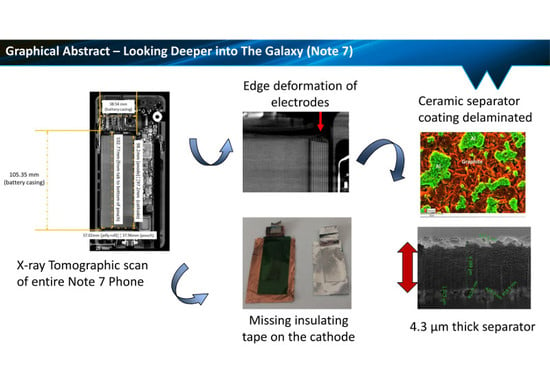
Looking Deeper into the Galaxy (Note 7)
Abstract
:1. Introduction
1.1. The Thermal Runaway Cascade
1.2. How was Battery Manufacturing the Cause of the Faults?
- (i)
- Insufficient insulation material within the batteries;
- (ii)
- Not enough room to safely accommodate the battery;
- (iii)
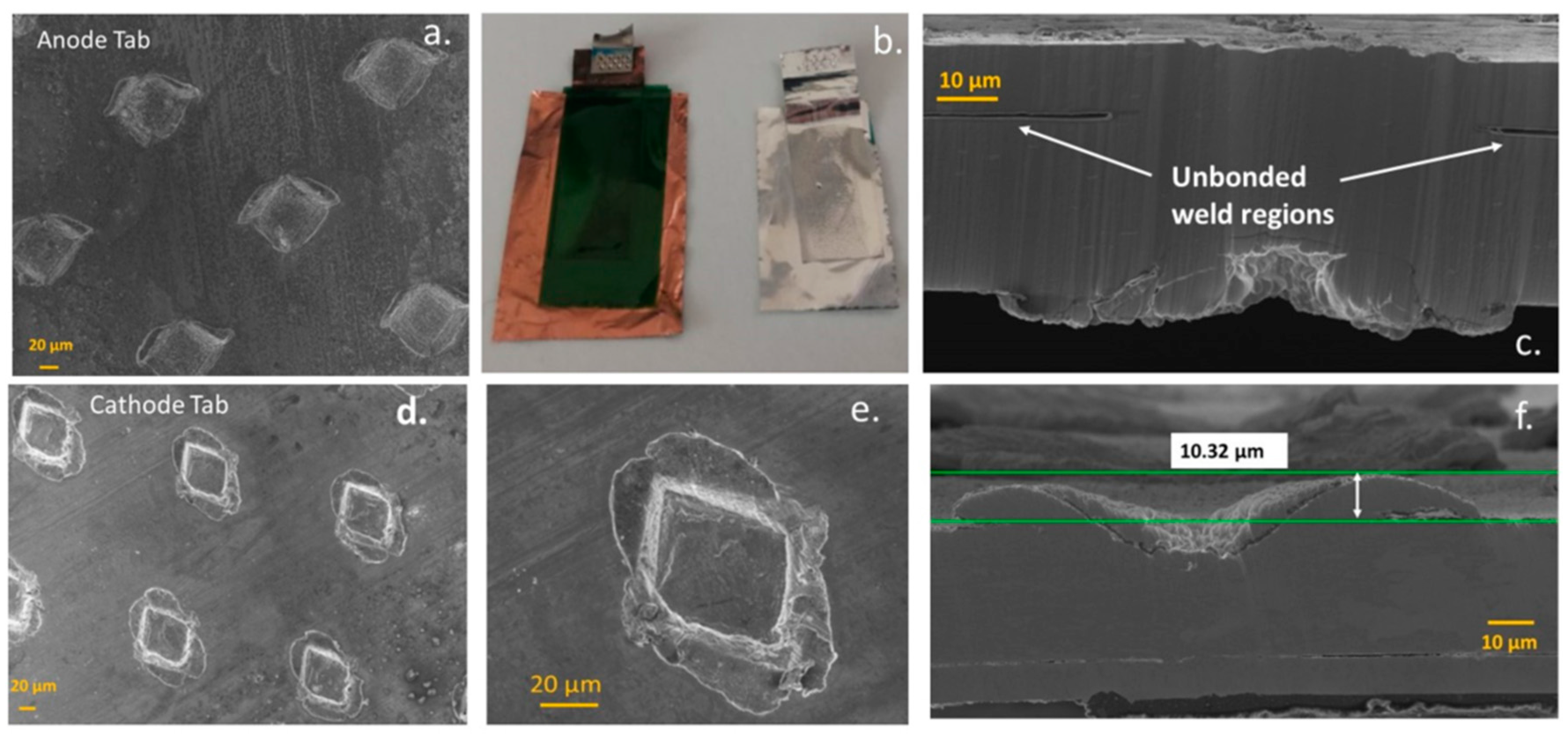
- Welding burrs on the positive electrode resulting in penetration of the insulating tape (on the tabs) and separator.
1.3. Reported Failure Suspicions and Battery Component Causes
- (i)
- Internal short circuit (ISC) at the upper right corner of the cells;
- (ii)
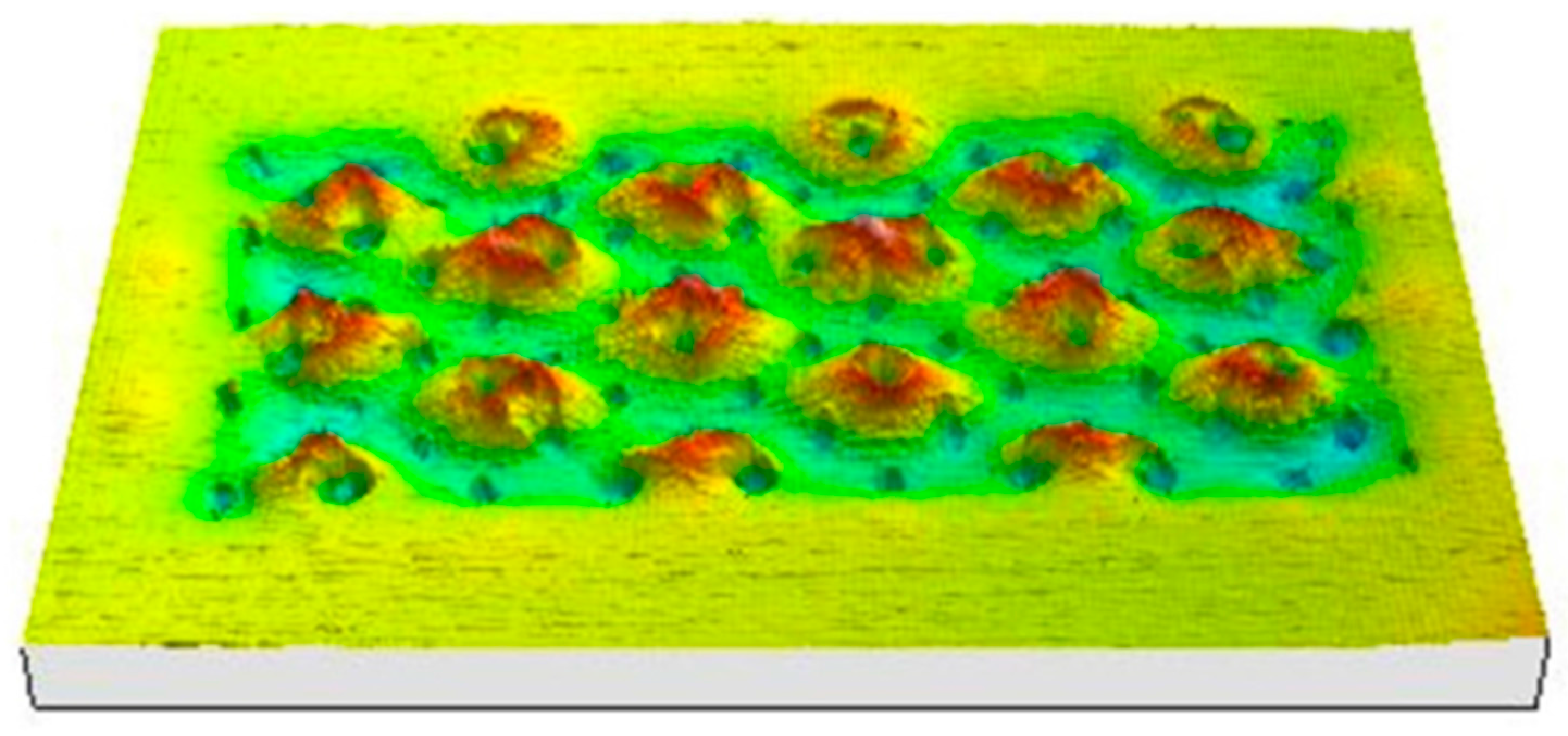
- Repeated deformation of the separator at corner locations;
- (iii)
- Missing insulating tape on the cathode tab;
- (iv)
- Poor alignment of components;
- (v)
- Uneven charge status and;
- (vi)
- Thinner separator compared with others used in previous devices.
2. Results and Discussion
2.1. Electrochemical Characterisation
2.2. X-ray CT Characterisation of Device and Internal Battery Features
2.2.1. Battery Cell Characterisation
2.2.2. Cell Component Characterisation
2.3. Characterisation of Welded Joints in Battery Tabs
3. Materials and Methods
3.1. Electrochemical Characterisation
3.2. Charging Limitation From “Safety Software Patch”
3.3. X-ray Computed Tomography
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Jacoby, M. Safer Lithium-Ion Batteries. Chem. Eng. News Arch. 2013, 91, 33–37. [Google Scholar]
- Kolly, B.J.; Panagiotou, M.J.; Czech, B.A. The Investigation of a Lithium-Ion Battery Fire Onboard a Boeing 787 by the US National Transportation Safety Board; Safety Research Corporation of America: Dothan, AL, USA, 2013; pp. 1–18. [Google Scholar]
- Christman, J. The case of the burning laptops. J. Case Stud. 2012, 30, 88–97. [Google Scholar]
- National Transportation Safety Board. Aircraft Incident Report: Auxiliary Power Unit Battery Fire, Japan Airlines Being 787-8, JA828j, NTSB/AIR-14/01; National Transportation Safety Board: Washington, DC, USA, 2013; pp. 1–95.
- Rourke, J.O.; Carrillo, A.; Harville, L.; Portilla, D.; Rourke, J.S.O. The Boeing Company: The Grounding of the 787 Dreamliner. J. Organ. Behav. Educ. 2015, 8, 1–12. [Google Scholar]
- Warren, S. Computed Tomography Specialist’s Factual Report; Office of Aviation Safety: Washington, DC, USA, 2013; pp. 1–112.
- Norihiro, G. Emergency Evacuation Using Slides All Nippon Airways Co., Ltd. Boeing 787-8, JA804A, Takamatsu AirportJTSB; Aircraft Serious Incident Investigation Report; Japan Transport Safety Board: Chiyoda, Tokyo, 2014.
- Finegan, D.P.; Scheel, M.; Robinson, J.B.; Tjaden, B.; Hunt, I.; Mason, T.J.; Millichamp, J.; Michiel, M.D.; Offer, G.J.; Hinds, G. Lithium-Ion Batteries During Thermal Runaway. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.F.; Jeevarajan, J.A.; Mukherjee, P.P. Charaterisation of Lithium-Ion Battery Thermal Abuse Behaviour Using Experimental and Computational Analysis. J. Electrochem. Soc. 2015, 162, A2163–A2173. [Google Scholar] [CrossRef]
- Xu, F.; He, H.; Dun, C.; Liu, Y.; Wang, M.; Liu, Q.; Ren, Y.; Xie, J. Failure Investigation of LiFePO4 Cells under Overcharge Conditions. ECS Trans. 2012, 41, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Gong, J.; Liu, K.; Wang, H.; Guo, L. Experimental Study of Thermal Runaway Process of 18650 Lithium-Ion Battery. Materials 2017, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, R.; Franklin, J. Abuse behaviour of lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Hewson, J.C.; Domino, S.P. Thermal runaway of lithium-ion batteries and hazards of abnormal thermal environments. In Proceedings of the 9th U.S. National Combustion Meeting, Cincinnati, OH, USA, 17–20 May 2015; pp. 1–9. [Google Scholar]
- Nedjalkov, A.; Meyer, J.; Köhring, M.; Doering, A.; Angelmahr, M.; Dahle, S.; Sander, A.; Fischer, A. Wolfgang Schade. Toxic Gas Emissions from Damaged Lithium Ion Batteries—Analysis and Safety Enhancement Solution. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Jiang, J.; Dahn, J.R. Effects of particle size and electrolyte salt on the thermal stability of Li0.5CoO2. Electrochim. Acta 2004, 49, 2661–2666. [Google Scholar] [CrossRef]
- Doh, C.-H.; Veluchamy, A. Thermo-Chemical Process Associated with Lithium Cobalt Oxide Cathode in Lithium-Ion Batteries, Lithium-ion Batteries, 1st ed.; Park, C.R., Ed.; InTech: Rijeka, Croatia, 1987; pp. 35–57. [Google Scholar]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Sun, F.; Moroni, R.; Dong, K.; Marko, H.; Zhou, D.; Hilger, A. Study of the Mechanisms of Internal Short Circuit in a Li-Li Cell by Synchrotron X-ray Phase Contrast Tomography. ACS Energy Lett. 2017, 26, 94–104. [Google Scholar] [CrossRef]
- Dunn, R.P. Flame Retardant Incorporation into Lithium-Ion Batteries. Ph.D. Thesis, University of Rhode Island, Kingston, RI, USA, 2013. [Google Scholar]
- Reuters. Note 7 Fiasco Could Burn a $17 Billion Hole Samsung Accounts. Available online: https://www.reuters.com/article/us-samsung-elec-smartphones-costs/note-7-fiasco-could-burn-a-17-billion-hole-in-samsung-accounts-idUSKCN12B0FX (accessed on 20 November 2016).
- Jesudas, S. Samsung Electronics Anounces Cause of Galaxy Note 7 Incidents in Press Conference, Press Release. Available online: https://news.samsung.com/global/samsung-electronics-announces-cause-of-galaxy-note7-incidents-in-press-conference (accessed on 23 Janary 2017).
- White, K. Samsung Recall Support Note7 Investigation—Root Cause Analysis, Exponent Press Release. Available online: https://www.exponent.com/newsevents/announcements/2017/01/samsung-n7-press-conf (accessed on 25 January 2017).
- Cannarella, J.; Liu, X.; Leng, C.Z.; Sinko, P.D.; Gennady, Y.; Arnold, C.B. Mechanical Properties of a Battery Separator under Compression and Tension. J. Electrochem. Soc. 2014, 161, F3117–F3122. [Google Scholar] [CrossRef]
- Gor, G.Y.; Cannarella, J.; Leng, C.Z.; Vishnyakov, A.; Arnold, C.B. Swelling and softening of lithium-ion battery separators in electrolyte solvents. J. Power Sources 2015, 294, 167–172. [Google Scholar]
- Arora, P.; Zhang, Z. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Heum, S.; Kim, D. Enhancement of thermal stability and cycling performance in lithium-ion cells through the use of ceramics-coated separators. J. Power Sources 2010, 195, 6192–6196. [Google Scholar] [CrossRef]
- Woo, J.; Zhang, Z.; Rago, N.L.D.; Lu, W.; Amine, K. A high performance separator with improved thermal stability for Li-ion batteries. Polym. J. Mater. Chem. A 2013, 1, 8538–8540. [Google Scholar] [CrossRef]
- Shi, C.; Dai, J.; Li, C.; Shen, X.; Peng, L.; Zhang, P.; Wu, D.; Sun, D.; Zhao, J. A Modified Ceramic-Coating Separator with High-Temperature Stability for Lithium-ion Battery. Polymers 2017, 9, 159. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, T.H.; Hu, S.J.; Cai, W.W.; Abell, J.A. Characterisation of Joint Quality in Ultrasonic Welding of Battery Tabs. J. Manuf. Sci. Eng. 2017, 135, 1–13. [Google Scholar]
- Kang, B.; Cai, W.; Tan, C.-A. Dynamic Response of Battery Tabs under Ultrasonic Welding. ASME J. Manuf. Sci. Eng. 2012, 136. [Google Scholar] [CrossRef]
- Humrick, M. Samsung Reveals Root Cause of Galaxy Note 7 Battery Fires. Available online: https://www.anandtech.com/show/11060/samsung-reveals-root-cause-of-galaxy-note7-battery-fires (accessed on 23 January 2017).
- Zhao, N.; Li, W. Fatigue Life Prediction Model For Ultrasonically Welded Battery Tab Joints. J. Manuf. Sci. Eng. 2014, 136. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loveridge, M.J.; Remy, G.; Kourra, N.; Genieser, R.; Barai, A.; Lain, M.J.; Guo, Y.; Amor-Segan, M.; Williams, M.A.; Amietszajew, T.; et al. Looking Deeper into the Galaxy (Note 7). Batteries 2018, 4, 3. https://doi.org/10.3390/batteries4010003
Loveridge MJ, Remy G, Kourra N, Genieser R, Barai A, Lain MJ, Guo Y, Amor-Segan M, Williams MA, Amietszajew T, et al. Looking Deeper into the Galaxy (Note 7). Batteries. 2018; 4(1):3. https://doi.org/10.3390/batteries4010003
Chicago/Turabian StyleLoveridge, Melanie J., Guillaume Remy, Nadia Kourra, Ronny Genieser, Anup Barai, Mike J. Lain, Yue Guo, Mark Amor-Segan, Mark A. Williams, Tazdin Amietszajew, and et al. 2018. "Looking Deeper into the Galaxy (Note 7)" Batteries 4, no. 1: 3. https://doi.org/10.3390/batteries4010003
APA StyleLoveridge, M. J., Remy, G., Kourra, N., Genieser, R., Barai, A., Lain, M. J., Guo, Y., Amor-Segan, M., Williams, M. A., Amietszajew, T., Ellis, M., Bhagat, R., & Greenwood, D. (2018). Looking Deeper into the Galaxy (Note 7). Batteries, 4(1), 3. https://doi.org/10.3390/batteries4010003