Enhanced Sodium Storage Performance of Few-Layer Graphene-Encapsulated Hard Carbon Fiber Composite Electrodes
Abstract
1. Introduction
2. Experiment
2.1. Preparation
2.2. Structural Characterization
2.3. Electrochemical Testing
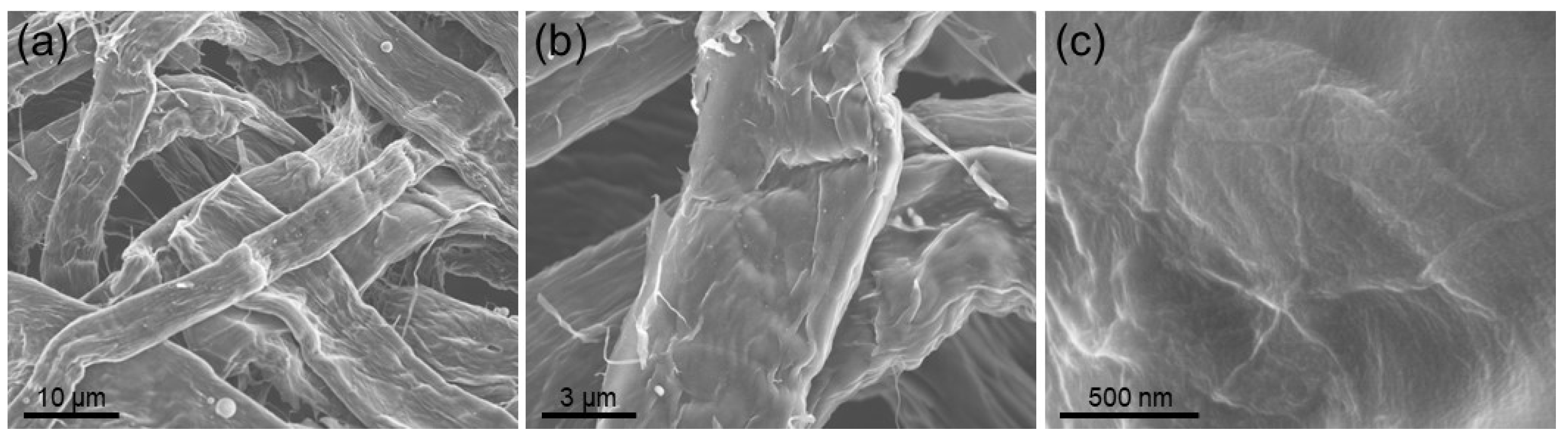
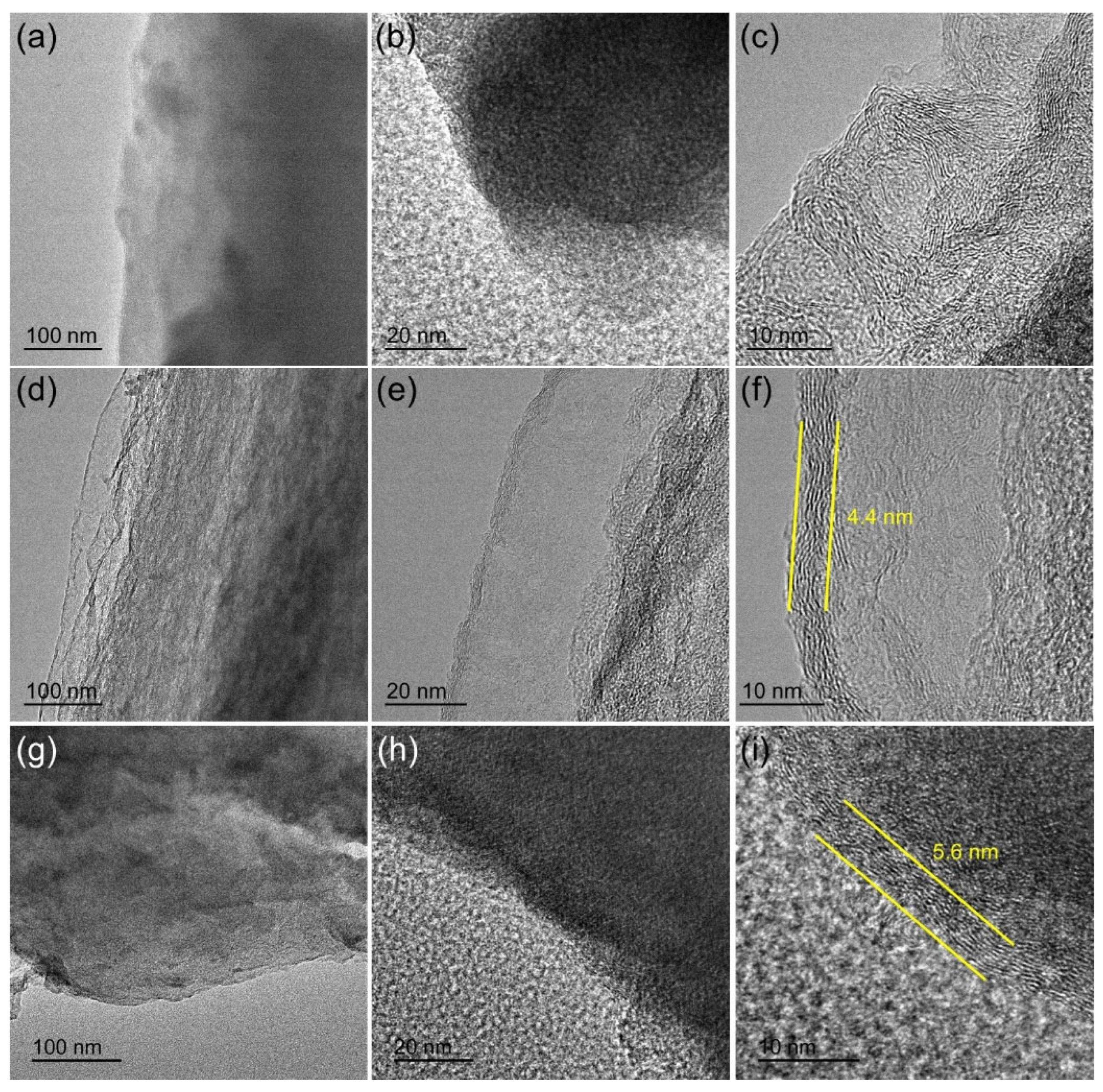
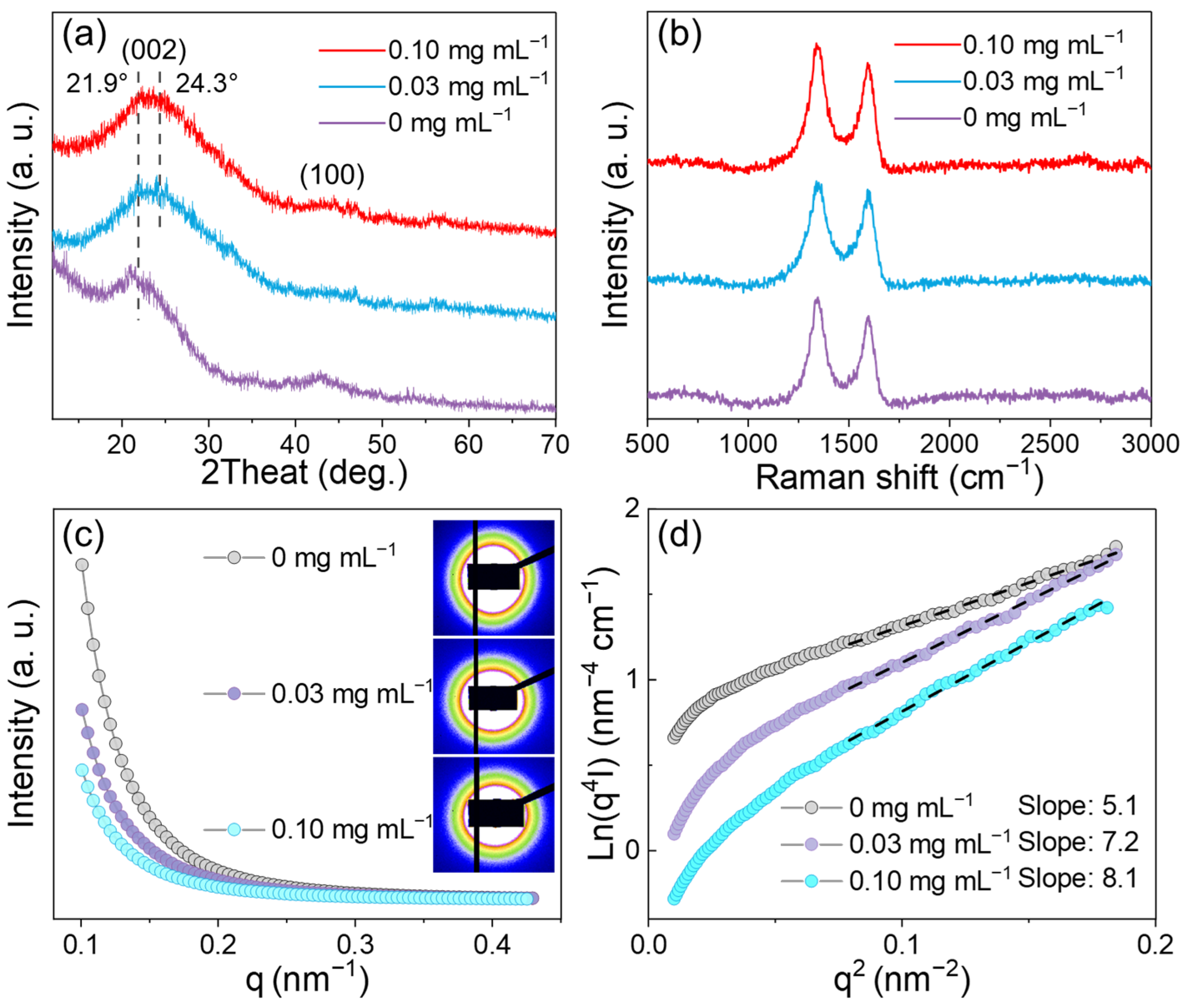
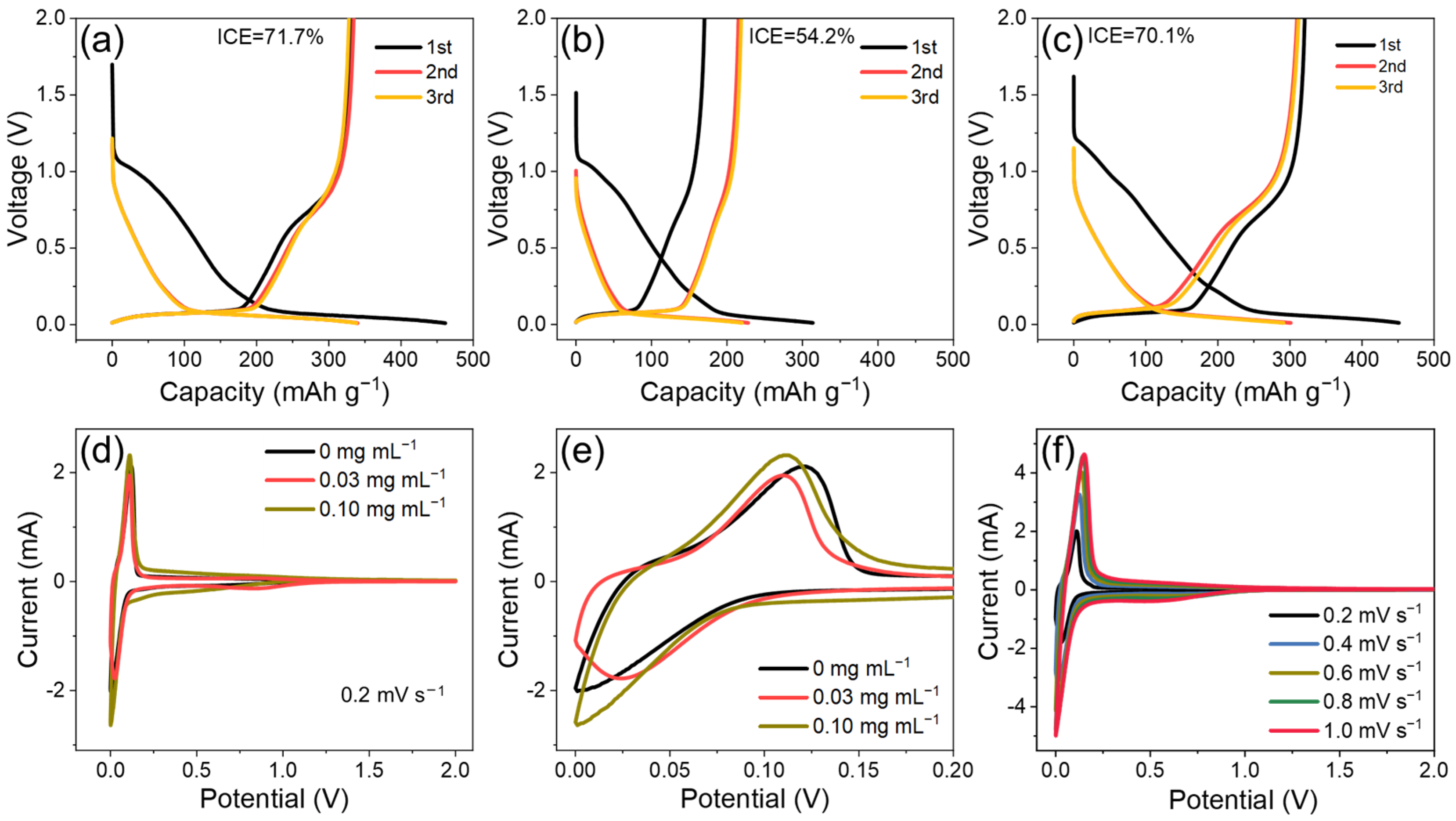
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Wang, Q.; Yao, Z.; Wang, J.; Sánchez-Lengeling, B.; Ding, F.; Qi, X.; Lu, Y.; Bai, X.; Li, B.; et al. Rational design of layered oxide materials for sodium-ion batteries. Science 2020, 370, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Deng, J.; Wang, Z.; Liang, Q.; Hu, L.; Ren, X.; Li, R.; Lin, Y.; Li, Y.; Wang, Q.; et al. Aggregate-Dominated Dilute Electrolytes with Low-Temperature-Resistant Ion-Conducting Channels for Highly Reversible Na Plating/Stripping. Adv. Mater. 2024, 36, 2408161. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Ji, T.; Zhang, M.; Yan, X.; Yao, M.; Sheng, D.; Li, S.; Ren, P.; Shen, Z. Potassium ion doped manganese oxide nanoscrolls enhanced the performance of aqueous zinc-ion batteries. Chin. Chem. Lett. 2025, 36, 109551. [Google Scholar] [CrossRef]
- Reddy, M.A.; Helen, M.; Groß, A.; Fichtner, M.; Euchner, H. Insight into Sodium Insertion and the Storage Mechanism in Hard Carbon. ACS Energy Lett. 2018, 3, 2851–2857. [Google Scholar] [CrossRef]
- Iglesias, L.K.; Antonio, E.N.; Martinez, T.D.; Zhang, L.; Zhuo, Z.; Weigand, S.J.; Guo, J.; Toney, M. Revealing the Sodium Storage Mechanisms in Hard Carbon Pores. Adv. Energy Mater. 2023, 13, 2302171. [Google Scholar] [CrossRef]
- Ji, T.; Liu, X.; Sheng, D.; Li, Y.; Ruan, H.; Guo, H.; Shen, Z.X.; Lai, L. Machine learning-assisted thermomechanical coupling fabrication of hard carbon for sodium-ion batteries. Energy Storage Mater. 2024, 71, 103563. [Google Scholar] [CrossRef]
- Li, Y.; Vasileiadis, A.; Zhou, Q.; Lu, Y.; Meng, Q.; Li, Y.; Ombrini, P.; Zhao, J.; Chen, Z.; Niu, Y.; et al. Origin of fast charging in hard carbon anodes. Nat. Energy 2024, 9, 134–142. [Google Scholar] [CrossRef]
- He, X.; Lai, W.; Liang, Y.; Zhao, J.-H.; Yang, Z.; Peng, J.; Liu, X.-H.; Wang, Y.-X.; Qiao, Y.; Li, L.; et al. Achieving All-Plateau and High-Capacity Sodium Insertion in Topological Graphitized Carbon. Adv. Mater. 2023, 35, 2302613. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, C.; He, X.; Zhao, J.; Yang, Z.; Li, L.; Wu, X.; Li, L.; Chou, S. Boosting the Development of Hard Carbon for Sodium-Ion Batteries: Strategies to Optimize the Initial Coulombic Efficiency. Adv. Funct. Mater. 2023, 34, 2302277. [Google Scholar] [CrossRef]
- Ji, T.; Liu, X.; Wang, T.; Shi, Y.; Sheng, D.; Hao, X.; He, C.; Shen, Z. Commercial Carbon Fibers as Host for Sodium Deposition to Achieve High Volumetric Capacity. Adv. Funct. Mater. 2024, 34, 2408880. [Google Scholar] [CrossRef]
- Li, W.; Guo, X.; Song, K.; Chen, J.; Zhang, J.; Tang, G.; Liu, C.; Chen, W.; Shen, C. Binder-Induced Ultrathin SEI for Defect-Passivated Hard Carbon Enables Highly Reversible Sodium-Ion Storage. Adv. Energy Mater. 2023, 13, 2300648. [Google Scholar] [CrossRef]
- Lu, Z.; Geng, C.; Yang, H.; He, P.; Wu, S.; Yang, Q.; Zhou, H. Step-by-step desolvation enables high-rate and ultra-stable sodium storage in hard carbon anodes. Proc. Natl. Acad. Sci. USA 2022, 119, e2210203119. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Chen, Z.; Xu, K.; Lu, J. New Concepts in Electrolytes. Chem. Rev. 2020, 120, 6783–6819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Rao, A.M.; Han, X.; Lu, B. Artificial SEI for Superhigh-Performance K-Graphite Anode. Adv. Sci. 2021, 8, 2003639. [Google Scholar] [CrossRef]
- Surace, Y.; Leanza, D.; Mirolo, M.; Kondracki, Ł.; Vaz, C.; El Kazzi, M.; Novák, P.; Trabesinger, S. Evidence for stepwise formation of solid electrolyte interphase in a Li-ion battery. Energy Storage Mater. 2022, 44, 156–167. [Google Scholar] [CrossRef]
- Yan, C.; Li, H.; Chen, X.; Zhang, X.; Cheng, X.-B.; Xu, R.; Huang, J.; Zhang, Q. Regulating the Inner Helmholtz Plane for Stable Solid Electrolyte Interphase on Lithium Metal Anodes. J. Am. Chem. Soc. 2019, 141, 9422–9429. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, F.; Li, Y.; Liu, M.; Feng, X.; Bai, Y.; Wu, C. Ether-based electrolytes for sodium ion batteries. Chem. Soc. Rev. 2022, 51, 4484–4536. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Wang, P.; Liu, H.; Zeng, W.; Chen, L.; Chen, Z.; Mu, S. NH2-MIL-125 (Ti) derived flower-like fine TiO2 nanoparticles implanted in N-doped porous carbon as an anode with high activity and long cycle life for lithium-ion batteries. Acta Phys.Chim. Sin. 2022, 38, 2106002. [Google Scholar]
- Lu, H.; Chen, X.; Jia, Y.; Chen, H.; Wang, Y.; Ai, X.; Yang, H.; Cao, Y. Engineering Al2O3 atomic layer deposition: Enhanced hard carbon-electrolyte interface towards practical sodium ion batteries. Nano Energy 2019, 64, 103903. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Wu, X.; Yu, J.; Wang, Y.; Hu, Y.-S.; Li, H.; Chen, L.; Huang, X. Amorphous monodispersed hard carbon micro-spherules derived from biomass as a high performance negative electrode material for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 71–77. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, Y.; Zhao, P.; Yuan, C.; Chen, M.; Wang, C. Commercial activated carbon as a novel precursor of the amorphous carbon for high-performance sodium-ion batteries anode. Carbon 2018, 129, 85–94. [Google Scholar] [CrossRef]
- Cheng, D.; Li, Z.; Zhang, M.; Duan, Z.; Wang, J.; Wang, C. Engineering Ultrathin Carbon Layer on Porous Hard Carbon Boosts Sodium Storage with High Initial Coulombic Efficiency. ACS Nano 2023, 17, 19063–19075. [Google Scholar] [CrossRef]
- Yu, Z.; Xin, S.; You, Y.; Yu, L.; Lin, Y.; Xu, D.; Qiao, C.; Huang, Z.; Yang, N.; Yu, S.; et al. Ion-Catalyzed Synthesis of Microporous Hard Carbon Embedded with Expanded Nanographite for Enhanced Lithium/Sodium Storage. J. Am. Chem. Soc. 2016, 138, 14915–14922. [Google Scholar] [CrossRef]
- Ji, T.; Liu, X.; Zhang, T.; Shi, Y.; Sheng, D.; Yin, H.; Shen, Z.X.; Chao, D. Potassium Metal Underpotential Deposition in Crystalline Carbon of Potassium—Ion Batteries. Adv. Energy Mater. 2024, 14, 2401908. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, W.; Luo, K.; Song, Y.; Zhong, Y.; Liu, Y.; Wang, G.; Zhong, B.; Wu, Z.; Guo, X. Hard carbon for sodium storage: Mechanism and optimization strategies toward commercialization. Energy Environ. Sci. 2021, 14, 2244–2262. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, Z.; Madhu, R.; Xie, F.; Chen, R.; Wang, J.; Tebyetekerwa, M.; Hu, Y.-S.; Titirici, M. Homogenous metallic deposition regulated by defect-rich skeletons for sodium metal batteries. Energy Environ. Sci. 2021, 14, 6381–6393. [Google Scholar] [CrossRef]
- Bommier, C.; Surta, T.W.; Dolgos, M.; Ji, X. New Mechanistic Insights on Na-Ion Storage in Nongraphitizable Carbon. Nano Lett. 2015, 15, 5888–5892. [Google Scholar] [CrossRef] [PubMed]
- Novko, D.; Zhang, Q.; Kaghazchi, P. Nonadiabatic Effects in Raman Spectra of AlCl4-graphite Based Batteries. Phys. Rev. Appl. 2019, 12, 024016. [Google Scholar] [CrossRef]
- Li, J.; Yin, J.; Feng, Y.; Liu, Y.; Zhao, H.; Li, Y.; Zhu, C.; Su, B.; Yue, D.; Liu, X. Role of interface between BNNS and LDPE in excellent electrical, thermal and mechanical properties of LDPE/BNNS composites. J. Mater. Sci. Mater. Electron. 2019, 30, 1531–1540. [Google Scholar] [CrossRef]
- Morikawa, Y.; Nishimura, S.; Hashimoto, R.; Ohnuma, M.; Yamada, A. Mechanism of Sodium Storage in Hard Carbon: An X-Ray Scattering Analysis. Adv. Energy Mater. 2020, 10, 1903176. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Gu, J.; Wang, H.; Liu, H.; Shen, Z. Inspired by nature: Self-fractal cobalt sulfate composite electrode for sodium ion storage. Chin. Chem. Lett. 2023, 34, 108471. [Google Scholar] [CrossRef]
- Chen, X.; Tian, J.; Li, P.; Fang, Y.; Fang, Y.; Liang, X.; Feng, J.; Dong, J.; Ai, X.; Yang, H.; et al. An Overall Understanding of Sodium Storage Behaviors in Hard Carbons by an “Adsorption-Intercalation/Filling” Hybrid Mechanism. Adv. Energy Mater. 2022, 12, 2200886. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, X.; Hu, X.; Li, Y.; Wang, K.; Tu, H.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Rationally Designing Closed Pore Structure by Carbon Dots to Evoke Sodium Storage Sites of Hard Carbon in Low-Potential Region. Adv. Funct. Mater. 2024, 34, 2308392. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, H.; Qiao, Y.; Zhu, X.; He, P.; Zhou, H. Tailoring the Solvation Sheath of Cations by Constructing Electrode Front-Faces for Rechargeable Batteries. Adv. Mater. 2022, 34, 2201339. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xue, G.; Li, C.; Zhao, S.; Zheng, Y.; He, Y.; Yuan, R.; Wang, K.; Mo, T.; Xiang, Y.; et al. Accelerating Ion Desolvation via Bioinspired Ion Channel Design in Nonconcentrated Aqueous Electrolytes. J. Am. Chem. Soc. 2025, 147, 5943–5954. [Google Scholar] [CrossRef]
- Zhang, X.; Song, T.; He, T.; Ma, Q.; Wu, Z.; Wang, Y.; Xiong, H. Sub-Nanometer Porous Carbon Materials for High-Performance Supercapacitors Using Carbon Dots as Self-templated Pore-Makers. Adv. Funct. Mater. 2024, 35, 2419219. [Google Scholar] [CrossRef]
- Zhou, C.; Li, A.; Cao, B.; Chen, X.; Jia, M.; Song, H. The Non-Ignorable Impact of Surface Oxygen Groups on the Electrochemical Performance of N/O Dual-Doped Carbon Anodes for Sodium Ion Batteries. J. Electrochem. Soc. 2018, 165, A1447. [Google Scholar] [CrossRef]
- Song, Z.; Di, M.; Chen, S.; Bai, Y. Three-dimensional N/O co-doped hard carbon anode enabled superior stabilities for sodium-ion batteries. Chem. Eng. J. 2023, 470, 144237. [Google Scholar] [CrossRef]
- Jialin, G.; Zheng, P. Graphene-coated micro/nanostructure hard carbon with improved electrochemical performance for sodium-ion battery. Appl. Phys. A 2021, 127, 509. [Google Scholar] [CrossRef]
- Gong, Y.; Yu, C.; Li, Y.; Qian, J.; Wu, C.; Bai, Y. Constructing Robust Solid Electrolyte Interface via ZrO2 Coating Layer for Hard Carbon Anode in Sodium-Ion Batteries. Batteries 2022, 8, 115. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Ji, T.; Liu, Q.; Li, L. Enhanced Sodium Storage Performance of Few-Layer Graphene-Encapsulated Hard Carbon Fiber Composite Electrodes. Batteries 2025, 11, 203. https://doi.org/10.3390/batteries11050203
Zhu B, Ji T, Liu Q, Li L. Enhanced Sodium Storage Performance of Few-Layer Graphene-Encapsulated Hard Carbon Fiber Composite Electrodes. Batteries. 2025; 11(5):203. https://doi.org/10.3390/batteries11050203
Chicago/Turabian StyleZhu, Bo, Tiany Ji, Qiong Liu, and Lixin Li. 2025. "Enhanced Sodium Storage Performance of Few-Layer Graphene-Encapsulated Hard Carbon Fiber Composite Electrodes" Batteries 11, no. 5: 203. https://doi.org/10.3390/batteries11050203
APA StyleZhu, B., Ji, T., Liu, Q., & Li, L. (2025). Enhanced Sodium Storage Performance of Few-Layer Graphene-Encapsulated Hard Carbon Fiber Composite Electrodes. Batteries, 11(5), 203. https://doi.org/10.3390/batteries11050203






