Covalently Bonded Ball-Milled Silicon/CNT Nanocomposite as Lithium-Ion Battery Anode Material
Abstract
1. Introduction
2. Results and Discussion
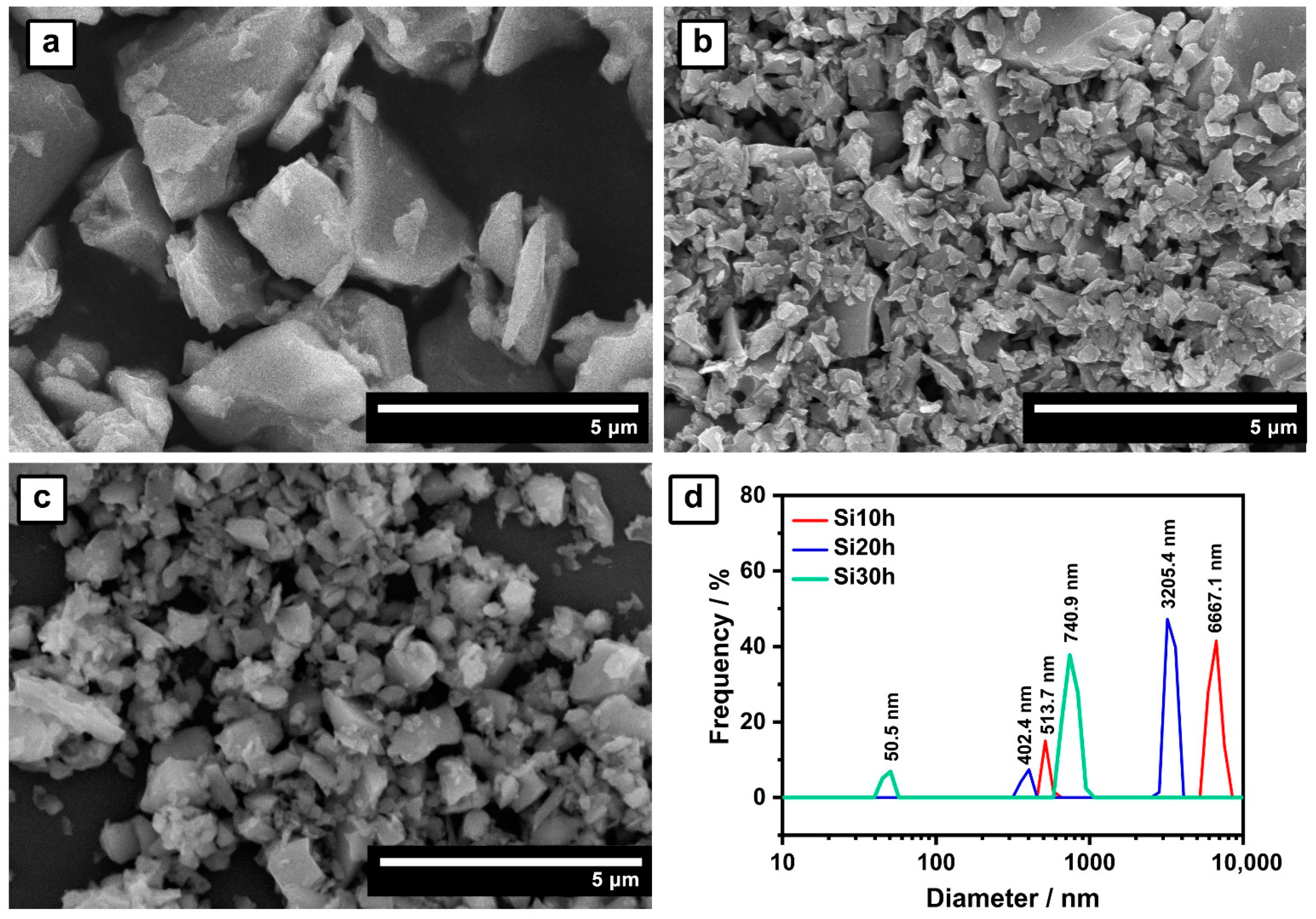
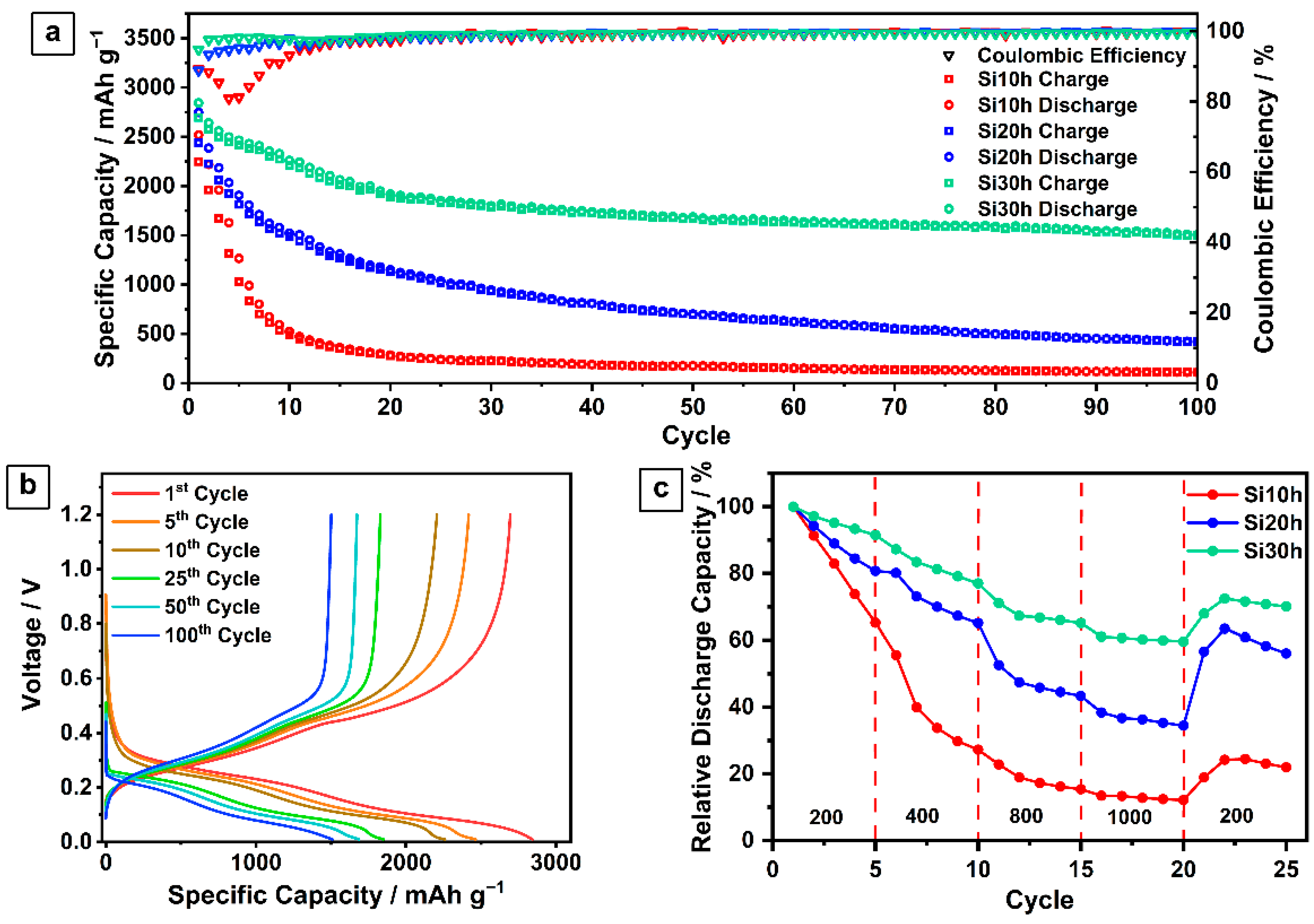
2.1. Ball-Milled Silicon Anode
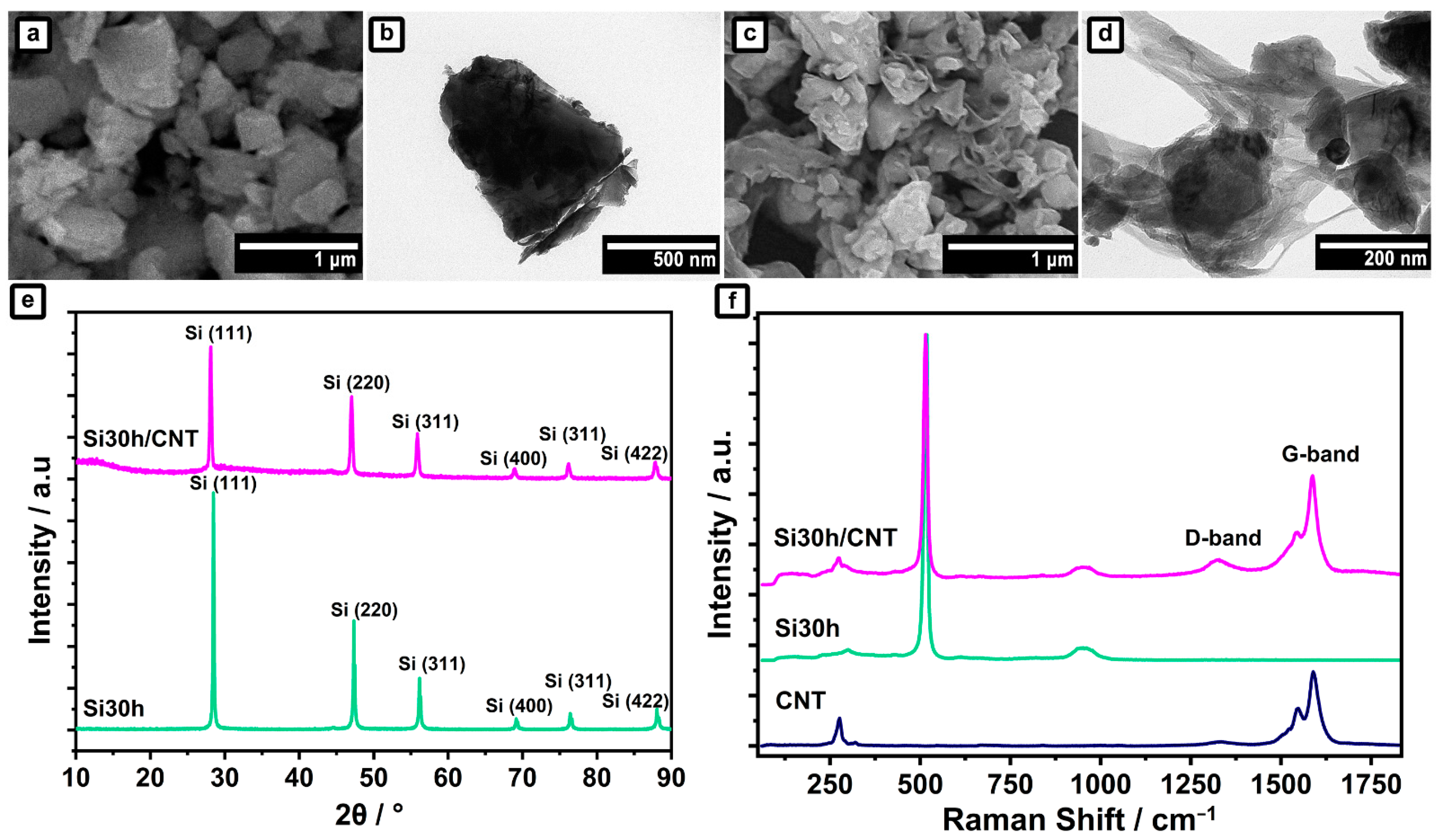
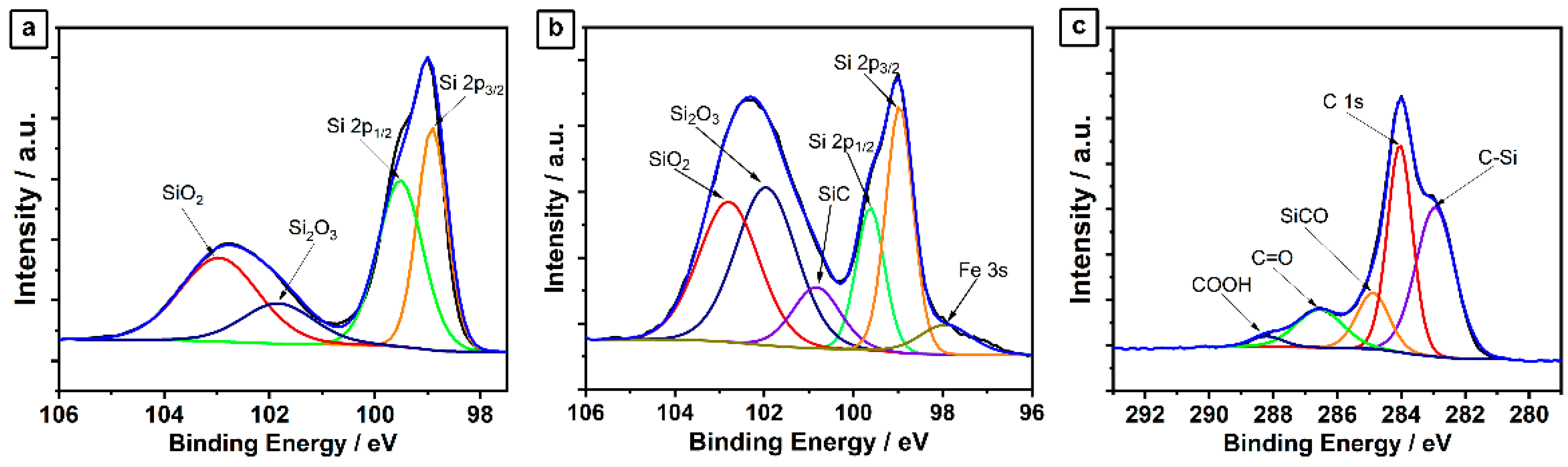
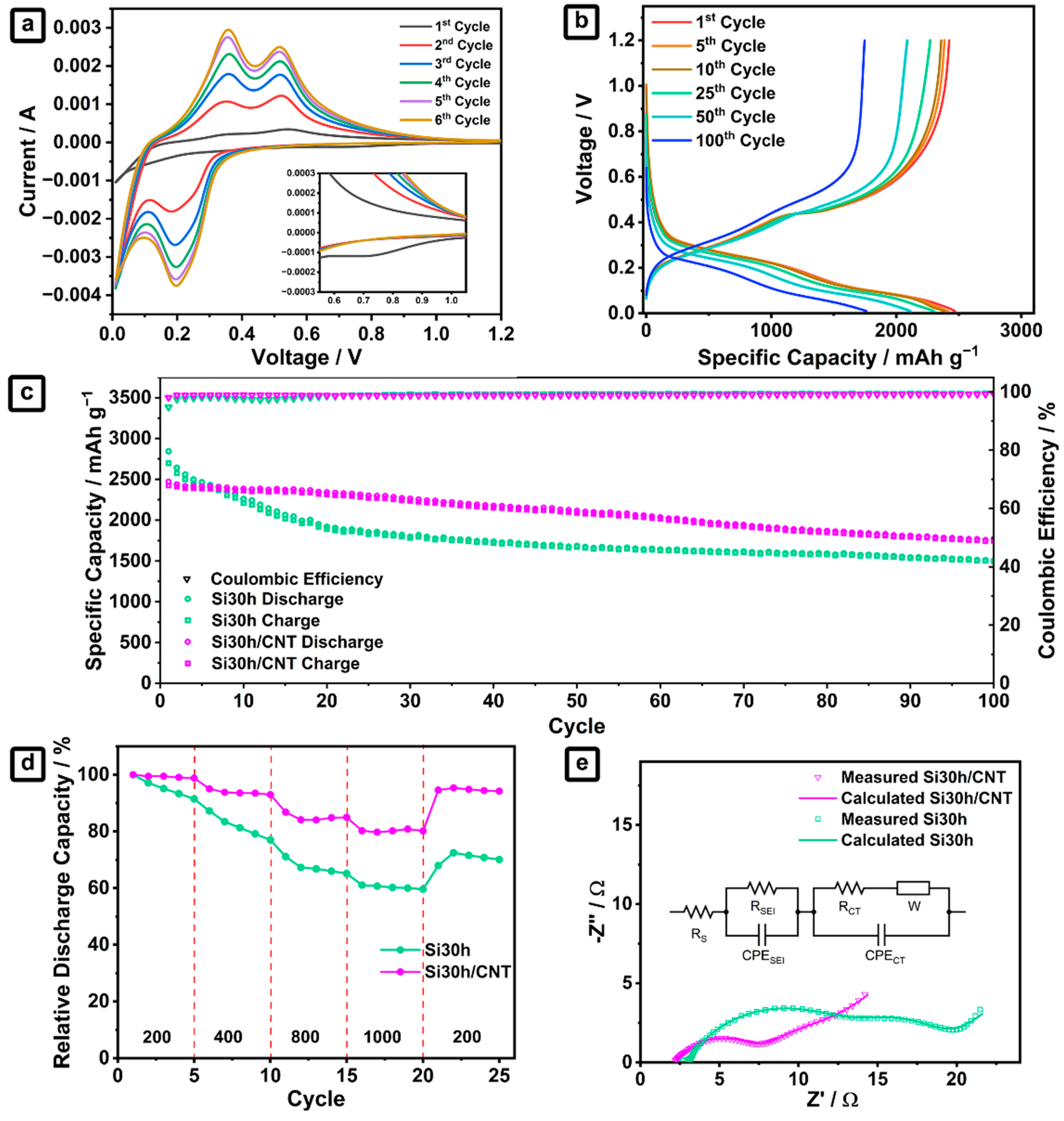
2.2. Ball-Milled Silicon/CNTs Nanocomposites Anode
3. Materials and Methods
3.1. Synthesis of Si/CNT Composite
3.2. Material Characterizations
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houache, M.S.E.; Yim, C.-H.; Karkar, Z.; Abu-Lebdeh, Y. On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review. Batteries 2022, 8, 70. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Liu, Z.; Zheng, J.; Zuo, Y.; Xue, C.; Li, C.; Cheng, B. High-performance ball-milled SiOx anodes for lithium ion batteries. J. Power Sources 2017, 339, 86–92. [Google Scholar] [CrossRef]
- Ping, W.; Yang, C.; Bao, Y.; Wang, C.; Xie, H.; Hitz, E.; Cheng, J.; Li, T.; Hu, L. A silicon anode for garnet-based all-solid-state batteries: Interfaces and nanomechanics. Energy Storage Mater. 2019, 21, 246–252. [Google Scholar] [CrossRef]
- Du, F.-H.; Wang, K.-X.; Chen, J.-S. Strategies to succeed in improving the lithium-ion storage properties of silicon nanomaterials. J. Mater. Chem. A 2016, 4, 32–50. [Google Scholar] [CrossRef]
- Kwon, H.-T.; Lee, C.K.; Jeon, K.-J.; Park, C.-M. Silicon diphosphide: A Si-based three-dimensional crystalline framework as a high-performance Li-ion battery anode. ACS Nano 2016, 10, 5701–5709. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, C.; Hao, S.; Zhu, K.; Zhang, P. An easy way for preparing high performance porous silicon powder by acid etching Al–Si alloy powder for lithium ion battery. Electrochim. Acta 2014, 115, 393–398. [Google Scholar] [CrossRef]
- Jo, H.; Kim, J.; Nguyen, D.-T.; Kang, K.K.; Jeon, D.-M.; Yang, A.-R.; Song, S.-W. Stabilizing the solid electrolyte interphase layer and cycling performance of silicon–graphite battery anode by using a binary additive of fluorinated carbonates. J. Phys. Chem. C 2016, 120, 22466–22475. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Wu, S.; Zhang, X.; Li, C.; Xue, C.; Cheng, B. High-columbic-efficiency lithium battery based on silicon particle materials. Nanoscale Res. Lett. 2015, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.P.; Hawari, N.H.; Prakoso, B.; Refino, A.D.; Yulianto, N.; Iskandar, F.; Kartini, E.; Peiner, E.; Wasisto, H.S.; Sumboja, A. Vertically Aligned n-Type Silicon Nanowire Array as a Free-Standing Anode for Lithium-Ion Batteries. Nanomaterials 2021, 11, 3137. [Google Scholar] [CrossRef] [PubMed]
- Refino, A.D.; Yulianto, N.; Syamsu, I.; Nugroho, A.P.; Hawari, N.H.; Syring, A.; Kartini, E.; Iskandar, F.; Voss, T.; Sumboja, A.; et al. Versatilely tuned vertical silicon nanowire arrays by cryogenic reactive ion etching as a lithium-ion battery anode. Sci. Rep. 2021, 11, 19779. [Google Scholar] [CrossRef] [PubMed]
- Nzabahimana, J.; Liu, Z.; Guo, S.; Wang, L.; Hu, X. Top-Down Synthesis of Silicon/Carbon Composite Anode Materials for Lithium-Ion Batteries: Mechanical Milling and Etching. Chem. Sus. Chem. 2020, 13, 1923–1946. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xu, Y.; Xian, X.; Liu, N.; Li, W. Fabrication of Porous Si@C Composites with Core-Shell Structure and Their Electrochemical Performance for Li-ion Batteries. Batteries 2019, 5, 27. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Hou, Z.; Wei, H. Poly (acrylic acid sodium) grafted carboxymethyl cellulose as a high performance polymer binder for silicon anode in lithium ion batteries. Sci. Rep. 2016, 6, 19583. [Google Scholar] [CrossRef]
- Porcher, W.; Chazelle, S.; Boulineau, A.; Mariage, N.; Alper, J.; Van Rompaey, T.; Bridel, J.-S.; Haon, C. Understanding polyacrylic acid and lithium polyacrylate binder behavior in silicon based electrodes for Li-ion batteries. J. Electrochem. Soc. 2017, 164, A3633. [Google Scholar] [CrossRef]
- Wang, Q.; Ban, Y.; Zhou, H.; Zhang, L.; Huang, Y.; Shao, W.; Chen, S. Preparation of Si/TiO2 Composite by the Sol-Gel Method Using As the Lithium-Ion Battery Anode. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 250, 012054. [Google Scholar] [CrossRef]
- Liu, B.; Huang, P.; Xie, Z.; Huang, Q. Large-Scale Production of a Silicon Nanowire/Graphite Composites Anode via the CVD Method for High-Performance Lithium-Ion Batteries. Energy Fuels 2021, 35, 2758–2765. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Huang, S.; Liu, L.; Wang, Y.; Jin, J.; Kong, D.; Zhang, L.; Schmidt, O.G. PVD customized 2D porous amorphous silicon nanoflakes percolated with carbon nanotubes for high areal capacity lithium ion batteries. J. Mater. Chem. A 2020, 8, 4836–4843. [Google Scholar] [CrossRef]
- Fasoli, A.; Milne, W. Overview and status of bottom-up silicon nanowire electronics. Mater. Sci. Semicond. Process. 2012, 15, 601–614. [Google Scholar] [CrossRef]
- Magasinski, A.; Dixon, P.; Hertzberg, B.; Kvit, A.; Ayala, J.; Yushin, G. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat. Mater. 2010, 9, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Yuda, A.P.; Koraag, P.Y.E.; Iskandar, F.; Wasisto, H.S.; Sumboja, A. Advances of the top-down synthesis approach for high-performance silicon anodes in Li-ion batteries. J. Mater. Chem. A 2021, 9, 18906–18926. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Morcrette, M.; Saint, J.; Aymard, L.; Janot, R. On the benefits of ball milling within the field of rechargeable Li-based batteries. C. R. Chim. 2005, 8, 17–26. [Google Scholar] [CrossRef]
- Yang, T.; Gao, Y.; Tang, Y.; Zhang, Y.; Li, X.; Liu, L. Porous silicon from industrial waste engineered for superior stability lithium-ion battery anodes. J. Nanopart. Res. 2021, 23, 209. [Google Scholar] [CrossRef]
- Mei, S.; Liu, Y.; Fu, J.; Guo, S.; Deng, J.; Peng, X.; Zhang, X.; Gao, B.; Huo, K.; Chu, P.K. Waste-glass-derived silicon/CNTs composite with strong Si-C covalent bonding for advanced anode materials in lithium-ion batteries. Appl. Surf. Sci. 2021, 563, 150280. [Google Scholar] [CrossRef]
- Eom, J.Y.; Kwon, H.S. Preparation of single-walled carbon nanotube/silicon composites and their lithium storage properties. ACS Appl. Mater. Interfaces 2011, 3, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Niu, X.; Wang, D.; Zhang, W.; Shi, H.; Yu, L.; Wang, C.; Xiong, Z.; Ji, Z.; Yan, X.; et al. Simple preparation of Si/CNTs/C composite derived from photovoltaic waste silicon powder as high-performance anode material for Li-ion batteries. Powder Technol. 2022, 408, 117744. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Yang, S.; Qu, Q.; Zheng, H. Correlation between the physical parameters and the electrochemical performance of a silicon anode in lithium-ion batteries. J. Mater. 2019, 5, 164–175. [Google Scholar] [CrossRef]
- Nachev, S.; Rango, P.; Skryabina, N.; Skachkov, A.; Aptukov, V.; Fruchart, D.; Marty, P. international journal of hydrogen energy. Int. J. Hydrog. Energy 2015, 40, 17065–17074. [Google Scholar] [CrossRef]
- Mu, G.; Wu, B.; Ma, C.; Wu, F. Dynamics analysis of Si electrode particle size effect employing accurate Si model. Electrochim. Acta 2021, 377, 138110. [Google Scholar] [CrossRef]
- Gauthier, M.; Mazouzi, D.; Reyter, D.; Lestriez, B.; Moreau, P.; Guyomard, D.; Roué, L. A low-cost and high performance ball-milled Si-based negative electrode for high-energy Li-ion batteries. Energy Environ. Sci. 2013, 6, 2145–2155. [Google Scholar] [CrossRef]
- Hubbard, C.; Swanson, H.; Mauer, F. A Silicon Powder Diffraction Standard Reference Material. J. Appl. Crystallogr. 1975, 8, 45–48. [Google Scholar] [CrossRef]
- Fu, J.; Liu, H.; Liao, L.; Fan, P.; Wang, Z.; Wu, Y.; Zhang, Z.; Hai, Y.; Lv, G.; Mei, L.; et al. Ultrathin Si/CNTs Paper-Like Composite for Flexible Li-Ion Battery Anode With High Volumetric Capacity. Front. Chem. 2018, 6, 624. [Google Scholar] [CrossRef]
- Chou, S.-L.; Zhao, Y.; Wang, J.-Z.; Chen, Z.-X.; Liu, H.-K.; Dou, S.-X. Silicon/Single-Walled Carbon Nanotube Composite Paper as a Flexible Anode Material for Lithium Ion Batteries. J. Phys. Chem. C 2010, 114, 15862–15867. [Google Scholar] [CrossRef]
- Roland, A.; Fullenwarth, J.; Ledeuil, J.-B.; Martinez, H.; Louvain, N.; Monconduit, L. How carbon coating or continuous carbon pitch matrix influence the silicon electrode/electrolyte interfaces and the performance in Li-ion batteries. Battery Energy 2022, 1, 20210009. [Google Scholar] [CrossRef]
- Futaba, D.N.; Yamada, T.; Kobashi, K.; Yumura, M.; Hata, K. Macroscopic Wall Number Analysis of Single-Walled, Double-Walled, and Few-Walled Carbon Nanotubes by X-ray Diffraction. J. Am. Chem. Soc. 2011, 133, 5716–5719. [Google Scholar] [CrossRef] [PubMed]
- Rotshteyn, V.M.; Turdaliev, T.K.; Ashurov, K.B. Analysis of Porous Nanosilicon by Raman Spectroscopy. J. Appl. Spectrosc. 2022, 89, 43–48. [Google Scholar] [CrossRef]
- Yan, X.; Suzuki, T.; Kitahama, Y.; Sato, H.; Itoh, T.; Ozaki, Y. A study on the interaction of single-walled carbon nanotubes (SWCNTs) and polystyrene (PS) at the interface in SWCNT–PS nanocomposites using tip-enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 20618–20624. [Google Scholar] [CrossRef]
- Huang, T.; Xu, Z.; Zeng, G.; Zhang, P.; Song, T.; Wang, Y.; Wang, T.; Huang, S.; Wang, T.; Zeng, H. Selective deposition of plasmonic copper on few layers graphene with specific defects for efficiently synchronous photocatalytic hydrogen production. Carbon 2019, 143, 257–267. [Google Scholar] [CrossRef]
- Telg, H.; Duque, J.G.; Staiger, M.; Tu, X.; Hennrich, F.; Kappes, M.M.; Zheng, M.; Maultzsch, J.; Thomsen, C.; Doorn, S.K. Chiral Index Dependence of the G+ and G– Raman Modes in Semiconducting Carbon Nanotubes. ACS Nano 2012, 6, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jiang, X.; Ban, B.; Li, J.; Chen, J. Carbon nanotubes-enhanced lithium storage capacity of recovered silicon/carbon anodes produced from solar-grade silicon kerf scrap. Electrochim. Acta 2021, 381, 138269. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, C.; Lin, X.; Chen, X.; Zhang, C.; Wei, F. Uniform coating of nano-carbon layer on SiOx in aggregated fluidized bed as high-performance anode material. Carbon 2019, 149, 462–470. [Google Scholar] [CrossRef]
- Cao, Y.; Dunlap, R.A.; Obrovac, M.N. Electrochemistry and Thermal Behavior of SiOx Made by Reactive Gas Milling. J. Electrochem. Soc. 2020, 167, 110501. [Google Scholar] [CrossRef]
- Palomino, J.; Varshney, D.; Weiner, B.R.; Morell, G. Study of the Structural Changes Undergone by Hybrid Nanostructured Si-CNTs Employed as an Anode Material in a Rechargeable Lithium-Ion Battery. J. Phys. Chem. C 2015, 119, 21125–21134. [Google Scholar] [CrossRef]
- Ohtsu, N.; Oku, M.; Satoh, K.; Wagatsuma, K. Dependence of core-level XPS spectra on iron silicide phase. Appl. Surf. Sci. 2013, 264, 219–224. [Google Scholar] [CrossRef]
- Cong, R.; Choi, J.-Y.; Song, J.-B.; Jo, M.; Lee, H.; Lee, C.-S. Characteristics and electrochemical performances of silicon/carbon nanofiber/graphene composite films as anode materials for binder-free lithium-ion batteries. Sci. Rep. 2021, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gao, Y.; Li, Q.; Wang, Y.; Zhao, D.; Xu, G.; Zhong, Z.; Su, F. Scalable synthesis of high-performance anode material SiOx/C for lithium-ion batteries by employing the Rochow reaction process. J. Alloys Compd. 2022, 902, 163668. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; He, R.; Luo, W.; Meng, J.; Yu, Q.; Zhao, D.; Zhou, L.; Mai, L. Yolk@Shell SiOx/C microspheres with semi-graphitic carbon coating on the exterior and interior surfaces for durable lithium storage. Energy Storage Mater. 2019, 19, 299–305. [Google Scholar] [CrossRef]
- Zeferino González, I.; Chiu, H.-C.; Gauvin, R.; Demopoulos, G.P.; Verde-Gómez, Y. Silicon doped carbon nanotubes as high energy anode for lithium-ion batteries. Mater. Today Commun. 2022, 30, 103158. [Google Scholar] [CrossRef]
- Ikonen, T.; Kalidas, N.; Lahtinen, K.; Isoniemi, T.; Toppari, J.J.; Vázquez, E.; Herrero-Chamorro, M.A.; Fierro, J.L.G.; Kallio, T.; Lehto, V.-P. Conjugation with carbon nanotubes improves the performance of mesoporous silicon as Li-ion battery anode. Sci. Rep. 2020, 10, 5589. [Google Scholar] [CrossRef]
- Parfeneva, A.V.; Rumyantsev, A.M.; Lozhkina, D.A.; Maximov, M.Y.; Astrova, E.V. Influence of Fluoroethylene Carbonate in the Composition of an Aprotic Electrolyte on the Electrochemical Characteristics of LIB’s Anodes Based on Carbonized Nanosilicon. Batteries 2022, 8, 91. [Google Scholar] [CrossRef]
- Sohn, M.; Park, H.I.; Kim, H. Foamed silicon particles as a high capacity anode material for lithium-ion batteries. Chem. Commun. 2017, 53, 11897–11900. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, J.; Xu, X.; Han, X.; Chen, M.; Yang, L.; Hirano, S.I. Large areal capacity all-in-one lithium-ion battery based on boron-doped silicon/carbon hybrid anode material and cellulose framework. J. Colloid Interface Sci. 2022, 612, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Choi, C.K.; Park, M.S.; Lee, S.M. Spherical Silicon/CNT/Carbon Composite Wrapped with Graphene as an Anode Material for Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2022, 13, 159–166. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Lu, D.; Guan, R.; Wang, J.; Bian, X. Si-based composite deriving from wok ash waste as high-performance anode for Li-ion battery. J. Alloys Compd. 2021, 858, 157680. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, C.S.; Hwang, J.Y.; Kim, S.J.; Maglia, F.; Lamp, P.; Myung, S.T.; Sun, Y.K. High-energy-density lithium-ion battery using a carbon-nanotube–Si composite anode and a compositionally graded Li[Ni0.85Co0.05Mn0.10]O2 cathode. Energy Environ. Sci. 2016, 9, 2152–2158. [Google Scholar] [CrossRef]
- Tugrul, C.; Mehmet, O.G.; Hatem, A. Enhancing electrochemical performance of silicon anodes by dispersing MWCNTs using planetary ball milling. Microelectron. Eng. 2013, 108, 169–176. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koraag, P.Y.E.; Firdaus, A.M.; Hawari, N.H.; Refino, A.D.; Dempwolf, W.; Iskandar, F.; Peiner, E.; Wasisto, H.S.; Sumboja, A. Covalently Bonded Ball-Milled Silicon/CNT Nanocomposite as Lithium-Ion Battery Anode Material. Batteries 2022, 8, 165. https://doi.org/10.3390/batteries8100165
Koraag PYE, Firdaus AM, Hawari NH, Refino AD, Dempwolf W, Iskandar F, Peiner E, Wasisto HS, Sumboja A. Covalently Bonded Ball-Milled Silicon/CNT Nanocomposite as Lithium-Ion Battery Anode Material. Batteries. 2022; 8(10):165. https://doi.org/10.3390/batteries8100165
Chicago/Turabian StyleKoraag, Pierre Yosia Edward, Arief Muhammad Firdaus, Naufal Hanif Hawari, Andam Deatama Refino, Wibke Dempwolf, Ferry Iskandar, Erwin Peiner, Hutomo Suryo Wasisto, and Afriyanti Sumboja. 2022. "Covalently Bonded Ball-Milled Silicon/CNT Nanocomposite as Lithium-Ion Battery Anode Material" Batteries 8, no. 10: 165. https://doi.org/10.3390/batteries8100165
APA StyleKoraag, P. Y. E., Firdaus, A. M., Hawari, N. H., Refino, A. D., Dempwolf, W., Iskandar, F., Peiner, E., Wasisto, H. S., & Sumboja, A. (2022). Covalently Bonded Ball-Milled Silicon/CNT Nanocomposite as Lithium-Ion Battery Anode Material. Batteries, 8(10), 165. https://doi.org/10.3390/batteries8100165







