Verification of Redox Flow Batteries’ Functionality by Electrochemical Impedance Spectroscopy Tests
Abstract
:1. Introduction
2. Experimental
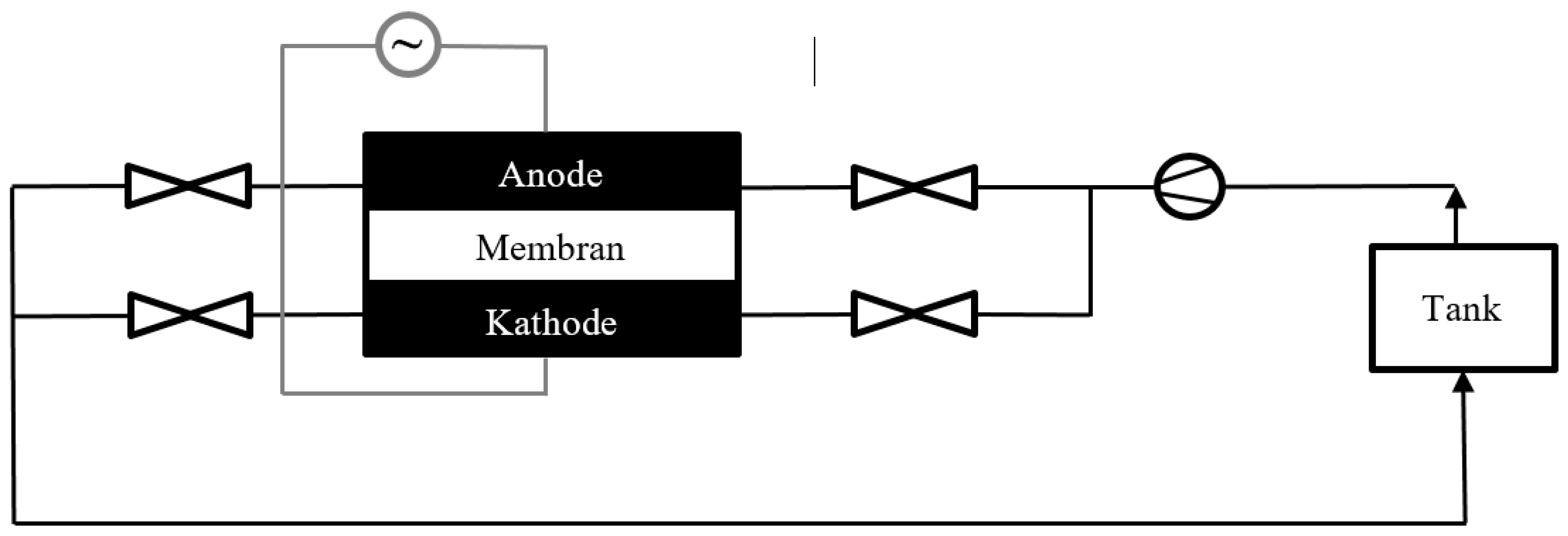
2.1. Set-Up of the Test Stand and the Battery
2.2. Measurement Parameters and Settings
- Hazard potential: How toxic and aggressive is the fluid?
- Repeatability: Is it possible to measure almost the same impedances several times? In general, the application of EIS requires that the following three conditions are fulfilled [16]:
- (1)
- Causality: the outgoing signal only reacts to the incoming signal (no outliers);
- (2)
- Stationarity: the system does not change with time;
- (3)
- Linearity: Linear systems should not generate higher harmonics. If there are no harmonics, the output signal should only have frequency components of the incoming signal. This can be proven by lowering the excitation amplitude until no changes of the impedance-spectrum can be measured.
- Distinguishability: do the evaluated diagrams (Bode- and Nyquist plots) have characteristics that are easy to distinguish (high points or turning points)?
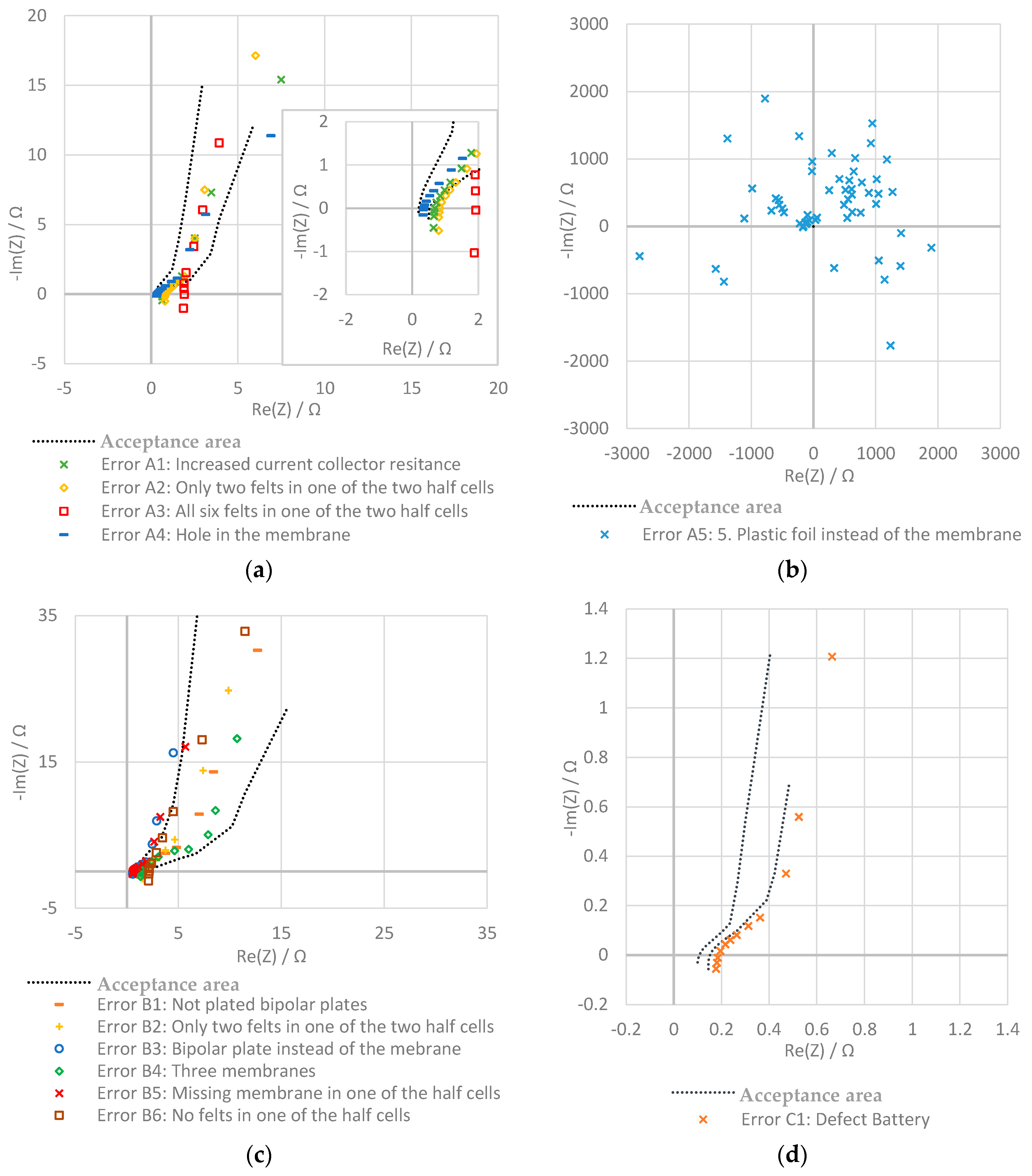
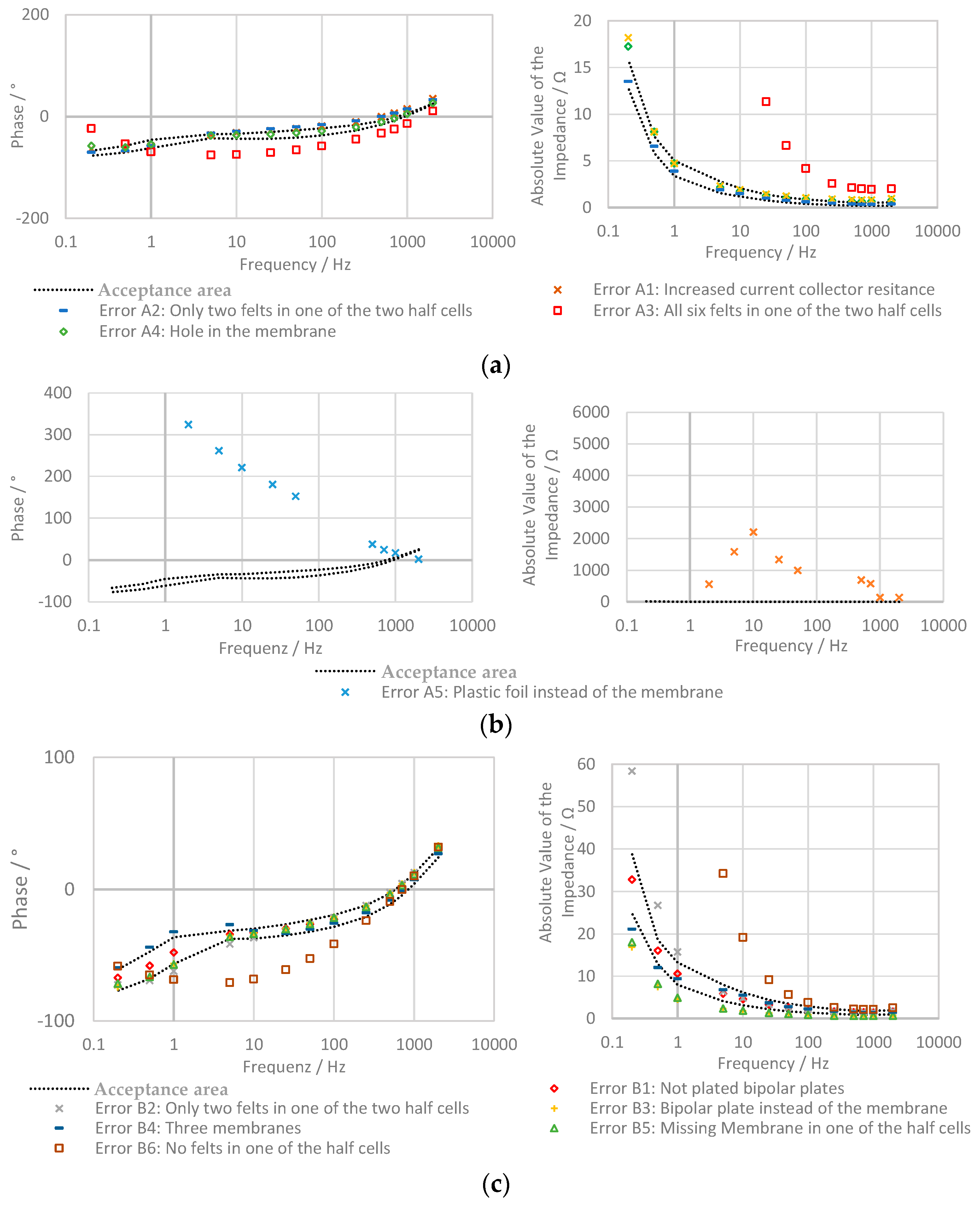
2.3. Possible Errors in the Battery
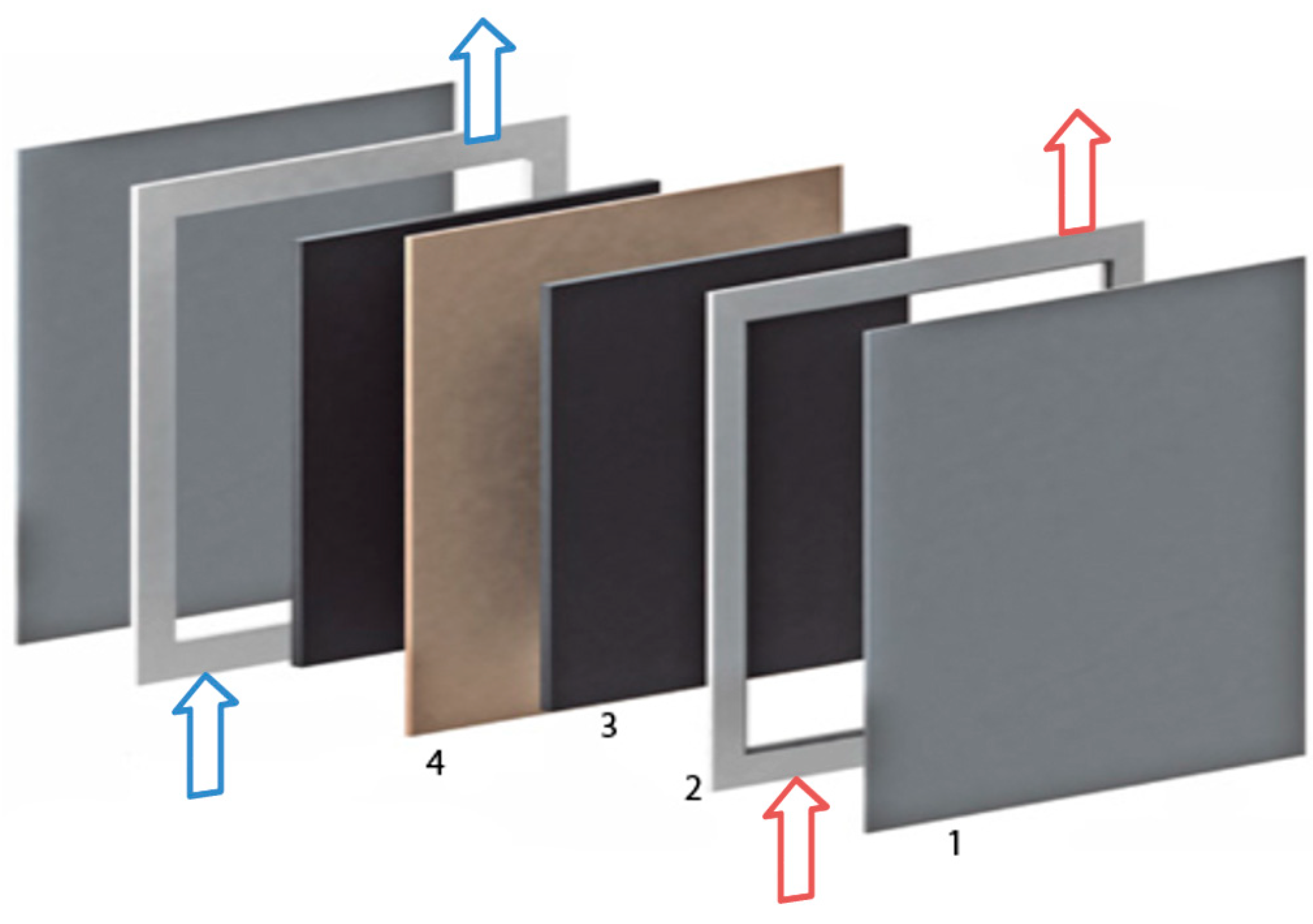
2.3.1. Battery A: One Cell, Electrode Surface: 64 cm2
- Increased current collector resistance: To decrease the ohmic resistance of the battery, the outer two bipolar plates with contact to the current collectors are normally nickle-plated. In this experiment, the impact of not plated bipolar plates was examined.
- Only two felts in one of the two half cells: in one of the two half cells, only two instead of three felts are used.
- All six felts in one of the two half cells: in this test, all felts are in the same half-cell of the stack and no felts are on the other side.
- Hole in the membrane: a hole with a diameter of about 2 mm was pierced into the membrane.
- Plastic foil instead of the membrane: in this experiment, the membrane is replaced by a non-conducting, tight plastic foil.
2.3.2. Battery B: Two Cells, Electrode Surface: 64 cm2
- Increased current collector resistance: see the section above.
- Only two felts in one of the two half cells: during this test, one of the four half cells only contains two felts.
- Bipolar plate instead of the membrane: in this experiment, one of the two membranes is replaced by a bipolar plate.
- Three membranes: the bipolar plate is replaced by a membrane.
- Missing Membrane in one of the half cells: one of the two membranes is removed.
- No felts in one of the half cells: in one of the half-cells, the felts are removed.
2.3.3. Battery C: One Cell, Electrode Surface: 493 cm2
2.3.4. Battery D: Ten Cells, Electrode Surface: 493 cm2
2.4. Measurement Analysis
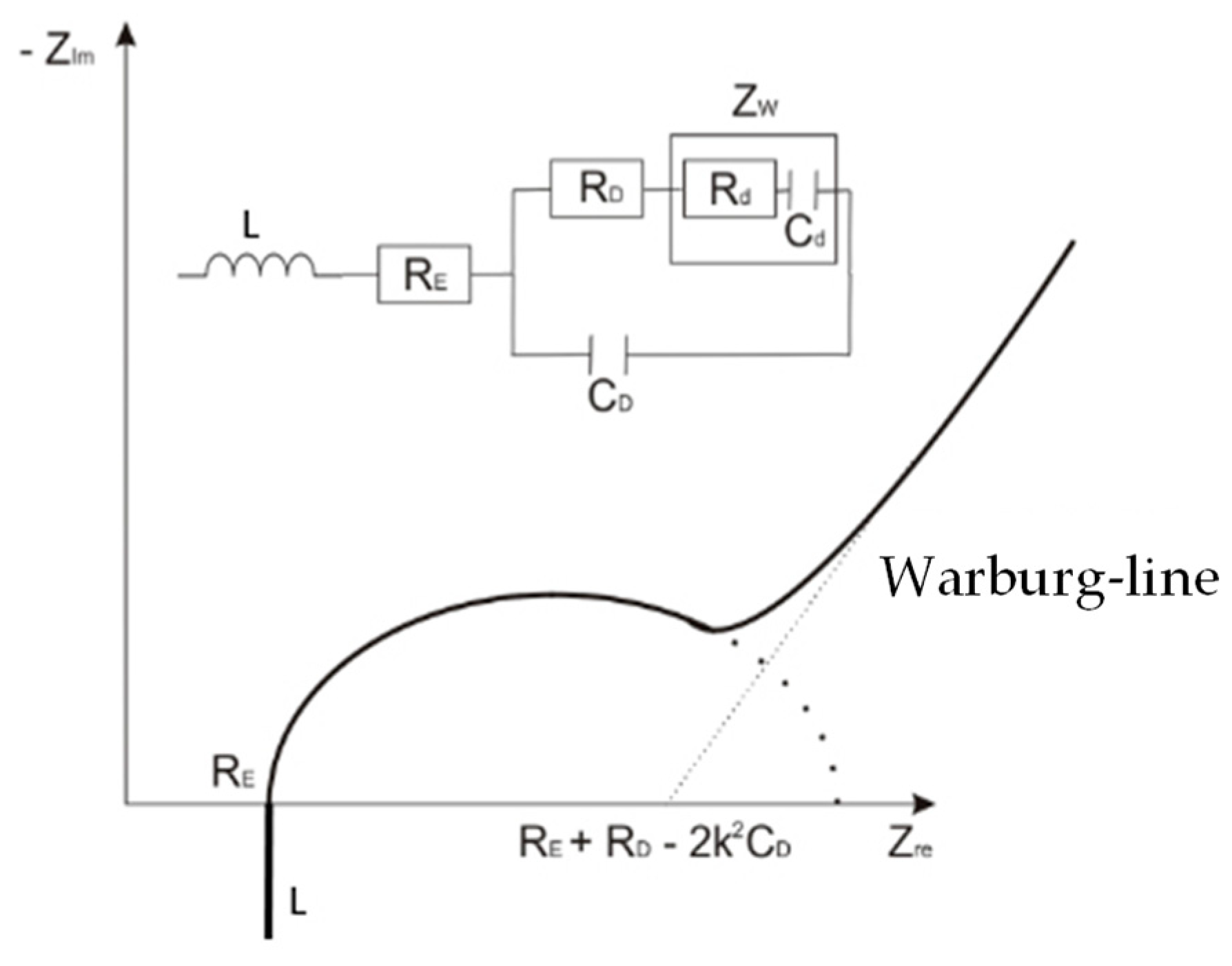
2.4.1. Nyquist Plot
2.4.2. Bode Plot
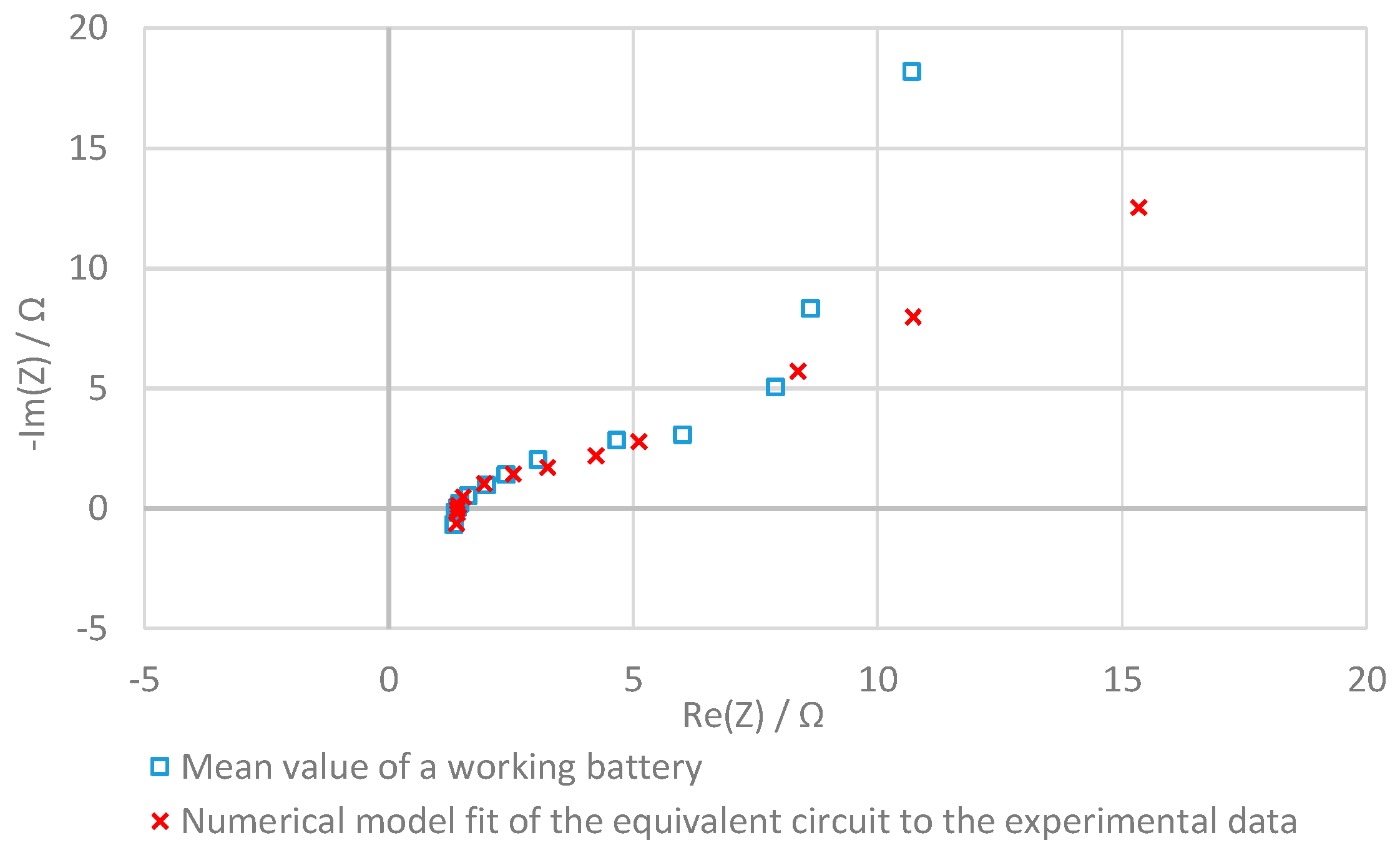
2.4.3. Analysis of the Equivalent Circuit
3. Results and Discussion
3.1. Tested Fluids
3.2. Evaluation with Nyquist Plot
3.3. Evaluation with Bode Plot
3.4. Evaluation with the Equivalent Circuit
4. Assessment of the Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roberts, B.P.; Sandberg, C. The Role of Energy Storage in Development of Smart Grids. Proc. IEEE 2011, 99, 1139–1144. [Google Scholar] [CrossRef]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Flow Batteries: Current Status and Trends. Chem. Rev. 2015, 115, 11533–11558. [Google Scholar] [CrossRef] [PubMed]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Kazacos, M. State of charge monitoring methods for vanadium redox flow battery control. J. Power Sources 2011, 196, 8822–8827. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Brandon, N.P.; Hajimolana, S.A.; Tariq, F.; Yufit, V.; Hashim, M.A.; Hussain, M.A.; Low, C.T.J.; Aravind, P.V. Application of carbon materials in redox flow batteries. J. Power Sources 2014, 253, 150–166. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137. [Google Scholar] [CrossRef]
- Blanke, H.; Bohlen, O.; Buller, S.; de Doncker, R.W.; Fricke, B.; Hammouche, A.; Linzen, D.; Thele, M.; Sauer, D.U. Impedance measurements on lead–acid batteries for state-of-charge, state-of-health and cranking capability prognosis in electric and hybrid electric vehicles. J. Power Sources 2005, 144, 418–425. [Google Scholar] [CrossRef]
- Lambert, S.M.; Armstrong, M.; Attidekou, P.S.; Christensen, P.A.; Widmer, J.D.; Wang, C.; Scott, K. Rapid Nondestructive-Testing Technique for In-Line Quality Control of Li-Ion Batteries. IEEE Trans. Ind. Electron. 2017, 64, 4017–4026. [Google Scholar] [CrossRef]
- Repp, S.; Harputlu, E.; Gurgen, S.; Castellano, M.; Kremer, N.; Pompe, N.; Wörner, J.; Hoffmann, A.; Thomann, R.; Emen, F.M.; et al. Synergetic effects of Fe3+ doped spinel Li4Ti5 O12 nanoparticles on reduced graphene oxide for high surface electrode hybrid supercapacitors. Nanoscale 2018, 10, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Kurzweil, P.; Shamonin, M. State-of-Charge Monitoring by Impedance Spectroscopy during Long-Term Self-Discharge of Supercapacitors and Lithium-Ion Batteries. Batteries 2018, 4, 35. [Google Scholar] [CrossRef]
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.G.; Ocakoglu, K.; Erdem, E. High-capacitance hybrid supercapacitor based on multi-colored fluorescent carbon-dots. Sci. Rep. 2017, 7, 11222. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, U.; Reinhard, G. Anwendung der elektrochemischen Impedanzspektroskopie (EIS) zur Beurteilung der Lochkorrosion und ihrer Inhibition. Mater. Corros. 1990, 41, 391–395. [Google Scholar] [CrossRef]
- Sun, C.-N.; Delnick, F.M.; Aaron, D.S.; Papandrew, A.B.; Mench, M.M.; Zawodzinski, T.A. Probing Electrode Losses in All-Vanadium Redox Flow Batteries with Impedance Spectroscopy. ECS Electrochem. Lett. 2013, 2, A43–A45. [Google Scholar] [CrossRef]
- Derr, I.; Przyrembel, D.; Schweer, J.; Fetyan, A.; Langner, J.; Melke, J.; Weinelt, M.; Roth, C. Electroless chemical aging of carbon felt electrodes for the all-vanadium redox flow battery (VRFB) investigated by Electrochemical Impedance and X-ray Photoelectron Spectroscopy. Electrochim. Acta 2017, 246, 783–793. [Google Scholar] [CrossRef]
- Sun, C.-N.; Delnick, F.M.; Aaron, D.S.; Papandrew, A.B.; Mench, M.M.; Zawodzinski, T.A. Resolving Losses at the Negative Electrode in All-Vanadium Redox Flow Batteries Using Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2014, 161, A981–A988. [Google Scholar] [CrossRef]
- SGL Group (2017). Available online: https://www.sglgroup.com/cms/international/products/product-groups/cfrc_felt/specialty-graphites-for-energy-storage/redox-flow-batteries.html?__locale=en (accessed on 5 November 2018).
- Manka, D. Elektrochemisch-Optische Impedanzspektroskopie; KIT Scientific Publishing: Karlsruhe, Germany, 2016. [Google Scholar]
- Dassisti, M.; Cozzolino, G.; Chimienti, M.; Rizzuti, A.; Mastrorilli, P.; L’Abbate, P. Sustainability of vanadium redox-flow batteries: Benchmarking electrolyte synthesis procedures. Int. J. Hydrog. Energy 2016, 41, 16477–16488. [Google Scholar] [CrossRef]
- Pezeshki, A.M.; Sacci, R.L.; Delnick, F.M.; Aaron, D.S.; Mench, M.M. Elucidating effects of cell architecture, electrode material, and solution composition on overpotentials in redox flow batteries. Electrochim. Acta 2017, 229, 261–270. [Google Scholar] [CrossRef]
- Pezeshki, A.M.; Clement, J.T.; Veith, G.M.; Zawodzinski, T.A.; Mench, M.M. High performance electrodes in vanadium redox flow batteries through oxygen-enriched thermal activation. J. Power Sources 2015, 294, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Zago, M.; Casalegno, A. Physically-based impedance modeling of the negative electrode in All-Vanadium Redox Flow Batteries: Insight into mass transport issues. Electrochim. Acta 2017, 248, 505–517. [Google Scholar] [CrossRef]
- Mielke, W. Modellierung von Kennlinien, Impedanzspektren und thermischem Verhalten einer Lithium-Eisenphosphat-Batterie. Master’s Thesis, Universität Stuttgart, Stuttgart, Germany, 2011. [Google Scholar]

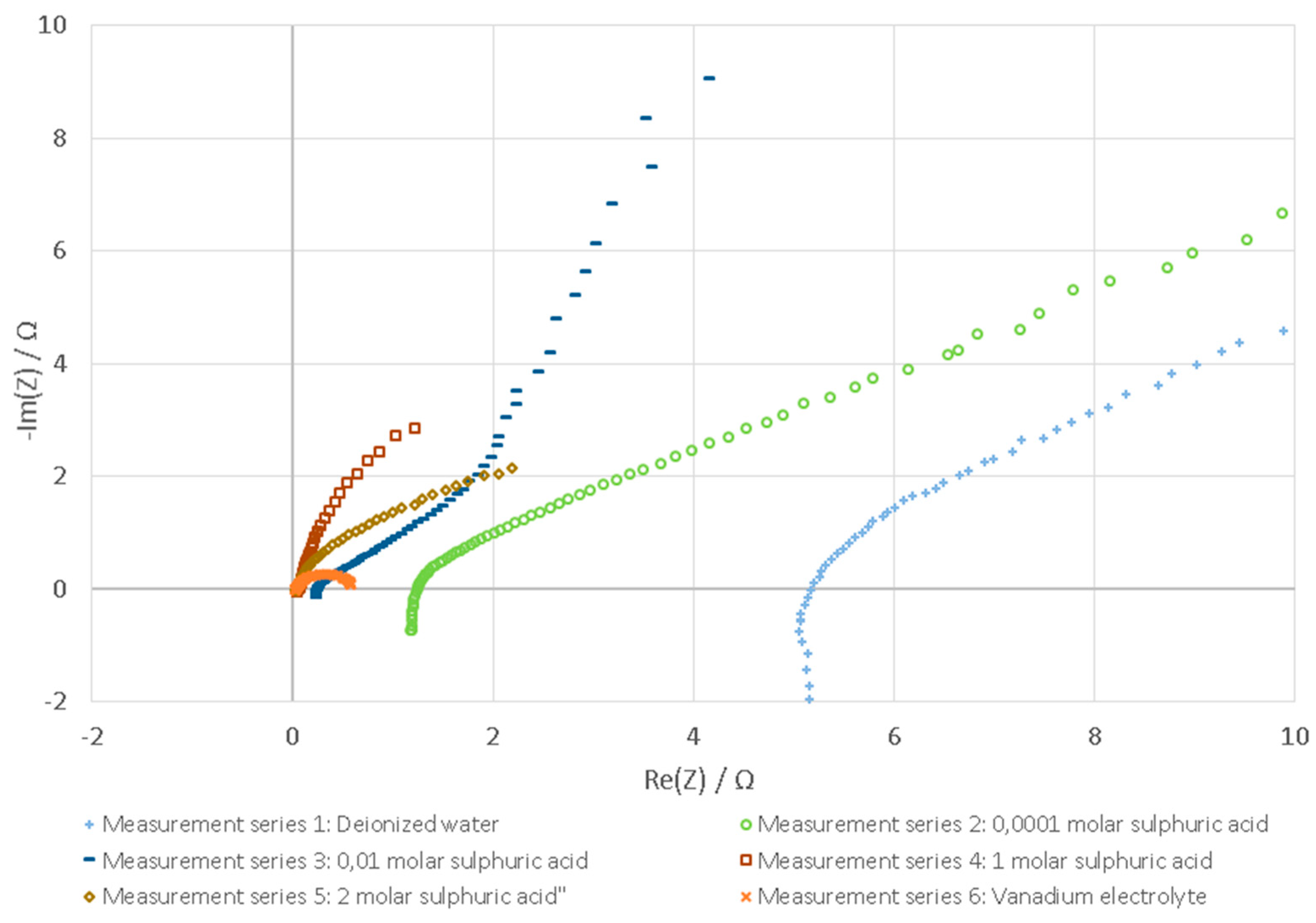

| Medium | Amplitude | Hazard Potential | Repeatability | Distinguishability |
|---|---|---|---|---|
| Air | 0.5 VAC | |||
| Deionized water | 0.5 VAC | |||
| 0.0001 molar sulphuric acid | 0.5 VAC | |||
| 0.01 molar sulphuric acid | 0.5 VAC | |||
| 1 molar sulphuric acid | 0.5 VAC | |||
| 2 molar sulphuric acid | 0.5 VAC | |||
| Vanadium electrolyte | 0.08 VAC |
| Battery | Error | Values Outside the Acceptance Area | ||
|---|---|---|---|---|
| Phase Shift | Impedance | |||
| Battery A One cell, 64 cm2 | Error-free | Mean value of the error-free battery | 0.4% | 2% |
| Error A1 | Increased current collector resistance | 70% | 55% | |
| Error A2 | Only two felts in one of the two half cells | 78% | 73% | |
| Error A3 | All six felts in one of the two half cells | 92% | 99% | |
| Error A4 | Hole in the membrane | 17% | 4% | |
| Error A5 | Plastic foil instead of the membrane | 100% | 100% | |
| Battery B Two cells, 64 cm2 | Error-free | Mean value of the error-free battery | 0.4% | 0.9% |
| Error B1 | Increased current collector resistance | 6% | 5% | |
| Error B2 | Only two felts in one of the half cells | 61% | 24% | |
| Error B3 | Bipolar plate instead of the membrane | 32% | 100% | |
| Error B4 | With three membranes | 33% | 13% | |
| Error B5 | Missing membrane in one of the half cells | 21% | 100% | |
| Error B6 | No felts in one of the half cells | 74% | 100% | |
| Battery C Two cells, 493 cm2 | Error-free | Mean value of the error-free battery | 0% | 4% |
| Error C1 | Damaged battery | 0% | 100% | |
| Battery | Error | L1 | R1 | C2 | R2 | W1 | |
|---|---|---|---|---|---|---|---|
| Battery A One cell, 64 cm2 | No Error | Mean value of the error-free battery | |||||
| Error A1 | Increased current collector resistance | ||||||
| Error A2 | Only two felts in one of the two half cells | ||||||
| Error A3 | All six felts in one of the two half cells | ||||||
| Error A4 | Hole in the membrane | ||||||
| Error A5 | Plastic foil instead of the membrane | ||||||
| Battery B Two cells, 64 cm2 | No Error | Mean value of the error-free battery | |||||
| Error B1 | Increased current collector resistance | ||||||
| Error B2 | Only two felts in one of the half cells | ||||||
| Error B3 | Bipolar plate instead of the membrane | ||||||
| Error B4 | With three membranes | ||||||
| Error B5 | Missing membrane in one of the half cells | ||||||
| Error B6 | No felts in one of the half cells | ||||||
| Battery C Two cells, 493 cm2 | No Error | Mean value of the error-free battery | |||||
| Error C1 | Damaged battery | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manschke, D.; Seipp, T.; Berthold, S.; Friedrich, K.A. Verification of Redox Flow Batteries’ Functionality by Electrochemical Impedance Spectroscopy Tests. Batteries 2018, 4, 58. https://doi.org/10.3390/batteries4040058
Manschke D, Seipp T, Berthold S, Friedrich KA. Verification of Redox Flow Batteries’ Functionality by Electrochemical Impedance Spectroscopy Tests. Batteries. 2018; 4(4):58. https://doi.org/10.3390/batteries4040058
Chicago/Turabian StyleManschke, Daniel, Thorsten Seipp, Sascha Berthold, and K. Andreas Friedrich. 2018. "Verification of Redox Flow Batteries’ Functionality by Electrochemical Impedance Spectroscopy Tests" Batteries 4, no. 4: 58. https://doi.org/10.3390/batteries4040058
APA StyleManschke, D., Seipp, T., Berthold, S., & Friedrich, K. A. (2018). Verification of Redox Flow Batteries’ Functionality by Electrochemical Impedance Spectroscopy Tests. Batteries, 4(4), 58. https://doi.org/10.3390/batteries4040058





