An In-Situ Reference Electrode Insertion Method for Commercial 18650-Type Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Coin Cells Production
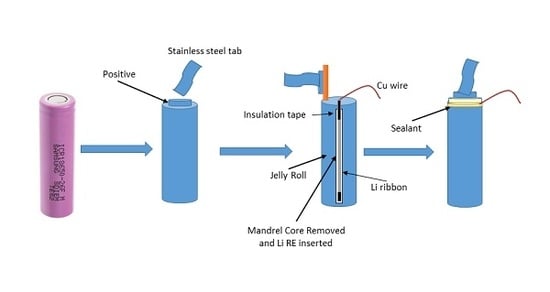
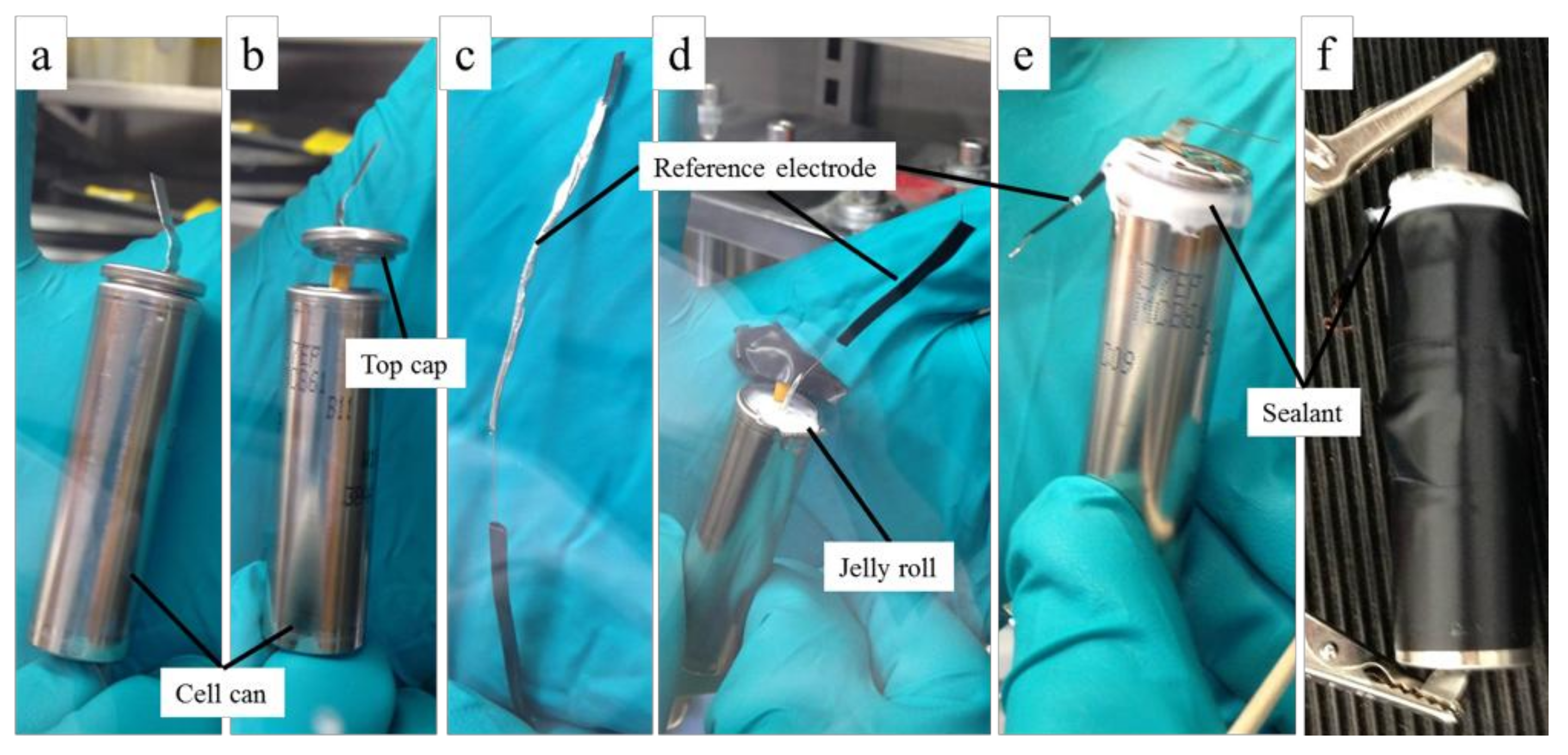
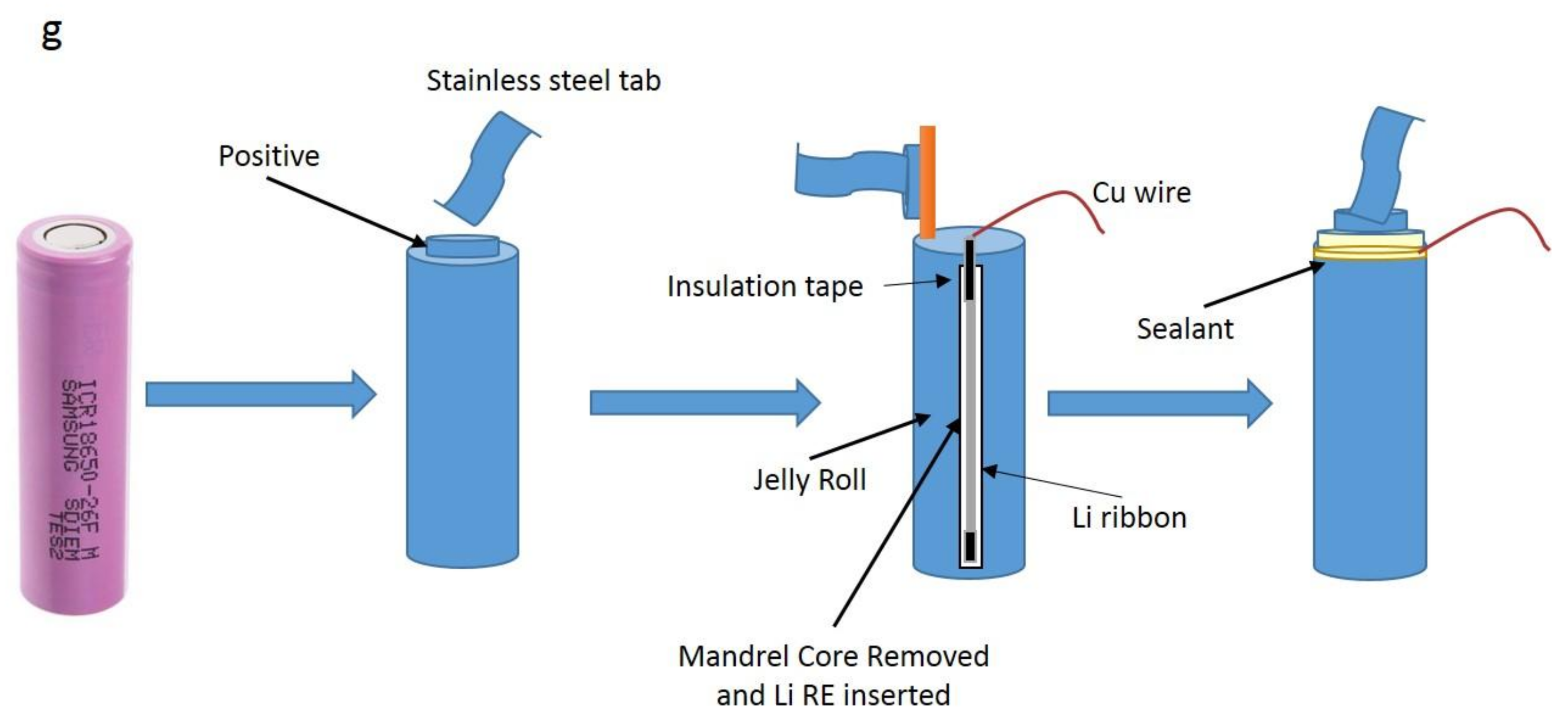
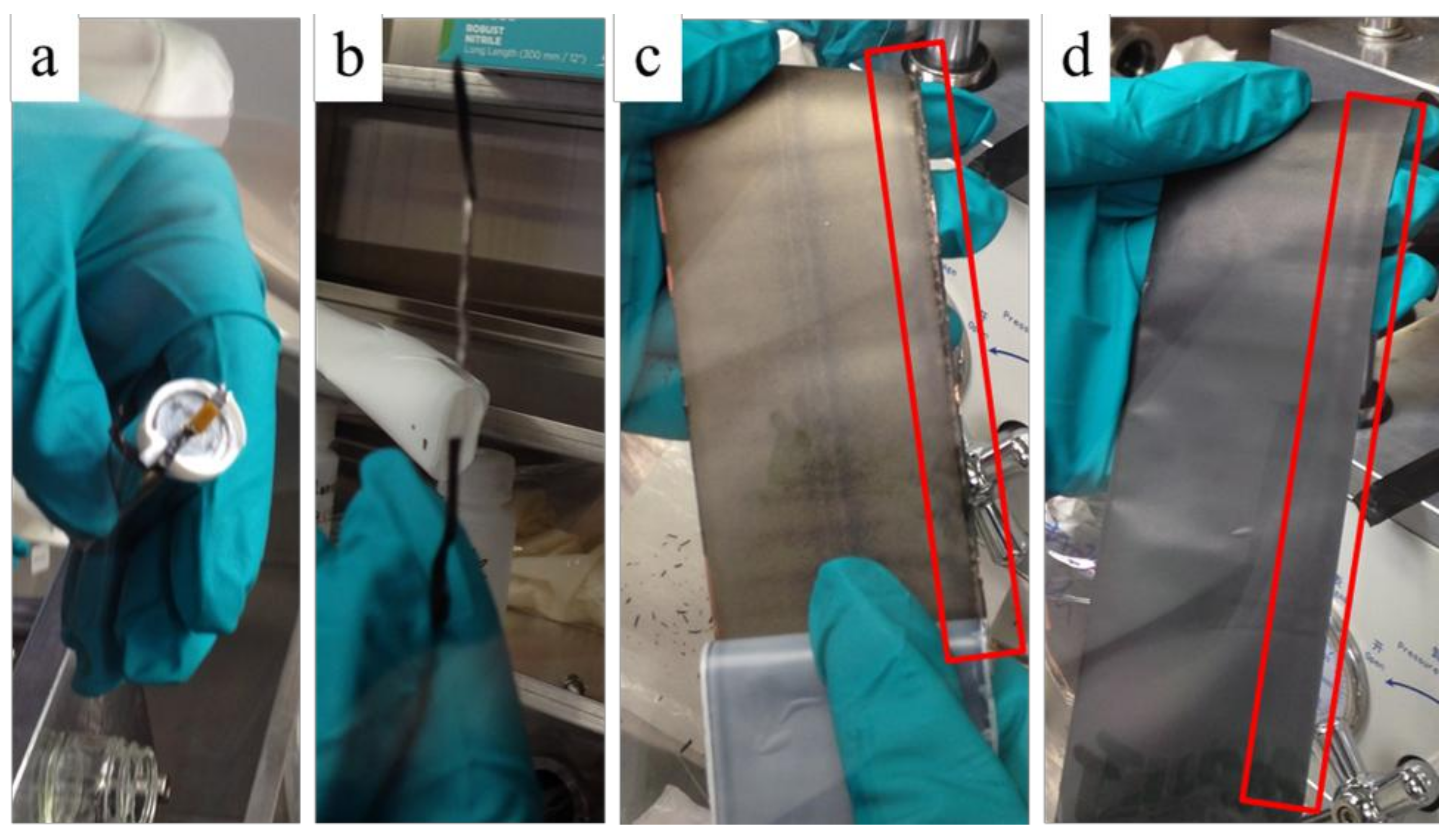
2.3. RE Insertion
2.4. Electrical Characterization
3. Results and Discussion
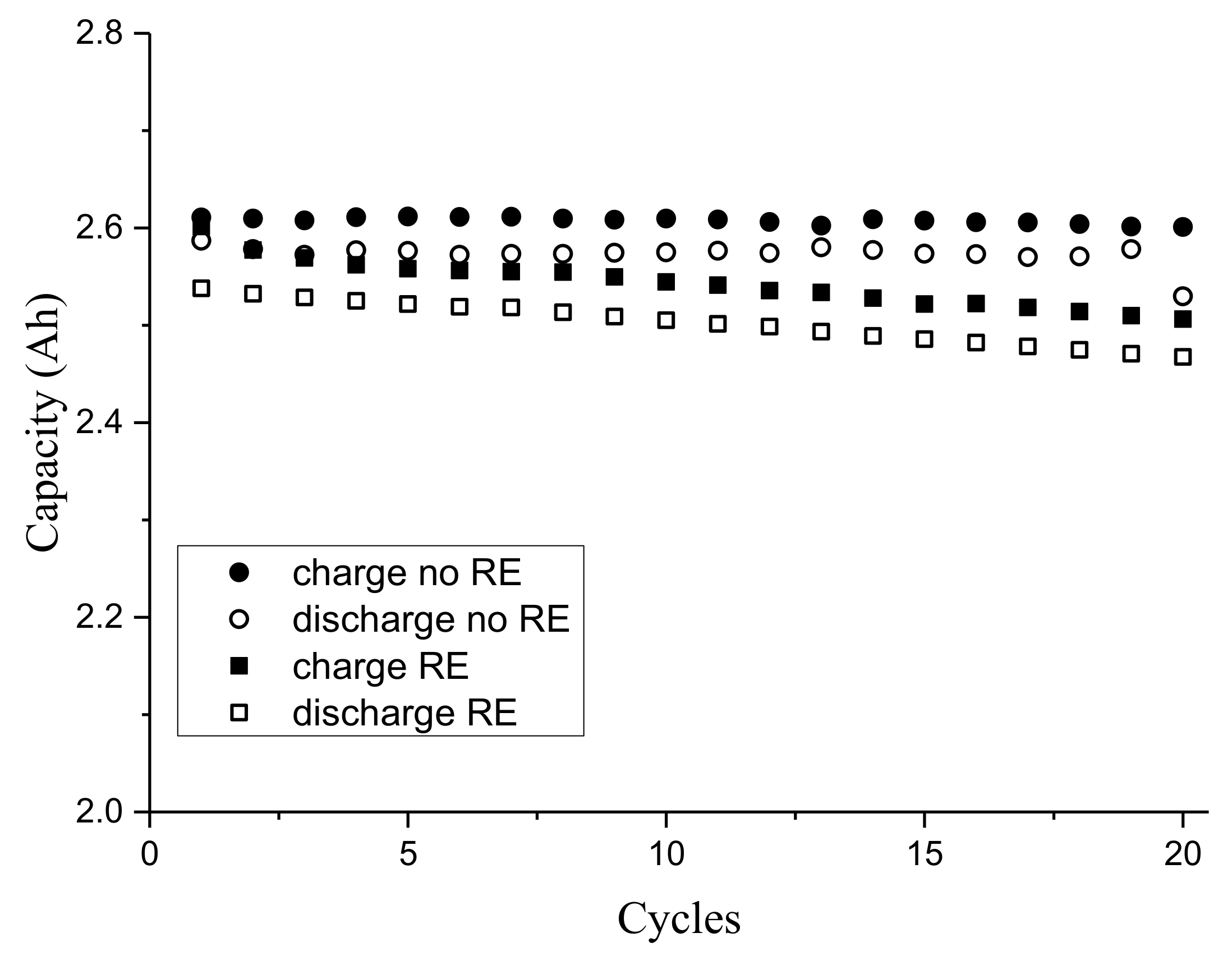
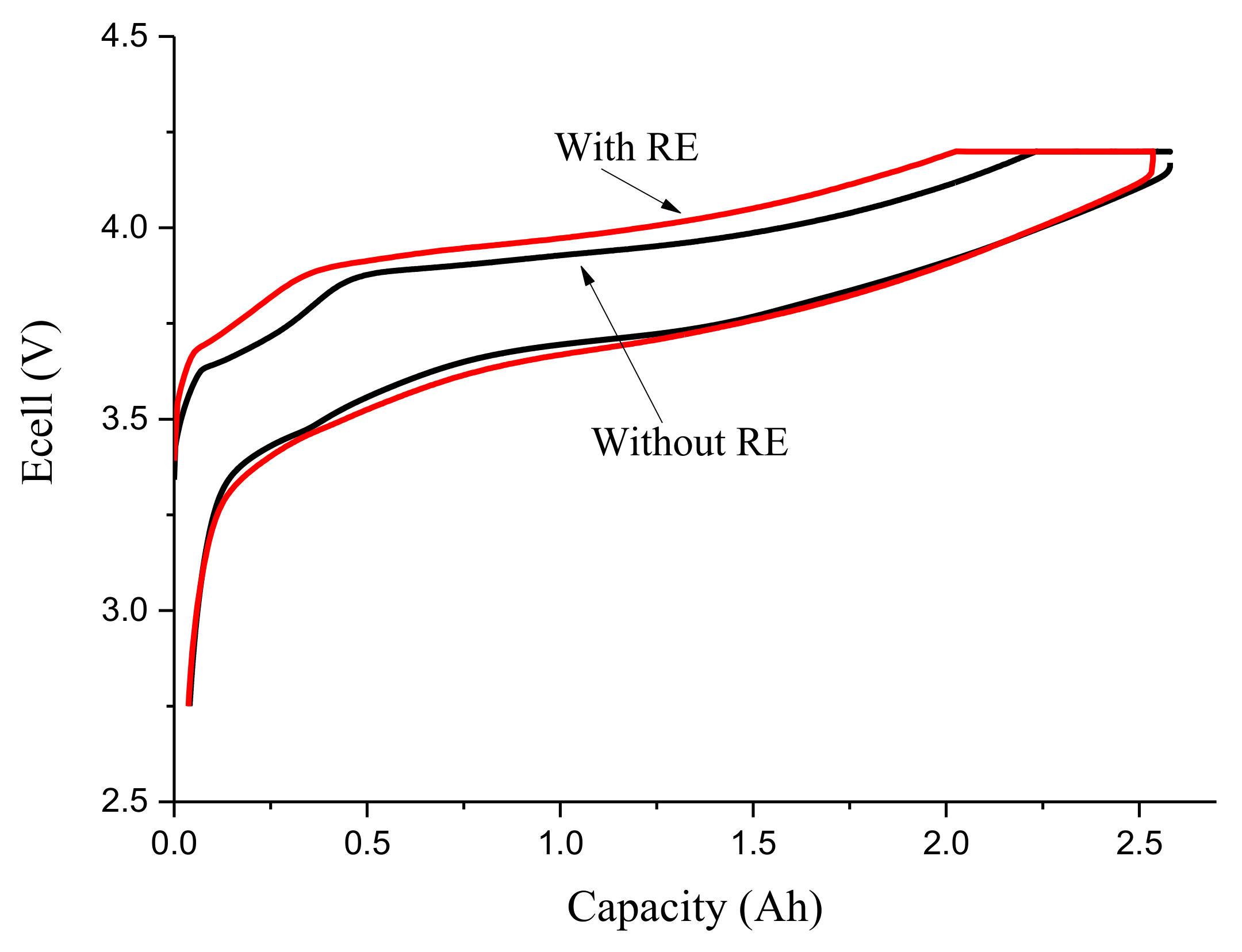
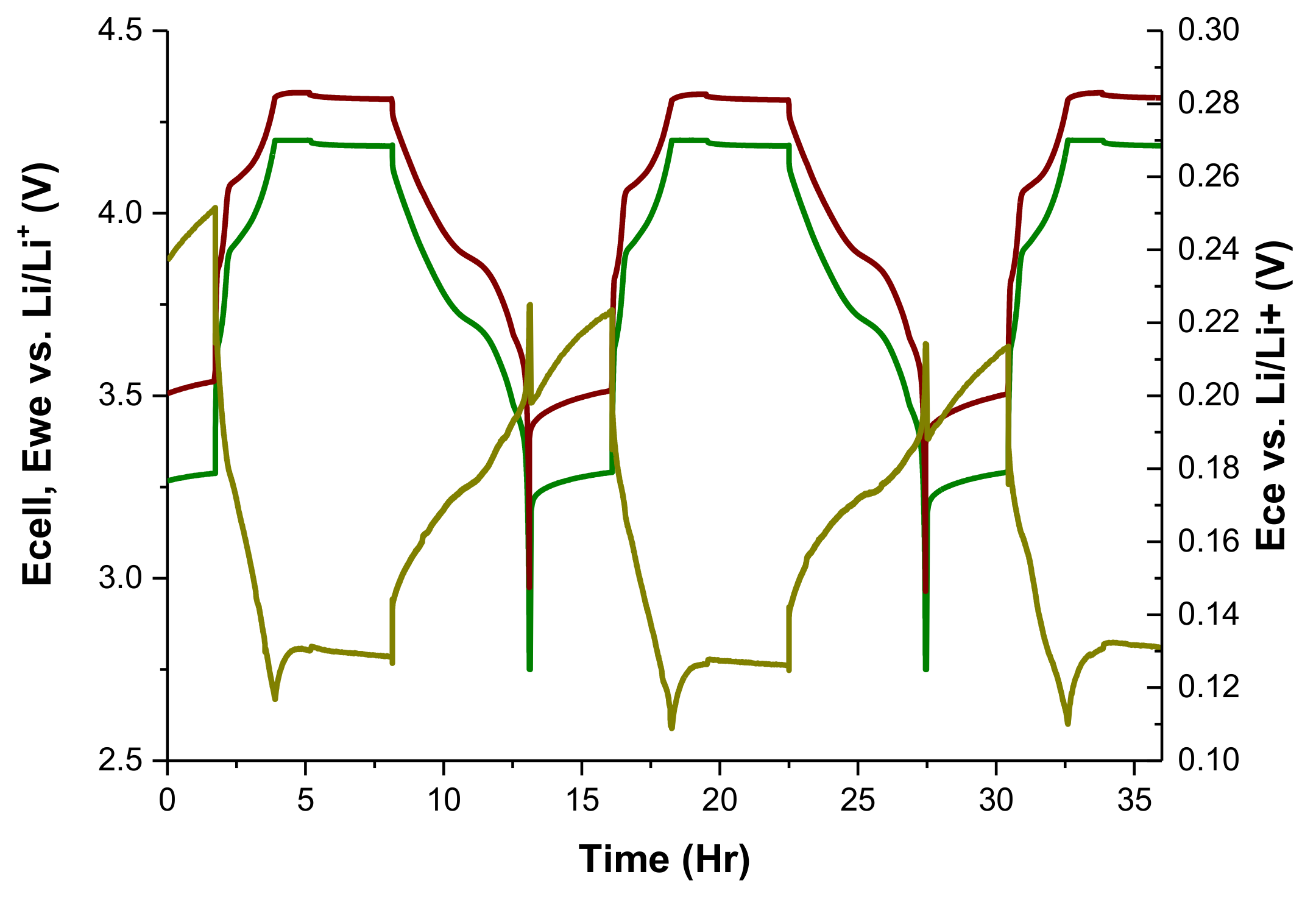
3.1. Third Electrode 18650 Results
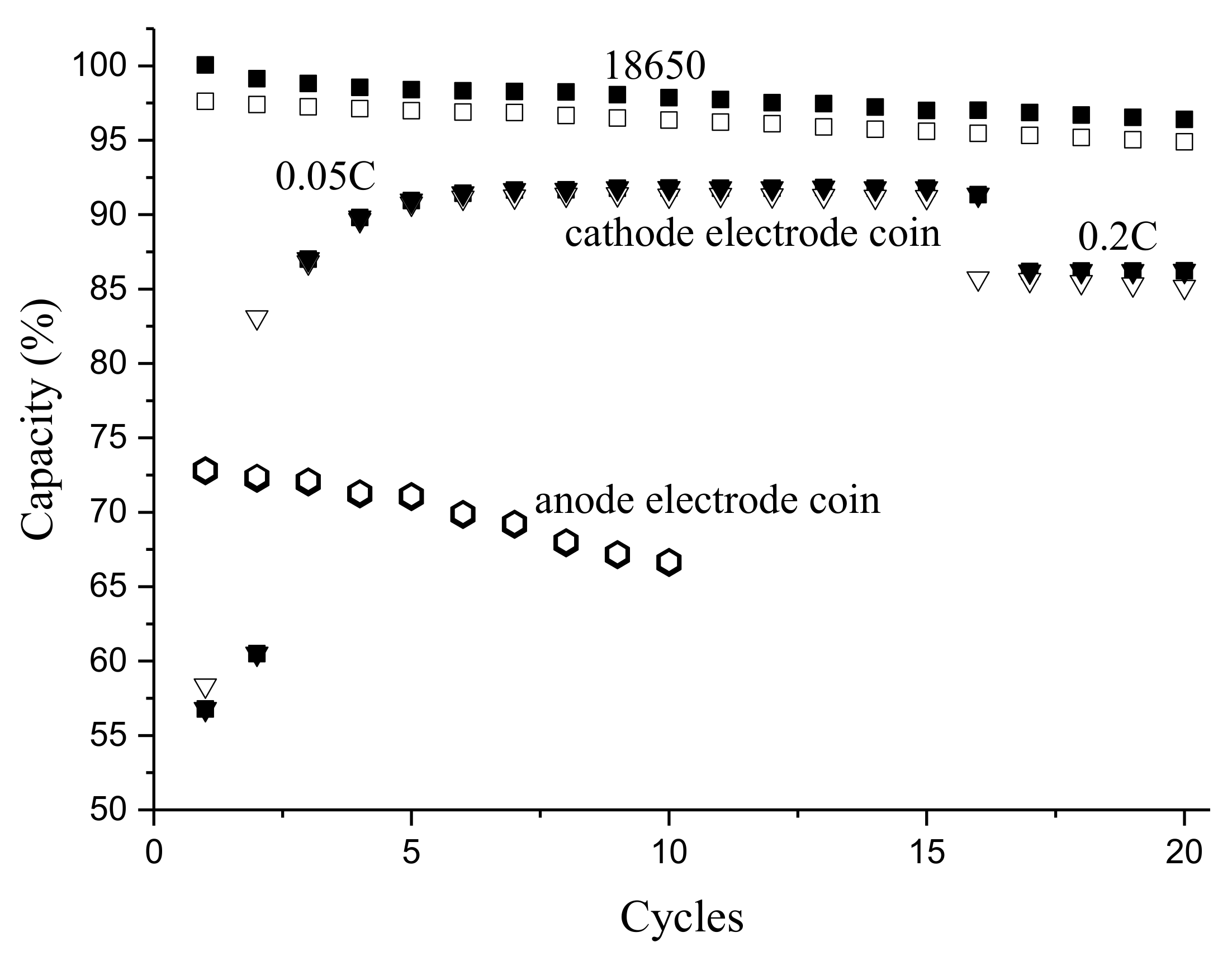
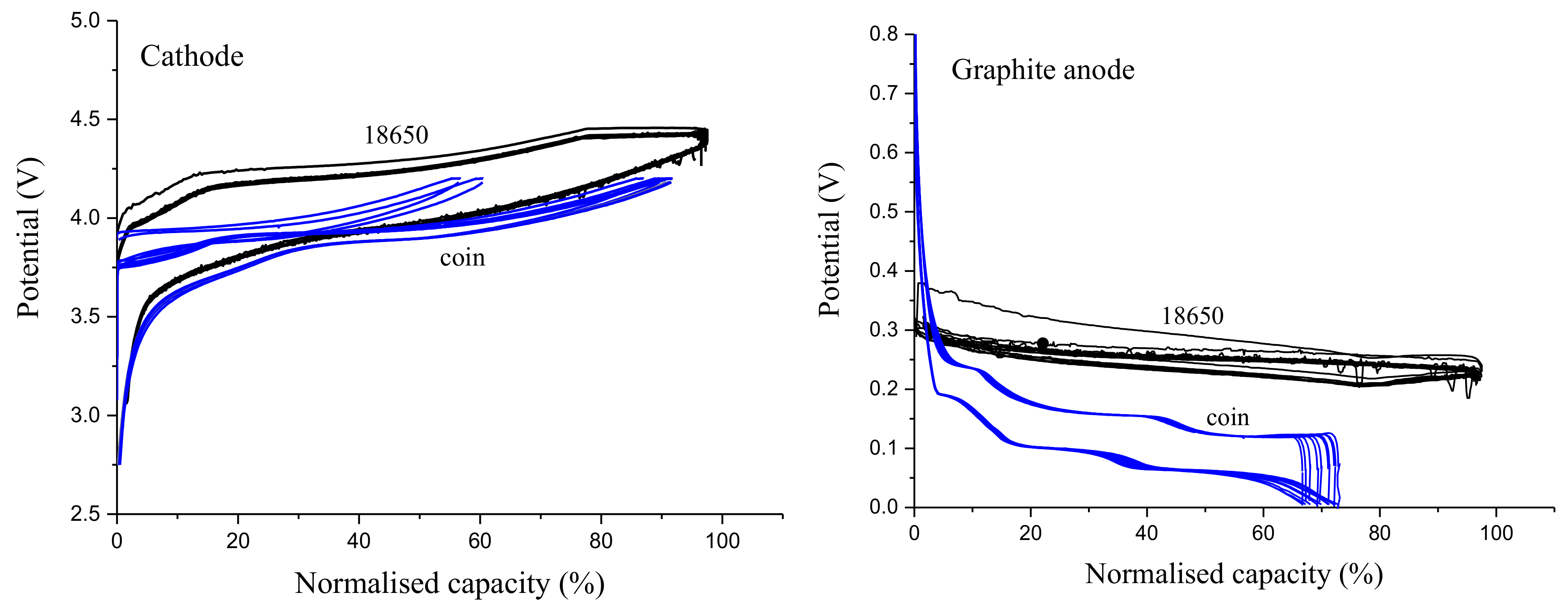
3.2. Coin Cells
3.3. Effect of RE Insertion Procedure on Internal Cell Components
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.; McGrory, J.; Kong, J.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Cycling degradation testing and analysis of a LiFePO4 battery at actual conditions. Int. J. Environ. Res. 2017, 41, 2565–2575. [Google Scholar] [CrossRef]
- Panchal, S.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Uneven temperature and voltage distributions due to rapid discharge rates and different boundary conditions for series-connected LiFePO4 batteries. Int. Commun. Heat Mass 2017, 81, 210–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.-Y.; Tang, X. Cycling degradation of an automotive LiFePO4 lithium-ion battery. J. Power Sources 2011, 196, 1513–1520. [Google Scholar] [CrossRef]
- Broussely, M.; Biensan, P.; Bonhomme, F.; Blanchard, P.; Herreyre, S.; Nechev, K.; Staniewicz, R. Main aging mechanisms in Li ion batteries. J. Power Sources 2005, 146, 90–96. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Blyr, A.; Sigala, C.; Amatucci, G.; Guyomard, D.; Chabre, Y.; Tarascon, J.M. Self-discharge of LiMn2O4/C Li-ion cells in their discharged state understanding by means of three-electrode measurements. J. Electrochem. Soc. 1998, 145, 194–209. [Google Scholar] [CrossRef]
- Abraham, D.; Reynolds, E.; Sammann, E.; Jansen, A.; Dees, D. Aging characteristics of high-power lithium-ion cells with LiNi0.8Co0.15Al0.05O2 and Li4/3Ti5/3O4 electrodes. Electrochim. Acta 2005, 51, 502–510. [Google Scholar] [CrossRef]
- Ives, D.J.; Janz, G.J.; King, C. Reference electrodes: Theory and practice. J. Electrochem. Soc. 1961, 108, 246C–247C. [Google Scholar] [CrossRef]
- McTurk, E.; Birkl, C.; Roberts, M.; Howey, D.; Bruce, P. Minimally invasive insertion of reference electrodes into commercial lithium-ion pouch cells. ECS Electrochem. Lett. 2015, 4, A145–A147. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Talyossef, Y.; Salitra, G.; Kim, H.-J.; Choi, S. Studies of cycling behavior, ageing, and interfacial reactions of LiNi0.5Mn1.5O4 and carbon electrodes for lithium-ion 5-V cells. J. Power Sources 2006, 162, 780–789. [Google Scholar]
- Abraham, D.; Knuth, J.; Dees, D.; Bloom, I.; Christophersen, J. Performance degradation of high-power lithium-ion cells—Electrochemistry of harvested electrodes. J. Power Sources 2007, 170, 465–475. [Google Scholar] [CrossRef]
- Waldmann, T.; Kasper, M.; Wohlfahrt-Mehrens, M. Optimization of charging strategy by prevention of lithium deposition on anodes in high-energy lithium-ion batteries–electrochemical experiments. Electrochim. Acta 2015, 178, 525–532. [Google Scholar] [CrossRef]
- Malmgren, S.; Ciosek, K.; Lindblad, R.; Plogmaker, S.; Kühn, J.; Rensmo, H.; Edström, K.; Hahlin, M. Consequences of air exposure on the lithiated graphite SEI. Electrochim. Acta 2013, 105, 83–91. [Google Scholar] [CrossRef]
- Somerville, L.; Bareño, J.; Jennings, P.; McGordon, A.; Lyness, C.; Bloom, I. The effect of pre-analysis washing on the surface film of graphite electrodes. Electrochim. Acta 2016, 206, 70–76. [Google Scholar] [CrossRef]
- Belt, J.R.; Bernardi, D.M.; Utgikar, V. Development and use of a Lithium-metal reference electrode in aging studies of Lithium-ion batteries. J. Electrochem. Soc. 2014, 161, A1116–A1126. [Google Scholar] [CrossRef]
- Liu, P.; Wang, J.; Hicks-Garner, J.; Sherman, E.; Soukiazian, S.; Verbrugge, M.; Tataria, H.; Musser, J.; Finamore, P. Aging mechanisms of LiFePO4 batteries deduced by electrochemical and structural analyses. J. Electrochem. Soc. 2010, 157, A499–A507. [Google Scholar] [CrossRef]
- Nagasubramanian, G. Two-and three-electrode impedance studies on 18650 Li-ion cells. J. Power Sources 2000, 87, 226–229. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.-Y. Cycle-life characterization of automotive lithium-ion batteries with LiNiO2 cathode. J. Electrochem. Soc. 2009, 156, A527–A535. [Google Scholar] [CrossRef]
- Burrows, B.; Jasinski, R.J. The Li/Li+ reference electrode in propylene carbonate. J. Electrochem. Soc. 1968, 115, 365–367. [Google Scholar] [CrossRef]
- Isutzu, K. Reference Electrodes for Use in Nonaqueous Solutions. In Handbook of Reference Electrodes; Inzelt, G., Lewenstam, A., Scholz, F., Eds.; Springer: Berlin, Germany, 2013; pp. 145–187. [Google Scholar]
- Aurbach, D.; Markovsky, B.; Weissman, I.; Levi, E.; Ein-Eli, Y. Correlation between surface chemistry and performance of graphite negative electrodes for Li ion batteries. Electrochim. Acta 1999, 45, 67–86. [Google Scholar] [CrossRef]
- Illig, J.; Schmidt, J.; Weiss, M.; Weber, A.; Ivers-Tiffée, E. Understanding the impedance spectrum of 18650 LiFePO4-cells. J. Power Sources 2013, 239, 670–679. [Google Scholar] [CrossRef]
- Klett, M.; Eriksson, R.; Groot, J.; Svens, P.; Högström, K.C.; Lindström, R.W.; Berg, H.; Gustafson, T.; Lindbergh, G.; Edström, K. Non-uniform aging of cycled commercial LiFePO4//graphite cylindrical cells revealed by post-mortem analysis. J. Power Sources 2014, 257, 126–137. [Google Scholar] [CrossRef]
- Prezas, P.D.; Somerville, L.; Jennings, P.; McGordon, A.; Basco, J.; Duong, T.; Bloom, I. Effect of Fast Charging of Lithium-Ion Cells: Performance and Post-Test Results; SAE Technical Paper; SAE International: Warrendale, PA, USA, 5 April 2016. [Google Scholar]
- Waldmann, T.; Bisle, G.; Hogg, B.-I.; Stumpp, S.; Danzer, M.A.; Kasper, M.; Axmann, P.; Wohlfahrt-Mehrens, M. Influence of cell design on temperatures and temperature gradients in Lithium-ion cells: An in operando study. J. Electrochem. Soc. 2015, 162, A921–A927. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somerville, L.; Ferrari, S.; Lain, M.J.; McGordon, A.; Jennings, P.; Bhagat, R. An In-Situ Reference Electrode Insertion Method for Commercial 18650-Type Cells. Batteries 2018, 4, 18. https://doi.org/10.3390/batteries4020018
Somerville L, Ferrari S, Lain MJ, McGordon A, Jennings P, Bhagat R. An In-Situ Reference Electrode Insertion Method for Commercial 18650-Type Cells. Batteries. 2018; 4(2):18. https://doi.org/10.3390/batteries4020018
Chicago/Turabian StyleSomerville, Limhi, Stefania Ferrari, Michael J. Lain, Andrew McGordon, Paul Jennings, and Rohit Bhagat. 2018. "An In-Situ Reference Electrode Insertion Method for Commercial 18650-Type Cells" Batteries 4, no. 2: 18. https://doi.org/10.3390/batteries4020018
APA StyleSomerville, L., Ferrari, S., Lain, M. J., McGordon, A., Jennings, P., & Bhagat, R. (2018). An In-Situ Reference Electrode Insertion Method for Commercial 18650-Type Cells. Batteries, 4(2), 18. https://doi.org/10.3390/batteries4020018