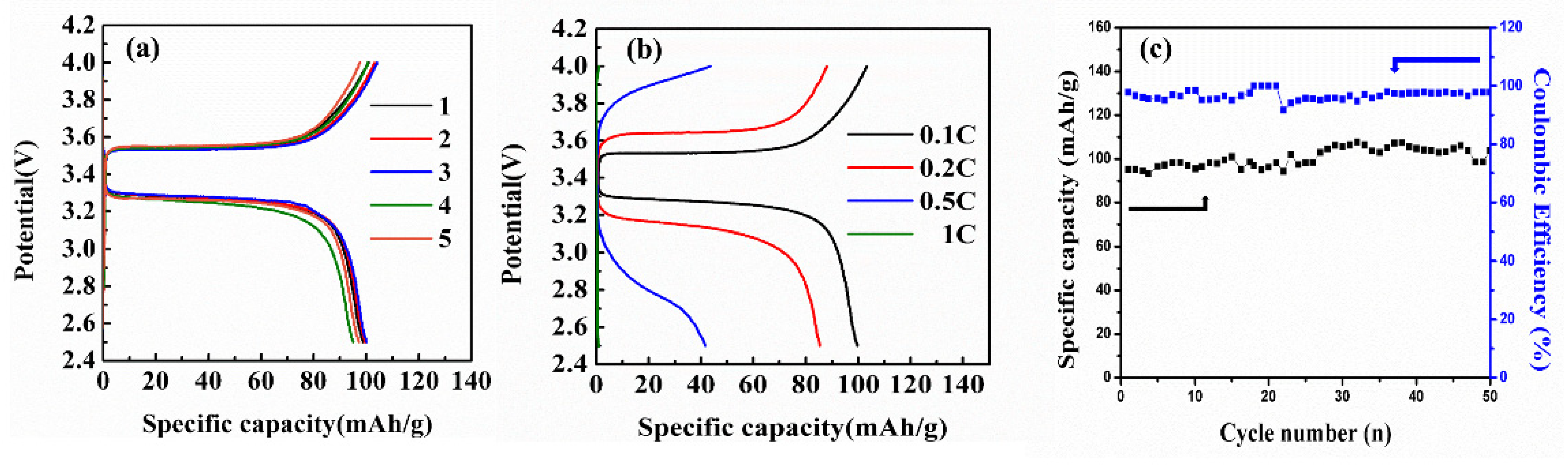
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al Shaqsi, A.Z.; Sopian, K.; Al-Hinai, A. Review of energy storage services, applications, limitations, and benefits. Energy Rep. 2020, 6, 288–306. [Google Scholar] [CrossRef]
- Divya, M.L.; Praneetha, S.; Lee, Y.-S.; Aravindan, V. Next-generation Li-ion capacitor with high energy and high power by limiting alloying-intercalation process using SnO2@Graphite composite as battery type electrode. Compos. Part B Eng. 2022, 230, 109487–109493. [Google Scholar] [CrossRef]
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Zhu, C.; Han, K.; Geng, D.; Ye, H.; Meng, X. Achieving High-Performance Silicon Anodes of Lithium-Ion Batteries via Atomic and Molecular Layer Deposited Surface Coatings: An Overview. Electrochim. Acta 2017, 251, 710–728. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational design on materials for developing next generation lithium-ion secondary battery. Prog. Solid State Chem. 2021, 62, 100298–100325. [Google Scholar] [CrossRef]
- Tsai, S.H.; Tsou, Y.L.; Yang, C.W.; Chen, T.Y.; Lee, C.Y. Applications of different nano-sized conductive materials in high energy density pouch type lithium ion batteries. Electrochim. Acta 2020, 362, 137166–137174. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Wang, Y.; Huang, W.; Lv, L.; Zhu, G.; Qu, Q.; Liang, Y.; Zheng, W.; Zheng, H. A novel covalently grafted binder through in-situ polymerization for high-performance Si-based lithium-ion batteries. Electrochim. Acta 2021, 400, 139442–139451. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X. A novel non-experiment-based reconstruction method for the relationship between open-circuit-voltage and state-of-charge/state-of-energy of lithium-ion battery. Electrochim. Acta 2022, 403, 139637–139656. [Google Scholar] [CrossRef]
- Fang, H. Challenges with the Ultimate Energy Density with Li-ion Batteries. IOP Conf. Ser. Earth Environ. Sci. 2021, 781, 42023–42029. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Zhang, H.; Judez, X.; Adenusi, H.; Armand, M.; Passerini, S.; Figgemeier, E. Production of high-energy Li-ion batteries comprising silicon-containing anodes and insertion-type cathodes. Nat. Commun. 2021, 12, 5459–5473. [Google Scholar] [CrossRef]
- Kwon, S.J.; Lee, S.E.; Lim, J.H.; Choi, J.; Kim, J. Performance and Life Degradation Characteristics Analysis of NCM LIB for BESS. Electronics 2018, 7, 406. [Google Scholar] [CrossRef]
- Greim, P.; Solomon, A.A.; Breyer, C. Assessment of lithium criticality in the global energy transition and addressing policy gaps in transportation. Nat. Commun. 2020, 11, 4570–4581. [Google Scholar] [CrossRef] [PubMed]
- Bobba, S.; Mathieux, F.; Blengini, G.A. How will second-use of batteries affect stocks and flows in the EU? A model for traction Li-ion batteries. Resour. Conserv. Recycl. 2019, 145, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Sanguesa, J.A.; Torres-Sanz, V.; Garrido, P.; Martinez, F.J.; Marquez-Barja, J.M. A Review on Electric Vehicles: Technologies and Challenges. Smart Cities 2021, 4, 372–404. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Wang, M.S.; Wang, Z.Q.; Yang, Z.L.; Huang, Y.; Zheng, J.; Li, X. Carbon nanotube-graphene nanosheet conductive framework supported SnO2 aerogel as a high performance anode for lithium ion battery. Electrochim. Acta 2017, 240, 7–15. [Google Scholar] [CrossRef]
- Kong, L.; Yang, Y.; Li, R.; Li, Z. Phenylalanine-functionalized graphene quantum dot-silicon nanoparticle composite as an anode material for lithium ion batteries with largely enhanced electrochemical performance. Electrochim. Acta 2016, 198, 144–155. [Google Scholar] [CrossRef]
- Thayumanasundaram, S.; Rangasamy, V.S.; Seo, J.W.; Locquet, J.P. Electrochemical performance of polymer electrolytes based on Poly(vinyl alcohol)/Poly(acrylic acid) blend and Pyrrolidinium ionic liquid for lithium rechargeable batteries. Electrochim. Acta 2017, 240, 371–378. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Tong, Z.; Wang, S.B.; Liao, Y.K.; Hu, S.F.; Liu, R.S. Interface Between Solid-State Electrolytes and Li-Metal Anodes: Issues, Materials, and Processing Routes. ACS Appl. Mater. Interfaces 2020, 12, 47181–47196. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, S.; Nakagawa, K.; Vásquez, F.A.; Fujii, Y.; Wang, Y.; Miura, A.; Calderón, J.A.; Rosero-Navarro, N.C.; Tadanaga, K. Preparation of Composite Electrodes for All-Solid-State Batteries Based on Sulfide Electrolytes: An Electrochemical Point of View. Batteries 2021, 7, 77. [Google Scholar] [CrossRef]
- Chen, X.; Guan, Z.; Chu, F.; Xue, Z.; Wu, F.; Yu, Y. Air-stable inorganic solid-state electrolytes for high energy density lithium batteries: Challenges, strategies, and prospects. InfoMat 2021, 4, e12248–e12268. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Yim, C.H.; Karkar, Z.; Abu-Lebdeh, Y. On the Current and Future Outlook of Battery Chemistries for Electric Vehicles-Mini Review. Batteries 2022, 8, 70. [Google Scholar] [CrossRef]
- Verdier, N.; Foran, G.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. Challenges in Solvent-Free Methods for Manufacturing Electrodes and Electrolytes for Lithium-Based Batteries. Polymers 2021, 13, 323. [Google Scholar] [CrossRef]
- Liu, F.; Bin, F.; Xue, J.; Wang, L.; Yang, Y.; Huo, H.; Zhou, J.; Li, L. Polymer Electrolyte Membrane with High Ionic Conductivity and Enhanced Interfacial Stability for Lithium Metal Battery. ACS Appl. Mater. Interfaces 2020, 12, 22710–22720. [Google Scholar] [CrossRef]
- Costa, C.M.; Lizundia, E.; Lanceros-Méndez, S. Polymers for advanced lithium-ion batteries: State of the art and future needs on polymers for the different battery components. Prog. Energy Combust. Sci. 2020, 79, 100846–100880. [Google Scholar] [CrossRef]
- Ye, F.; Liao, K.; Ran, R.; Shao, Z. Recent Advances in Filler Engineering of Polymer Electrolytes for Solid-State Li-Ion Batteries: A Review. Energy Fuels 2020, 34, 9189–9207. [Google Scholar] [CrossRef]
- Yao, Z.; Zhu, K.; Li, X.; Zhang, J.; Chen, J.; Wang, J.; Yan, K.; Liu, J. 3D poly(vinylidene fluoride–hexafluoropropylen) nanofiber-reinforced PEO-based composite polymer electrolyte for high-voltage lithium metal batteries. Electrochim. Acta 2022, 404, 139769. [Google Scholar] [CrossRef]
- Feng, C.; Kyu, T. Role of dinitrile plasticizer chain lengths in electrochemical performance of highly conductive polymer electrolyte membrane for lithium ion battery. Electrochim. Acta 2020, 330, 135320–135331. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO based polymer-ceramic hybrid solid electrolytes: A review. Nano Converg. 2021, 8, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, T.; Pu, Y.; Guan, M.; Tang, Z.; Ding, F.; Xu, Z.; Li, Y. Facile synthesis of NASICON-type Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte and its application for enhanced cyclic performance in lithium ion batteries through the introduction of an artificial Li3PO4 SEI layer. RSC Adv. 2017, 7, 46545–46552. [Google Scholar] [CrossRef]
- Yang, L.; Song, Y.; Liu, H.; Wang, Z.; Yang, K.; Zhao, Q.; Cui, Y.; Wen, J.; Luo, W.; Pan, F. Stable Interface between Lithium and Electrolyte Facilitated by a Nanocomposite Protective Layer. Small Methods 2020, 4, 1900751. [Google Scholar] [CrossRef]
- Ling, S.G.; Peng, J.Y.; Yang, Q.; Qiu, J.L.; Lu, J.Z.; Li, H. Enhanced ionic conductivity in LAGP/LATP composite electrolyte. Chin. Phys. B 2018, 27, 038201–038208. [Google Scholar] [CrossRef]
- Yang, H.; Bright, J.; Chen, B.; Zheng, P.; Gao, X.; Liu, B.; Kasani, S.; Zhang, X.; Wu, N. Chemical interaction and enhanced interfacial ion transport in a ceramic nanofiber-polymer composite electrolyte for all-solid-state lithium metal batteries. J. Mater. Chem. A 2020, 8, 7261–7272. [Google Scholar] [CrossRef]
- Polu, A.R.; Rhee, H.W. Ionic liquid doped PEO-based solid polymer electrolytes for lithium-ion polymer batteries. Int. J. Hydrogen Energy 2017, 42, 7212–7219. [Google Scholar] [CrossRef]
- Shi, X.; Ma, N.; Wu, Y.; Lu, Y.; Xiao, Q.; Li, Z.; Lei, G. Fabrication and electrochemical properties of LATP/PVDF composite electrolytes for rechargeable lithium-ion battery. Solid State Ion. 2018, 325, 112–119. [Google Scholar] [CrossRef]
- Chen, S.Y.; Hsieh, C.T.; Zhang, R.S.; Mohanty, D.; Gandomi, Y.A.; Hung, I.M. Hybrid solid state electrolytes blending NASICON-type Li1+xAlxTi2–x(PO4)3 with poly(vinylidene fluoride-co-hexafluoropropene) for lithium metal batteries. Electrochim. Acta 2022, 427, 140903–140912. [Google Scholar] [CrossRef]

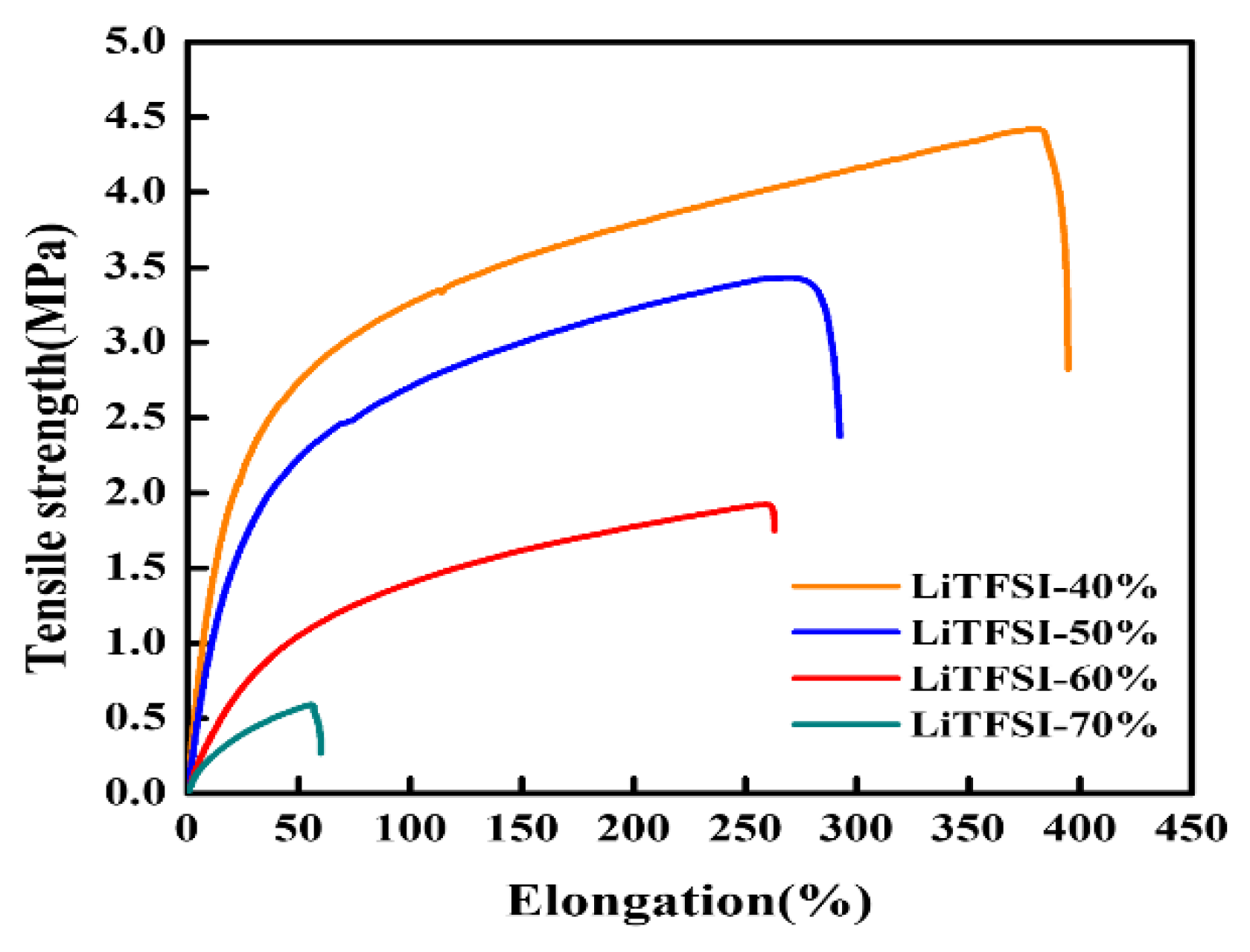
| Sample | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|
| LiTFSI-40% | 4.42 | 394.8 |
| LiTFSI-50% | 3.34 | 293.1 |
| LiTFSI-60% | 2.06 | 263.4 |
| LiTFSI-70% | 0.32 | 60.89 |
| Sample | Rb (Ω) | Rinf (Ω) | Rtotal (Ω) | σ (S cm−1) |
|---|---|---|---|---|
| LiTFSI-40% | 342.2 | 805.3 | 1147.5 | 6.85 × 10−5 |
| LiTFSI-50% | 99.4 | 375.1 | 474.5 | 1.28 × 10−4 |
| LiTFSI-60% | 59.5 | 175.5 | 235.1 | 2.14 × 10−4 |
| LiTFSI-70% | 34.8 | 285.3 | 320.2 | 3.22 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, D.; Chen, S.-Y.; Hung, I.-M. Effect of Lithium Salt Concentration on Materials Characteristics and Electrochemical Performance of Hybrid Inorganic/Polymer Solid Electrolyte for Solid-State Lithium-Ion Batteries. Batteries 2022, 8, 173. https://doi.org/10.3390/batteries8100173
Mohanty D, Chen S-Y, Hung I-M. Effect of Lithium Salt Concentration on Materials Characteristics and Electrochemical Performance of Hybrid Inorganic/Polymer Solid Electrolyte for Solid-State Lithium-Ion Batteries. Batteries. 2022; 8(10):173. https://doi.org/10.3390/batteries8100173
Chicago/Turabian StyleMohanty, Debabrata, Shu-Yu Chen, and I-Ming Hung. 2022. "Effect of Lithium Salt Concentration on Materials Characteristics and Electrochemical Performance of Hybrid Inorganic/Polymer Solid Electrolyte for Solid-State Lithium-Ion Batteries" Batteries 8, no. 10: 173. https://doi.org/10.3390/batteries8100173
APA StyleMohanty, D., Chen, S.-Y., & Hung, I.-M. (2022). Effect of Lithium Salt Concentration on Materials Characteristics and Electrochemical Performance of Hybrid Inorganic/Polymer Solid Electrolyte for Solid-State Lithium-Ion Batteries. Batteries, 8(10), 173. https://doi.org/10.3390/batteries8100173