Fe-Substitution for Ni in Misch Metal-Based Superlattice Hydrogen Absorbing Alloys—Part 2. Ni/MH Battery Performance and Failure Mechanisms
Abstract
:1. Introduction
2. Experimental Setup
3. Results and Discussion
3.1. Alloy Properties
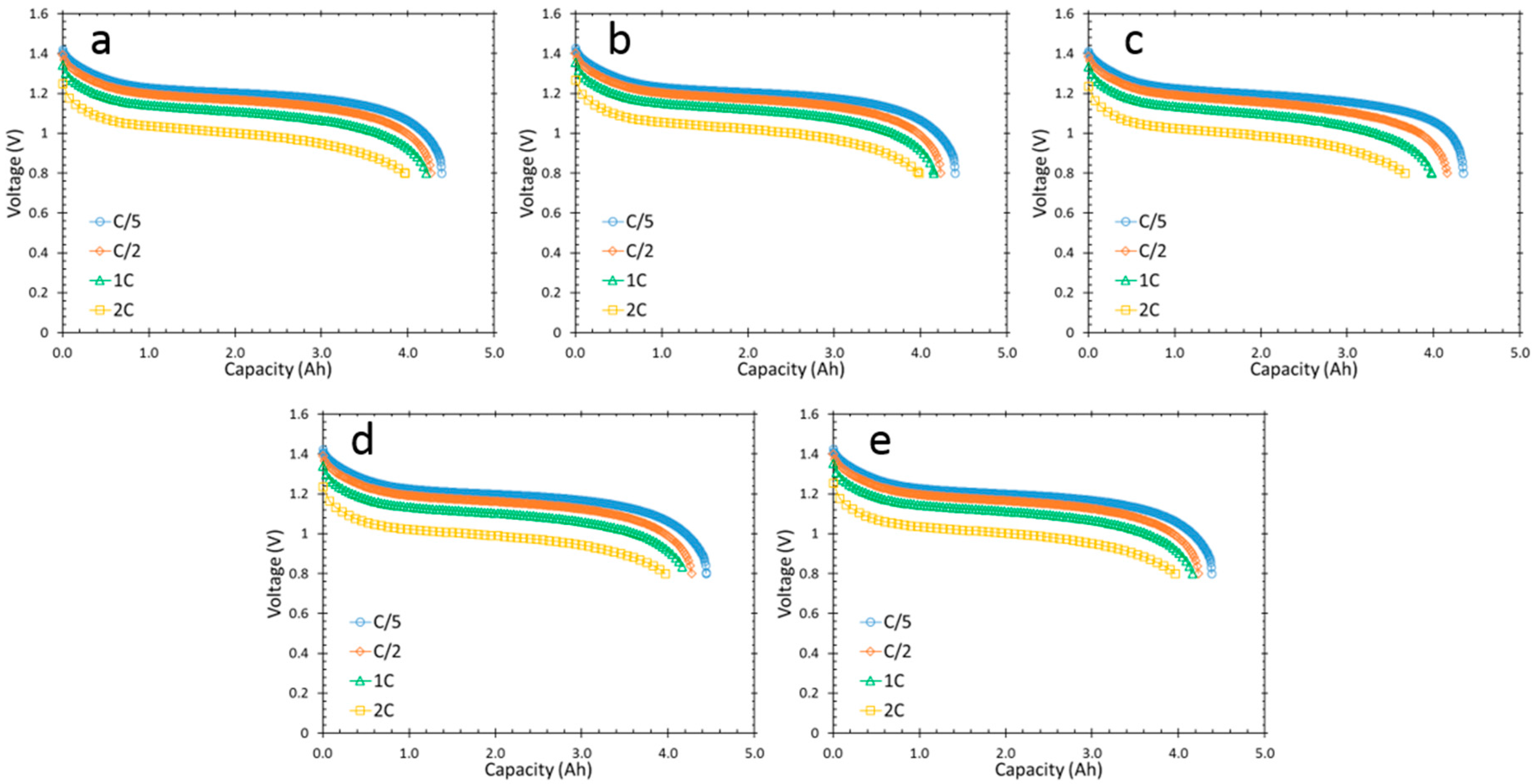
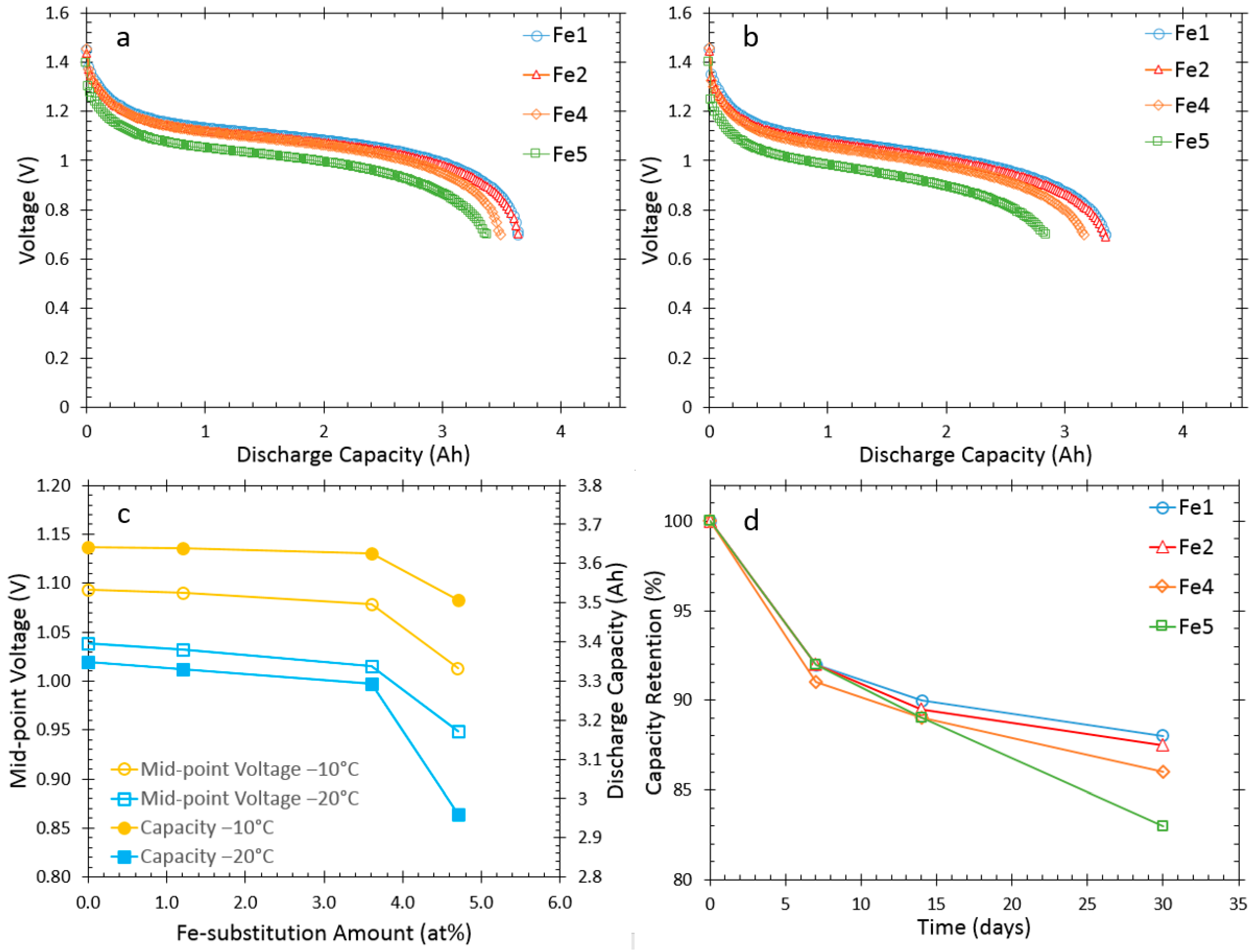
3.2. Cell Capacities at Room Temperature and Low Temperature
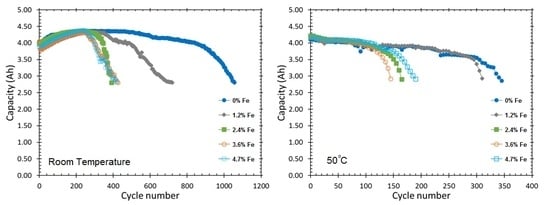
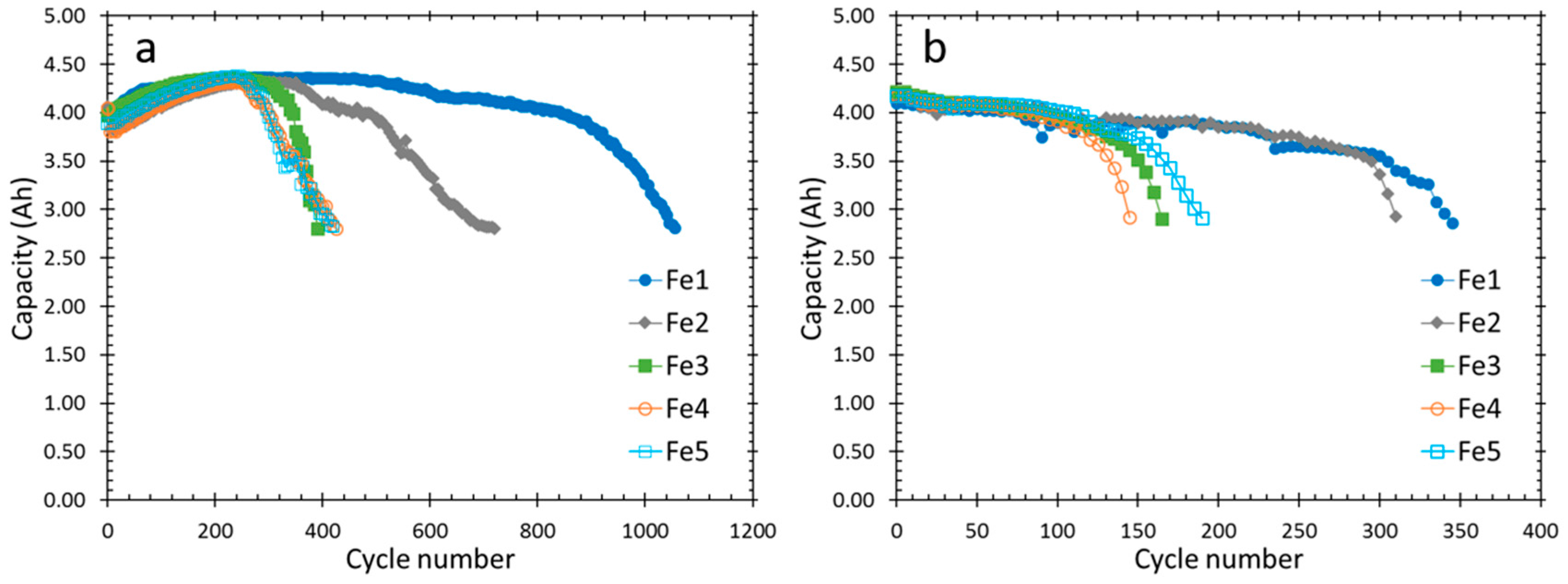
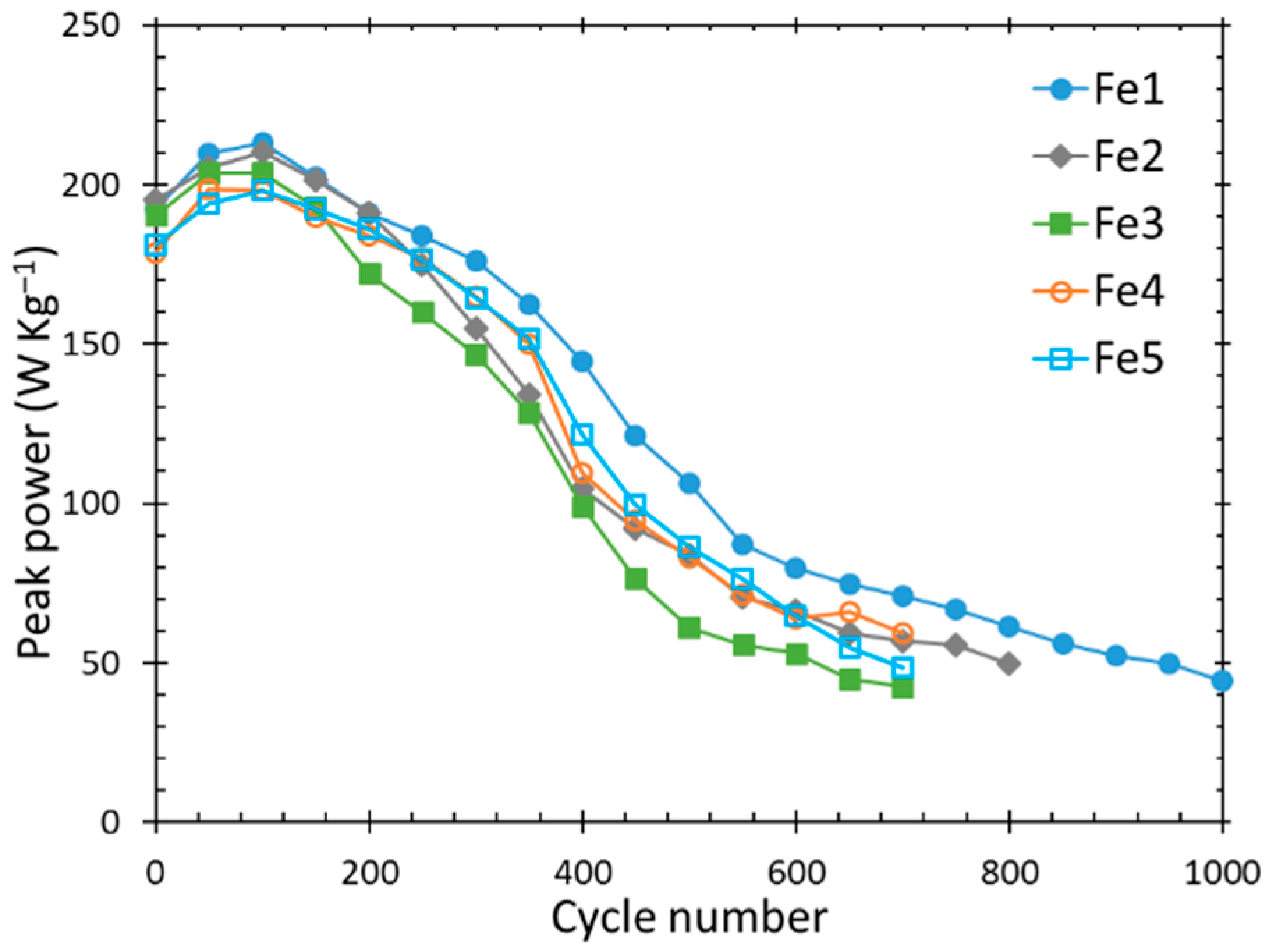
3.3. Cycle Life and Peak Power
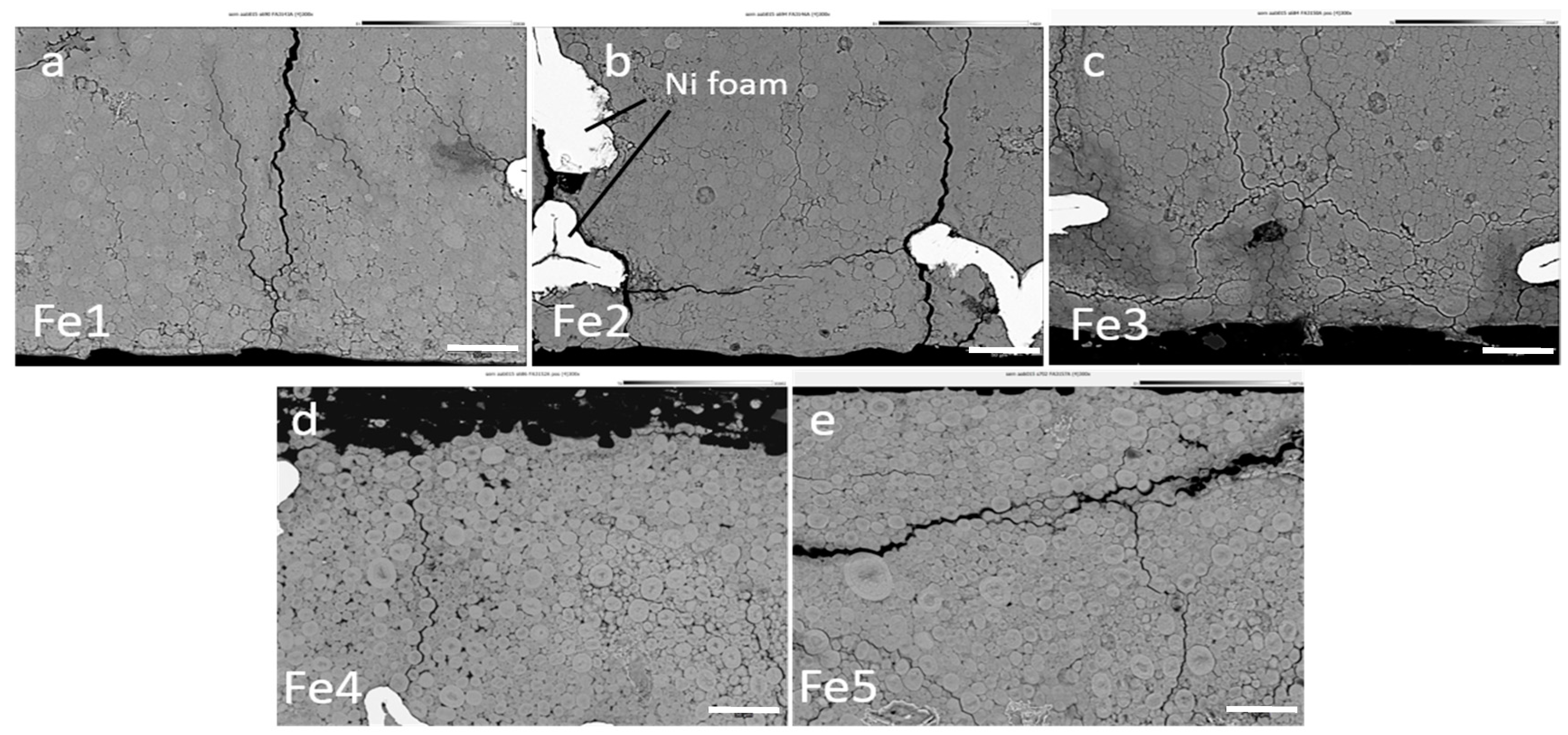
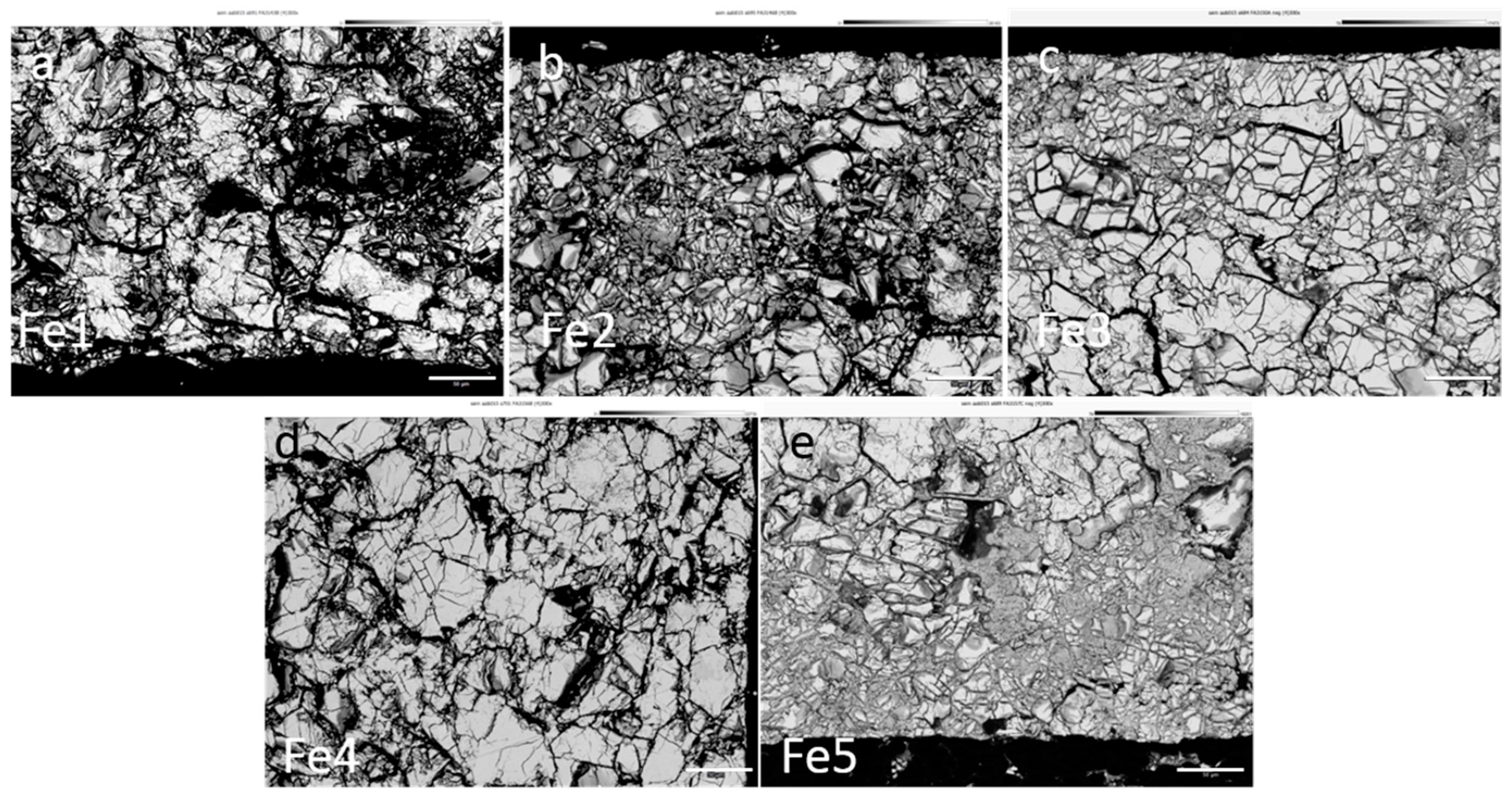
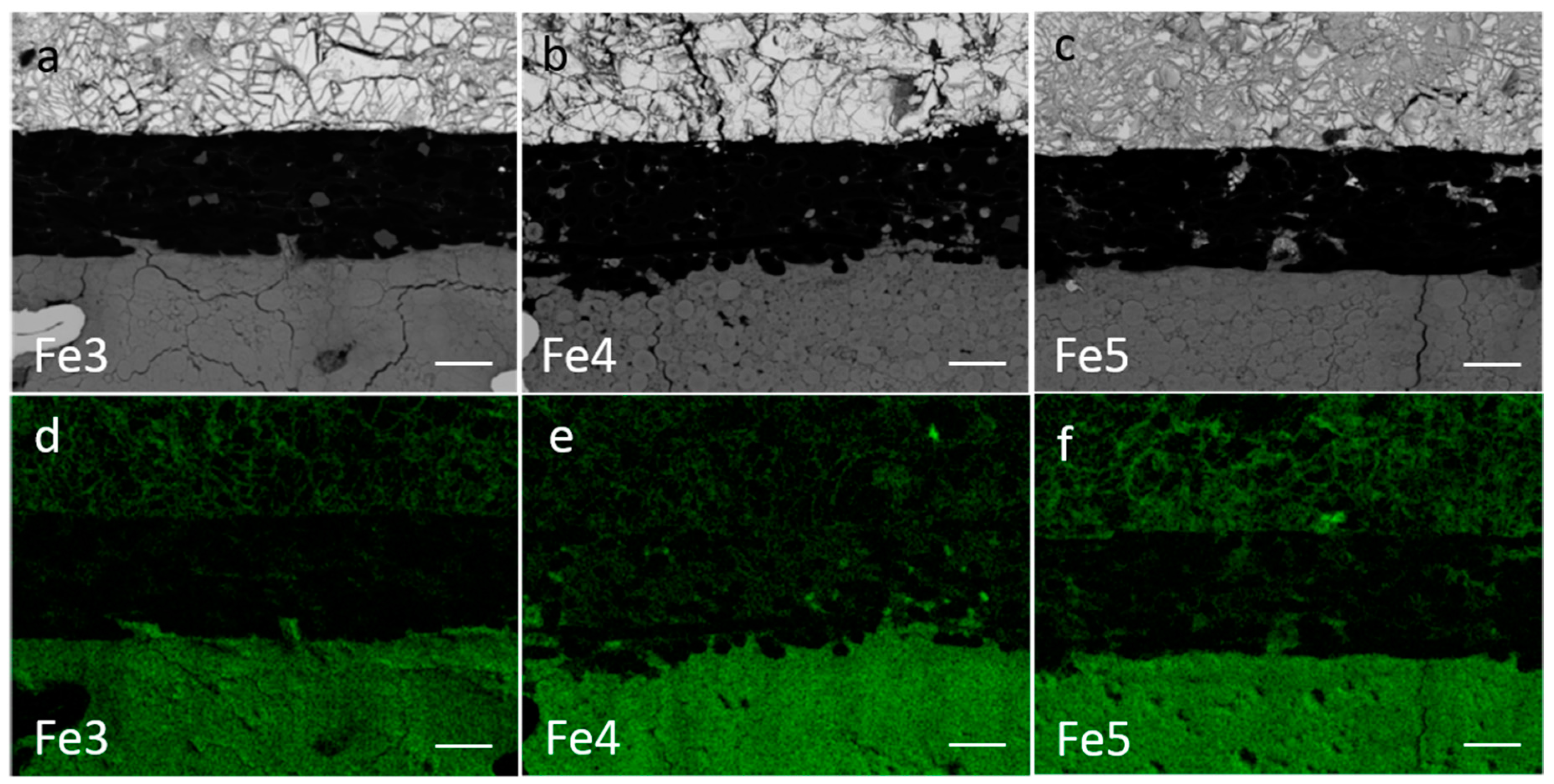
3.4. Failure Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Mm | Misch metal |
| HAA | Hydrogen absorbing alloy |
| Ni/MH | Nickel/metal hydride |
| HRD | High-rate dischargeability |
| RT | Room temperature |
| DOD | Depth-of-discharge |
| SEM | Scanning electron microscope |
| EDS | Energy dispersive spectroscopy |
| MS | Saturated magnetic susceptibility |
| C | Double-layer capacitance |
| LT | Low temperature |
| R | Charge-transfer resistance |
| BEI | Backscattered electron images |
| PCT | Pressure-composition-temperature |
| O-EDS | Oxygen-energy dispersive spectroscopy |
References
- Yasuoka, S.; Magari, Y.; Murata, T.; Tanaka, T.; Ishida, J.; Nakamura, H.; Nohma, T.; Kihara, M.; Baba, Y.; Teraoka, H. Development of high-capacity nickel-metal hydride batteries using superlattice hydrogen-absorbing alloys. J. Power Sources 2006, 156, 662–666. [Google Scholar]
- Takasaki, T.; Nishimura, K.; Saito, M.; Fukunaga, H.; Iwaki, T.; Sakai, T. Cobalt-free nickel-metal hydride battery for industrial applications. J. Alloy. Compd. 2013, 580, S378–S381. [Google Scholar]
- Liu, J.; Han, S.; Li, Y.; Yang, S.; Chen, X.; We, C.; Ma, C. Effect of Pr on phase structure and cycling stability of La-Mg-Ni-based alloys with A2B7- and A5B19-type superlattice structure. Electrochim. Acta 2015, 184, 257–263. [Google Scholar]
- Kai, T.; Ishida, J.; Yasuoka, S.; Takeno, K. The effect of nickel-metal hydride battery’s characteristics with structure of the alloy. In Proceedings of the 54th Battery Symposium in Japan, Osaka, Japan, 7–9 Octorber 2013. [Google Scholar]
- Young, K.; Wong, D.F.; Wang, L.; Nei, J.; Ouchi, T.; Yasuoka, S. Mn in misch-metal based superlattice metal hydride alloy—Part 1 Structural, hydrogen storage and electrochemical properties. J. Power Sources 2015, 277, 426–432. [Google Scholar]
- Young, K.; Wong, D.F.; Wang, L.; Nei, J.; Ouchi, T.; Yasuoka, S. Mn in misch-metal based superlattice metal hydride alloy—Part 2 Ni/MH battery performance and failure mechanism. J. Power Sources 2015, 277, 433–442. [Google Scholar]
- Wang, L.; Young, K.; Meng, T.; Ouchi, T.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy—Part 1 Structural, hydrogen storage and electrochemical properties. J. Alloy. Compd. 2016, 660, 407–415. [Google Scholar]
- Wang, L.; Young, K.; Meng, T.; English, N.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy—Part 2 Battery performance and failure mechanism. J. Alloy. Compd. 2016, 664, 417–427. [Google Scholar]
- Yasuoka, S.; Ishida, J.; Kichida, K.; Iniu, H. Effects of cerium on the hydrogen absorption-desorption properties of rare Earth-Mg-Ni hydrogen-absorbing alloys. J. Power Sources 2017, 346, 56–62. [Google Scholar]
- Young, K.; Ouchi, T.; Nei, J.; Yasuoka, S. Fe-substitution for Ni in misch metal-based superlattice hydrogen absorbing alloys—Part 1 Structural, hydrogen storage, and electrochemical properties. Batteries 2016, 2, 34. [Google Scholar]
- Kong, L.; Chen, B.; Young, K.; Koch, J.; Chan, A.; Li, W. Effects of Al- and Mn-contents in the negative MH alloy on the self-discharge and long-term storage properties of Ni/MH battery. J. Power Sources 2013, 213, 128–139. [Google Scholar]
- Young, K.; Wu, A.; Qiu, Z.; Tan, J.; Mays, W. Effects of H2O2 addition to the cell balance and self-discharge of Ni/MH batteries with AB5 and A2B7 alloys. Int. J. Hydrog. Energy 2012, 37, 9882–9891. [Google Scholar]
- Young, K.; Yasuoka, S. Past, Present, and Future of Metal Hydride Alloys in Nickel-Metal Hydride Batteries. In Proceedings of the 14th International Symposium on Metal-Hydrogen Systems, Manchester, UK, 21–25 July 2014. [Google Scholar]
- Yan, H.; Xiong, W.; Wang, L.; Li, B.; Li, J.; Zhao, X. Investigations on AB3-, A2B7- and A5B19-type La-Y-Ni system hydrogen storage alloys. Int. J. Hydrog. Energy 2017, 42, 2257–2264. [Google Scholar]
- Young, K.; Ouchi, T.; Nei, J.; Koch, M.J.; Lien, Y. Comparison among constituent phases in superlattice metal hydride alloys for battery applications. Batteries 2017. submitted. [Google Scholar]
- Young, K.; Huang, B.; Regmi, R.K.; Lawes, G.; Liu, Y. Comparisons of metallic clusters imbedded in the surface of AB2, AB5, and A2B7 alloys. J. Alloy. Compd. 2010, 506, 831–840. [Google Scholar]
- Young, K.; Chang, S.; Lin, X. C14 Laves phase metal hydride alloys for Ni/MH batteries applications. Batteries 2017. accepted. [Google Scholar]
- Young, K.; Ouchi, T.; Huang, B.; Reichman, B.; Fetcenko, M.A. The structure, hydrogen storage, and electrochemical properties of Fe-doped C14-predominating AB2 metal hydride alloys. Int. J. Hydrog. Energy 2011, 36, 12296–12304. [Google Scholar]
- Young, K.; Ouchi, T.; Reichman, B.; Koch, J.; Fetcenko, M.A. Improvement in the low-temperature performance of AB5 metal hydride alloys by Fe-addition. J. Alloy. Compd. 2011, 509, 7611–7617. [Google Scholar]
- Shinyama, K.; Magari, Y.; Akita, H.; Kumagae, K.; Nakamura, H.; Matsuta, S.; Nohma, T.; Takee, M.; Ishiwa, K. Investigation into the deterioration in storage characteristics of nickel-metal hydride batteries during cycling. J. Power Sources 2005, 143, 265–269. [Google Scholar]
- Teraoka, H. Development of Highly Durable and Long Life Ni-MH Batteries for Energy Storage Systems. In Proceedings of the 32th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 9–12 March 2015. [Google Scholar]
- Young, K.; Yasuoka, S. Capacity degradation mechanisms in nickel/metal hydride batteries. Batteries 2016, 2, 3. [Google Scholar] [CrossRef]
- Meng, T.; Young, K.; Koch, J.; Ouchi, T.; Yasuoka, S. Failure mechanisms of nickel/metal hydride batteries with cobalt-substituted superlattice hydrogen-absorbing alloy anodes at 50 °C. Batteries 2016, 2, 20. [Google Scholar] [CrossRef]
- Yasuoka, S.; Ishida, J.; Kai, T.; Kajiwara, T.; Doi, S.; Yamazaki, T.; Kishida, K.; Inui, H. Function of aluminum in crystal structure of rare Earth-Mg-Ni hydrogen-absorbing alloy and deterioration mechanism of Nd0.9Mg0.1Ni3.5 and Nd0.9Mg0.1Ni3.3Al0.2 alloys. Int. J. Hydrog. Energy 2017, 42, 11574–11583. [Google Scholar]
- Young, K.; Wang, L.; Yan, S.; Liao, X.; Meng, T.; Shen, H.; Mays, W.C. Fabrications of high-capacity alpha-Ni(OH)2. Batteries 2017, 3, 6. [Google Scholar] [CrossRef]
- Singh, D. Characteristics and effects of γ-NiOOH on cell performance and a method to quantify it in nickel electrode. J. Electrochem. Soc. 1998, 145, 116–120. [Google Scholar]
- Yuan, A.; Cheng, S.; Zhang, J.; Cao, C. Effects of metallic cobalt addition on the performance of pasted nickel electrodes. J. Power Sources 1999, 77, 178–182. [Google Scholar]
- Jayashree, R.S.; Kamath, P.V. Suppression of the α-nickel hydroxide transformation in concentrated alkali: Role of dissolved cations. J. Appl. Electrochem. 2001, 31, 1315–1320. [Google Scholar]
- Tessier, C.; Guerlou-Demourgues, L.; Faure, C.; Denage, C.; Delatouche, B.; Delmas, C. Influence of zinc on the stability of the β(II)/β(III) nickel hydroxide system during electrochemical cycling. J. Power Sources 2001, 102, 105–111. [Google Scholar]
- Ravikumar, C.R.; Kotteeswaran, P.; Bheema Raju, V.; Murugan, A.; Santosh, M.S.; Nagaswarupa, H.P.; Prashantha, S.C.; Anil Kumar, M.R.; Shivakumar, M.S. Influence of zinc additive and pH on the electrochemical activities of β-nickel hydroxide materials and its applications in secondary batteries. J. Energy Storage 2017, 9, 12–24. [Google Scholar]
- Zhou, X.; Young, K.; West, J.; Regalado, J.; Cherisol, K. Degradation mechanisms of high-energy bipolar nickel metal hydride battery with AB5 and A2B7 alloys. J. Alloy. Compd. 2013, 560, S373–S377. [Google Scholar]
- Young, K.; Ouchi, T.; Koch, J.; Fetcenko, M.A. Compositional optimization of vanadium-free hypo-stoichiometric AB2 metal hydride alloy for Ni/MH battery application. J. Alloy. Compd. 2012, 510, 97–106. [Google Scholar]
- Young, K.; Ouchi, T.; Fetcenko, M.A. Pressure-composition-temperature hysteresis in C14 Laves phase alloys—Part 1 Simple ternary alloys. J. Alloy. Compd. 2009, 480, 428–433. [Google Scholar]

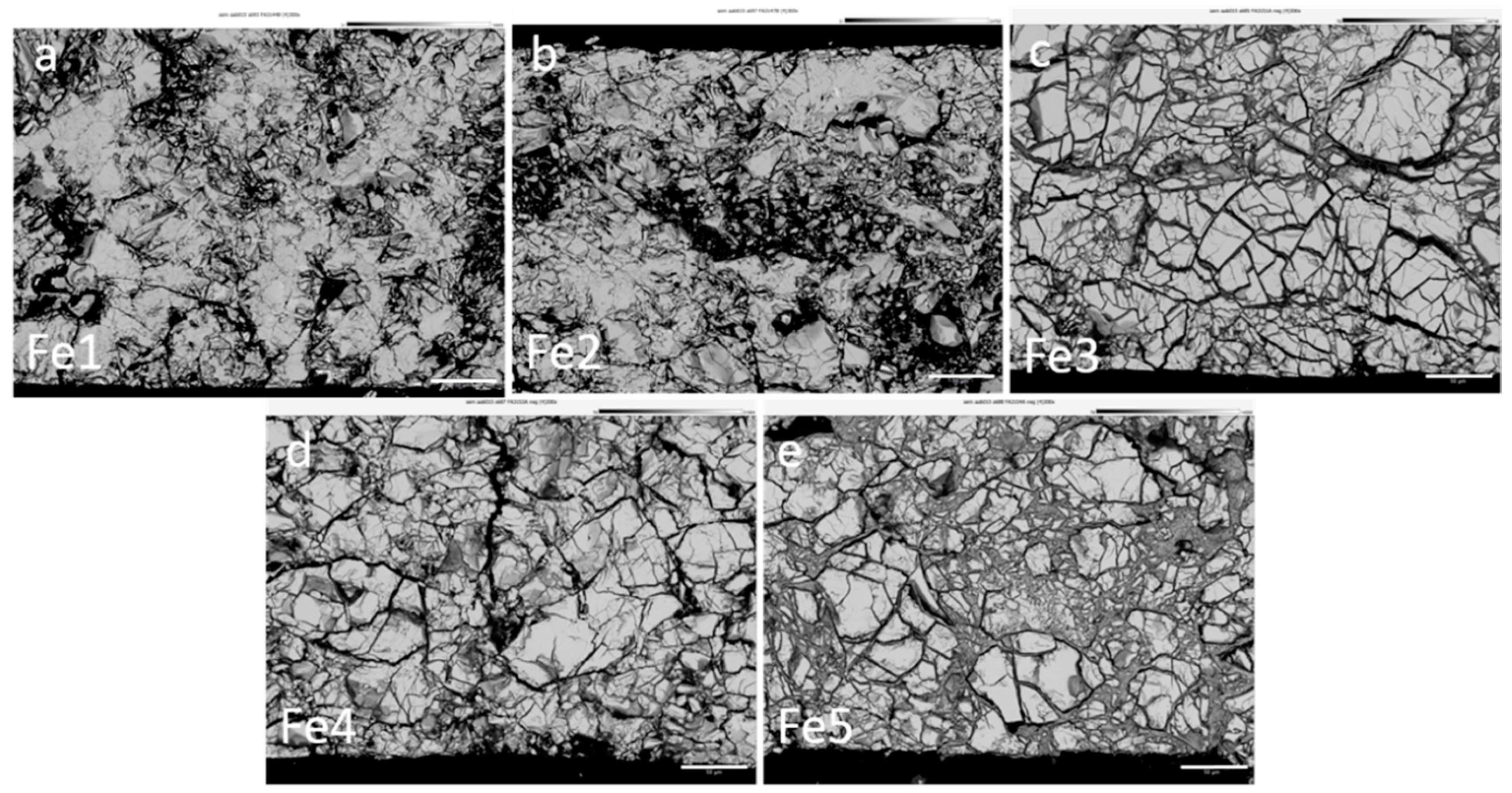
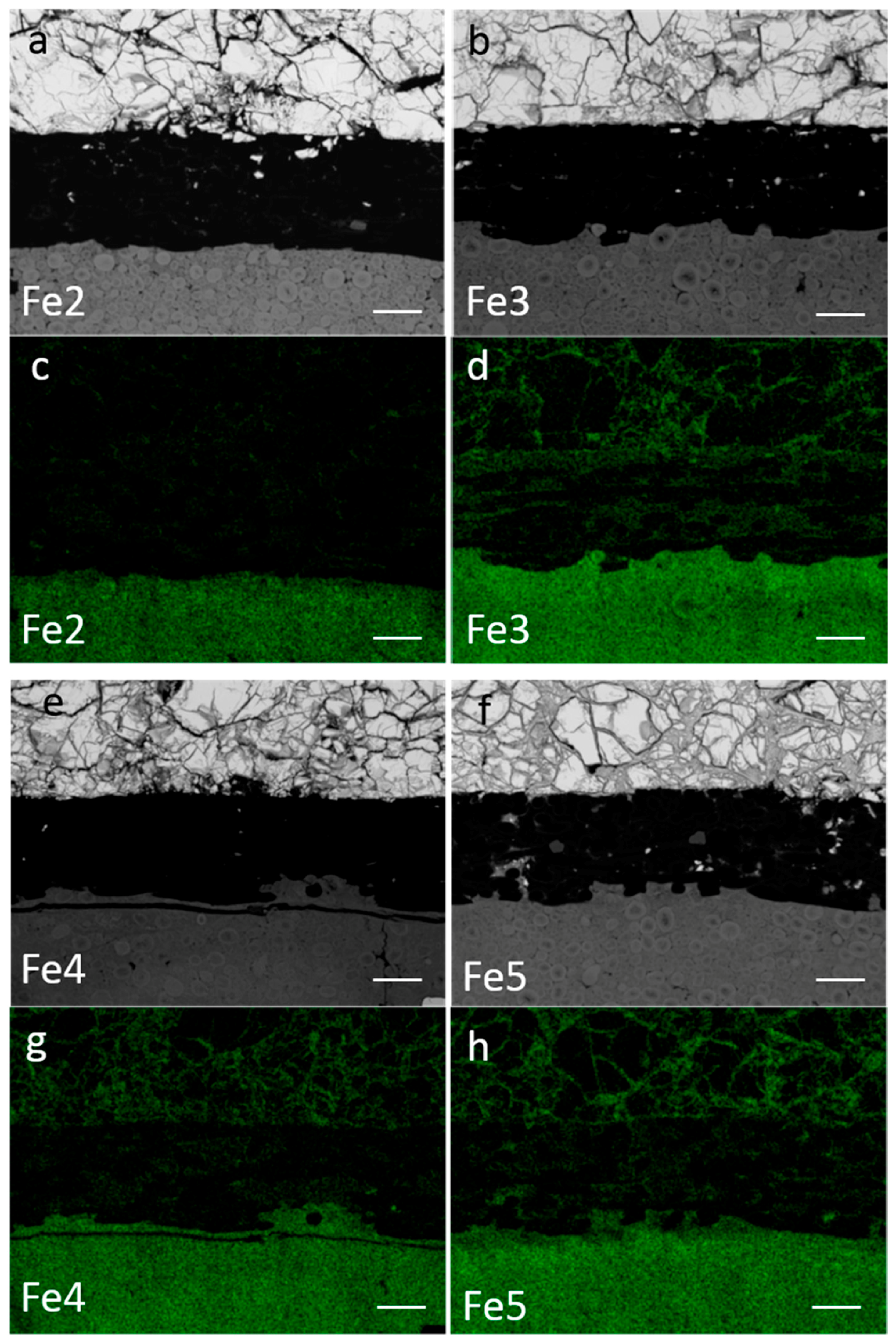
| Alloy | Fe-Content (at%) | Max H Storage Capacity at 30 °C (wt %) | Discharge Capacity at 8 mA g−1 (mAh g−1) | Electrochemical to Gaseous Phase Capacity Ratio (%) | HRD (%) | MS (Memu g−1) | C (Farad g−1) |
|---|---|---|---|---|---|---|---|
| Fe1 | 0.0 | 1.43 | 351 | 92 | 93 | 1016 | 0.30 |
| Fe2 | 1.2 | 1.39 | 347 | 93 | 93 | 835 | 0.42 |
| Fe3 | 2.4 | 1.37 | 346 | 94 | 94 | 718 | 0.37 |
| Fe4 | 3.6 | 1.39 | 335 | 90 | 92 | 481 | 0.71 |
| Fe5 | 4.7 | 1.41 | 345 | 91 | 87 | 341 | 0.82 |
| Alloy | QC/2 at RT (Ah) | Q2C at 20 °C (Ah) | QC/2 at −10 °C (Ah) | QC/2 at −20 °C (Ah) | 30-day Charge Retention (%) | Mid-Point Voltage at −10 °C (V) | Mid-Point Voltage at −20 °C (V) |
|---|---|---|---|---|---|---|---|
| Fe1 | 4.27 | 3.97 | 3.64 | 3.35 | 88 | 1.093 | 1.038 |
| Fe2 | 4.27 | 3.97 | 3.63 | 3.34 | 87 | 1.090 | 1.032 |
| Fe4 | 4.27 | 3.97 | 3.62 | 3.29 | 86 | 1.079 | 1.015 |
| Fe5 | 4.23 | 3.96 | 3.51 | 2.96 | 83 | 1.013 | 0.949 |
| Alloy | Cycle life at C/2, RT | Cycle life at C/2, 50 °C | Initial Peak Power at 50% DOD (W kg−1) | Cycle Life (Peak Power Reached 100 W kg−1) |
|---|---|---|---|---|
| Fe1 | 1055 | 345 | 192 | 550 |
| Fe2 | 720 | 310 | 195 | 450 |
| Fe3 | 390 | 165 | 190 | 450 |
| Fe4 | 425 | 145 | 179 | 450 |
| Fe5 | 420 | 190 | 181 | 450 |
| Cell | Ni | Co | Zn | Al |
|---|---|---|---|---|
| Fe1 | 76.5 | 18.0 | 2.3 | 3.2 |
| Fe2 | 75.7 | 18.5 | 2.6 | 3.2 |
| Fe3 | 76.6 | 18.0 | 2.8 | 2.6 |
| Fe4 | 74.9 | 20.2 | 2.7 | 2.2 |
| Fe5 | 75.1 | 20.2 | 2.6 | 2.1 |
| Cell | Mm | Ni | Co | Mg | Al | Fe |
|---|---|---|---|---|---|---|
| Fe1 | 19.1 | 69.5 | 4.6 | 3.7 | 3.1 | 0.0 |
| Fe2 | 18.5 | 68.3 | 4.9 | 3.7 | 3.2 | 1.4 |
| Fe3 | 19.2 | 66.7 | 4.6 | 3.8 | 3.3 | 2.4 |
| Fe4 | 18.6 | 66.0 | 5.1 | 3.7 | 3.2 | 3.5 |
| Fe5 | 19.2 | 64.3 | 4.6 | 3.8 | 3.0 | 5.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, T.; Young, K.-H.; Nei, J.; Koch, J.M.; Yasuoka, S. Fe-Substitution for Ni in Misch Metal-Based Superlattice Hydrogen Absorbing Alloys—Part 2. Ni/MH Battery Performance and Failure Mechanisms. Batteries 2017, 3, 28. https://doi.org/10.3390/batteries3030028
Meng T, Young K-H, Nei J, Koch JM, Yasuoka S. Fe-Substitution for Ni in Misch Metal-Based Superlattice Hydrogen Absorbing Alloys—Part 2. Ni/MH Battery Performance and Failure Mechanisms. Batteries. 2017; 3(3):28. https://doi.org/10.3390/batteries3030028
Chicago/Turabian StyleMeng, Tiejun, Kwo-Hsiung Young, Jean Nei, John M. Koch, and Shigekazu Yasuoka. 2017. "Fe-Substitution for Ni in Misch Metal-Based Superlattice Hydrogen Absorbing Alloys—Part 2. Ni/MH Battery Performance and Failure Mechanisms" Batteries 3, no. 3: 28. https://doi.org/10.3390/batteries3030028
APA StyleMeng, T., Young, K.-H., Nei, J., Koch, J. M., & Yasuoka, S. (2017). Fe-Substitution for Ni in Misch Metal-Based Superlattice Hydrogen Absorbing Alloys—Part 2. Ni/MH Battery Performance and Failure Mechanisms. Batteries, 3(3), 28. https://doi.org/10.3390/batteries3030028