Thermal Mapping of a Lithium Polymer Batteries Pack with FBGs Network
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fiber Bragg Grating Sensor Network
2.2. Experimental Setup
3. Results and Discussion
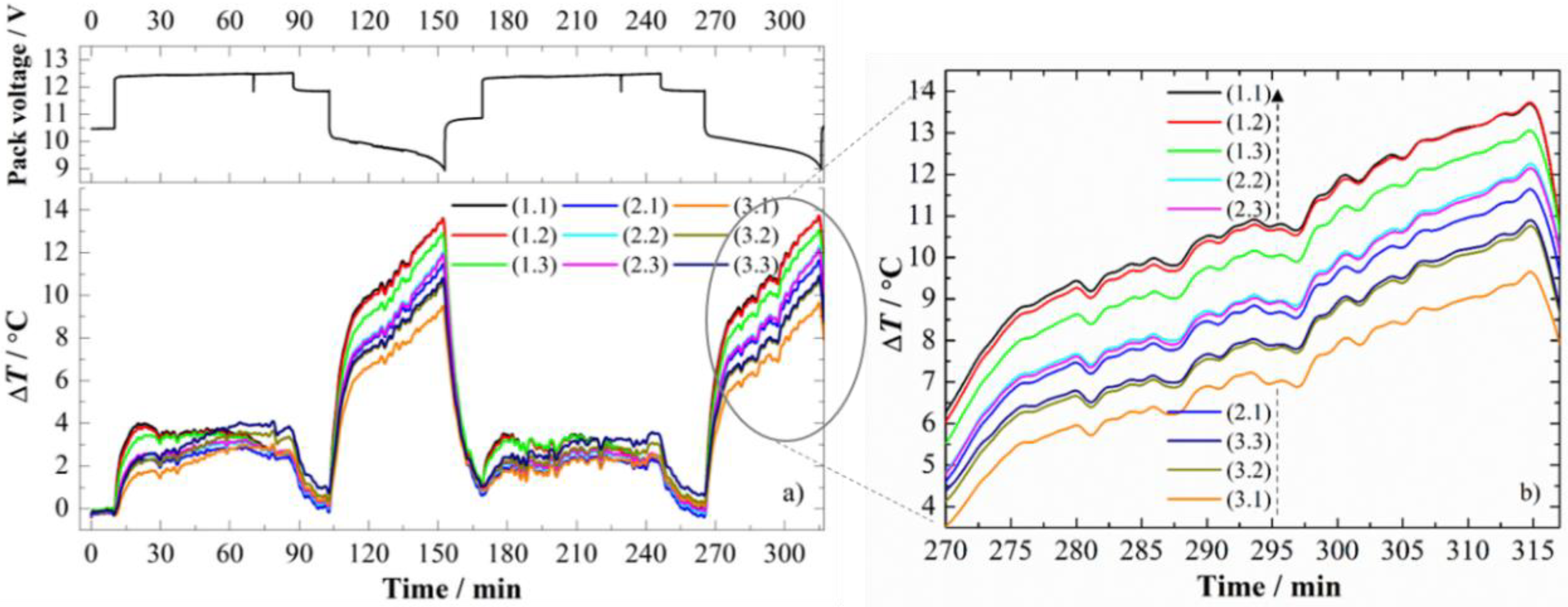
3.1. Cycling Protocols—Voltage and Temperature Curves
3.2. Spatial and Temporal Thermal Mapping
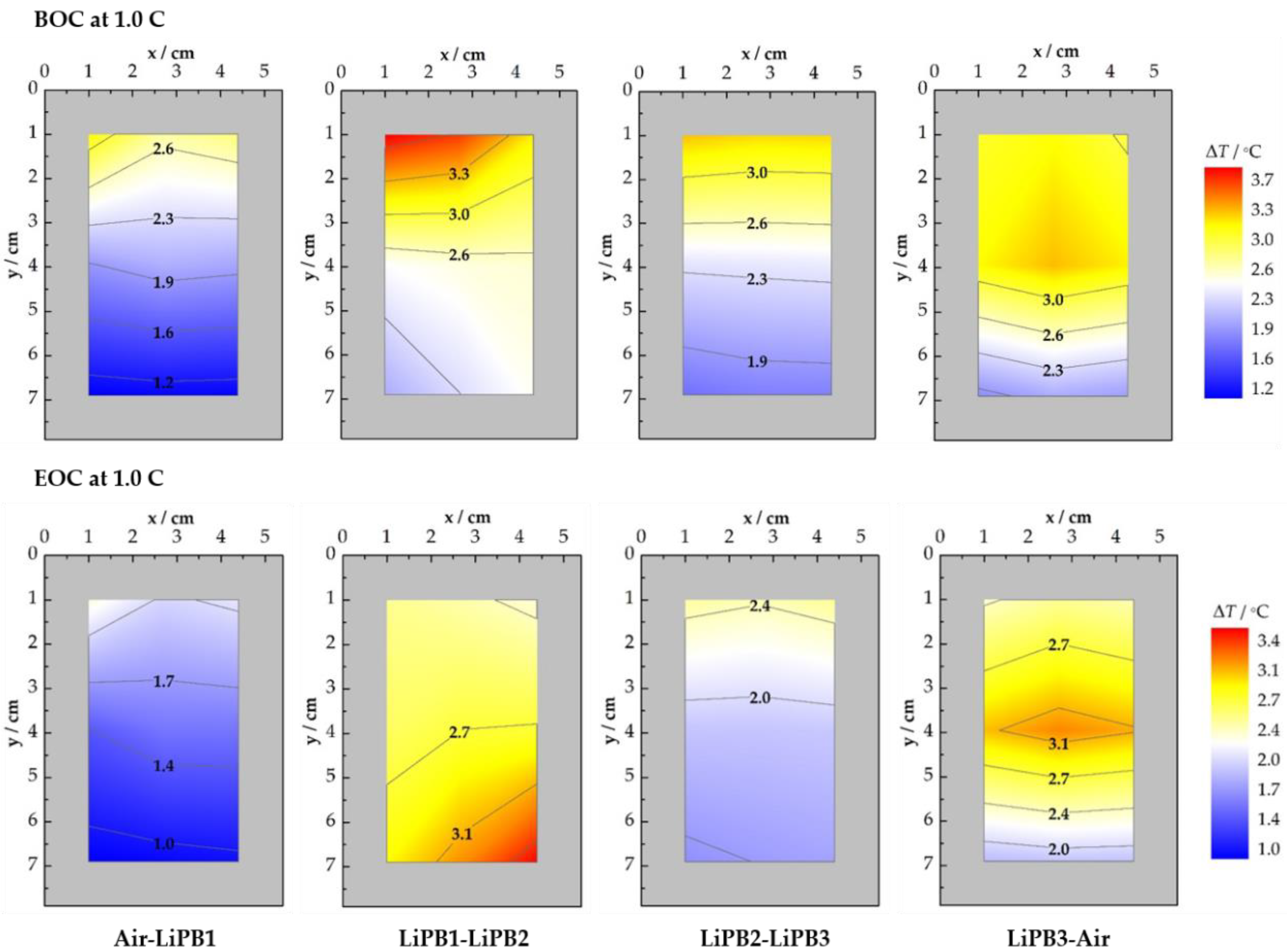
3.2.1. CC Charge at 1.0 C
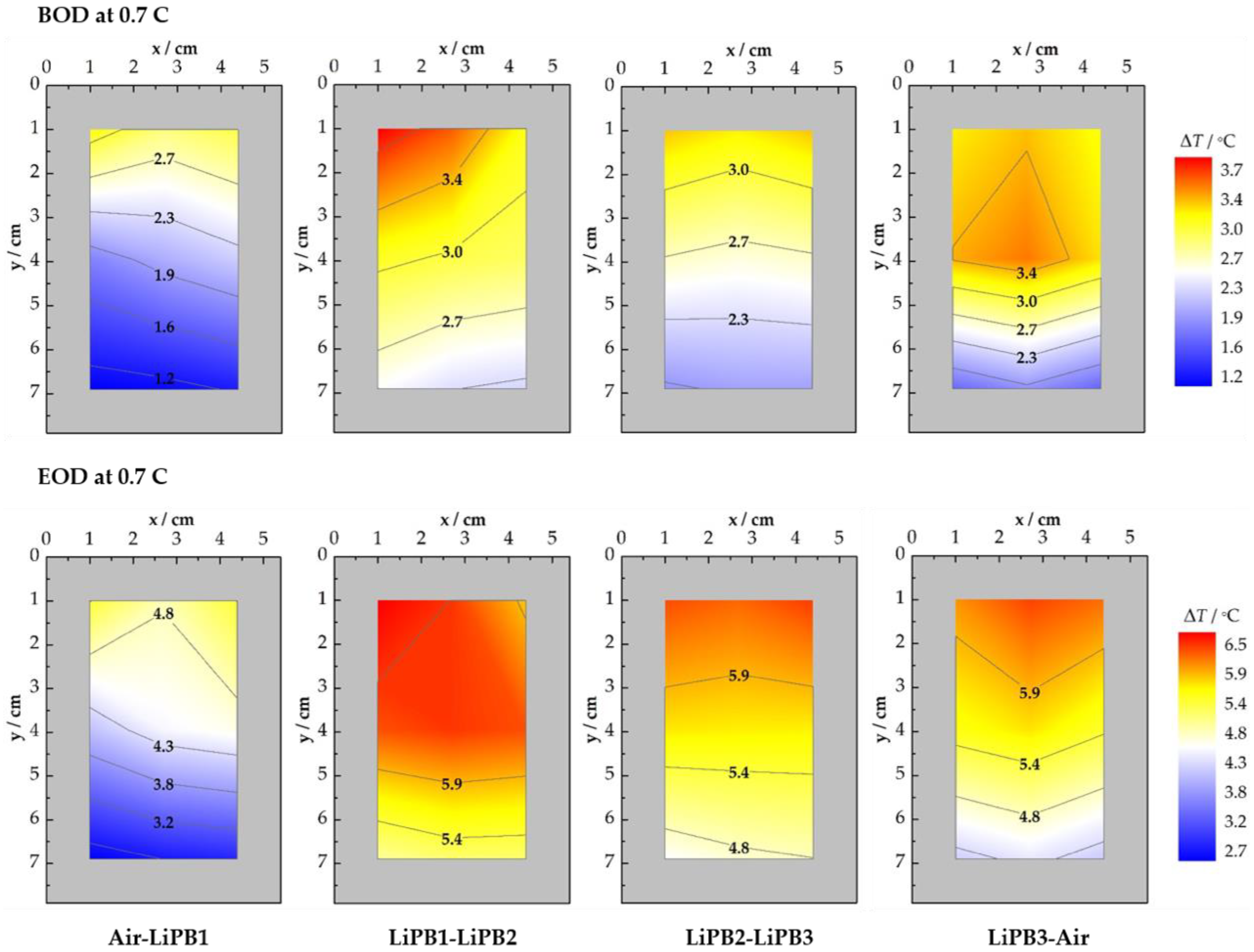
3.2.2. CC Discharge at 0.7 C
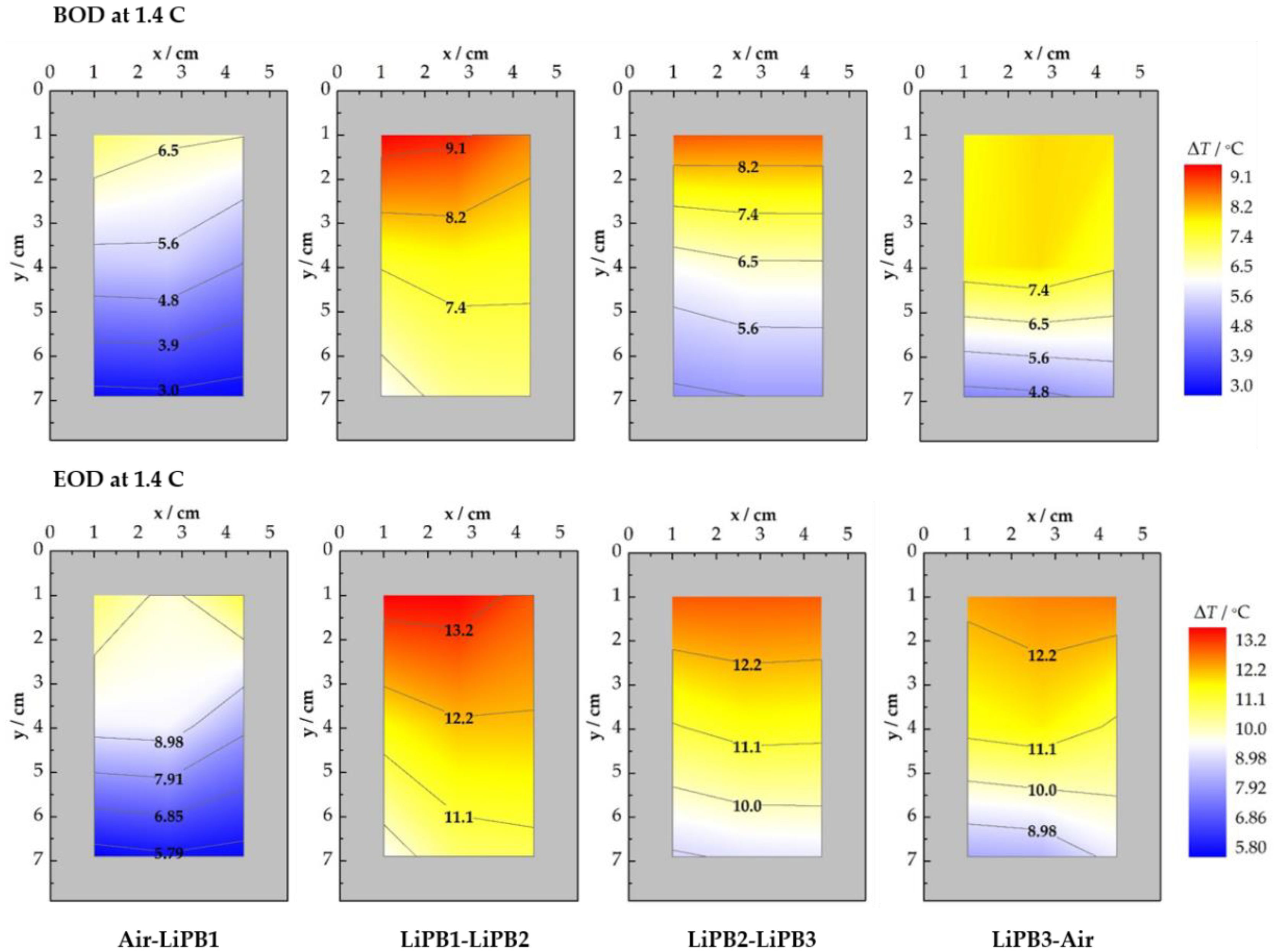
3.2.3. CC Discharge at 1.4 C
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Ramadass, P.; Fang, W. Safety of lithium-ion batteries. In Lithium-Ion Batteries: Advances and Applications; Pistoia, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 18, pp. 409–435. ISBN 978-0-444-59513-3. [Google Scholar]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Panchal, S.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Experimental temperature distributions in a prismatic lithium-ion battery at varying conditions. Int. Commun. Heat Mass Transf. 2016, 71, 35–43. [Google Scholar] [CrossRef]
- Xing, Y.; Miao, Q.; Tsui, K.-L.; Pecht, M. Prognostics and health monitoring for lithium-ion battery. In Proceedings of the IEEE International Conference on Intelligence and Security Informatics, Beijing, China, 10–12 July 2011. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Koch, S.; Birke, K.P.; Kuhn, R. Fast thermal runaway detection for lithium-ion cells in large scale traction batteries. Batteries 2018, 4, 16. [Google Scholar] [CrossRef]
- Lei, B.; Zhao, W.; Ziebert, C.; Uhlmann, N.; Rohde, M.; Seifert, H.J. Experimental analysis of thermal runaway in 18650 cylindrical Li-ion cells using an accelerating rate calorimeter. Batteries 2017, 3, 14. [Google Scholar] [CrossRef]
- Loveridge, M.J.; Remy, G.; Kourra, N.; Genieser, R.; Barai, A.; Lain, M.J.; Guo, Y.; Amor-Segan, M.; Williams, M.A.; Amietszajew, T.; et al. Looking deeper into the Galaxy (Note 7). Batteries 2018, 4, 3. [Google Scholar] [CrossRef]
- Bella, F.; Colò, F.; Nair, J.R.; Gerbaldi, C. Photopolymer electrolytes for sustainable upscalable, safe, and ambient-temperature sodium-ion secondary batteries. ChemSusChem 2015, 8, 3668–3676. [Google Scholar] [CrossRef]
- Radzir, N.N.H.; Hanifah, S.A.; Ahmad, A.; Hassan, N.H.; Bella, F. Effect of lithium bis(trifluoromethylsulfonyl)imide salt-doped UV-cured glycidyl methacrylate. Solid State Electrochem. 2015, 19, 3079–3085. [Google Scholar] [CrossRef]
- Suriyakumar, S.; Gopi, S.; Kathiresan, M.; Bose, S.; Gowd, E.B.; Nair, J.R.; Angulakshmi, N.; Meligrana, G.; Bella, F.; Gerbaldi, C.; et al. Metal organic framework laden poly(ethylene oxide) based composite electroytes for all-solid-state Li-S and Li-metal polymer batteries. Electrochem. Acta 2018, 285, 355–364. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Lin, H.; Min, Y.; Pan, F. Composite electrolytes of pyrrolidone-derivates-PEO enable to enhance performance of all solid-state lithium-ion batteries. Electrochem. Acta 2019, 293, 25–29. [Google Scholar] [CrossRef]
- Mankowski, P.J.; Kanevski, J.; Bakirtzian, P.; Cugno, S. Cellular phone collateral damage: A review of burns associated with lithium battery powered mobile devices. Burns 2016, 42, e61–e64. [Google Scholar] [CrossRef]
- Duh, Y.S.; Lin, K.H.; Kao, C.S. Experimental investigation and visualization on thermal runaway of hard prismatic lithium-ion batteries used in smart phones. J. Therm. Anal. Calorim. 2018, 132, 1677–1692. [Google Scholar] [CrossRef]
- Panchal, S.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Thermal modeling and validation of temperature distributions in a prismatic lithium-ion battery at different discharge rates and varying boundary conditions. Appl. Therm. Eng. 2015, 96, 190–199. [Google Scholar] [CrossRef]
- Panchal, S.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Experimental and theoretical investigation of temperature distributions in a prismatic lithium-ion battery. Int. J. Therm. Sci. 2015, 99, 204–2015. [Google Scholar] [CrossRef]
- Mutyala, M.S.K.; Zhao, J.; Li, J.; Pan, H.; Yuan, C.; Li, X. In situ temperature measurement in lithium-ion battery by flexible thin film thermocouples. J. Power Sources 2014, 260, 43–49. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, S.; Li, K.; Liu, C.; Cheng, X.; Zhang, H. An experimental study on burning behaviors of 18650 lithium ion batteries using a cone calorimeter. J. Power Sources 2015, 273, 216–222. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Wu, B.; Huang, J.; Nie, Z.; Sun, Y.; An, F.; Wu, N. Examining temporal and spatial variations of internal temperature in large-format laminated battery with embedded thermocouples. J. Power Sources 2013, 241, 536–553. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, C.Y. Heating strategies for Li-ion batteries operated from subzero temperatures. Electrochem. Acta 2013, 107, 664–674. [Google Scholar] [CrossRef]
- Tippmann, S.; Wapler, D.; Balboa, L.; Spier, B.; Bessler, W.G. Low temperature charging of lithium-ion cells part I: Eletrochemical modeling and experimental investigation of degradation behavior. J. Power Sources 2014, 252, 305–316. [Google Scholar] [CrossRef]
- Yildiz, M.; Karakoc, H.; Dincer, I. Modelling and validation of temperature changes in pouch lithium-ion battery at various discharge rates. Int. Commun. Heat Mass Transf. 2016, 75, 311–314. [Google Scholar] [CrossRef]
- Panchal, S.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Uneven temperature and voltage distributions due to rapid discharge rates and different boundary conditions for series-connected LiFePO4 batteries. Int. Commun. Heat Mass Transf. 2017, 81, 210–217. [Google Scholar] [CrossRef]
- Mathew, M.; Mastali, M.; Catton, J.; Samadani, E.; Janhunen, S.; Fowler, M. Development of an electro-thermal model for electric vehicles using a design of experiments approach. Batteries 2018, 4, 29. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Weng, F.-B.; Huang, Y.-P.; Chang, C.-P.; Cheng, C.-K. Real time monitoring of internal temperature and voltage of high-temperature fuel cell stack. Electrochim. Acta 2015, 161, 413–419. [Google Scholar] [CrossRef]
- Yi, J.; Kim, U.S.; Chee, S.; Han, T.; Park, S. Modelling the temperature dependence of the discharge behavior of a lithium-ion battery in low environmental temperature. J. Power Sources 2013, 244, 143–148. [Google Scholar] [CrossRef]
- Kim, U.S.; Yi, J.; Chee, S.; Han, T.; Park, S. Modelling the thermal bahaviour of a lithium-ion battery during charge. J. Power Sources 2011, 196, 5115–5121. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Yang, L.; Zhang, X.; Yang, M.; Chen, H.; Fang, D. Real-time monitoring of internal temperature evolution of Li-ion coin cell battery during the charge and discharge process. Extreme Mech. Lett. 2016, 9, 459–466. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lee, S.-J.; Hung, Y.-M.; Hsieh, C.-T.; Chang, Y.-M.; Huang, Y.-T.; Lin, J.-T. Integrated microsensor for real-time microscopic monitoring of local temperature, voltage and current inside lithium ion battery. Sens. Actuators A Phys. 2017, 253, 59–68. [Google Scholar] [CrossRef]
- Grattan, K.T.V.; Meggitt, B.T. Optical Fiber Sensor Technology: Applications and Systems; Kluwer Academic Publishers: London, UK, 1999; Volume 3, pp. 357–363. ISBN 978-1-4757-6077-4. [Google Scholar]
- Othonos, A.; Kalli, K. Fiber Bragg Gratings: Fundamentals and Applications in Telecommunications and Sensing; Artech House: New York, NY, USA, 1999. [Google Scholar]
- Yang, G.; Leitão, C.; Lib, Y.; Pinto, J.L.; Jiang, X. Real-time temperature measurement with fiber Bragg sensors in lithium batteries for safety usage. Measurement 2013, 46, 3166–3172. [Google Scholar] [CrossRef]
- Sommer, L.W.; Kiesel, P.; Ganguli, A.; Lochbaum, A.; Saha, B.; Schwartz, J.; Bae, C.-J.; Alamgir, M. Fast and slow ion diffusion processes in lithium-ion pouch cells during cycling observed with fiber optic strain sensors. J. Power Sources 2015, 296, 46–52. [Google Scholar] [CrossRef]
- Novais, S.; Nascimento, M.; Grande, L.; Domingues, M.F.; Antunes, P.; Alberto, A.; Leitão, C.; Oliveira, R.; Koch, S.; Kim, G.T.; et al. Internal and external temperature monitoring of a Li-ion battery with fiber Bragg grating sensors. Sensors 2016, 16, 1394. [Google Scholar] [CrossRef]
- Nascimento, M.; Ferreira, M.; Pinto, J.L. Real time thermal monitoring of lithium batteries with fiber sensors and thermocouples: A comparative study. Measurement 2017, 111, 260–263. [Google Scholar] [CrossRef]
- Gao, H.; Li, H.; Liu, B.; Zhang, H.; Luo, J.; Cao, Y.; Yuan, S.; Zhang, W.; Kai, G.; Dong, X. A novel fiber Bragg grating sensor multiplexing technique. Opt. Commun. 2005, 251, 361–366. [Google Scholar] [CrossRef]
- Mihailov, S.J. Fiber Bragg Grating Sensors for Harsh Environments. Sensors 2012, 12, 1898–1918. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Mizunami, T.; Yamao, T.; Shimomura, T. Fiber Bragg grating cryogenic temperature sensors. Appl. Opt. 1996, 35, 5202–5205. [Google Scholar] [CrossRef]
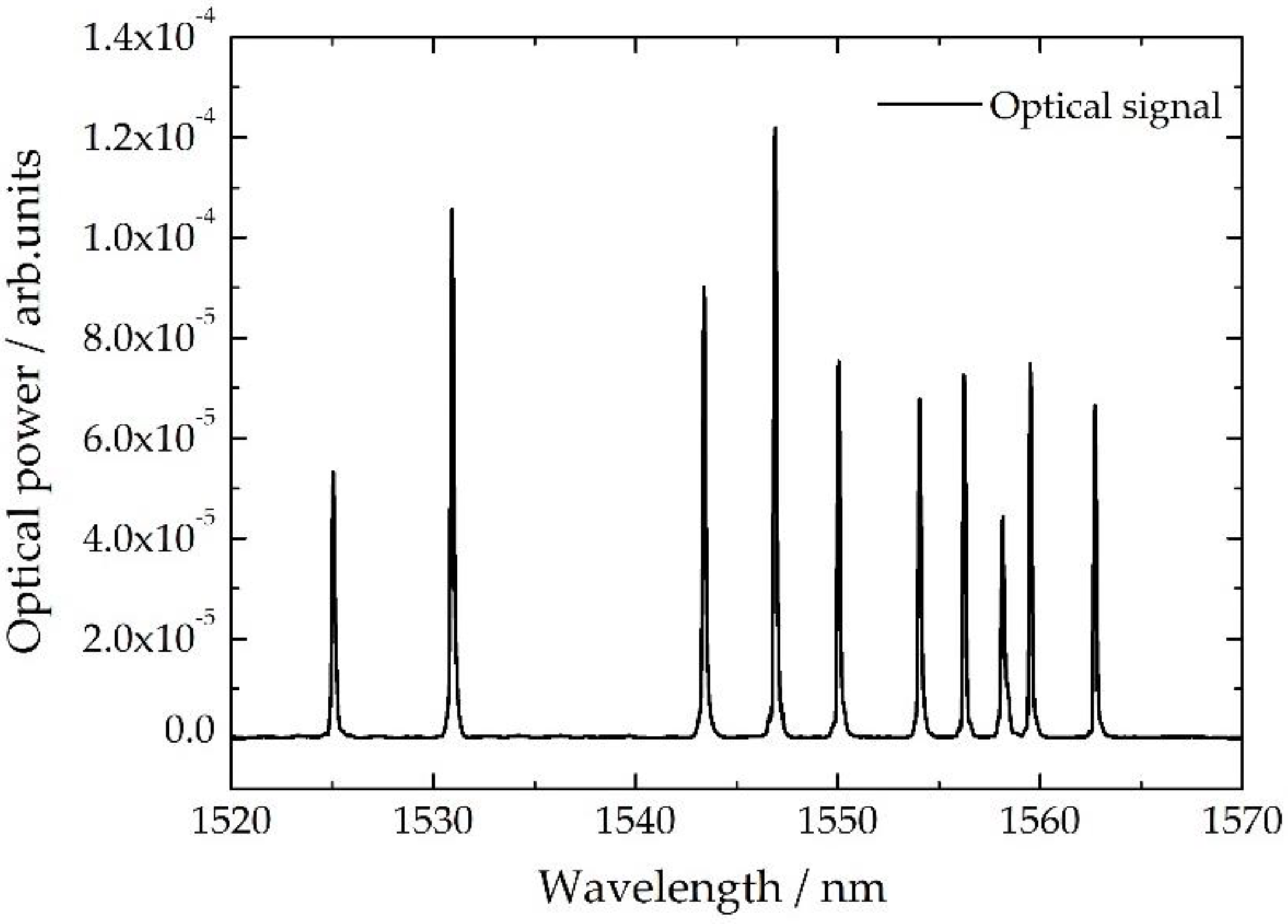
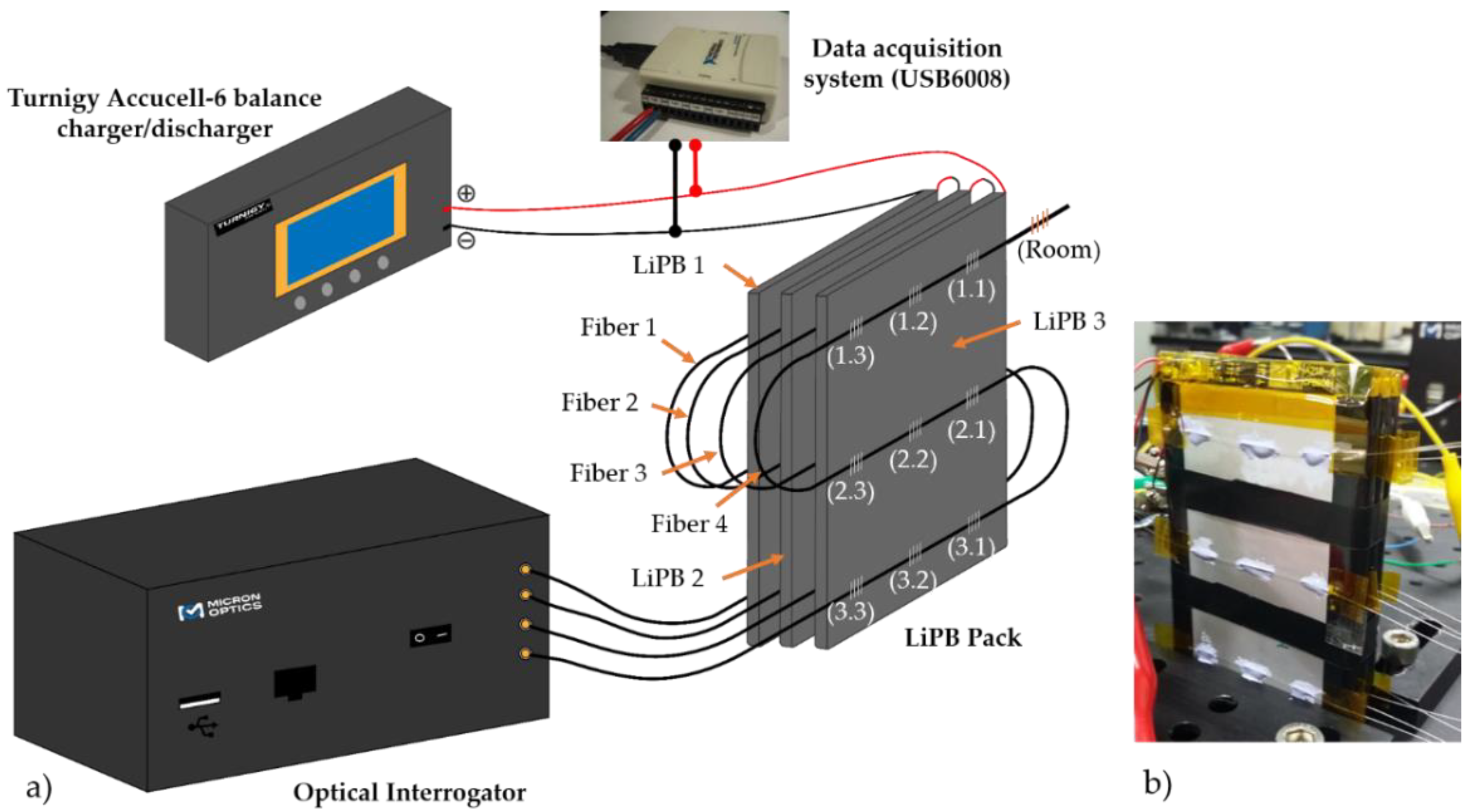
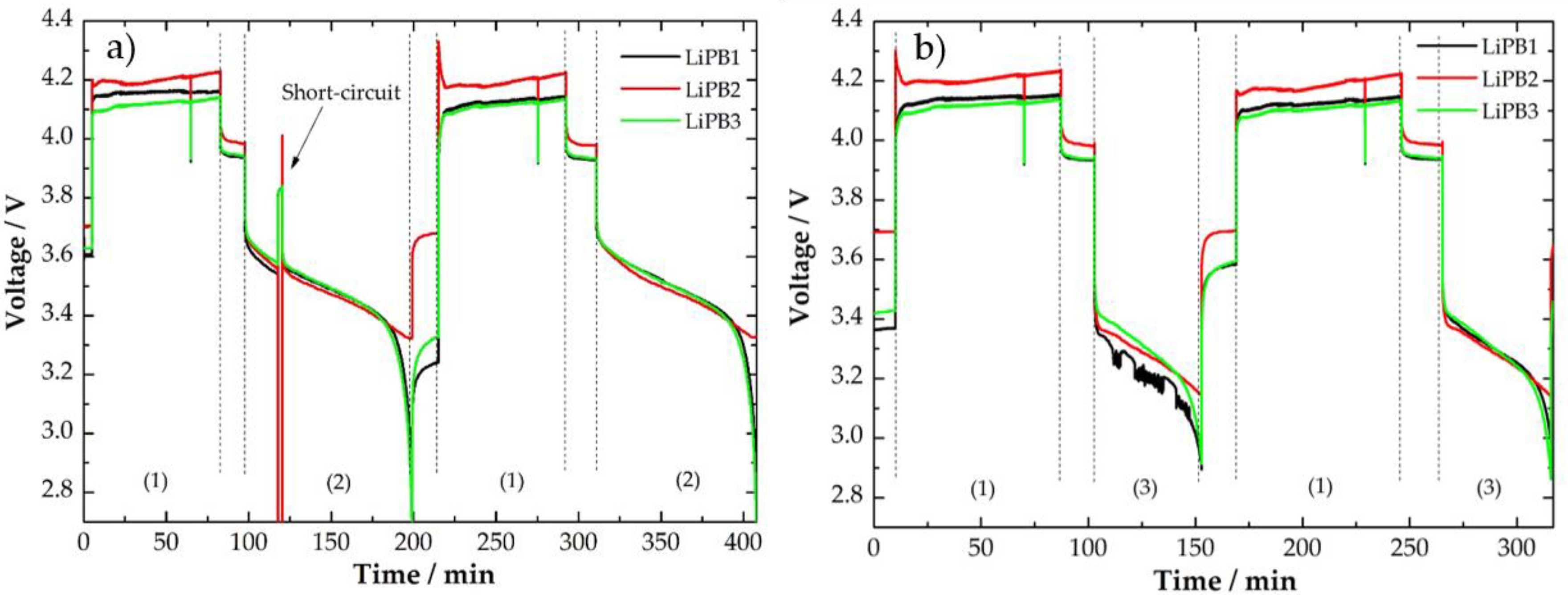
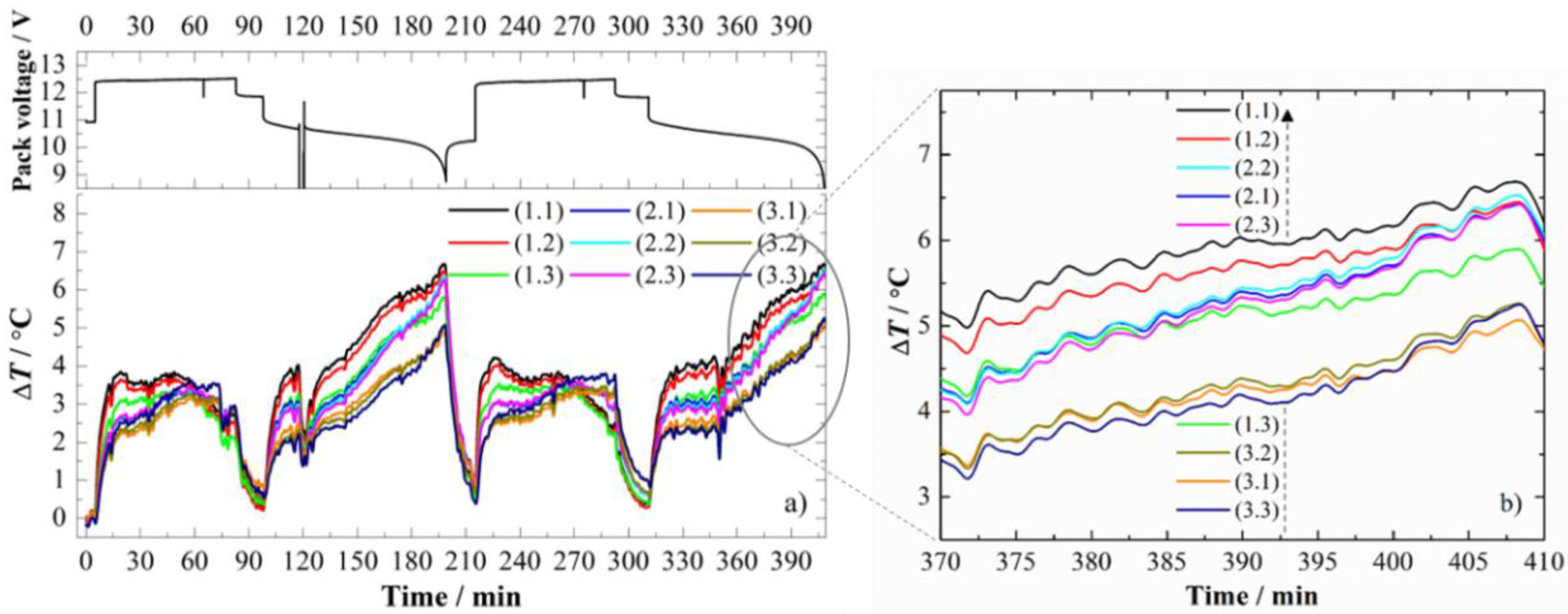
| FBG Position | λB/nm | KT ± 0.1/pm/°C | FBG Position | λB/nm | KT ± 0.1/pm/°C |
|---|---|---|---|---|---|
| Room | 1525.05 | 10.0 | (2.2) | 1554.06 | 10.2 |
| (1.1) | 1530.92 | 10.0 | (2.3) | 1556.25 | 10.2 |
| (1.2) | 1543.39 | 10.1 | (3.1) | 1558.17 | 10.3 |
| (1.3) | 1546.91 | 10.1 | (3.2) | 1559.52 | 10.3 |
| (2.1) | 1550.05 | 10.2 | (3.3) | 1562.71 | 10.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, M.; Paixão, T.; Ferreira, M.S.; Pinto, J.L. Thermal Mapping of a Lithium Polymer Batteries Pack with FBGs Network. Batteries 2018, 4, 67. https://doi.org/10.3390/batteries4040067
Nascimento M, Paixão T, Ferreira MS, Pinto JL. Thermal Mapping of a Lithium Polymer Batteries Pack with FBGs Network. Batteries. 2018; 4(4):67. https://doi.org/10.3390/batteries4040067
Chicago/Turabian StyleNascimento, Micael, Tiago Paixão, Marta S. Ferreira, and João L. Pinto. 2018. "Thermal Mapping of a Lithium Polymer Batteries Pack with FBGs Network" Batteries 4, no. 4: 67. https://doi.org/10.3390/batteries4040067
APA StyleNascimento, M., Paixão, T., Ferreira, M. S., & Pinto, J. L. (2018). Thermal Mapping of a Lithium Polymer Batteries Pack with FBGs Network. Batteries, 4(4), 67. https://doi.org/10.3390/batteries4040067






