The Evolution of Lithium-Ion Cell Thermal Safety with Aging Examined in a Battery Testing Calorimeter
Abstract
:1. Introduction
2. Experiment
2.1. Accelerated Aging under Storage Condition
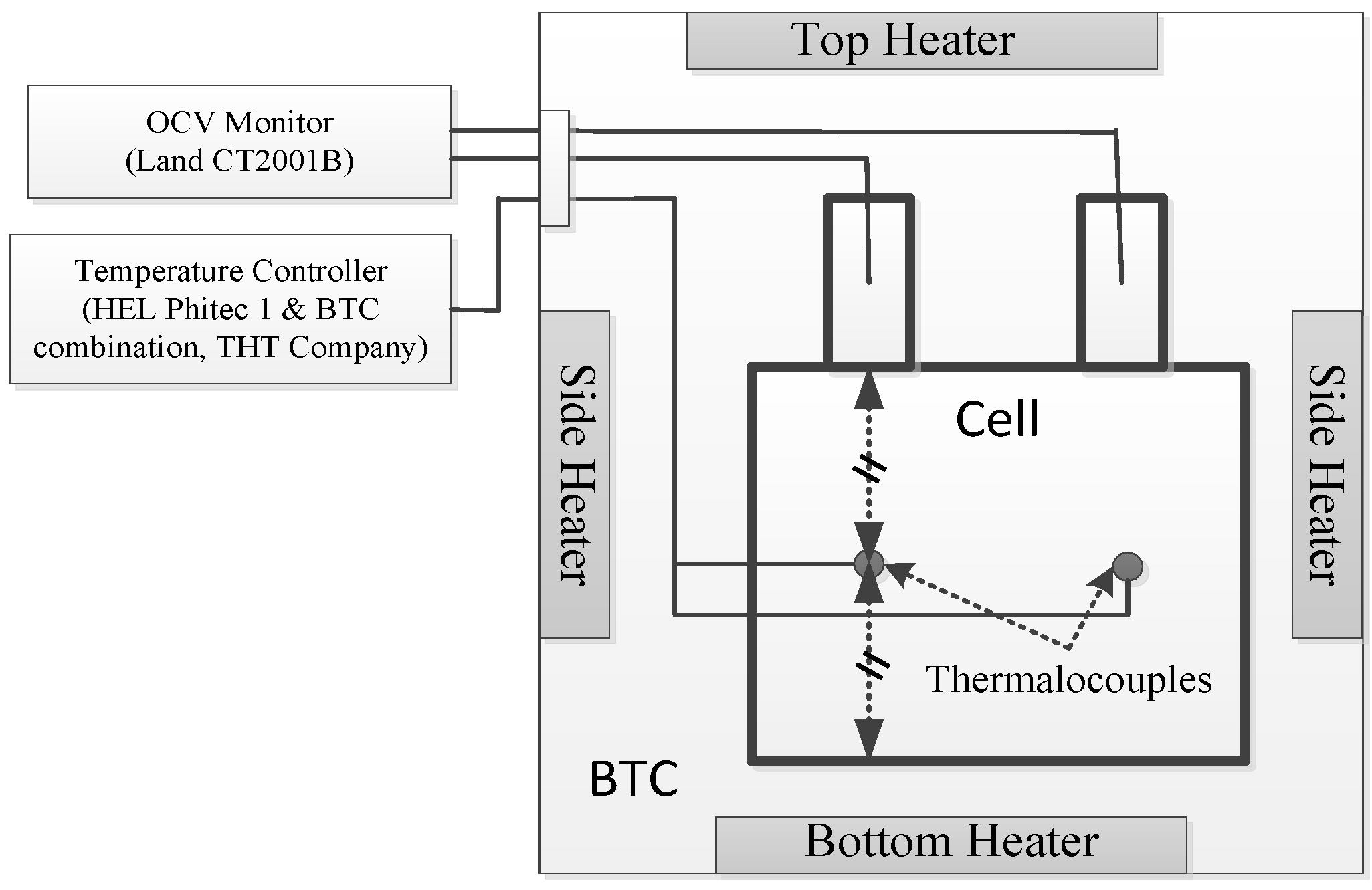
2.2. Thermal Safety Test
3. Results and Discussion
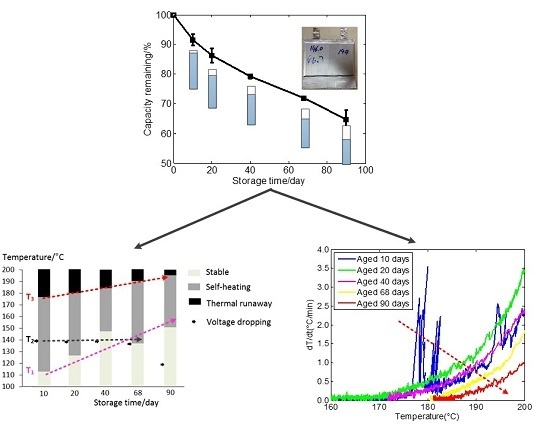
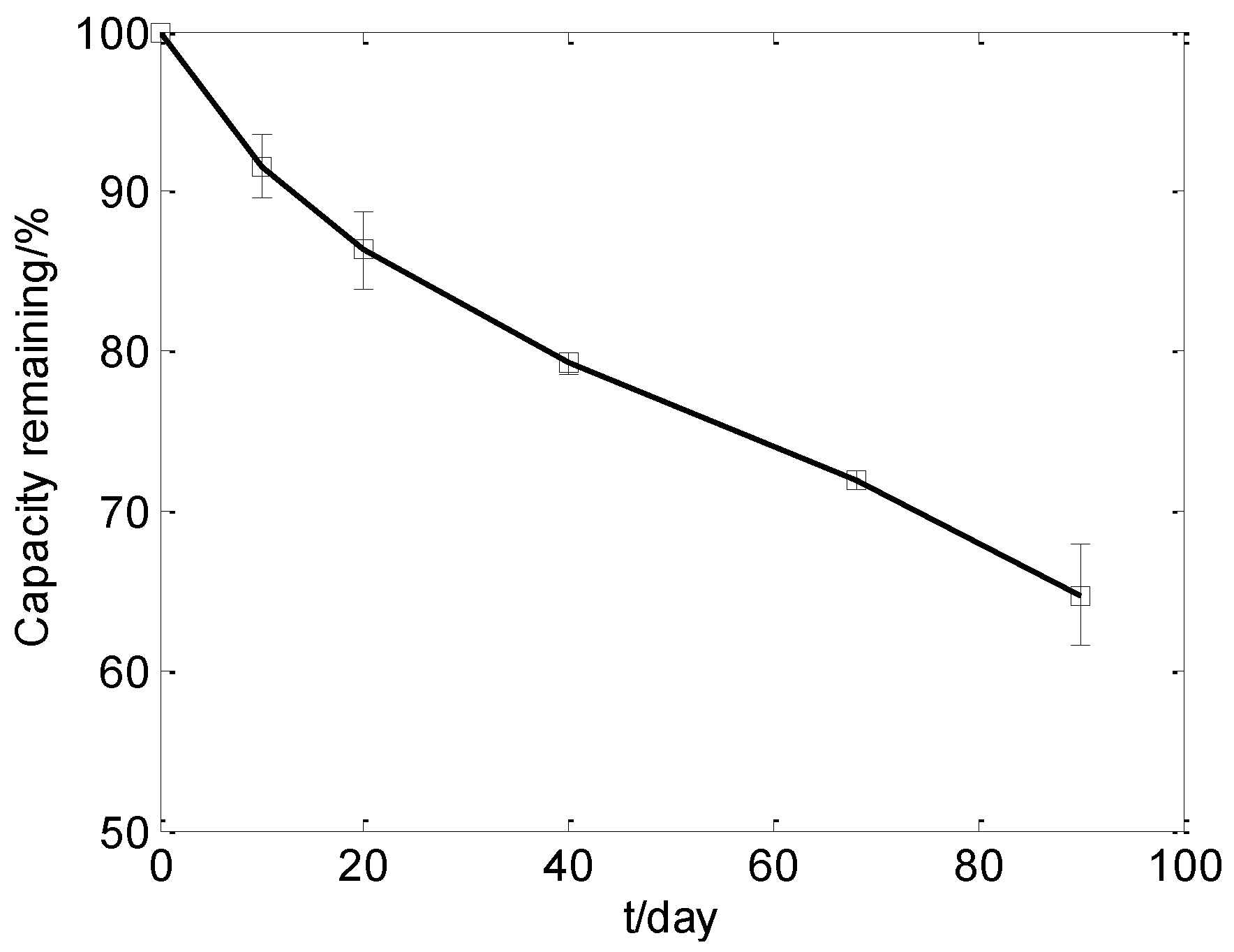
3.1. Cell Capacity Fade during the Storage Test
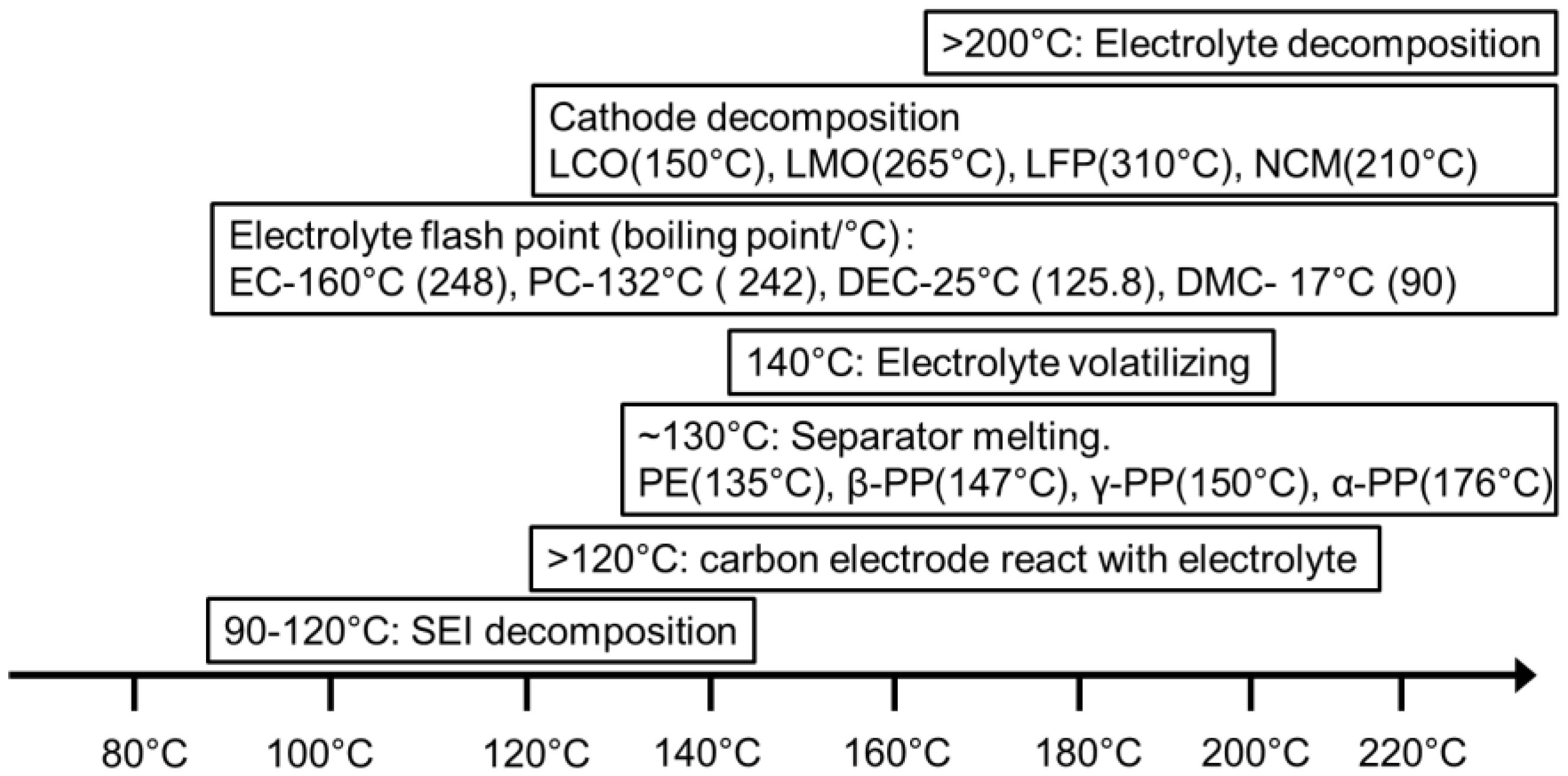
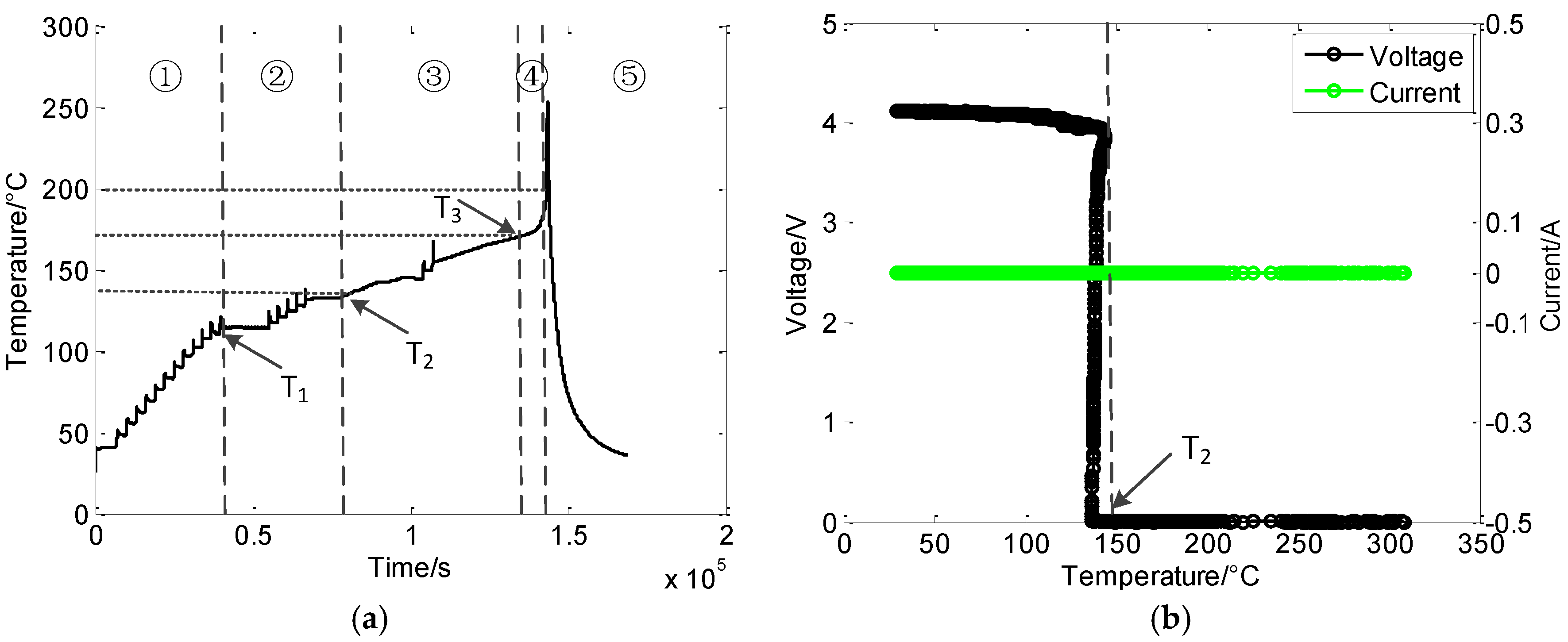
3.2. Thermal Safety Test
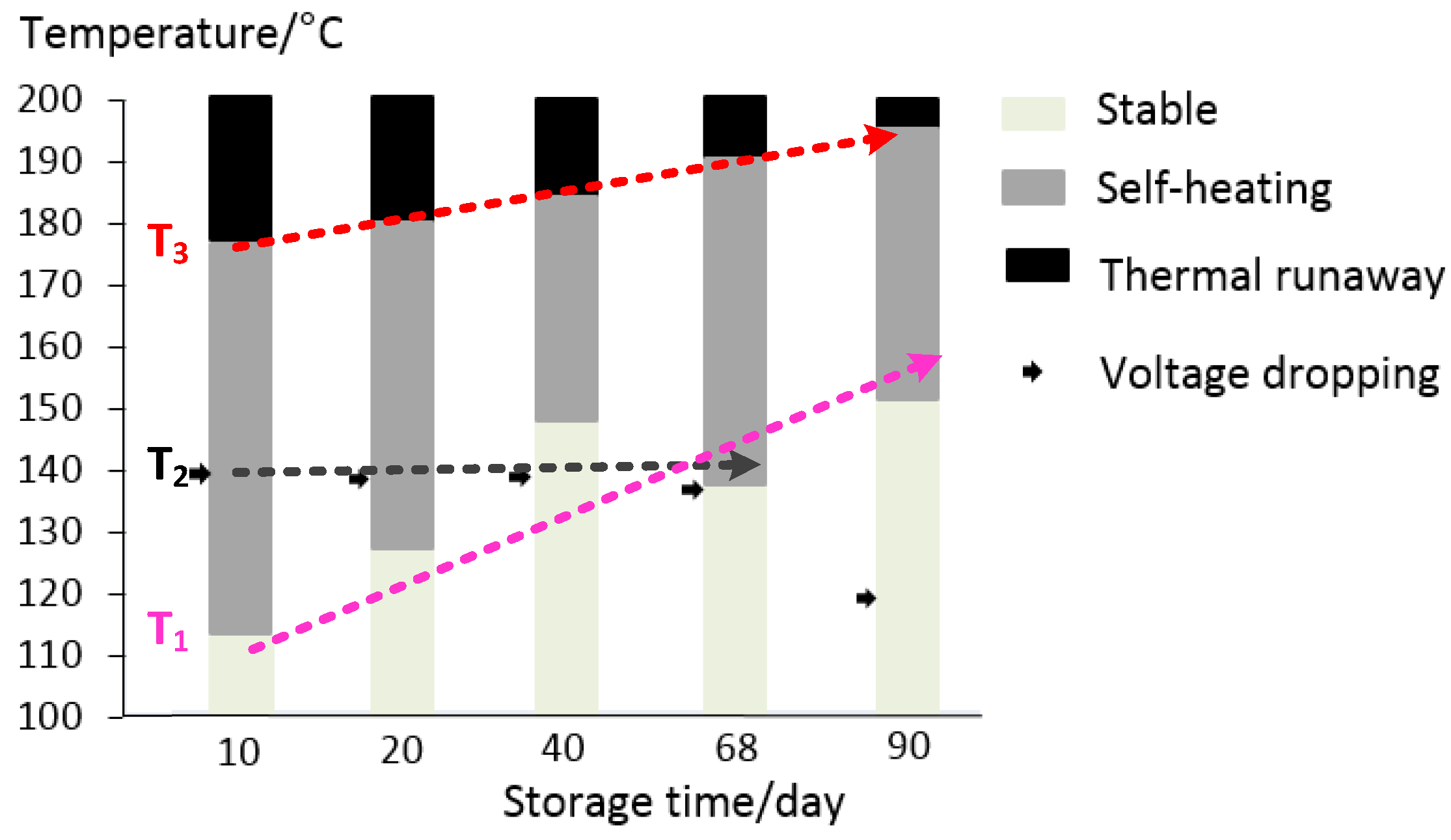
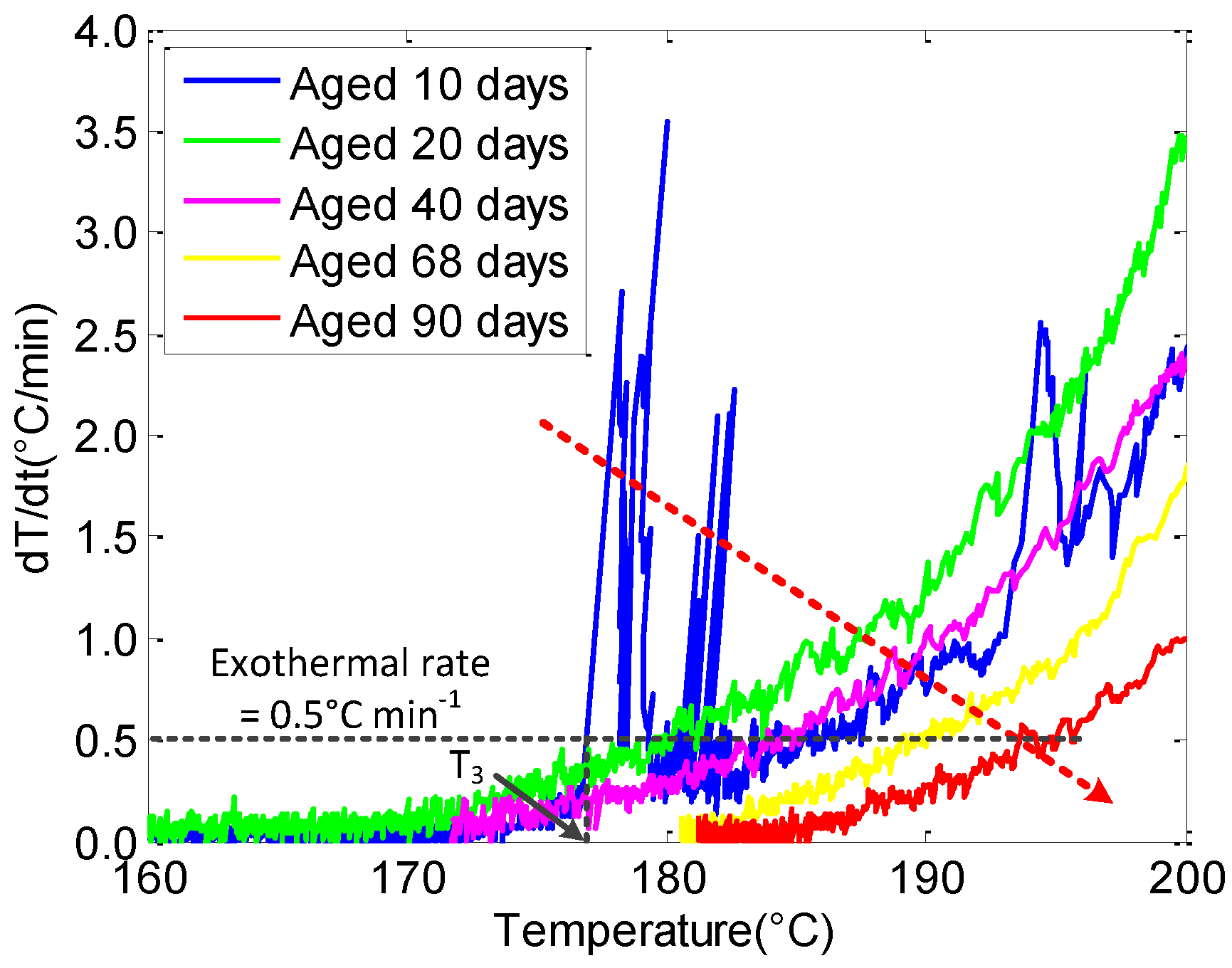
3.3. The Effect of Aging on Cell Thermal Safety
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, N.Q.; Sun, K.N.; Yang, T.Y.; Zhu, X.D. Influencing factors on safety characteristics of Li-ion batteries. Rare Met. Mater. Eng. 2010, 39, 936–940. [Google Scholar]
- Wen, J.W.; Yu, Y.; Chen, C.H. A review on lithium-ion batteries safety issues: Existing problems and possible solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Mendoza-Hernandez, O.S.; Ishikawa, H.; Nishikawa, Y.; Maruyama, Y.; Umeda, M. Cathode material comparison of thermal runaway behavior of Li-ion cells at different state of charges including over charge. J. Power Sources 2015, 280, 499–504. [Google Scholar] [CrossRef]
- Chen, J.J.; Li, Z.D.; Xiang, H.F.; Wu, W.W.; Cheng, S.; Zhang, L.J.; Wang, Q.S.; Wu, Y.C. Enhanced electrochemical performance and thermal stability of a CePO4-coated Li1.2Ni0.13Co0.13Mn0.54O2 cathode material for lithium-ion batteries. RSC Adv. 2015, 5, 3031–3038. [Google Scholar] [CrossRef]
- Zhuang, W.; Lu, L.H.; Wu, X.B.; Jin, W.; Meng, M.; Zhu, Y.D.; Lu, X.H. TiO2-B nanofibers with high thermal stability as improved anodes for lithium ion batteries. Electrochem. Commun. 2012, 27, 124–127. [Google Scholar] [CrossRef]
- Dileo, R.A.; Ganter, M.J.; Thone, M.N.; Forney, M.W.; Staub, J.W.; Rogers, R.E.; Landi, B.J. Balanced approach to safety of high capacity silicon-germanium-carbon nanotube free-standing lithium ion battery anodes. Nano Energy 2013, 2, 268–275. [Google Scholar] [CrossRef]
- Furuya, Y.; Zhao, W.W.; Iida, T.; Unno, M.; Noguchi, H. Influence of preparation temperature of precursor on the thermal stability of TiO2(B) and its electrochemical property as an anode material in the lithium-ion battery. Electrochemistry 2014, 82, 7–13. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Lambert, T.N.; Chavez, C.A.; Bencomo, M.; Fenton, K.R. Polyester separators for lithium-ion cells: Improving thermal stability and abuse tolerance. Adv. Energy Mater. 2013, 3, 314–320. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Cao, Y.L.; Yang, H.X.; Lu, S.G.; Ai, X.P. A redox-active polythiophene-modified separator for safety control of lithium-ion batteries. J. Polym. Sci. Polym. Phys. 2013, 51, 1487–1493. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, S.M.; Kim, S.H.; Kim, H.S. Electrochemical and safety performances of polyimide nano fiber-based nonwoven separators for Li-ion batteries. J. Electrochem. Sci. Technol. 2015, 6, 26–33. [Google Scholar] [CrossRef]
- Choi, K.H.; Cho, S.J.; Kim, S.H.; Kwon, Y.H.; Kim, J.Y.; Lee, S.Y. Thin, Deformable, and safety-reinforced plastic crystal polymer electrolytes for high-performance flexible lithium-ion batteries. Adv. Funct. Mater. 2014, 24, 44–52. [Google Scholar] [CrossRef]
- Hofmann, A.; Migeot, M.; Thissen, E.; Schulz, M.; Heinzmann, R.; Indris, S.; Bergfeldt, T.; Lei, B.; Ziebert, C.; Hanemann, T. Electrolyte mixtures based on ethylene carbonate and dimethyl sulfone for Li-ion batteries with improved safety characteristics. ChemSusChem 2015, 8, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Dahn, J.R. Effects of solvents and salts on the thermal stability of LiC6. Electrochim. Acta 2004, 49, 4599–4604. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Yang, H.; Amiruddin, S.; Bang, H.J.; Sun, Y.K.; Prakash, J. A review of Li-ion cell chemistries and their potential use in hybrid electric vehicles. J. Ind. Eng. Chem. 2006, 12, 12–38. [Google Scholar]
- Wang, Q.S.; Ping, P.; Zhao, X.J.; Chu, G.Q.; Sun, J.H.; Chen, C.H. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Roth, E.P.; Doughty, D.H. Thermal abuse performance of high-power 18650 Li-ion cells. J. Power Sources 2004, 128, 308–318. [Google Scholar] [CrossRef]
- Roder, P.; Stiaszny, B.; Ziegler, J.C.; Baba, N.; Lagaly, P.; Wiemhofer, H.D. The impact of calendar aging on the thermal stability of a LiMn2O4-Li(Ni1/3Mn1/3Co1/3)O2/graphite lithium-ion cell. J. Power Sources 2014, 268, 315–325. [Google Scholar] [CrossRef]
- Fleischhammer, M.; Waldmann, T.; Bisle, G.; Hogg, B.I.; Wohlfahrt-Mehrens, M. Interaction of cyclic ageing at high-rate and low temperatures and safety in lithium-ion batteries. J. Power Sources 2015, 274, 432–439. [Google Scholar] [CrossRef]
- Doughty, D.; Roth, E.P. A general discussion of Li Ion battery safety. Electrochem. Soc. Interface 2012, 21, 37–44. [Google Scholar]
- Ishikawa, H.; Mendoza, O.; Sone, Y.; Umeda, M. Study of thermal deterioration of lithium-ion secondary cell using an accelerated rate calorimeter (ARC) and AC impedance method. J. Power Sources 2012, 198, 236–242. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y.; Gering, K.; Sazhin, S.; Jamison, D.; Michelbacher, C. Evaluation of commercial lithium-ion cells based on composite positive electrode for plug-in hybrid electric vehicle applications. Part II. Degradation mechanism under 2C cycle aging. J. Power Sources 2011, 196, 10336–10343. [Google Scholar] [CrossRef]
- Ploehn, H.J.; Ramadass, P.; White, R.E. Solvent diffusion model for aging of lithium-ion battery cells. J. Electrochem. Soc. 2004, 151, A456–A462. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Lu, Z.; Chen, Z.; Dahn, J.R. A comparison of the electrode/electrolyte reaction at elevated temperatures for various Li-ion battery cathodes. J. Power Sources 2002, 108, 8–14. [Google Scholar] [CrossRef]
- Feng, X.N.; Fang, M.; He, X.M.; Ouyang, M.G.; Lu, L.G.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Battery and Energy Technologies. Available online: http://www.mpoweruk.com/lithium_failures.htm (accessed on 26 February 2016).
- Waldmann, T.; Gorse, S.; Samtleben, T.; Schneider, G.; Knoblauch, V.; Wohlfahrt-Mehrens, M. A mechanical aging mechanism in lithium-ion batteries. J. Electrochem. Soc. 2014, 161, A1742–A1747. [Google Scholar] [CrossRef]

| No. | Item | Specification |
|---|---|---|
| 1 | Cathode material | LMO |
| 2 | Anode material | Graphite |
| 3 | Electrolyte | EC + LiPF6 |
| 4 | Nominal capacity | 4.6 Ah |
| 5 | Weight | 146 g |
| 6 | Cross section area | 120 × 180 mm2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Su, L.; Li, Z.; Sun, Y.; Wu, N. The Evolution of Lithium-Ion Cell Thermal Safety with Aging Examined in a Battery Testing Calorimeter. Batteries 2016, 2, 12. https://doi.org/10.3390/batteries2020012
Zhang J, Su L, Li Z, Sun Y, Wu N. The Evolution of Lithium-Ion Cell Thermal Safety with Aging Examined in a Battery Testing Calorimeter. Batteries. 2016; 2(2):12. https://doi.org/10.3390/batteries2020012
Chicago/Turabian StyleZhang, Jianbo, Laisuo Su, Zhe Li, Ying Sun, and Ningning Wu. 2016. "The Evolution of Lithium-Ion Cell Thermal Safety with Aging Examined in a Battery Testing Calorimeter" Batteries 2, no. 2: 12. https://doi.org/10.3390/batteries2020012
APA StyleZhang, J., Su, L., Li, Z., Sun, Y., & Wu, N. (2016). The Evolution of Lithium-Ion Cell Thermal Safety with Aging Examined in a Battery Testing Calorimeter. Batteries, 2(2), 12. https://doi.org/10.3390/batteries2020012