Fabrication of a Flexible Current Collector for Lithium Ion Batteries by Inkjet Printing
Abstract
1. Introduction
2. Experiment
2.1. Materials
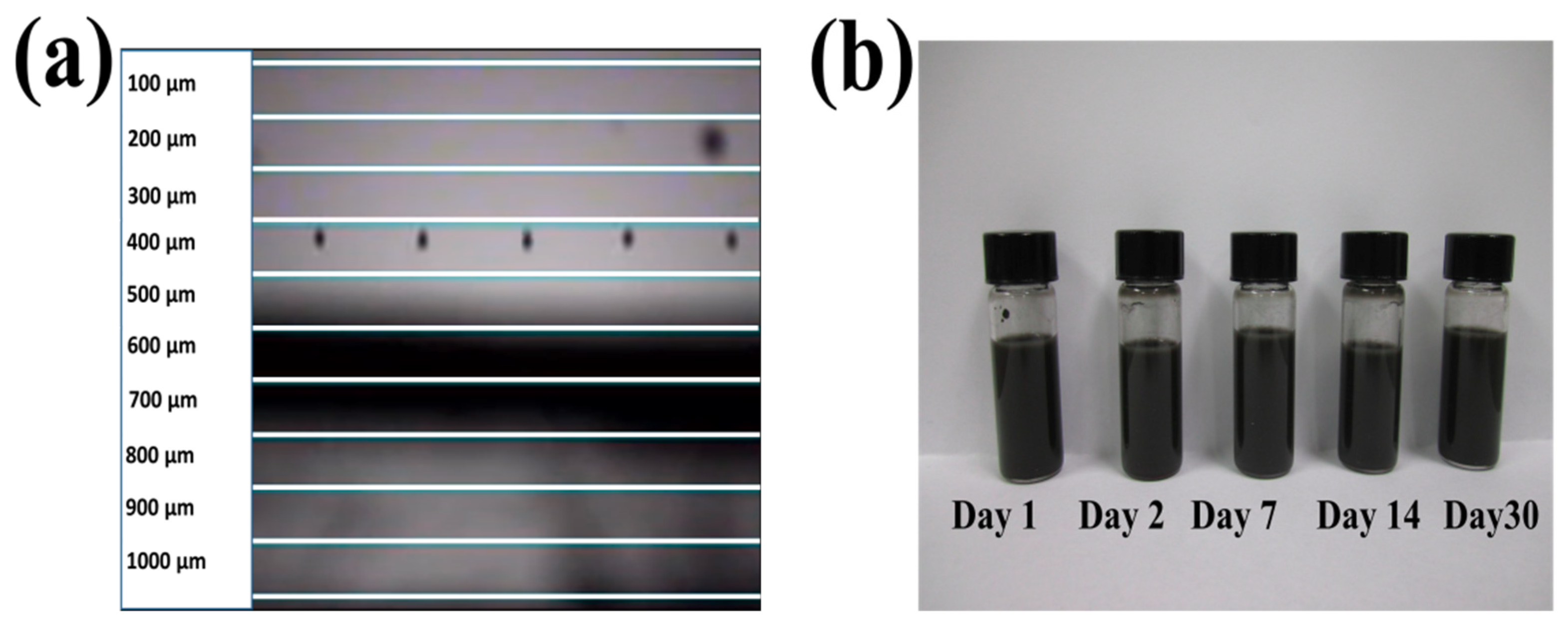
2.2. Synthesis of Nickel Nanoparticle and Battery Inks
2.3. Fabrication of Cathode and Anode for Battery
2.4. Characterization
3. Results and Discussion
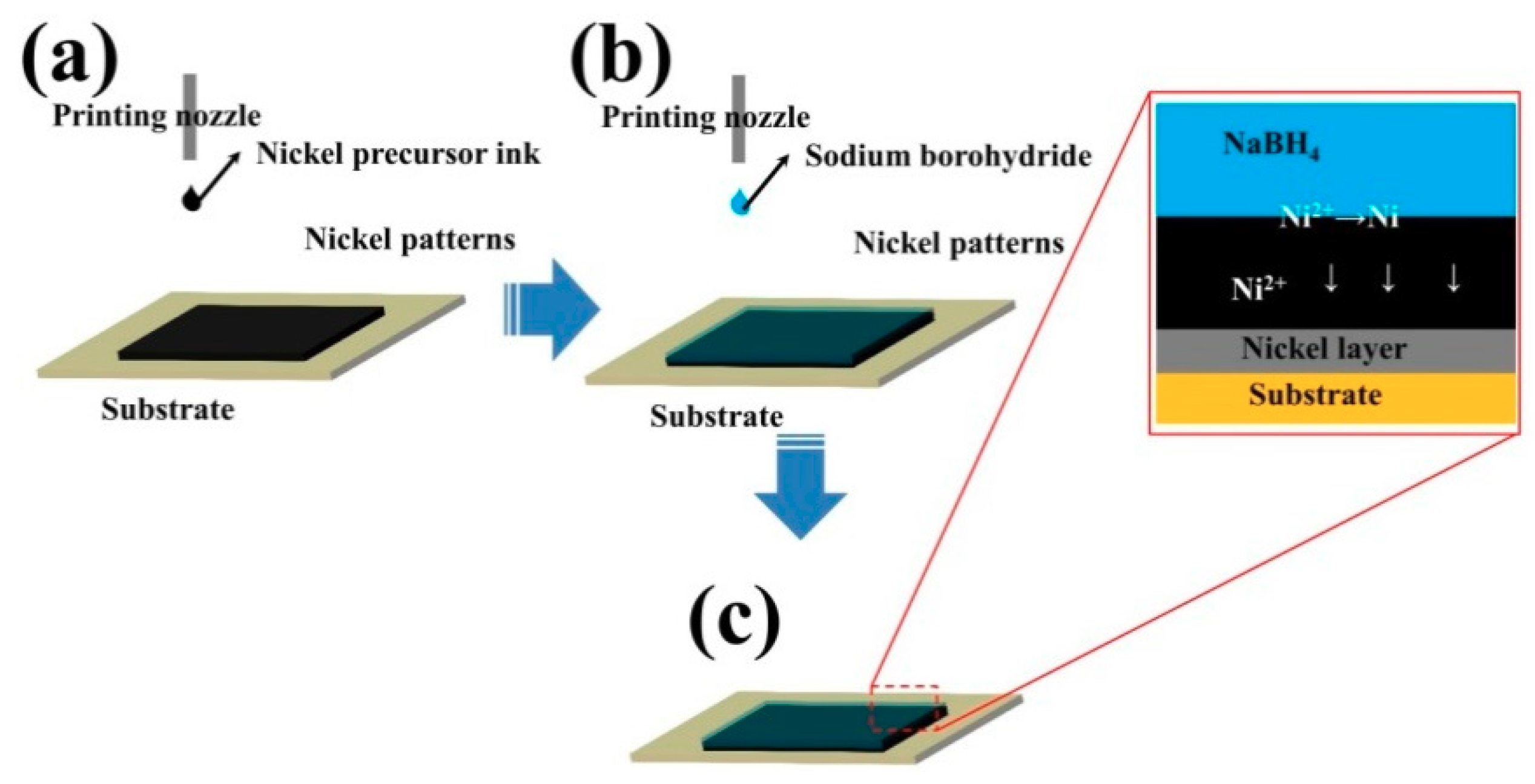
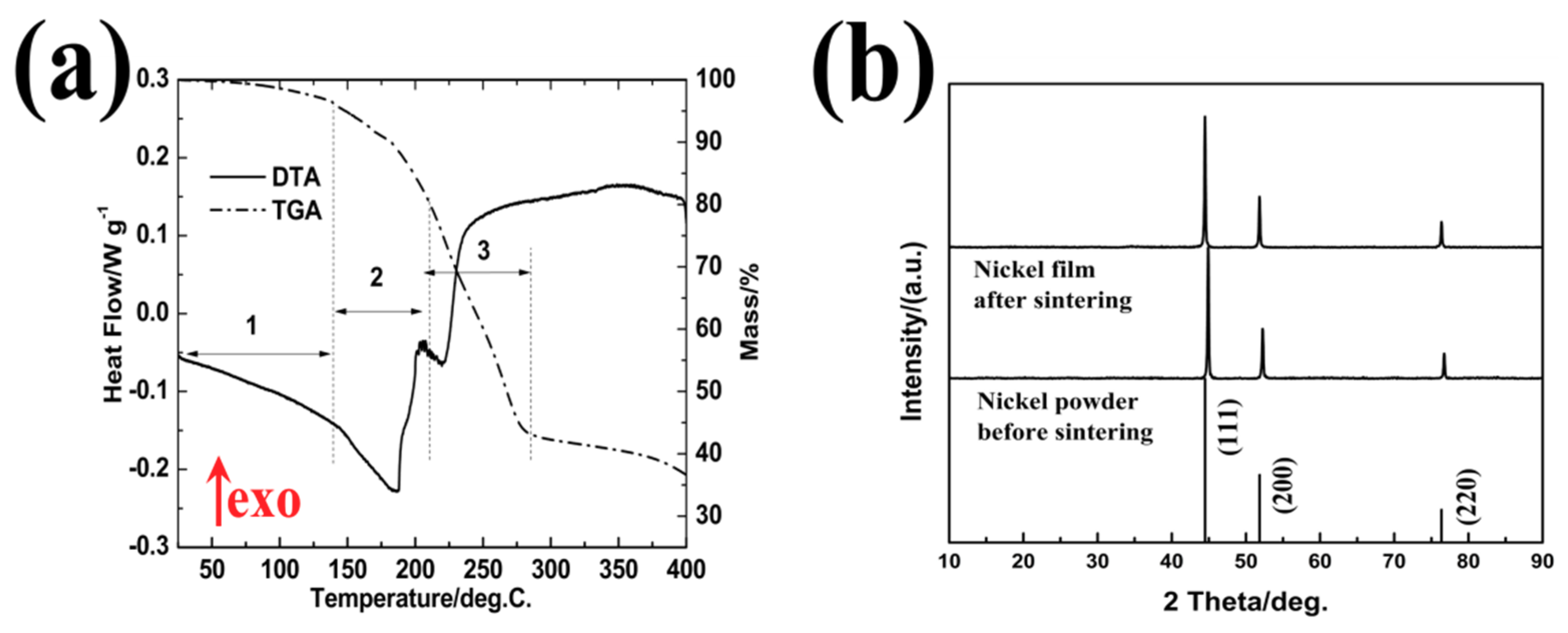
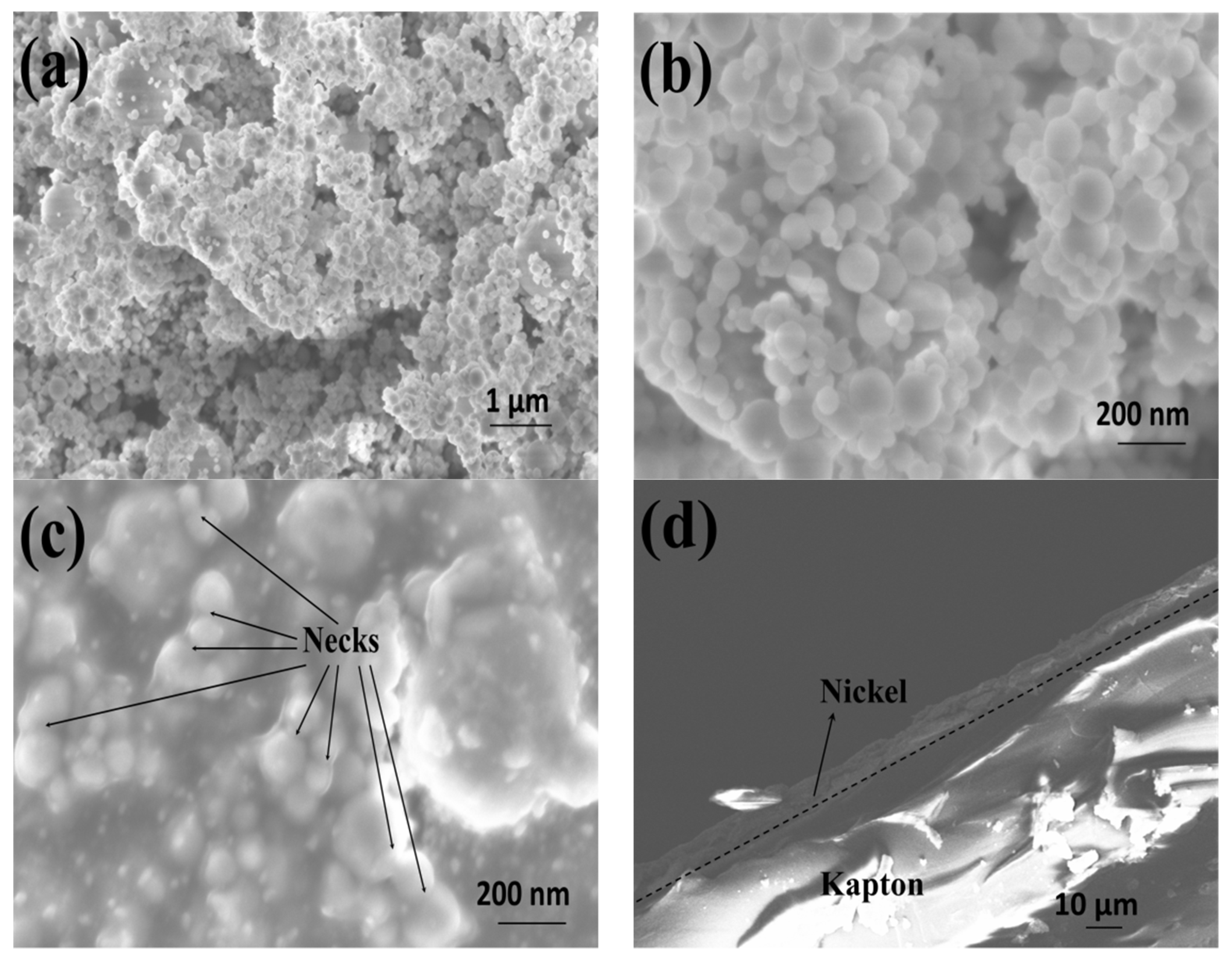
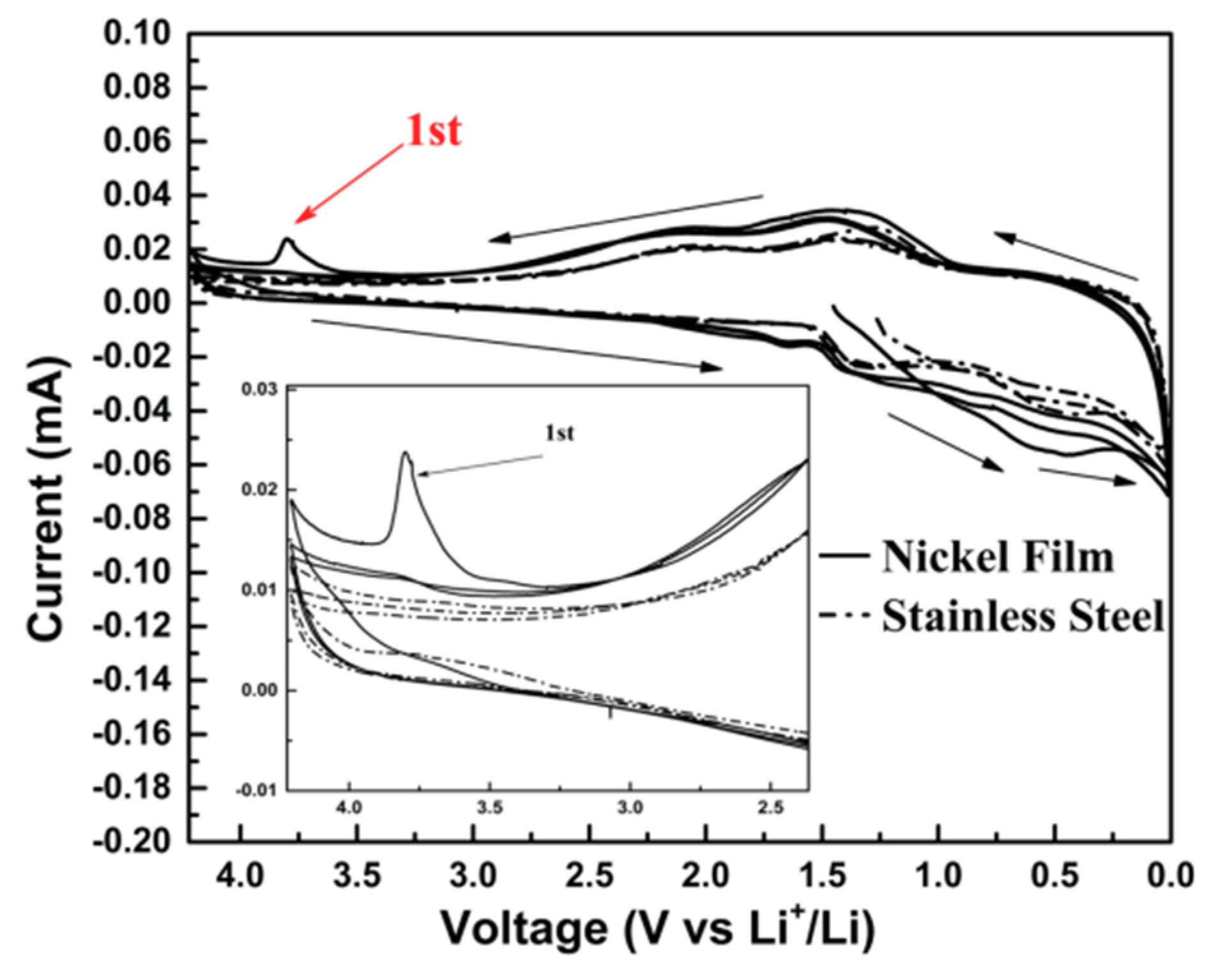
3.1. Nickel Current Collectors for Flexible Batteries
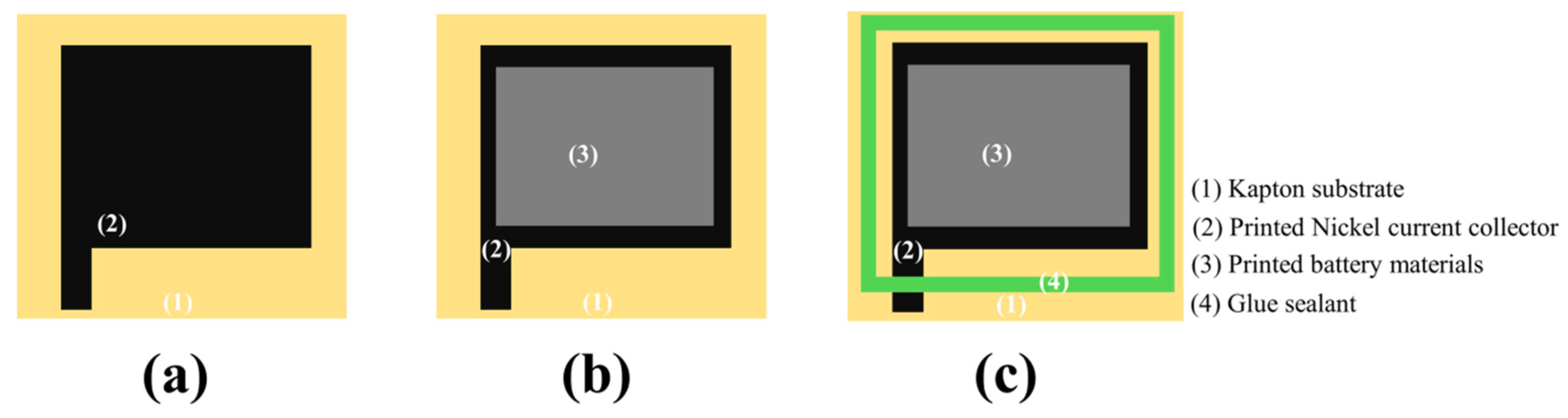
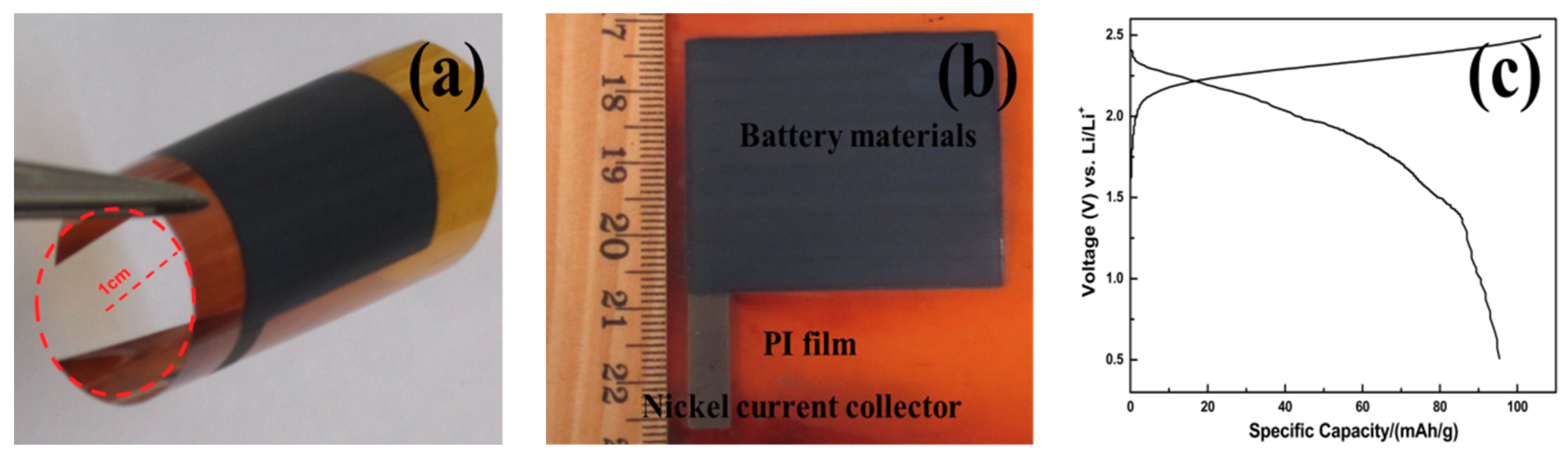
3.2. Flexible Battery with Nickel Current Collector
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le Borgne, B.; De Sagazan, O.; Crand, S.; Jacques, E.; Harnois, M. Conformal electronics wrapped around daily life objects using an original method: Water transfer printing. ACS Appl. Mater. Interfaces 2017, 9, 29424–29429. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Brenckle, M.A.; Yang, M.; Zhang, J.; Liu, M.; Siebert, S.M.; Averitt, R.D.; Mannoor, M.S.; McAlpine, M.C.; Rogers, J.A. Silk-based conformal, adhesive, edible food sensors. Adv. Mater. 2012, 24, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Seifert, T.; Sowade, E.; Roscher, F.; Wiemer, M.; Gessner, T.; Baumann, R.R. Additive manufacturing technologies compared: Morphology of deposits of silver ink using inkjet and aerosol jet printing. Ind. Eng. Chem. Res. 2015, 54, 769–779. [Google Scholar] [CrossRef]
- Rahman, T.; Renaud, L.; Heo, D.; Renn, M.; Panat, R. Aerosol based direct-write micro-additive fabrication method for sub-mm 3D metal-dielectric structures. J. Micromech. Microeng. 2015, 25, 107002. [Google Scholar] [CrossRef]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Wee, G.; Salim, T.; Lam, Y.M.; Mhaisalkar, S.G.; Srinivasan, M. Printable photo-supercapacitor using single-walled carbon nanotubes. Energy Environ. Sci. 2011, 4, 413–416. [Google Scholar] [CrossRef]
- Xu, Y.; Schwab, M.G.; Strudwick, A.J.; Hennig, I.; Feng, X.; Wu, Z.; Müllen, K. Screen-printable thin film supercapacitor device utilizing graphene/polyaniline inks. Adv. Energy Mater. 2013, 3, 1035–1040. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; et al. A hole-conductor–free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Park, S.J.; Cho, J.W.; Gwak, J.; Oh, M.-K.; Min, B.K. Nearly carbon-free printable cigs thin films for solar cell applications. Solar Energy Mater. Solar Cells 2011, 95, 2928–2932. [Google Scholar] [CrossRef]
- Lee, H.M.; Choi, S.Y.; Kim, K.T.; Yun, J.Y.; Jung, D.S.; Park, S.B.; Park, J. A novel solution-stamping process for preparation of a highly conductive aluminum thin film. Adv. Mater. 2011, 23, 5524–5528. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wei, T.S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D printing of interdigitated li-ion microbattery architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.H.; Schreiber, M. Current collectors for positive electrodes of lithium-based batteries. J. Electrochem. Soc. 2005, 152, A2105–A2113. [Google Scholar] [CrossRef]
- Pikul, J.H.; Zhang, H.G.; Cho, J.; Braun, P.V.; King, W.P. High-power lithium ion microbatteries from interdigitated three-dimensional bicontinuous nanoporous electrodes. Nat. Commun. 2013, 4, 1732. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sutton, D.; Burgess, A.; Graham, D.; Calvert, P.D. Conductive copper and nickel lines via reactive inkjet printing. J. Mater. Chem. 2009, 19, 3719–3724. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Gao, C. Preparation of nanometer nickel powder from spent electroless nickel plating baths by using thiourea dioxide as a green reductant. J. Clean. Prod. 2014, 84, 701–706. [Google Scholar] [CrossRef]
- Chew, S.Y.; Ng, S.H.; Wang, J.; Novák, P.; Krumeich, F.; Chou, S.L.; Chen, J.; Liu, H.K. Flexible free-standing carbon nanotube films for model lithium-ion batteries. Carbon 2009, 47, 2976–2983. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; La Mantia, F.; Yang, Y.; Cui, Y. Thin, flexible secondary li-ion paper batteries. ACS Nano 2010, 4, 5843–5848. [Google Scholar] [CrossRef] [PubMed]
- Krebs, F.C. All solution roll-to-roll processed polymer solar cells free from indium-tin-oxide and vacuum coating steps. Org. Electron. 2009, 10, 761–768. [Google Scholar] [CrossRef]
- Krebs, F.C. Roll-to-roll fabrication of monolithic large-area polymer solar cells free from indium-tin-oxide. Solar Energy Mater. Solar Cells 2009, 93, 1636–1641. [Google Scholar] [CrossRef]
- Schmidt, H.; Flügge, H.; Winkler, T.; Bülow, T.; Riedl, T.; Kowalsky, W. Efficient semitransparent inverted organic solar cells with indium tin oxide top electrode. Appl. Phys. Lett. 2009, 94, 243302. [Google Scholar] [CrossRef]
- Fan, Z.; Wei, T.; Luo, G.; Wei, F. Fabrication and characterization of multi-walled carbon nanotubes-based ink. J. Mater. Sci. 2005, 40, 5075–5077. [Google Scholar] [CrossRef]
- Vaillancourt, J.; Zhang, H.; Vasinajindakaw, P.; Xia, H.; Lu, X.; Han, X.; Janzen, D.C.; Shih, W.-S.; Jones, C.S.; Stroder, M.; et al. All ink-jet-printed carbon nanotube thin-film transistor on a polyimide substrate with an ultrahigh operating frequency of over 5 GHz. Appl. Phys. Lett. 2008, 93, 243301. [Google Scholar] [CrossRef]
- Beecher, P.; Servati, P.; Rozhin, A.; Colli, A.; Scardaci, V.; Pisana, S.; Hasan, T.; Flewitt, A.J.; Robertson, J.; Hsieh, G.W.; et al. Ink-jet printing of carbon nanotube thin film transistors. J. Appl. Phys. 2007, 102, 043710. [Google Scholar] [CrossRef]
- Grande, L.; Chundi, V.T.; Wei, D.; Bower, C.; Andrew, P.; Ryhänen, T. Graphene for energy harvesting/storage devices and printed electronics. Particuology 2012, 10, 1–8. [Google Scholar] [CrossRef]
- Blake, P.; Brimicombe, P.D.; Nair, R.R.; Booth, T.J.; Jiang, D.; Schedin, F.; Ponomarenko, L.A.; Morozov, S.V.; Gleeson, H.F.; Hill, E.W.; et al. Graphene-based liquid crystal device. Nano Lett. 2008, 8, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Lemme, M.C.; Echtermeyer, T.J.; Baus, M.; Kurz, H. A graphene field-effect device. IEEE Electron Device Lett. 2007, 28, 282–284. [Google Scholar] [CrossRef]
- Tan, Y.W.; Zhang, Y.; Bolotin, K.; Zhao, Y.; Adam, S.; Hwang, E.H.; Das Sarma, S.; Stormer, H.L.; Kim, P. Measurement of scattering rate and minimum conductivity in graphene. Phys. Rev. Lett. 2007, 99, 246803. [Google Scholar] [CrossRef] [PubMed]
- Heusing, S.; de Oliveira, P.W.; Kraker, E.; Haase, A.; Palfinger, C.; Veith, M. Wet chemical deposited ito coatings on flexible substrates for organic photodiodes. Thin Solid Films 2009, 518, 1164–1169. [Google Scholar] [CrossRef]
- Ederth, J.; Johnsson, P.; Niklasson, G.A.; Hoel, A.; Hultåker, A.; Heszler, P.; Granqvist, C.G.; van Doorn, A.R.; Jongerius, M.J.; Burgard, D. Electrical and optical properties of thin films consisting of tin-doped indium oxide nanoparticles. Phys. Rev. B 2003, 68, 155410. [Google Scholar] [CrossRef]
- Redinger, D.; Molesa, S.; Shong, Y.; Farschi, R.; Subramanian, V. An ink-jet-deposited passive component process for rfid. IEEE Trans. Electron Devices 2004, 51, 1978–1983. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, L.; Wang, D.; Zhu, J.; Wang, B.; Guo, C. Corrosion resistance of nickel foam modified with electroless ni-p alloy as positive current collector in a lithium ion battery. RSC Adv. 2013, 3, 25648–25651. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Federici, J.F. Fabrication of a Flexible Current Collector for Lithium Ion Batteries by Inkjet Printing. Batteries 2018, 4, 42. https://doi.org/10.3390/batteries4030042
Gu Y, Federici JF. Fabrication of a Flexible Current Collector for Lithium Ion Batteries by Inkjet Printing. Batteries. 2018; 4(3):42. https://doi.org/10.3390/batteries4030042
Chicago/Turabian StyleGu, Yuan, and John F. Federici. 2018. "Fabrication of a Flexible Current Collector for Lithium Ion Batteries by Inkjet Printing" Batteries 4, no. 3: 42. https://doi.org/10.3390/batteries4030042
APA StyleGu, Y., & Federici, J. F. (2018). Fabrication of a Flexible Current Collector for Lithium Ion Batteries by Inkjet Printing. Batteries, 4(3), 42. https://doi.org/10.3390/batteries4030042




