Ionic Liquids and Ammoniates as Electrolytes for Advanced Sodium-Based Secondary Batteries
Abstract
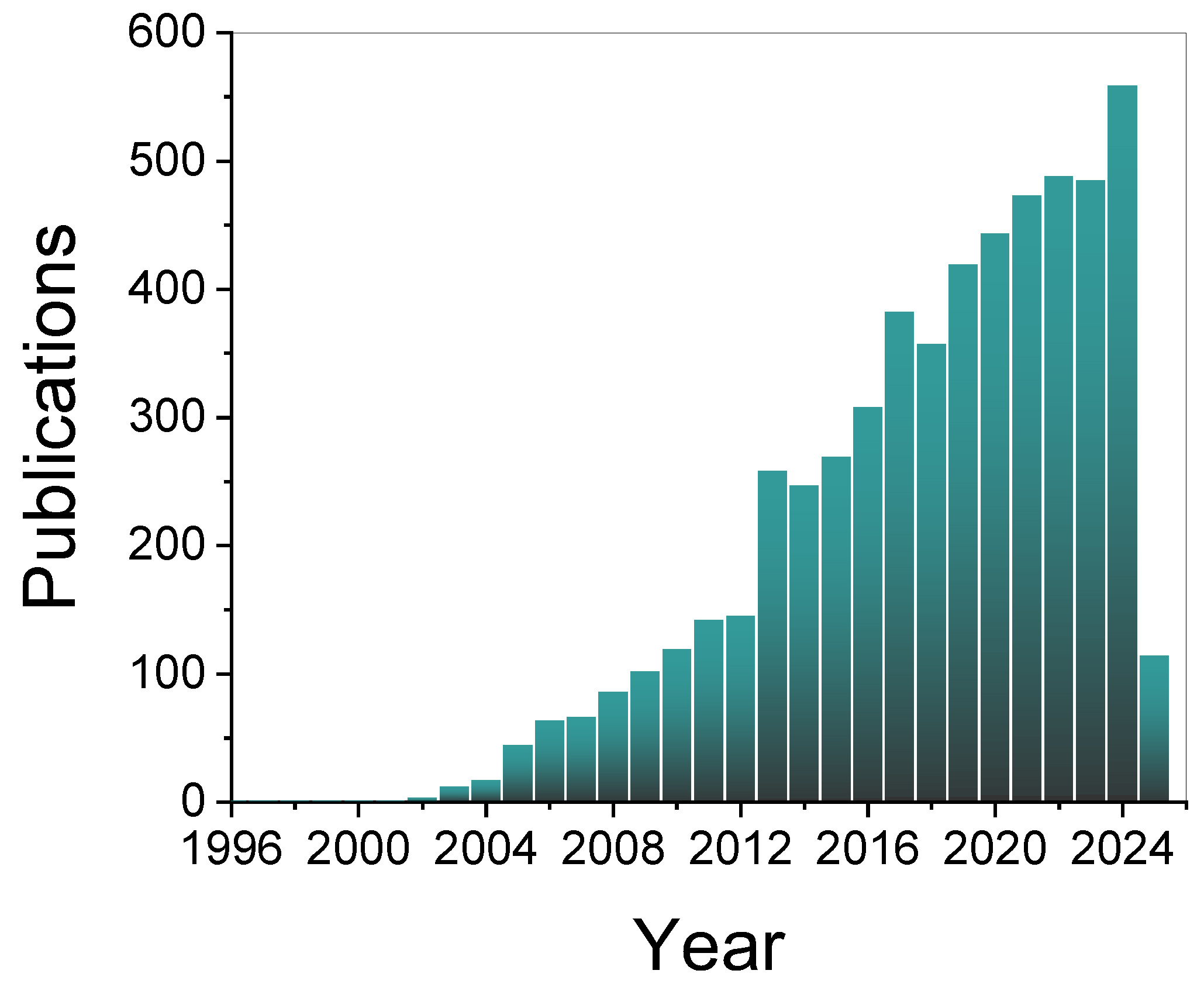
1. Introduction
| Role/Impact |
|
| Ideal Properties |
|
| Role/impact |
|
| Ideal Properties |
|
| Role/Impact |
|
| Ideal Properties |
|
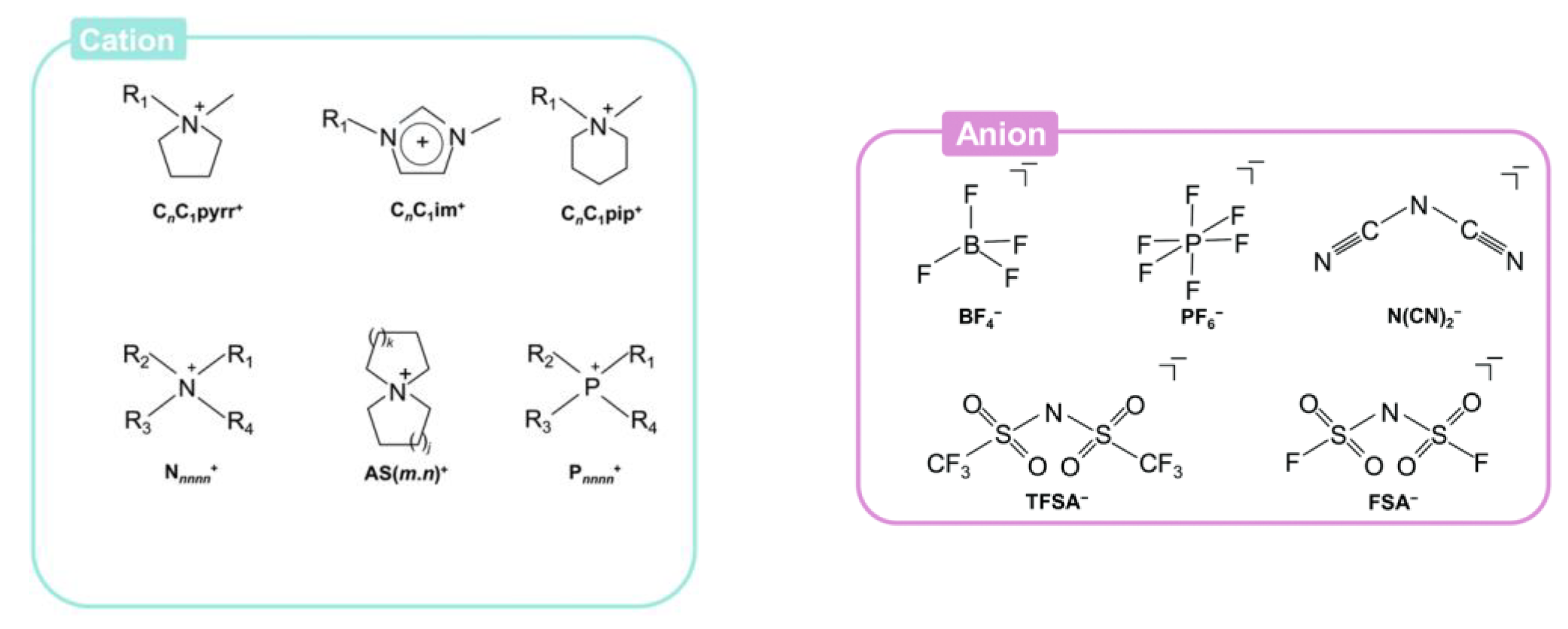
2. Ionic Liquids (ILS)
2.1. Classification of ILs
2.2. Properties of ILs
2.2.1. Thermal Stability
2.2.2. Conductivity and Viscosity
2.2.3. Electrochemical Stability Window (ESW)
2.3. ILs in Sodium-Based Batteries
2.3.1. One-Anion-Type Electrolytes
NaPF6-[X+][PF6−]-Based Electrolytes
NaBF4-[X+][BF4−]-Based Electrolytes
Sulfonylamide-Based Electrolytes
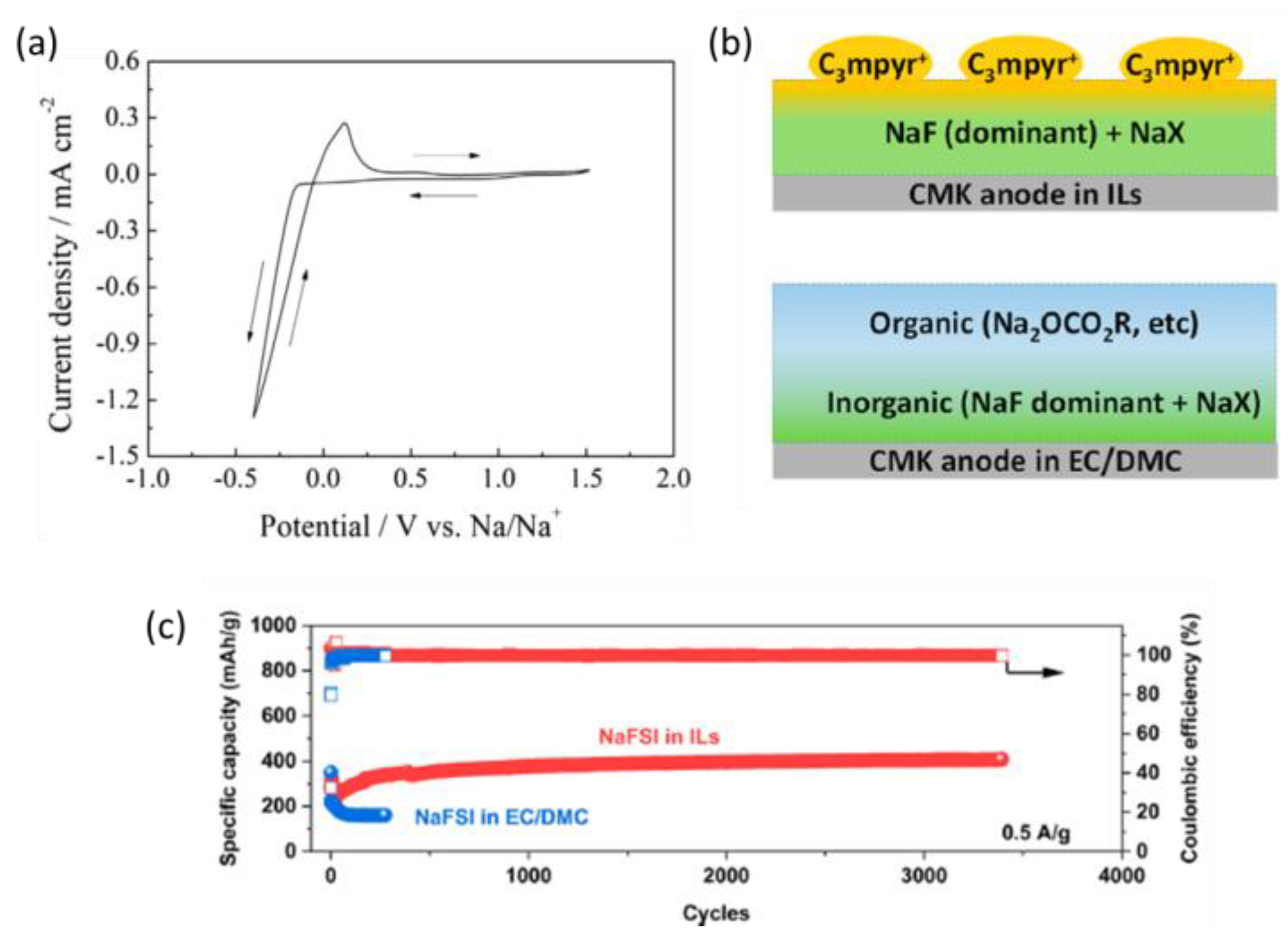
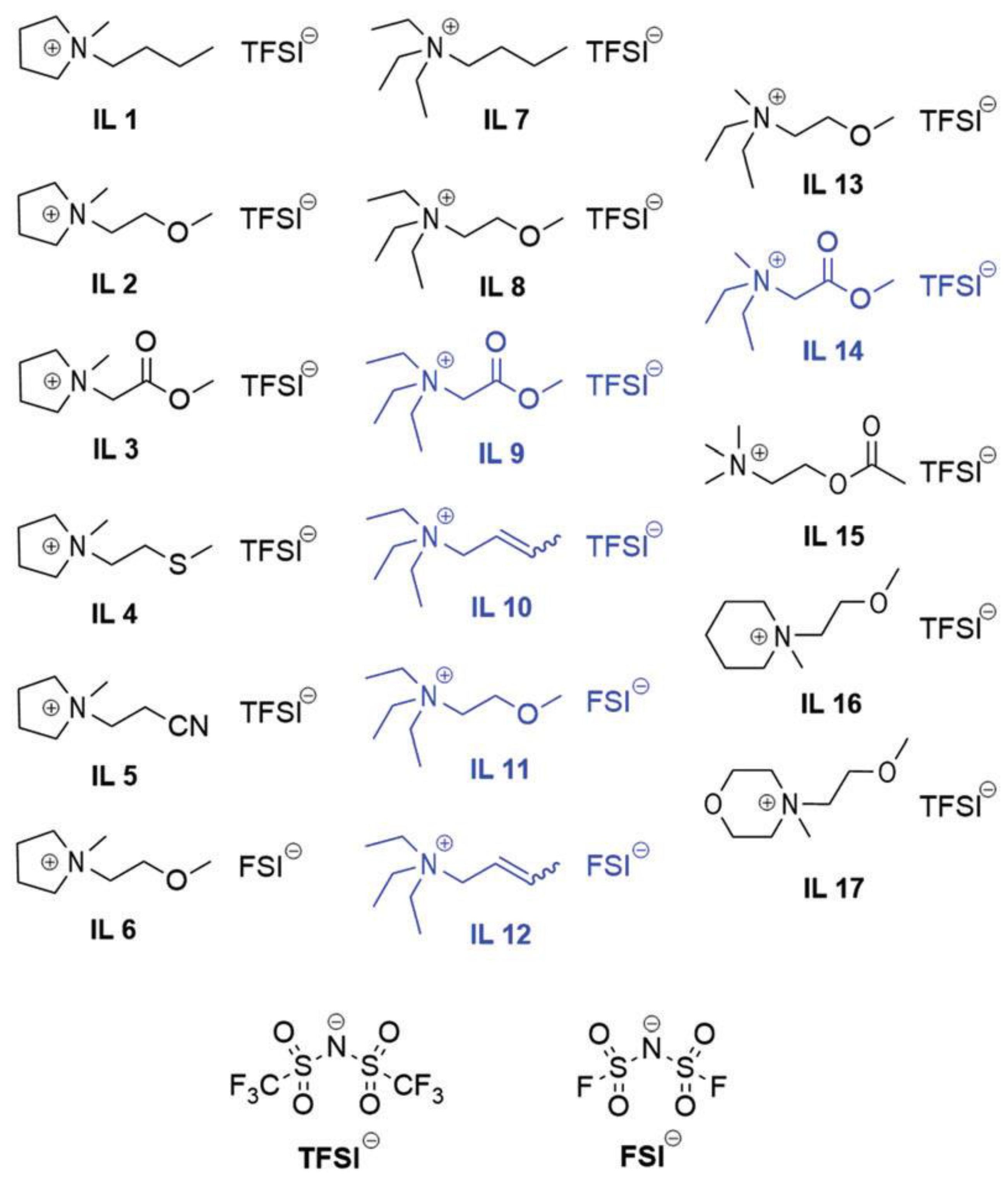
2.3.2. Two-Anion-Type Electrolytes
3. Ammoniates
3.1. Properties of Ammoniates
Physical Properties
| Application field | Ammoniate | State | Ref. |
|---|---|---|---|
| Electrochromic devices | NH4NO3·1.5NH3 | Liquid | [177] |
| LiNO3·2NH3 | Liquid | [157] | |
| LiSO3CF3·2NH3 | Liquid | [157] | |
| Cu (SO3CF3)2 | Liquid | [157] | |
| NaSO3CF3·2.9NH3 | Liquid | [157] | |
| Thermal storage | NH4SCN·xNH3 | Liquid | [159] |
| NiCl2·xNH3 | Solid | [158] | |
| BaCl2·xNH3 | Solid | [158] | |
| CoCl2·xNH3 | Solid | [158] | |
| MnCl2·xNH3 | Solid | [158] | |
| SrCl2·xNH3 | Solid | [158] | |
| CaCl2·xNH3 | Solid | [158] | |
| NaBr·xNH3 | Solid | [158] | |
| Hydrogen storage | Mg(BH4)2·6NH3 | Solid | [170] |
| LiBH4·NH3 | Solid | [169] | |
| Ca(BH4)2·2NH3 | Solid | [171] | |
| Al(BH4)2·6NH3 | Solid | [172] | |
| Li2Al(BH4)5·6NH3 | Solid | [175] | |
| NaZn(BH4)3·2NH3 | Solid | [176] | |
| Batteries | LiClO4·4NH3 | Liquid | [145] |
| NaI·3.3NH3 | Liquid | [145,146,178,179,180] | |
| LiNO3·xNH3 | Liquid | [181] | |
| LiSO3CF3·xNH3 | Liquid | [181] | |
| NaBF4·2.5NH3, | Liquid | [146] | |
| NaBH4·1.5NH3 | Liquid | ||
| Li(NH3)nBH4 | Solid | [141] | |
| LiTFSI·xNH3 | Liquid | [182] | |
| BeF2(NH3)2 | Solid | [143] |
3.2. Ammoniates for Batteries
| Ammoniate | Cathode/Anode | Capacity/ mAh g−1 | Specific Energy /Wh kg−1 | Cyclability | Working Voltages/V | Ref |
|---|---|---|---|---|---|---|
| LiClO4·4NH3 (liq.) | (SN)x/Li | - | 300 | - | From OCP (2.8, 3 and 2.6) to 1 | [145] |
| MnO2/Li | 320 | |||||
| CuO/Li | 92 | |||||
| LiNO3·xNH3 (liq.) | MnO2/Li | - | ≈650 | - | From OCP (3.5) to 2 | [181] |
| LiSO3CF3·xNH3 (liq.) | MnO2/Li | - | ≈650 | - | From OCP (3.9) to 2 | [181] |
| LiTFSI·xNH3 (liq.) | MnO2/Li | - | ≈600 | - | - | [182] |
| NaBF4·2.5NH3 (liq.) | IB/Na | 188 | 275 | 90 at 8C (4 °C) | 3–1.2 | [179] |
| NaI·3.3NH3 (liq.) | (SN)x/Li | - | 190 | - | - | [145] |
| MnO2/Li | 213 | |||||
| CuO/Li | 72 | |||||
| TiO2/Na | 145 | 73 | 400 at 14C | 2.6–0.5 | [178] | |
| IB/Na | ≈100 | 210 | 1000 at 10C | 2.5–1.3 | [179] | |
| PAQS/Na | 218 | 320 (after 100 cycles) | 300 at 11C | 2.6–1.2 | [180] | |
| (IEP-11-SR)/Na | 100 at 1C | - | 4000 at 15C | 2.5–1.1 | [193] | |
| Li(NH3)0.5BH4@SiO2 (s.) | SPAN/Li | 800 at 0.2C | - | <10 | 0–2.4 V | [197] |
| Mg(BH4)2·2NH3 (s.) | TiS2/Mg | 141 (1st cycle) at 0.05C | - | 30 | 0–1.4 V | [198] |
4. Summary and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Ionic liquid |
| MEIC | 1-methyl-3-ethylimidazolium chloride |
| CnC1pyrr+ | N-alkyl-N-methylpyrrolidinium |
| CnC1im+ | N-alkyl-N-methylimidazolium |
| CnC1pip+ | N-alkyl-N-methylpiperidinium |
| Nnnnn+ | Tetraalkylammonium |
| AS(m.n)+ | Azoniaspiro[m,n]nonane |
| Pnnnn+ | Tetraalkylphosphonium. |
| BF4− | Tetrafluoroborate |
| PF6− | Hexafluorophosphate |
| N(CN)2− | Dicyanamide |
| TFSI− | Bis(trifluoromethanesulfonyl)imide |
| FSI− | Bis(fluorosulfonyl)imide |
| [C4Hpyrr][TFSI] | N-butylpyrrolidinium-bis(trifluoromethanesulfonyl)imide |
| LiTFSI | Lithium bis(trifluoromethanesulfonyl)imide |
| NaTFSI [C4Pyrr][TFSI] | Sodium bis(trifluoromethanesulfonyl)imide N-butylpyrrolidinium-Bis(trifluoromethanesulfonyl)imide |
| NaBF4 | Sodium tetrafluoroborate |
| NaPF6 | Sodium hexafluorophosphate |
| NaClO4 | Sodium perchlorate |
| NaTFSI | Sodium bis(trifluoromethanesulfonyl)imide |
| NaFSI [C2C1im][FSI] | Sodium bis(fluoromethanesulfonyl)imide [1-ethyl-3methylimidazolium][bis(fluorosulfonyl)imide] |
| [C2C1im][BF4] | 1-ethyl-3-methylimidazolium tetrafluoroborate |
| [C4C1im][OTf] | 1-butyl-3-methylimidazolium trifluoromethanesulfonate |
| [CnC1im]+ | N-alkyl-N-methylimidazolium |
| [CnC1im][TFSI] | N-alkyl-N-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [CnC1pyrr][TFSI] | N-alkyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide |
| [CnCnpip][TFSI] | N-alkyl-N-alkylpiperidinum bis(trifluoromethansulfonyl)imide |
| [C4C1im][TFSI] | 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide |
| [C4C1im][FSI] | 1-butyl-3-methylimidazolium bis(fluorosulfonyl)imide |
| [C4C1im][BF4] | 1-butyl-3-methylimidazolium tetrafluoroborate |
| [C4C1im][PF6] | 1-butyl-3-methylimidazolium hexafluorophosphate |
| [C4C1im][DCA] | 1-butyl-3-methylimidazolium dicyanamide |
| [C2C1im][TFSI] | 1-ethyl, 3-methylimidazolium bis(trifluoromethanesulfonyl)imide |
| [C2C1im][FSI] | 1-ethyl, 3-methylimidazolium bis(fluorosulfonyl)imide |
| [C2C1im][DCA] | 1-ethyl, 3-methylimidazolium dicyanamide |
| [C2C1im][OTf] | 1-ethyl, 3-methylimidazolium trifluoromethanesulfonate |
| [C4C1pyrr][TFSI] | N-butyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide |
| [C4C1pyrr][FSI] | N-butyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide |
| [C4C1pyrr][DCA] | N-butyl-N-methylpyrrolidinium Dicyanamide |
| [C3C1pyrr][TFSI] | N-propyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide |
| [C3C1pyrr][FSI] | N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide |
| [N2,1,1,3][TFSI] | N-ethyldimethylpropylammonium bis(trifluoromethylsulfonyl)imide |
| [N2,2,2,5][TFSI] | Triethylpentylammonium bis(trifluoromethylsulfonyl)imide |
| [N4441][FSI] | Tributylmethylammonium bis(fluorosulfonyl)imide |
| [N1114][TFSI] | Butyltrimethylammonium bis(trifluoromethylsulfonyl)imide |
| [C2C1im][BF4] | 1-ethyl, 3-methylimidazolium tetrafluoroborate |
| [C4C1pyrr][TFSI] | N-butyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide |
| [C3C1pyrr][DCA] | N-methyl,N-propyl pyrrolidinium dicyanamide |
| [N1114][FSI] | N-trimethyl-N-butyl-ammonium bis(fluorosulfonyl)imide |
| [N2,1,1,3][TFSI] | N-ethyldimethylpropylammonium bis(trifluoromethylsulfonyl)imide |
| [N6,2,2,2][TFSI] | N-hexyltriethylammonium bis(trifluoromethylsulfonyl)imide |
| [P1i4i4i4][FSI] | Tri(isobutyl)methylphosphonium bis(fluorosulfonyl)imide |
| NaN(CN)2 | Sodium dicyanamide |
| MM26py | 1-methylpyridinum 2,6-dicarboxylate |
References
- “Data Page: Share of Electricity Generated by Renewables”. Part of the Following Publication: Hannah Ritchie, Pablo Rosado and Max Roser (2023)—“Energy”. Data adapted from Ember, Energy Institute. Available online: https://ourworldindata.org/grapher/share-electricity-renewables (accessed on 21 October 2024).
- Electricity Generation by Source 2023 | Statista. Available online: https://www.statista.com/statistics/269811/world-electricity-production-by-energy-source/ (accessed on 21 October 2024).
- El Pacto Verde Europeo—Comisión Europea. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_es (accessed on 21 October 2024).
- REPowerEU. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_22_3131 (accessed on 21 October 2024).
- Renewables Take the Lead in Power Generation in 2023—Eurostat. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/ddn-20240627-1 (accessed on 21 October 2024).
- Balali, M.H.; Nouri, N.; Omrani, E.; Nasiri, A.; Otieno, W. An Overview of the Environmental, Economic, and Material Developments of the Solar and Wind Sources Coupled with the Energy Storage Systems. Int. J. Energy Res. 2017, 41, 1948–1962. [Google Scholar] [CrossRef]
- Mahmoud, M.; Ramadan, M.; Olabi, A.G.; Pullen, K.; Naher, S. A Review of Mechanical Energy Storage Systems Combined with Wind and Solar Applications. Energy Convers. Manag. 2020, 210, 112670. [Google Scholar] [CrossRef]
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A Comprehensive Review of Stationary Energy Storage Devices for Large Scale Renewable Energy Sources Grid Integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Li, C.; Xu, H.; Ni, L.; Qin, B.; Ma, Y.; Jiang, H.; Xu, G.; Zhao, J.; Cui, G. Nonaqueous Liquid Electrolytes for Sodium-Ion Batteries: Fundamentals, Progress and Perspectives. Adv. Energy Mater. 2023, 13, 2301758. [Google Scholar] [CrossRef]
- CATL Unveils Its Latest Breakthrough Technology by Releasing Its First Generation of Sodium-Ion Batteries. Available online: https://www.catl.com/en/news/665.html (accessed on 21 October 2024).
- Peters, J.F.; Baumann, M.; Binder, J.R.; Weil, M. On the Environmental Competitiveness of Sodium-Ion Batteries under a Full Life Cycle Perspective—A Cell-Chemistry Specific Modelling Approach. Sustain. Energy Fuels 2021, 5, 6414–6429. [Google Scholar] [CrossRef]
- International Energy Agency. Global EV Outlook 2023: Catching Up with Climate Ambitions; International Energy Agency: Paris, France, 2023. [Google Scholar]
- Sodium Ion VS LiFePO4 Battery Compared: Pros, Cons, and Differences—ECO Teardown. Available online: https://ecoteardown.top/sodium-ion-vs-lifepo4-battery-compared-pros-cons-and-differences/ (accessed on 21 October 2024).
- Sodium-Ion vs. Lithium-Ion Battery: Comparison, Challenges & Alternative | GEP Blog. Available online: https://www.gep.com/blog/strategy/lithium-ion-vs-sodium-ion-battery (accessed on 21 October 2024).
- Perveen, T.; Siddiq, M.; Shahzad, N.; Ihsan, R.; Ahmad, A.; Shahzad, M.I. Prospects in Anode Materials for Sodium Ion Batteries—A Review. Renew. Sustain. Energy Rev. 2020, 119, 109549. [Google Scholar] [CrossRef]
- Qiao, S.; Zhou, Q.; Ma, M.; Liu, H.K.; Dou, S.X.; Chong, S. Advanced Anode Materials for Rechargeable Sodium-Ion Batteries. ACS Nano 2023, 17, 11220–11252. [Google Scholar] [CrossRef]
- Kumar Prajapati, A.; Bhatnagar, A. A Review on Anode Materials for Lithium/Sodium-Ion Batteries. J. Energy Chem. 2023, 83, 509–540. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Wang, S.; Miao, Z.; Liu, H.K.; Chou, S. Structural Design of Anode Materials for Sodium-Ion Batteries. J. Mater. Chem. A Mater. 2018, 6, 6183–6205. [Google Scholar] [CrossRef]
- Gupta, P.; Pushpakanth, S.; Haider, M.A.; Basu, S. Understanding the Design of Cathode Materials for Na-Ion Batteries. ACS Omega 2022, 7, 5605–5614. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. Recent Advances in Sodium-Ion Batteries: Cathode Materials. Materials 2023, 16, 6869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, Y.; Liu, X.; Zhou, L.; Li, J.; Xiao, Y.; Peng, J.; Wang, J.; Chou, S.L. Long-Cycle-Life Cathode Materials for Sodium-Ion Batteries toward Large-Scale Energy Storage Systems. Adv. Energy Mater. 2023, 13, 2300149. [Google Scholar] [CrossRef]
- Xu, S.; Dong, H.; Yang, D.; Wu, C.; Yao, Y.; Rui, X.; Chou, S.; Yu, Y. Promising Cathode Materials for Sodium-Ion Batteries from Lab to Application. ACS Cent. Sci. 2023, 9, 2012–2035. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhu, D.; Shan, M.; Wang, H.; Zhang, J.; Cui, G.; Hu, Z.; Gordon, K.C.; Xu, G.; Zhu, M. Functional Separator Materials of Sodium-Ion Batteries: Grand Challenges and Industry Perspectives. Nano Today 2024, 55, 102175. [Google Scholar] [CrossRef]
- Yi, M.; Jing, M.; Deng, W.; Zou, G.; Hou, H.; Wu, T.; Ji, X. Electrolyte, Separator, Binder and Other Devices of Sodium Ion Batteries. In Sodium-Ion Batteries: Technologies and Applications; Wiley: Hoboken, NJ, USA, 2023; pp. 171–245. [Google Scholar] [CrossRef]
- Luo, W.; Cheng, S.; Wu, M.; Zhang, X.; Yang, D.; Rui, X. A Review of Advanced Separators for Rechargeable Batteries. J. Power Sources 2021, 509, 230372. [Google Scholar] [CrossRef]
- Hashmi, M.M.; Arif, N.A.; Ali, S.M.; Khan, M.B.; Singh, M.P.; Khan, Z.H. Recent Progress in Separators for Rechargeable Batteries. In Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2022; pp. 417–498. [Google Scholar] [CrossRef]
- Huang, Z.X.; Zhang, X.L.; Zhao, X.X.; Zhao, Y.Y.; Aravindan, V.; Liu, Y.H.; Geng, H.; Wu, X.L. Electrode/Electrolyte Additives for Practical Sodium-Ion Batteries: A Mini Review. Inorg. Chem. Front. 2022, 10, 37–48. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.; Li, L.; Xie, M.; Wu, F.; Chen, R. Electrolytes and Electrolyte/Electrode Interfaces in Sodium-Ion Batteries: From Scientific Research to Practical Application. Adv. Mater. 2019, 31, 1808393. [Google Scholar] [CrossRef]
- Usiskin, R.; Lu, Y.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.S.; Maier, J. Fundamentals, Status and Promise of Sodium-Based Batteries. Nat. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Tian, Z.; Zou, Y.; Liu, G.; Wang, Y.; Yin, J.; Ming, J.; Alshareef, H.N. Electrolyte Solvation Structure Design for Sodium Ion Batteries. Adv. Sci. 2022, 9, 2201207. [Google Scholar] [CrossRef]
- Gao, X.; Xing, Z.; Wang, M.; Nie, C.; Shang, Z.; Bai, Z.; Dou, S.X.; Wang, N. Comprehensive Insights into Solid-State Electrolytes and Electrode-Electrolyte Interfaces in All-Solid-State Sodium-Ion Batteries. Energy Storage Mater. 2023, 60, 102821. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Ali, A.; Aljawrneh, B.; Al-Othman, A. Progress in Safe Nano-Structured Electrolytes for Sodium Ion Batteries: A Comprehensive Review. Nano-Struct. Nano-Objects 2024, 39, 101311. [Google Scholar] [CrossRef]
- Ahmad, H.; Kubra, K.T.; Butt, A.; Nisar, U.; Iftikhar, F.J.; Ali, G. Recent Progress, Challenges, and Perspectives in the Development of Solid-State Electrolytes for Sodium Batteries. J. Power Sources 2023, 581, 233518. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Xu, C.; Hu, N.; Molenda, J.; Lu, L. Development of Solid-State Electrolytes for Sodium-Ion Battery—A Short Review. Nano Mater. Sci. 2019, 1, 91–100. [Google Scholar] [CrossRef]
- Domingues, L.S.; de Melo, H.G.; Martins, V.L. Ionic Liquids as Potential Electrolytes for Sodium-Ion Batteries: An Overview. Phys. Chem. Chem. Phys. 2023, 25, 12650–12667. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, M.; Kong, X.; Huang, W.; Zhang, Q. Recent Advance in Ionic-Liquid-Based Electrolytes for Rechargeable Metal-Ion Batteries. Adv. Sci. 2021, 8, 2004490. [Google Scholar] [CrossRef]
- Xu, C.; Yang, G.; Wu, D.; Yao, M.; Xing, C.; Zhang, J.; Zhang, H.; Li, F.; Feng, Y.; Qi, S.; et al. Roadmap on Ionic Liquid Electrolytes for Energy Storage Devices. Chem. Asian J. 2021, 16, 549–562. [Google Scholar] [CrossRef]
- Walden, P. Molecular Weights and Electrical Conductivity of Several Fused Salts. Bull. Acad. Imper. Sci. 1914, 1800, 405–422. [Google Scholar]
- Welton, T. Ionic Liquids: A Brief History. Biophys. Rev. 2018, 10, 691–706. [Google Scholar]
- Poole, C.F.; Furton, K.G.; Kersten, B.R. Liquid Organic Salt Phases for Gas Chromatography. J. Chromatogr. Sci. 1986, 24, 400–409. [Google Scholar]
- Hussey, C.L. Room Temperature Molten Salt Systems. Adv. Molten Salt Chem. 1983, 5, 185–230. [Google Scholar]
- Pacholec, F.; Butler, H.T.; Poole, C.F. Molten Organic Salt Phase for Gas-Liquid Chromatography. Anal. Chem. 1982, 54, 1938–1941. [Google Scholar] [CrossRef]
- Magnuson, D.K.; Bodley, J.W.; Evans, D.F. The Activity and Stability of Alkaline Phosphatase in Solutions of Water and the Fused Salt Ethylammonium Nitrate. J. Solut. Chem. 1984, 13, 583–587. [Google Scholar] [CrossRef]
- Evans, D.F.; Chen, S.H.; Schriver, G.W.; Arnett, E.M. Thermodynamics of Solution of Nonpolar Gases in a Fused Salt. “Hydrophobic Bonding” Behavior in a Nonaqueous System. J. Am. Chem. Soc. 1981, 103, 481–482. [Google Scholar] [CrossRef]
- Schubert, T.J.S. Current and Future Ionic Liquid Markets. In Ionic Liquids: Current State and Future Directions; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2017; Volume 1250, pp. 35–65. ISBN 9780841232136. [Google Scholar]
- Sood, K.; Saini, Y.; Thakur, K.K. Ionic Liquids in Catalysis: A Review. Mater. Today Proc. 2023, 81, 739–744. [Google Scholar] [CrossRef]
- Singh, V.V.; Nigam, A.K.; Batra, A.; Boopathi, M.; Singh, B.; Vijayaraghavan, R. Applications of Ionic Liquids in Electrochemical Sensors and Biosensors. Int. J. Electrochem. 2012, 2012, 165683. [Google Scholar] [CrossRef]
- Jónsson, E. Ionic Liquids as Electrolytes for Energy Storage Applications—A Modelling Perspective. Energy Storage Mater. 2020, 25, 827–835. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef]
- Lim, J.R.; Chua, L.S.; Mustaffa, A.A. Ionic Liquids as Green Solvent and Their Applications in Bioactive Compounds Extraction from Plants. Process Biochem. 2022, 122, 292–306. [Google Scholar] [CrossRef]
- Gray, G.E.; Kohl, P.A.; Winnick, J. Stability of Sodium Electrodeposited from a Room Temperature Chloroaluminate Molten Salt. J. Electrochem. Soc. 1995, 142, 3636–3642. [Google Scholar] [CrossRef]
- Scordilis-Kelleya, C.; Carlin, R.T. Stability and Electrochemistry of Lithium in Room Temperature Chloroaluminate Molten Salts. J. Electrochem. Soc. 1994, 141, 873–875. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Jeong, S.; Pandard, P.; Lecocq, A.; Marlair, G.; Passerini, S. Comprehensive Insights into the Thermal Stability, Biodegradability, and Combustion Chemistry of Pyrrolidinium-Based Ionic Liquids. ChemSusChem 2017, 10, 3146–3159. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Hwang, J.; Kaushik, S.; Chen, C.Y.; Hagiwara, R. Advances in Sodium Secondary Batteries Utilizing Ionic Liquid Electrolytes. Energy Environ. Sci. 2019, 12, 3247–3287. [Google Scholar] [CrossRef]
- Stettner, T.; Balducci, A. Protic Ionic Liquids in Energy Storage Devices: Past, Present and Future Perspective. Energy Storage Mater. 2021, 40, 402–414. [Google Scholar] [CrossRef]
- Brutti, S.; Simonetti, E.; De Francesco, M.; Sarra, A.; Paolone, A.; Palumbo, O.; Fantini, S.; Lin, R.; Falgayrat, A.; Choi, H.; et al. Ionic Liquid Electrolytes for High-Voltage, Lithium-Ion Batteries. J. Power Sources 2020, 479, 228791. [Google Scholar] [CrossRef]
- Gao, X.; Wu, F.; Mariani, A.; Passerini, S. Concentrated Ionic-Liquid-Based Electrolytes for High-Voltage Lithium Batteries with Improved Performance at Room Temperature. ChemSusChem 2019, 12, 4185–4193. [Google Scholar] [CrossRef]
- Freemantle, M. An Introduction to Ionic Liquids; Royal Society of Chemistry: London, UK, 2010. [Google Scholar]
- Brandt, A.; Pires, J.; Anouti, M.; Balducci, A. An Investigation about the Cycling Stability of Supercapacitors Containing Protic Ionic Liquids as Electrolyte Components. Electrochim. Acta 2013, 108, 226–231. [Google Scholar] [CrossRef]
- Arnaiz, M.; Huang, P.; Ajuria, J.; Rojo, T.; Goikolea, E.; Balducci, A. Protic and Aprotic Ionic Liquids in Combination with Hard Carbon for Lithium-Ion and Sodium-Ion Batteries. Batter. Supercaps 2018, 1, 204–208. [Google Scholar] [CrossRef]
- Menne, S.; Schroeder, M.; Vogl, T.; Balducci, A. Carbonaceous Anodes for Lithium-Ion Batteries in Combination with Protic Ionic Liquids-Based Electrolytes. J. Power Sources 2014, 266, 208–212. [Google Scholar] [CrossRef]
- Lingua, G.; Falco, M.; Stettner, T.; Gerbaldi, C.; Balducci, A. Enabling Safe and Stable Li Metal Batteries with Protic Ionic Liquid Electrolytes and High Voltage Cathodes. J. Power Sources 2021, 481, 228979. [Google Scholar] [CrossRef]
- Vogl, T.; Vaalma, C.; Buchholz, D.; Secchiaroli, M.; Marassi, R.; Passerini, S.; Balducci, A. The Use of Protic Ionic Liquids with Cathodes for Sodium-Ion Batteries. J. Mater. Chem. A Mater. 2016, 4, 10472–10478. [Google Scholar] [CrossRef]
- Basile, A.; Hilder, M.; Makhlooghiazad, F.; Pozo-Gonzalo, C.; MacFarlane, D.R.; Howlett, P.C.; Forsyth, M. Ionic Liquids and Organic Ionic Plastic Crystals: Advanced Electrolytes for Safer High Performance Sodium Energy Storage Technologies. Adv. Energy Mater. 2018, 8, 1703491. [Google Scholar] [CrossRef]
- Wang, C.H.; Yeh, Y.W.; Wongittharom, N.; Wang, Y.C.; Tseng, C.J.; Lee, S.W.; Chang, W.S.; Chang, J.K. Rechargeable Na/Na0.44MnO2 Cells with Ionic Liquid Electrolytes Containing Various Sodium Solutes. J. Power Sources 2015, 274, 1016–1023. [Google Scholar] [CrossRef]
- Babucci, M.; Akçay, A.; Balci, V.; Uzun, A. Thermal Stability Limits of Imidazolium Ionic Liquids Immobilized on Metal-Oxides. Langmuir 2015, 31, 9163–9176. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hosokawa, T.; Nohira, T.; Hagiwara, R.; Fukunaga, A.; Numata, K.; Itani, E.; Sakai, S.; Nitta, K.; Inazawa, S. The Na[FSA]–[C2C1im][FSA] (C2C1im+:1-Ethyl-3-Methylimidazolium and FSA−:Bis(Fluorosulfonyl)Amide) Ionic Liquid Electrolytes for Sodium Secondary Batteries. J. Power Sources 2014, 265, 36–39. [Google Scholar] [CrossRef]
- Mondal, T.; Samanta, P. Study of Physicochemical Properties of Ionic Liquids. In Handbook of Ionic Liquids; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2024; pp. 51–67. ISBN 9783527839520. [Google Scholar]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical Properties of Ionic Liquids: Database and Evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar]
- Coadou, E.; Goodrich, P.; Neale, A.R.; Timperman, L.; Hardacre, C.; Jacquemin, J.; Anouti, M. Synthesis and Thermophysical Properties of Ether-Functionalized Sulfonium Ionic Liquids as Potential Electrolytes for Electrochemical Applications. ChemPhysChem 2016, 17, 3992–4002. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, W.; Nie, J.; Zhou, Z. Recent Progresses on Electrolytes of Fluorosulfonimide Anions for Improving the Performances of Rechargeable Li and Li-Ion Battery. J. Fluor. Chem. 2015, 174, 49–61. [Google Scholar] [CrossRef]
- Kazemiabnavi, S.; Zhang, Z.; Thornton, K.; Banerjee, S. Electrochemical Stability Window of Imidazolium-Based Ionic Liquids as Electrolytes for Lithium Batteries. J. Phys. Chem. B 2016, 120, 5691–5702. [Google Scholar] [CrossRef]
- Mousavi, M.P.S.; Wilson, B.E.; Kashefolgheta, S.; Anderson, E.L.; He, S.; Bühlmann, P.; Stein, A. Ionic Liquids as Electrolytes for Electrochemical Double-Layer Capacitors: Structures That Optimize Specific Energy. ACS Appl. Mater. Interfaces 2016, 8, 3396–3406. [Google Scholar] [CrossRef]
- Fitchett, B.D.; Rollins, J.B.; Conboy, J.C. 1-Alkyl-3-Methylimidazolium Bis(Perfluoroalkylsulfonyl)Imide Water-Immiscible Ionic Liquids. J. Electrochem. Soc. 2005, 152, E251. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Montanino, M.; Zane, D.; Carewska, M.; Alessandrini, F.; Passerini, S. Effect of the Alkyl Group on the Synthesis and the Electrochemical Properties of N-Alkyl-N-Methyl-Pyrrolidinium Bis(Trifluoromethanesulfonyl)Imide Ionic Liquids. Electrochim. Acta 2009, 54, 1325–1332. [Google Scholar] [CrossRef]
- Montanino, M.; Carewska, M.; Alessandrini, F.; Passerini, S.; Appetecchi, G.B. The Role of the Cation Aliphatic Side Chain Length in Piperidinium Bis(Trifluoromethansulfonyl)Imide Ionic Liquids. Electrochim. Acta 2011, 57, 153–159. [Google Scholar] [CrossRef]
- Hwang, J.; Matsumoto, K.; Hagiwara, R. Electrolytes toward High-Voltage Na3V2(PO4)2F3 Positive Electrode Durable against Temperature Variation. Adv. Energy Mater. 2020, 10, 2001880. [Google Scholar] [CrossRef]
- Rogers, E.I.; Šljukic, B.; Hardacre, C.; Compton, R.G. Electrochemistry in Room-Temperature Ionic Liquids: Potential Windows at Mercury Electrodes. J. Chem. Eng. Data 2009, 54, 2049–2053. [Google Scholar] [CrossRef]
- Randström, S.; Appetecchi, G.B.; Lagergren, C.; Moreno, A.; Passerini, S. The Influence of Air and Its Components on the Cathodic Stability of N-Butyl-N-Methylpyrrolidinium Bis(Trifluoromethanesulfonyl)Imide. Electrochim. Acta 2007, 53, 1837–1842. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, D.; Kaushik, S.; Zhang, S.; Wada, T.; Hwang, J.; Matsumoto, K.; Hagiwara, R. Ionic Liquid Electrolytes for Next-Generation Electrochemical Energy Devices. EnergyChem 2022, 4, 100075. [Google Scholar] [CrossRef]
- Musiał, M.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Musiol, R.; Zorȩbski, E.; Dzida, M. Pyrrolidinium-Based Ionic Liquids as Sustainable Media in Heat-Transfer Processes. ACS Sustain. Chem. Eng. 2017, 5, 11024–11033. [Google Scholar] [CrossRef]
- Jamil, R.; Loomba, S.; Kar, M.; Collis, G.E.; Silvester, D.S.; Mahmood, N. Metal Anodes Meet Ionic Liquids: An Interfacial Perspective. Appl. Phys. Rev. 2024, 11, 011307. [Google Scholar] [CrossRef]
- Ma, L.A.; Naylor, A.J.; Nyholm, L.; Younesi, R. Strategies for Mitigating Dissolution of Solid Electrolyte Interphases in Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 4855–4863. [Google Scholar] [CrossRef]
- Mogensen, R.; Brandell, D.; Younesi, R. Solubility of the Solid Electrolyte Interphase (SEI) in Sodium Ion Batteries. ACS Energy Lett. 2016, 1, 1173–1178. [Google Scholar] [CrossRef]
- Bao, C.; Wang, B.; Liu, P.; Wu, H.; Zhou, Y.; Wang, D.; Liu, H.; Dou, S.; Bao, C.Y.; Wang, B.; et al. Solid Electrolyte Interphases on Sodium Metal Anodes. Adv. Funct. Mater. 2020, 30, 2004891. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, D.; Wang, Z.; Wang, H.; Liu, J.; Guo, K.; Liu, Q.; Ma, J.; Zhu, C.; Wu, D.; et al. Optimizing NaF-Rich Solid Electrolyte Interphase for Stabilizing Sodium Metal Batteries by Electrolyte Additive. Adv. Funct. Mater. 2024, 34, 2214195. [Google Scholar] [CrossRef]
- Lin, Y.; Peng, Q.; Chen, L.; Zuo, Q.; Long, Q.; Lu, F.; Huang, S.; Chen, Y.; Meng, Y. Organic Liquid Electrolytes in Sodium-Based Batteries: Actualities and Perspectives. Energy Storage Mater. 2024, 67, 103211. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J.; Lin, D.; Wang, D.W.; Li, B.; Lv, W.; Sun, S.; He, Y.B.; Kang, F.; Yang, Q.H.; et al. Evolution of the Electrochemical Interface in Sodium Ion Batteries with Ether Electrolytes. Nat. Commun. 2019, 10, 725. [Google Scholar] [CrossRef]
- Cui, R.; Ma, Y.; Gao, X.; Wang, W.; Wang, J.; Xing, Z.; Ju, Z. Research Progress of Co-Intercalation Mechanism Electrolytes in Sodium-Ion Batteries. Energy Storage Mater. 2024, 71, 103627. [Google Scholar] [CrossRef]
- Cheng, F.; Cao, M.; Li, Q.; Fang, C.; Han, J.; Huang, Y. Electrolyte Salts for Sodium-Ion Batteries: NaPF6 or NaClO4? ACS Nano 2023, 17, 18608–18615. [Google Scholar] [CrossRef]
- Barnes, P.; Smith, K.; Parrish, R.; Jones, C.; Skinner, P.; Storch, E.; White, Q.; Deng, C.; Karsann, D.; Lau, M.L.; et al. A Non-Aqueous Sodium Hexafluorophosphate-Based Electrolyte Degradation Study: Formation and Mitigation of Hydrofluoric Acid. J. Power Sources 2020, 447, 227363. [Google Scholar] [CrossRef]
- Ponrouch, A.; Monti, D.; Boschin, A.; Steen, B.; Johansson, P.; Palacín, M.R. Non-Aqueous Electrolytes for Sodium-Ion Batteries. J. Mater. Chem. A Mater. 2014, 3, 22–42. [Google Scholar] [CrossRef]
- Seki, S.; Kobayashi, T.; Kobayashi, Y.; Takei, K.; Miyashiro, H.; Hayamizu, K.; Tsuzuki, S.; Mitsugi, T.; Umebayashi, Y. Effects of Cation and Anion on Physical Properties of Room-Temperature Ionic Liquids. J. Mol. Liq. 2010, 152, 9–13. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Elia, G.A.; Armand, M.; Forsyth, M.; Komaba, S.; Rojo, T.; Passerini, S. Electrolytes and Interphases in Sodium-Based Rechargeable Batteries: Recent Advances and Perspectives. Adv. Energy Mater. 2020, 10, 2000093. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are Ionic Liquids Chemically Stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef] [PubMed]
- Galiński, M.; Lewandowski, A.; Stepniak, I. Ionic Liquids as Electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, Electrochemical and Radiolytic Stabilities of Ionic Liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef]
- Ding, C.; Nohira, T.; Hagiwara, R.; Matsumoto, K.; Okamoto, Y.; Fukunaga, A.; Sakai, S.; Nitta, K.; Inazawa, S. Na[FSA]-[C3C1pyrr][FSA] Ionic Liquids as Electrolytes for Sodium Secondary Batteries: Effects of Na Ion Concentration and Operation Temperature. J. Power Sources 2014, 269, 124–128. [Google Scholar] [CrossRef]
- Hilder, M.; Howlett, P.C.; Saurel, D.; Gonzalo, E.; Basile, A.; Armand, M.; Rojo, T.; Kar, M.; MacFarlane, D.R.; Forsyth, M. The Effect of Cation Chemistry on Physicochemical Behaviour of Superconcentrated NaFSI Based Ionic Liquid Electrolytes and the Implications for Na Battery Performance. Electrochim. Acta 2018, 268, 94–100. [Google Scholar] [CrossRef]
- Golding, J.; Hamid, N.; MacFarlane, D.R.; Forsyth, M.; Forsyth, C.; Collins, C.; Huang, J. N-Methyl-N-Alkylpyrrolidinium Hexafluorophosphate Salts: Novel Molten Salts and Plastic Crystal Phases. Chem. Mater. 2001, 13, 558–564. [Google Scholar] [CrossRef]
- Harris, K.R.; Woolf, L.A.; Kanakubo, M. Temperature and Pressure Dependence of the Viscosity of the Ionic Liquid 1-Butyl-3-Methylimidazolium Hexafluorophosphate. J. Chem. Eng. Data 2005, 50, 1777–1782. [Google Scholar] [CrossRef]
- Wang, D.; Takiyama, M.; Hwang, J.; Matsumoto, K.; Hagiwara, R. A Hexafluorophosphate-Based Ionic Liquid as Multifunctional Interfacial Layer between High Voltage Positive Electrode and Solid-State Electrolyte for Sodium Secondary Batteries. Adv. Energy Mater. 2023, 13, 2301020. [Google Scholar] [CrossRef]
- Plashnitsa, L.S.; Kobayashi, E.; Noguchi, Y.; Okada, S.; Yamaki, J. Performance of NASICON Symmetric Cell with Ionic Liquid Electrolyte. J. Electrochem. Soc. 2010, 157, A536. [Google Scholar] [CrossRef]
- Wu, F.; Zhu, N.; Bai, Y.; Liu, L.; Zhou, H.; Wu, C. Highly Safe Ionic Liquid Electrolytes for Sodium-Ion Battery: Wide Electrochemical Window and Good Thermal Stability. ACS Appl. Mater. Interfaces 2016, 8, 21381–21386. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.J.; Do, M.P.; Reich, R.M.; Nagasubramanian, A.; Srinivasan, M.; Kühn, F.E. Synthesis and Physicochemical Characterization of Room Temperature Ionic Liquids and Their Application in Sodium Ion Batteries. Phys. Chem. Chem. Phys. 2018, 20, 29412–29422. [Google Scholar] [CrossRef] [PubMed]
- Bazito, F.F.C.; Kawano, Y.; Torresi, R.M. Synthesis and Characterization of Two Ionic Liquids with Emphasis on Their Chemical Stability towards Metallic Lithium. Electrochim. Acta 2007, 52, 6427–6437. [Google Scholar] [CrossRef]
- Hosokawa, T.; Matsumoto, K.; Nohira, T.; Hagiwara, R.; Fukunaga, A.; Sakai, S.; Nitta, K. Stability of Ionic Liquids against Sodium Metal: A Comparative Study of 1-Ethyl-3-Methylimidazolium Ionic Liquids with Bis(Fluorosulfonyl)Amide and Bis(Trifluoromethylsulfonyl)Amide. J. Phys. Chem. C 2016, 120, 9628–9636. [Google Scholar] [CrossRef]
- Wibowo, R.; Aldous, L.; Rogers, E.I.; Ward Jones, S.E.; Compton, R.G. A Study of the Na/Na+ Redox Couple in Some Room Temperature Ionic Liquids. J. Phys. Chem. C 2010, 114, 3618–3626. [Google Scholar] [CrossRef]
- Wu, S.; Wada, T.; Shionoya, H.; Hwang, J.; Matsumoto, K.; Hagiwara, R. Practical Level of Low-N/P Ratio Sodium Metal Batteries: On the Basis of Deposition/Dissolution Efficiency in the Aspects of Electrolytes and Temperature. Energy Storage Mater. 2023, 61, 102897. [Google Scholar] [CrossRef]
- Ding, C.; Nohira, T.; Kuroda, K.; Hagiwara, R.; Fukunaga, A.; Sakai, S.; Nitta, K.; Inazawa, S. NaFSA-C1C3pyrFSA Ionic Liquids for Sodium Secondary Battery Operating over a Wide Temperature Range. J. Power Sources 2013, 238, 296–300. [Google Scholar] [CrossRef]
- Basile, A.; Makhlooghiazad, F.; Yunis, R.; MacFarlane, D.R.; Forsyth, M.; Howlett, P.C. Extensive Sodium Metal Plating and Stripping in a Highly Concentrated Inorganic−Organic Ionic Liquid Electrolyte through Surface Pretreatment. ChemElectroChem 2017, 4, 986–991. [Google Scholar] [CrossRef]
- Basile, A.; Ferdousi, S.A.; Makhlooghiazad, F.; Yunis, R.; Hilder, M.; Forsyth, M.; Howlett, P.C. Beneficial Effect of Added Water on Sodium Metal Cycling in Super Concentrated Ionic Liquid Sodium Electrolytes. J. Power Sources 2018, 379, 344–349. [Google Scholar] [CrossRef]
- Ferdousi, S.A.; O’Dell, L.A.; Hilder, M.; Barlow, A.J.; Armand, M.; Forsyth, M.; Howlett, P.C. SEI Formation on Sodium Metal Electrodes in Superconcentrated Ionic Liquid Electrolytes and the Effect of Additive Water. ACS Appl. Mater. Interfaces 2021, 13, 5706–5720. [Google Scholar] [CrossRef]
- Sun, J.; Rakov, D.; Wang, J.; Hora, Y.; Laghaei, M.; Byrne, N.; Wang, X.; Howlett, P.C.; Forsyth, M. Sustainable Free-Standing Electrode from Biomass Waste for Sodium-Ion Batteries. ChemElectroChem 2022, 9, e202200382. [Google Scholar] [CrossRef]
- Sun, J.; O’Dell, L.A.; Armand, M.; Howlett, P.C.; Forsyth, M. Anion-Derived Solid-Electrolyte Interphase Enables Long Life Na-Ion Batteries Using Superconcentrated Ionic Liquid Electrolytes. ACS Energy Lett. 2021, 6, 2481–2490. [Google Scholar] [CrossRef]
- Wongittharom, N.; Wang, C.H.; Wang, Y.C.; Yang, C.H.; Chang, J.K. Ionic Liquid Electrolytes with Various Sodium Solutes for Rechargeable Na/NaFePO4 Batteries Operated at Elevated Temperatures. ACS Appl. Mater. Interfaces 2014, 6, 17564–17570. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Matsumoto, K.; Nohira, T.; Hagiwara, R.; Orikasa, Y.; Uchimoto, Y. Pyrophosphate Na2FeP2O7 as a Low-Cost and High-Performance Positive Electrode Material for Sodium Secondary Batteries Utilizing an Inorganic Ionic Liquid. J. Power Sources 2014, 246, 783–787. [Google Scholar] [CrossRef]
- Oh, J.A.S.; He, H.; Sun, J.; Cao, X.; Chua, B.; Huang, Y.; Zeng, K.; Lu, L. Dual-Nitrogen-Doped Carbon Decorated on Na3V2(PO4)3 to Stabilize the Intercalation of Three Sodium Ions. ACS Appl. Energy Mater. 2020, 3, 6870–6879. [Google Scholar] [CrossRef]
- Ding, C.; Nohira, T.; Hagiwara, R. Charge-Discharge Performance of Na2/3Fe1/3Mn2/3O2 Positive Electrode in an Ionic Liquid Electrolyte at 90 °C for Sodium Secondary Batteries. Electrochim. Acta 2017, 231, 412–416. [Google Scholar] [CrossRef]
- Hasa, I.; Passerini, S.; Hassoun, J. Characteristics of an Ionic Liquid Electrolyte for Sodium-Ion Batteries. J. Power Sources 2016, 303, 203–207. [Google Scholar]
- Wang, X.; Shang, Z.; Yang, A.; Zhang, Q.; Cheng, F.; Jia, D.; Chen, J. Combining Quinone Cathode and Ionic Liquid Electrolyte for Organic Sodium-Ion Batteries. Chem 2019, 5, 364–375. [Google Scholar] [CrossRef]
- Forsyth, M.; Yoon, H.; Chen, F.; Zhu, H.; MacFarlane, D.R.; Armand, M.; Howlett, P.C. Novel Na+ Ion Diffusion Mechanism in Mixed Organic-Inorganic Ionic Liquid Electrolyte Leading to High Na+ Transference Number and Stable, High Rate Electrochemical Cycling of Sodium Cells. J. Phys. Chem. C 2016, 120, 4276–4286. [Google Scholar] [CrossRef]
- Matsumoto, K.; Okamoto, Y.; Nohira, T.; Hagiwara, R. Thermal and Transport Properties of Na[N(SO2F)2]-[ N -Methyl- N -Propylpyrrolidinium][N(SO2F)2] Ionic Liquids for Na Secondary Batteries. J. Phys. Chem. C 2015, 119, 7648–7655. [Google Scholar] [CrossRef]
- Massaro, A.; Lingua, G.; Bozza, F.; Piovano, A.; Prosini, P.P.; Muñoz-García, A.B.; Pavone, M.; Gerbaldi, C. P2-Type Na0.84Li0.1Ni0.27Mn0.63O2-Layered Oxide Na-Ion Battery Cathode: Structural Insights and Electrochemical Compatibility with Room-Temperature Ionic Liquids. Chem. Mater. 2024, 36, 7046–7055. [Google Scholar] [CrossRef] [PubMed]
- Do, M.P.; Bucher, N.; Nagasubramanian, A.; Markovits, I.; Bingbing, T.; Fischer, P.J.; Loh, K.P.; Kühn, F.E.; Srinivasan, M. Effect of Conducting Salts in Ionic Liquid Electrolytes for Enhanced Cyclability of Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 23972–23981. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhu, N.; Bai, Y.; Li, Y.; Wang, Z.; Ni, Q.; Wang, H.; Wu, C. Unveil the Mechanism of Solid Electrolyte Interphase on Na3V2(PO4)3 Formed by a Novel NaPF6/BMITFSI Ionic Liquid Electrolyte. Nano Energy 2018, 51, 524–532. [Google Scholar] [CrossRef]
- Chagas, L.G.; Jeong, S.; Hasa, I.; Passerini, S. Ionic Liquid-Based Electrolytes for Sodium-Ion Batteries: Tuning Properties to Enhance the Electrochemical Performance of Manganese-Based Layered Oxide Cathode. ACS Appl. Mater. Interfaces 2019, 11, 22278–22289. [Google Scholar] [CrossRef]
- Hilder, M.; Gras, M.; Pope, C.R.; Kar, M.; Macfarlane, D.R.; Forsyth, M.; O’Dell, L.A. Effect of Mixed Anions on the Physicochemical Properties of a Sodium Containing Alkoxyammonium Ionic Liquid Electrolyte. Phys. Chem. Chem. Phys. 2017, 19, 17461–17468. [Google Scholar] [CrossRef]
- Hosseini-Bab-Anari, E.; Navarro-Suárez, A.M.; Moth-Poulsen, K.; Johansson, P. Ionic Liquid Based Battery Electrolytes Using Lithium and Sodium Pseudo-Delocalized Pyridinium Anion Salts. Phys. Chem. Chem. Phys. 2019, 21, 18393–18399. [Google Scholar] [CrossRef]
- Maresca, G.; Ottaviani, M.; Ryan, K.M.; Brutti, S.; Appetecchi, G.B. Physicochemical Investigation on the Hard Carbon Interface in Ionic Liquid Electrolyte. Electrochim. Acta 2024, 498, 144631. [Google Scholar] [CrossRef]
- Chagas, L.G.; Buchholz, D.; Wu, L.; Vortmann, B.; Passerini, S. Unexpected Performance of Layered Sodium-Ion Cathode Material in Ionic Liquid-Based Electrolyte. J. Power Sources 2014, 247, 377–383. [Google Scholar] [CrossRef]
- Forsyth, M.; Hilder, M.; Zhang, Y.; Chen, F.; Carre, L.; Rakov, D.A.; Armand, M.; Macfarlane, D.R.; Pozo-Gonzalo, C.; Howlett, P.C. Tuning Sodium Interfacial Chemistry with Mixed-Anion Ionic Liquid Electrolytes. ACS Appl. Mater. Interfaces 2019, 11, 43093–43106. [Google Scholar] [CrossRef]
- Basile, A.; Yoon, H.; MacFarlane, D.R.; Forsyth, M.; Howlett, P.C. Investigating Non-Fluorinated Anions for Sodium Battery Electrolytes Based on Ionic Liquids. Electrochem. Commun. 2016, 71, 48–51. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, G.; Xu, X.; Liao, M.; Li, Y.Y.; Angell, M.; Gu, M.; Zhu, Y.; Hung, W.H.; Li, J.; et al. A Safe and Non-Flammable Sodium Metal Battery Based on an Ionic Liquid Electrolyte. Nat. Commun. 2019, 10, 3302. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Koo, B.; Kang, S.; Lee, H.; Lee, H. Hydrofluoroether-Assisted Dilution of Na-Ion Concentrated Ionic Liquid Electrolyte for Safe, Stable Cycling of High-Voltage Na-Metal Batteries. Chem. Eng. J. 2021, 425, 130612. [Google Scholar] [CrossRef]
- Noor, S.A.M.; Su, N.C.; Khoon, L.T.; Mohamed, N.S.; Ahmad, A.; Yahya, M.Z.A.; Zhu, H.; Forsyth, M.; MacFarlane, D.R. Properties of High Na-Ion Content N-Propyl-N-Methylpyrrolidinium Bis(Fluorosulfonyl)Imide -Ethylene Carbonate Electrolytes. Electrochim. Acta 2017, 247, 983–993. [Google Scholar] [CrossRef]
- Quan, P.; Linh, L.T.M.; Tuyen, H.T.K.; Van Hoang, N.; Thanh, V.D.; Van Man, T.; Phung, L.M.L. Safe Sodium-Ion Battery Using Hybrid Electrolytes of Organic Solvent/Pyrrolidinium Ionic Liquid. Vietnam. J. Chem. 2021, 59, 17–26. [Google Scholar] [CrossRef]
- Benchakar, M.; Naéjus, R.; Damas, C.; Santos-Peña, J. Exploring the Use of EMImFSI Ionic Liquid as Additive or Co-Solvent for Room Temperature Sodium Ion Battery Electrolytes. Electrochim. Acta 2020, 330, 135193. [Google Scholar] [CrossRef]
- Arkhipova, E.A.; Levin, M.M.; Ivanov, A.S.; Zakharov, M.A.; Kozhatkin, I.D.; Maslakov, K.I.; Singh, P.K.; Savilov, S.V. Electroactive Ferrocene-Based Ionic Liquids: Transport and Electrochemical Properties of Their Acetonitrile Solutions. Electrochim. Acta 2025, 512, 145443. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Song, T.; Miyaoka, H.; Shinzato, K.; Miyaoka, H.; Ichikawa, T.; Shi, S.; Zhang, X.; Isobe, S.; et al. Ammonia, a Switch for Controlling High Ionic Conductivity in Lithium Borohydride Ammoniates. Joule 2018, 2, 1522–1533. [Google Scholar] [CrossRef]
- Yan, Y.; Grinderslev, J.B.; Lee, Y.S.; Jørgensen, M.; Cho, Y.W.; Černý, R.; Jensen, T.R. Ammonia-Assisted Fast Li-Ion Conductivity in a New Hemiammine Lithium Borohydride, LiBH4·1/2NH3. Chem. Commun. 2020, 56, 3971–3974. [Google Scholar] [CrossRef]
- Kraus, F.; Fichtl, M.B.; Baer, S.A. Beryllium Diammine Difluoride [BeF2(NH3)2]. Z. Fur Naturforschung—Sect. B J. Chem. Sci. 2009, 64, 257–262. [Google Scholar] [CrossRef]
- Goncalves, A.; Tran-Van, P.; Herlem, G.; Kwa, E.; Fahys, B.; Herlem, M. New Potential Candidates for Redox Battery Using Liquid Ammoniates: Na+/Na and Ag+/Ag. Port. Electrochem. Acta 2006, 26, 117–127. [Google Scholar]
- Badoz-Lambling, J.; Bardin, M.; Bernard, C.; Fahys, B.; Herlem, M.; Thiebault, A.; Robert, G. New Battery Electrolytes for Low and High Temperatures: Liquid and Solid Ammoniates for High Energy Batteries I. Sodium Iodide and Lithium Perchlorate Ammoniates. Electrochem. Soc. 1988, 135, 587. [Google Scholar]
- Ruiz-Martínez, D.; Kovacs, A.; Gómez, R. Development of Novel Inorganic Electrolytes for Room Temperature Rechargeable Sodium Metal Batteries. Energy Environ. Sci. 2017, 10, 1936–1941. [Google Scholar] [CrossRef]
- Geng, C.; Buchholz, D.; Kim, G.T.; Carvalho, D.V.; Zhang, H.; Chagas, L.G.; Passerini, S. Influence of Salt Concentration on the Properties of Sodium-Based Electrolytes. Small Methods 2019, 3, 1800208. [Google Scholar] [CrossRef]
- Song, B.; Xiong, X.; Peng, Y.; Liu, X.; Gao, W.; Wang, T.; Wang, F.; Ma, Y.; Zhong, Y.; Cheng, X.B.; et al. Review of Electrolyte Additives for Secondary Sodium Batteries. Adv. Energy Mater. 2024, 14, 2401407. [Google Scholar]
- Tapia-Ruiz, N.; Armstrong, A.R.; Alptekin, H.; Amores, M.A.; Au, H.; Barker, J.; Boston, R.; Brant, W.R.; Brittain, J.M.; Chen, Y.; et al. 2021 Roadmap for Sodium-Ion Batteries. J. Phys. Energy 2021, 3, 31503. [Google Scholar] [CrossRef]
- Wei Seh, Z.; Sun, J.; Sun, Y.; Cui, Y. A Highly Reversible Room-Temperature Sodium Metal Anode. ACS Cent. Sci. 2015, 1, 449–455. [Google Scholar] [CrossRef]
- Yang, Z.; He, J.; Lai, W.-H.; Peng, J.; Liu, X.-H.; He, X.-X.; Guo, X.-F.; Li, L.; Qiao, Y.; Ma, J.-M.; et al. Fire-Retardant, Stable-Cycling and High-Safety Sodium Ion Battery. Angew. Chem. 2021, 133, 27292–27300. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A Review on the Key Issues for Lithium-Ion Battery Management in Electric Vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, R.; Yang, J.; Xin, Y.; Singh, P.; Wu, F.; Qian, Y.; Gao, H. The Safety Aspect of Sodium Ion Batteries for Practical Applications. J. Energy Chem. 2024, 95, 407–427. [Google Scholar] [CrossRef]
- Yamada, Y.; Wang, J.; Ko, S.; Watanabe, E.; Yamada, A. Advances and Issues in Developing Salt-Concentrated Battery Electrolytes. Nat. Energy 2019, 4, 269–280. [Google Scholar]
- Ye, Z.; Li, J.; Li, Z. Recent Progress in Nonflammable Electrolytes and Cell Design for Safe Li-Ion Batteries. J. Mater. Chem. A Mater. 2023, 11, 15576–15599. [Google Scholar] [CrossRef]
- Gond, R.; Van Ekeren, W.; Mogensen, R.; Naylor, A.J.; Younesi, R. Non-Flammable Liquid Electrolytes for Safe Batteries. Mater. Horiz. 2021, 8, 2913–2928. [Google Scholar] [CrossRef] [PubMed]
- Herlem, M.; Székely, M.; Sutter, E.; Mathieu, C.; Gonçalves, A.M.; Caillot, E.; Herlem, G.; Fahys, B. Liquid Ammoniates: Nonaqueous Electrolytes for Electrochromism. Electrochim. Acta 2001, 46, 2967–2973. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A Review of Promising Candidate Reactions for Chemical Heat Storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Fujiwara, I.; Nakashima, Y.; Goto, T. Thermal energy storage with chemical reaction of NaI/NH3 system-I. Region of liquid phase. Energy Convers. Manag. 1981, 21, 157–162. [Google Scholar] [CrossRef]
- Osman, A.I.; Nasr, M.; Eltaweil, A.S.; Hosny, M.; Farghali, M.; Al-Fatesh, A.S.; Rooney, D.W.; Abd El-Monaem, E.M. Advances in Hydrogen Storage Materials: Harnessing Innovative Technology, from Machine Learning to Computational Chemistry, for Energy Storage Solutions. Int. J. Hydrog. Energy 2024, 67, 1270–1294. [Google Scholar] [CrossRef]
- Vittetoe, A.W.; Niemann, M.U.; Srinivasan, S.S.; McGrath, K.; Kumar, A.; Goswami, D.Y.; Stefanakos, E.K.; Thomas, S. Destabilization of LiAlH4 by Nanocrystalline MgH2. Int. J. Hydrog. Energy 2009, 34, 2333–2339. [Google Scholar] [CrossRef]
- Iosub, V.; Matsunaga, T.; Tange, K.; Ishikiriyama, M. Direct Synthesis of Mg(AlH4)2 and CaAlH5 Crystalline Compounds by Ball Milling and Their Potential as Hydrogen Storage Materials. Int. J. Hydrog. Energy 2009, 34, 906–912. [Google Scholar] [CrossRef]
- Srinivasan, S.; Escobar, D.; Goswami, Y.; Stefanakos, E. Effects of Catalysts Doping on the Thermal Decomposition Behavior of Zn(BH4)2. Int. J. Hydrog. Energy 2008, 33, 2268–2272. [Google Scholar] [CrossRef]
- Arnbjerg, L.M.; Ravnsbæk, D.B.; Filinchuk, Y.; Vang, R.T.; Cerenius, Y.; Besenbacher, F.; Jørgensen, J.E.; Jakobsen, H.J.; Jensen, T.R. Structure and Dynamics for LiBH4—LiCl Solid Solutions. Chem. Mater. 2009, 21, 5772–5782. [Google Scholar] [CrossRef]
- Bogdanović, B.; Felderhoff, M.; Streukens, G. Hydrogen Storage in Complex Metal Hydrides[Russian Source]. J. Serbian Chem. Soc. 2009, 74, 183–196. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia Borane, a Material with Exceptional Properties for Chemical Hydrogen Storage. Int. J. Hydrog. Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a New Hydrogen Storage Material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible Storage of Hydrogen in Destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef]
- Guo, Y.; Xia, G.; Zhu, Y.; Gao, L.; Yu, X. Hydrogen Release from Amminelithium Borohydride, LiBH4·NH3. Chem. Commun. 2010, 46, 2599–2601. [Google Scholar] [CrossRef]
- Soloveichik, G.; Her, J.H.; Stephens, P.W.; Gao, Y.; Rijssenbeek, J.; Andrus, M.; Zhao, J.C. Ammine Magnesium Borohydride Complex as a New Material for Hydrogen Storage: Structure and Properties of Mg(BH4)2· 2NH3. Inorg. Chem. 2008, 47, 4290–4298. [Google Scholar] [CrossRef]
- Chua, Y.S.; Wu, G.; Xiong, Z.; He, T.; Chen, P. Calcium Amidoborane Ammoniate-Synthesis, Structure, and Hydrogen Storage Properties. Chem. Mater. 2009, 21, 4899–4904. [Google Scholar] [CrossRef]
- Tang, Z.; Tan, Y.; Wu, H.; Gu, Q.; Zhou, W.; Jensen, C.M.; Yu, X. Metal Cation-Promoted Hydrogen Generation in Activated Aluminium Borohydride Ammoniates. Acta Mater. 2013, 61, 4787–4796. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, G.; Li, W.; Xiong, Z.; He, T.; Guo, J.; Chen, H.; Chen, P. Releasing 17.8 Wt% H2 from Lithium Borohydride Ammoniate. Energy Environ. Sci. 2011, 4, 3593–3600. [Google Scholar] [CrossRef]
- Wang, M.; Ouyang, L.; Peng, C.; Zhu, X.; Zhu, W.; Shao, H.; Zhu, M. Synthesis and Hydrolysis of NaZn(BH4)3 and Its Ammoniates. J. Mater. Chem. A Mater. 2017, 5, 17012–17020. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Zhou, W.; Yu, X. Dehydrogenation Tuning of Ammine Borohydrides Using Double-Metal Cations. J. Am. Chem. Soc. 2011, 133, 4690–4693. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Gu, Q.; Guo, Y.; Yu, X. Ammine Bimetallic (Na, Zn) Borohydride for Advanced Chemical Hydrogen Storage. J. Mater. Chem. 2012, 22, 7300–7307. [Google Scholar] [CrossRef]
- Divers, E., IX. On the Union of Ammonia Nitrate with Ammonia. Philos. Trans. R. Soc. Lond. 1873, 163, 359–376. [Google Scholar]
- Ruiz-Martínez, D.; Gómez, R. The Liquid Ammoniate of Sodium Iodide as an Alternative Electrolyte for Sodium Ion Batteries: The Case of Titanium Dioxide Nanotube Electrodes. Energy Storage Mater. 2019, 22, 424–432. [Google Scholar] [CrossRef]
- Ruiz-Martínez, D.; Orts, J.M.; Gómez, R. A Stable Anthraquinone-Derivative Cathode to Develop Sodium Metal Batteries: The Role of Ammoniates as Electrolytes. J. Energy Chem. 2023, 77, 572–580. [Google Scholar] [CrossRef]
- Ruiz-Martínez, D.; Lana-Villarreal, T.; Gómez, R. Liquid Ammoniates as Efficient Electrolytes for Room-Temperature Rechargeable Sodium-Metal Batteries Based on an Organic Cathode. ACS Appl. Energy Mater. 2021, 4, 6806–6814. [Google Scholar] [CrossRef]
- Fahys, B.; Herlem, M. Lithium Nitrate and Lithium Trifluoromethanesulfonate Ammoniates for Electrolytes in Lithium Batteries. J. Power Sources 1991, 34, 183–188. [Google Scholar] [CrossRef]
- Fahys, B.R.; Herlem, M.P.; Jaume, J. New Electrolytes from Concentrated Solutions of Lithium Salts in Mixtures of Ammonia And Amines. Electrochim. Acta 1994, 39, 2343–2346. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, Z.; An, X.; Yue, X.; Wang, J.; Abudula, A.; Guan, G. Anode-Free Rechargeable Lithium Metal Batteries: Progress and Prospects. Energy Storage Mater. 2020, 32, 386–401. [Google Scholar] [CrossRef]
- Genovese, M.; Louli, A.J.; Weber, R.; Hames, S.; Dahn, J.R. Measuring the Coulombic Efficiency of Lithium Metal Cycling in Anode-Free Lithium Metal Batteries. J. Electrochem. Soc. 2018, 165, A3321–A3325. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, L.; Wang, X.; Zheng, Q.; Han, C.; Li, W. Current Progress of Anode-Free Rechargeable Sodium Metal Batteries: Origin, Challenges, Strategies, and Perspectives. Adv. Funct. Mater. 2024, 34, 2313823. [Google Scholar]
- Cohn, A.P.; Muralidharan, N.; Carter, R.; Share, K.; Pint, C.L. Anode-Free Sodium Battery through in Situ Plating of Sodium Metal. Nano Lett. 2017, 17, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; An, Y.; Wei, C.; Jiang, H.; Xiong, S.; Feng, J.; Qian, Y. Recently Advances and Perspectives of Anode-Free Rechargeable Batteries. Nano Energy 2020, 78, 105344. [Google Scholar]
- Zhang, H.; Gao, Y.; Liu, X.H.; Yang, Z.; He, X.X.; Li, L.; Qiao, Y.; Chen, W.H.; Zeng, R.H.; Wang, Y.; et al. Organic Cathode Materials for Sodium-Ion Batteries: From Fundamental Research to Potential Commercial Application. Adv. Funct. Mater. 2022, 32, 2107718. [Google Scholar]
- Han, H.; Lu, H.; Jiang, X.; Zhong, F.; Ai, X.; Yang, H.; Cao, Y. Polyaniline Hollow Nanofibers Prepared by Controllable Sacrifice-Template Route as High-Performance Cathode Materials for Sodium-Ion Batteries. Electrochim. Acta 2019, 301, 352–358. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Tang, M.; Zhu, S.; Jiang, C.; Zhuo, S.; Wang, C. A Highly Conductive Conjugated Coordination Polymer for Fast-Charge Sodium-Ion Batteries: Reconsidering Its Structures. Chem. Commun. 2019, 55, 10856–10859. [Google Scholar] [CrossRef]
- Tang, W.; Liang, R.; Li, D.; Yu, Q.; Hu, J.; Cao, B.; Fan, C. Highly Stable and High Rate-Performance Na-Ion Batteries Using Polyanionic Anthraquinone as the Organic Cathode. ChemSusChem 2019, 12, 2181–2185. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, Q.; Shao, Q.; Li, Q.; Gao, B.; Duan, Q.; Wang, H.G. Free-Standing and Flexible Organic Cathode Based on Aromatic Carbonyl Compound/Carbon Nanotube Composite for Lithium and Sodium Organic Batteries. J. Colloid. Interface Sci. 2018, 517, 72–79. [Google Scholar] [CrossRef]
- Ruiz-Martinez, D.; Grieco, R.; Liras, M.; Patil, N.; Marcilla, R. A High Performing Conjugated Microporous Polymer Cathode for Practical Sodium Metal Batteries Using an Ammoniate as Electrolyte. Adv. Energy Mater. 2024, 14, 2400857. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, W.; Liu, Z.; Wei, S.; Yan, Y.; Chen, Y. Enhanced Room Temperature Ionic Conductivity of the LiBH4·1/2NH3-Al2O3 composite. Chem. Commun. 2021, 57, 2380–2383. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, R.; Li, H.; Zhang, Y.; Wang, Y.; Wu, C.; Yan, Y.; Chen, Y. Li-Ion Conductivity Enhancement of LiBH4 XNH3 with in Situ Formed Li2O Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 31635–31641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, H.; Wang, Q.; Wei, S.; Yan, Y.; Chen, Y. Size Effect of MgO on the Ionic Conduction Properties of a LiBH4·1/2NH3-MgO Nanocomposite. ACS Appl. Mater. Interfaces 2022, 14, 8947–8954. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, T.; Ju, S.; Ji, Y.; Hu, Z.; Lv, Y.; Xia, G.; Yu, X. Fast Ionic Migration from Bulk to Interface in the Li(NH3) XBH4@SiO2 Composite. ACS Appl. Energy Mater. 2022, 5, 14301–14310. [Google Scholar] [CrossRef]
- Pang, Y.; Nie, Z.; Xu, F.; Sun, L.; Yang, J.; Sun, D.; Fang, F.; Zheng, S. Borohydride Ammoniate Solid Electrolyte Design for All-Solid-State Mg Batteries. Energy Environ. Mater. 2024, 7, e12527. [Google Scholar] [CrossRef]
- Yan, Y.; Grinderslev, J.B.; Jo̷rgensen, M.; Skov, L.N.; Skibsted, J.; Jensen, T.R. Ammine Magnesium Borohydride Nanocomposites for All-Solid-State Magnesium Batteries. ACS Appl. Energy Mater. 2020, 3, 9264–9270. [Google Scholar] [CrossRef]
- Yan, Y.; Grinderslev, J.B.; Burankova, T.; Wei, S.; Embs, J.P.; Skibsted, J.; Jensen, T.R. Fast Room-Temperature Mg2+ Conductivity in Mg(BH4)2·1.6NH3- Al2O3 Nanocomposites. J. Phys. Chem. Lett. 2022, 13, 2211–2216. [Google Scholar] [CrossRef]

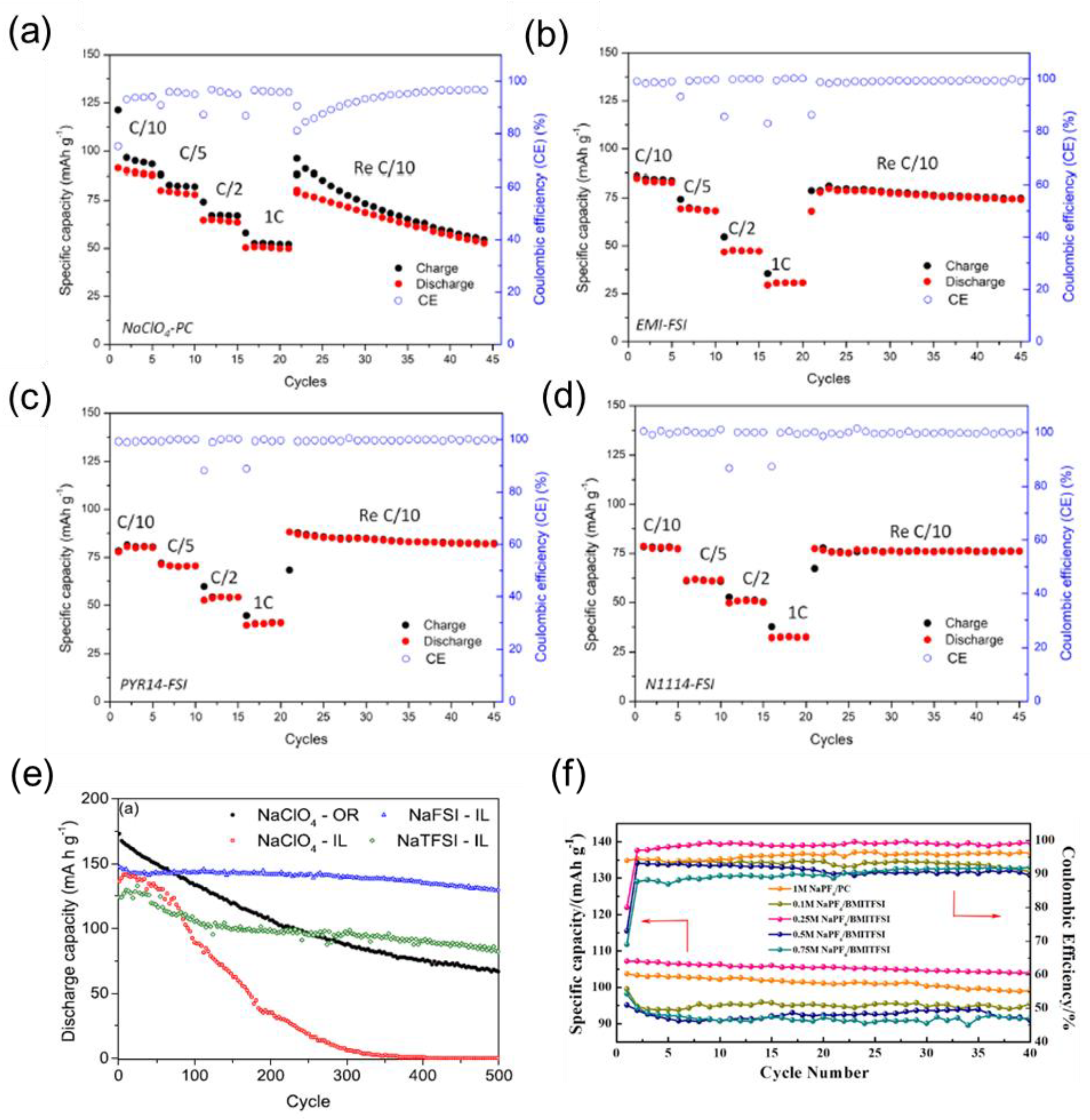
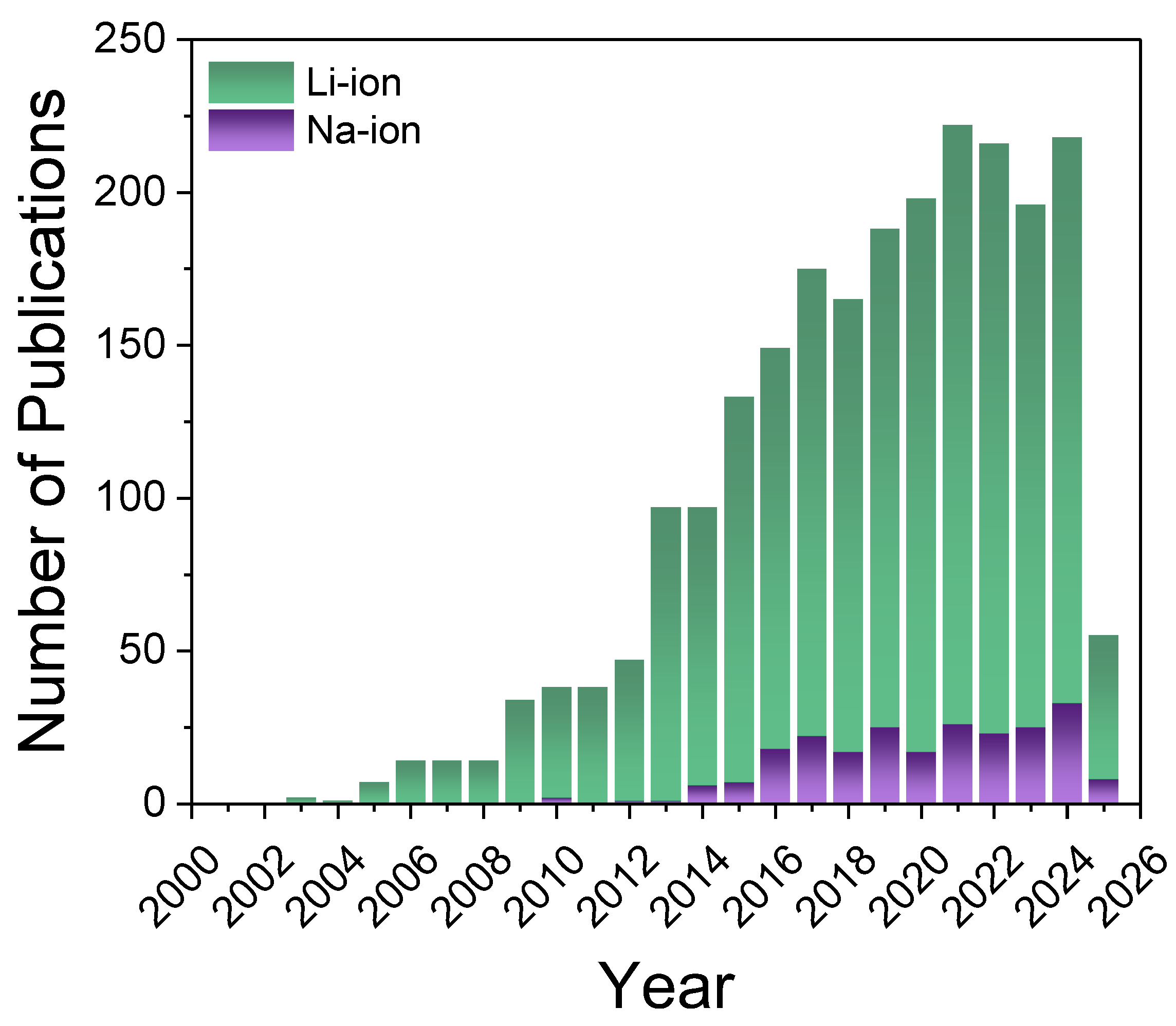
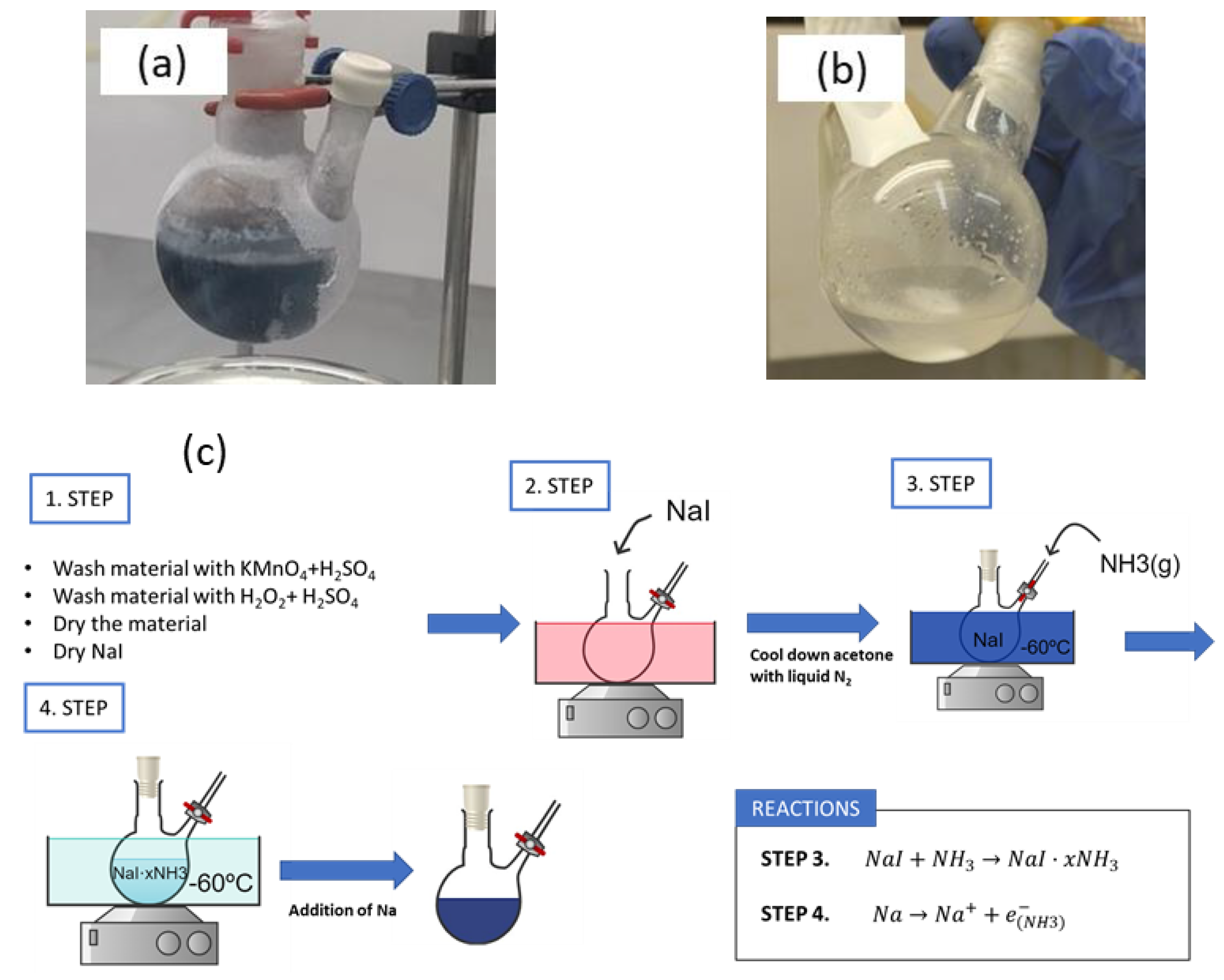
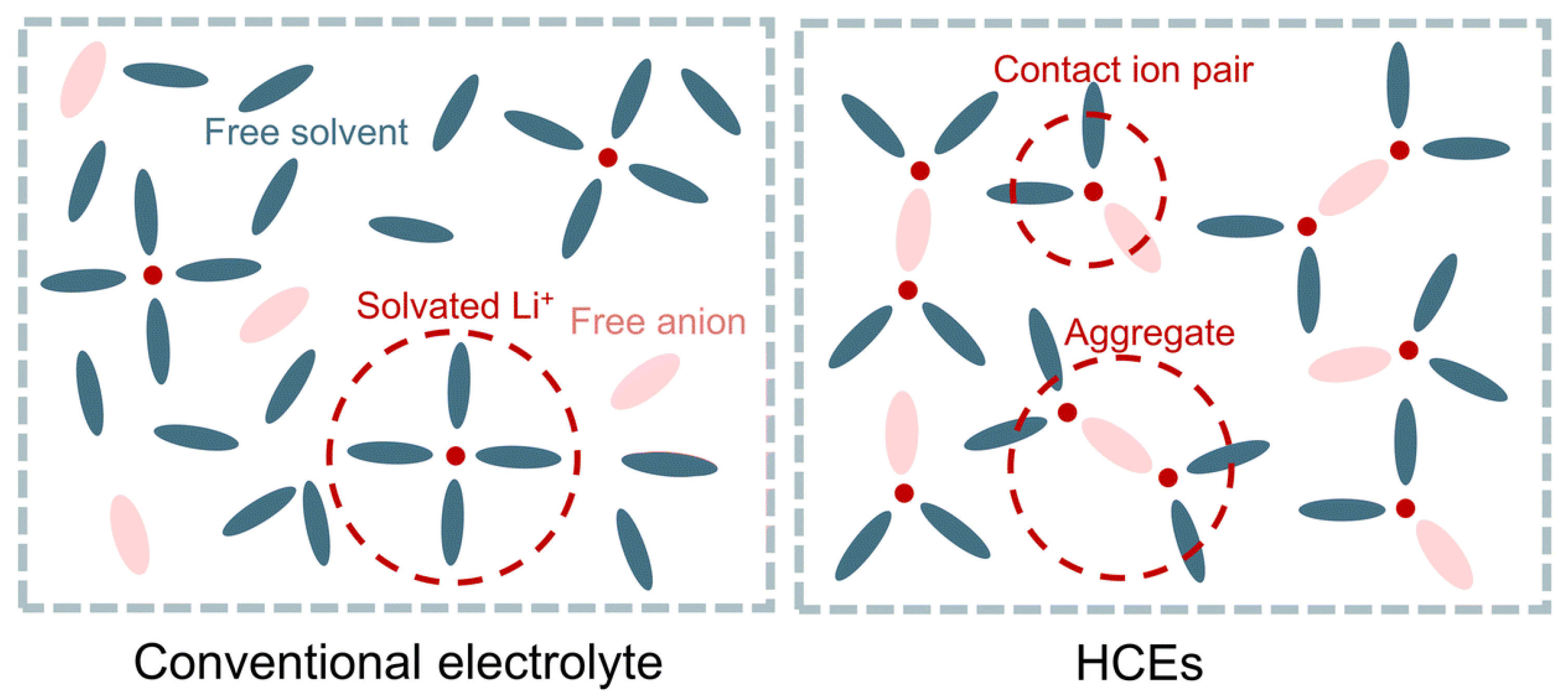
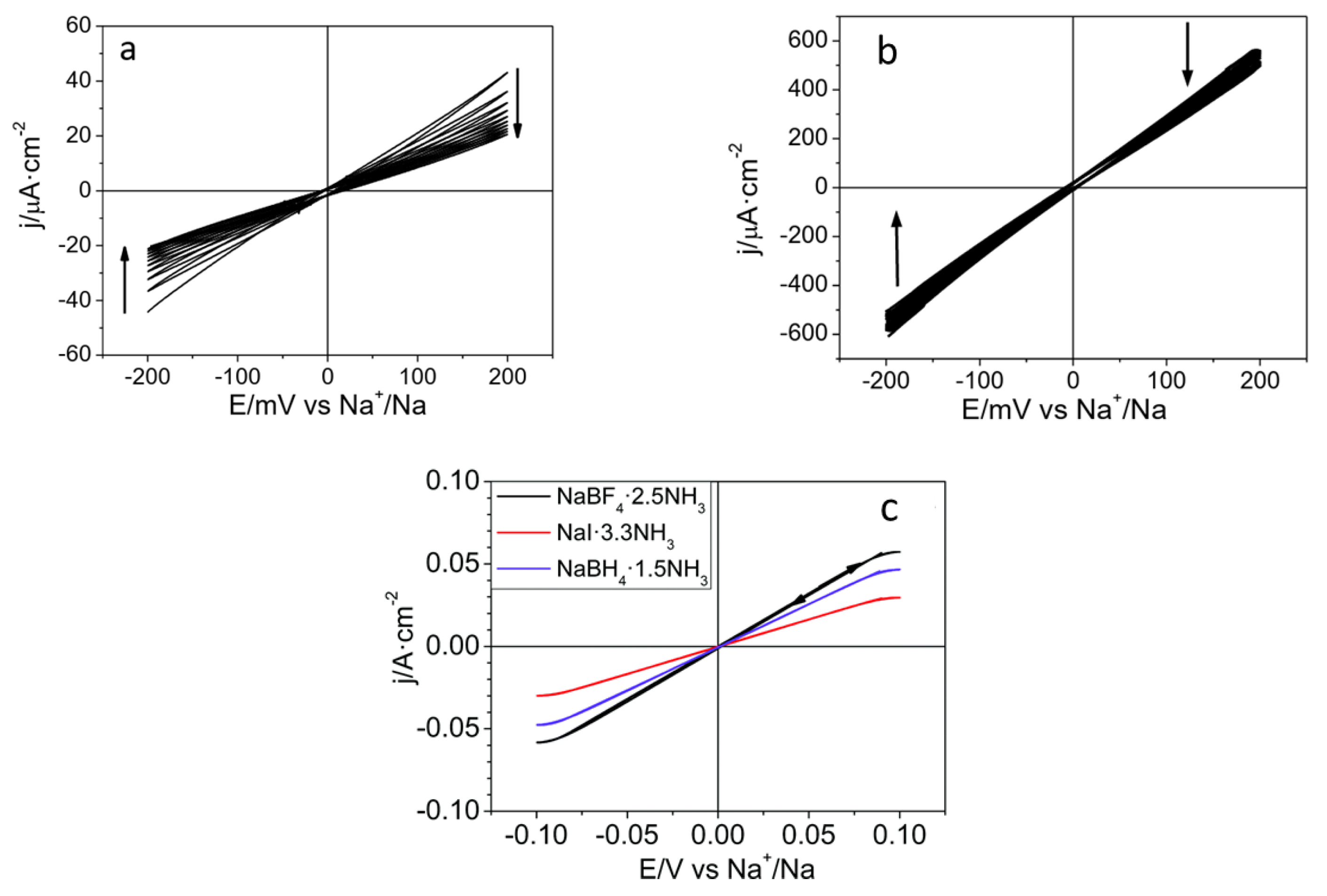
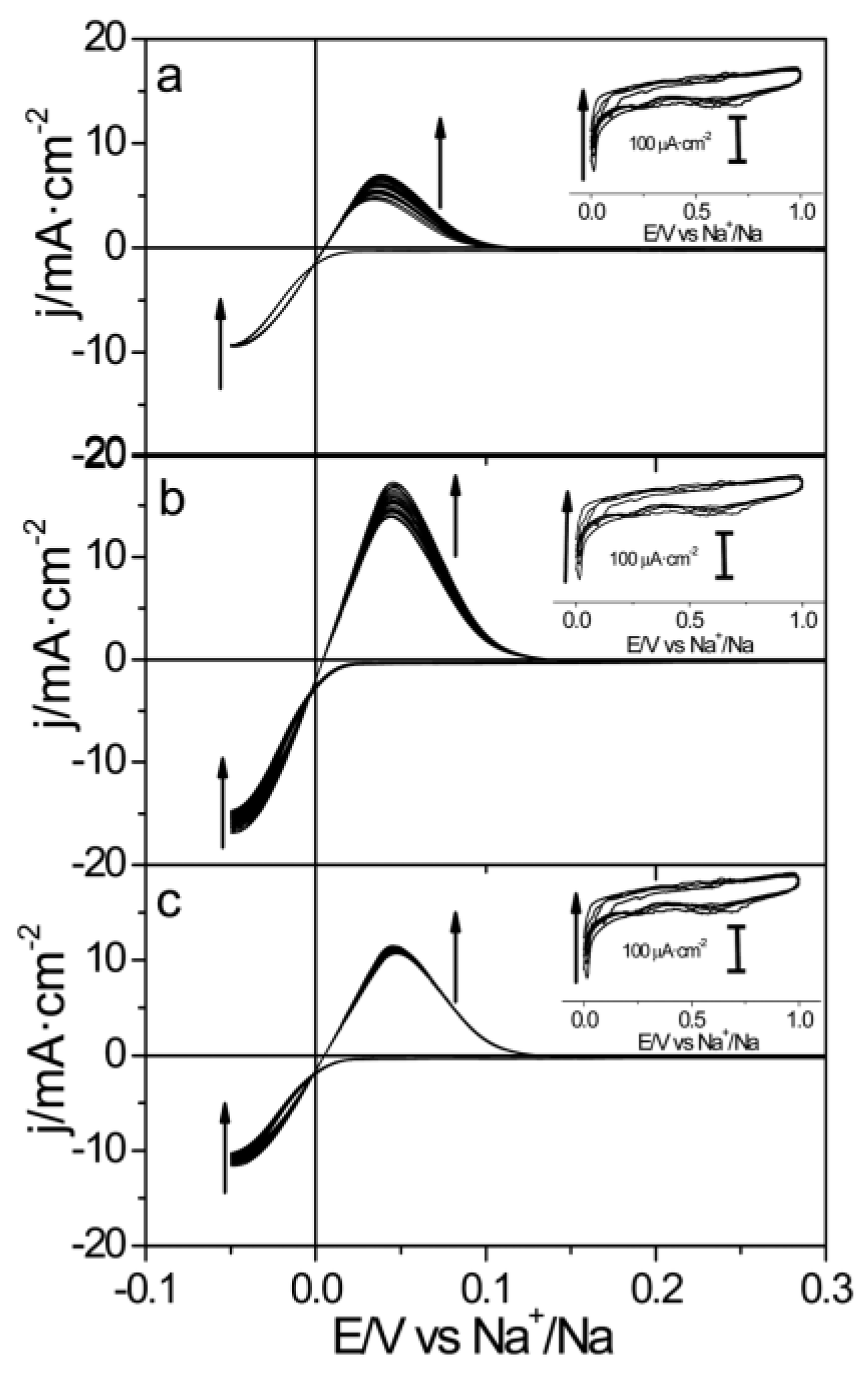

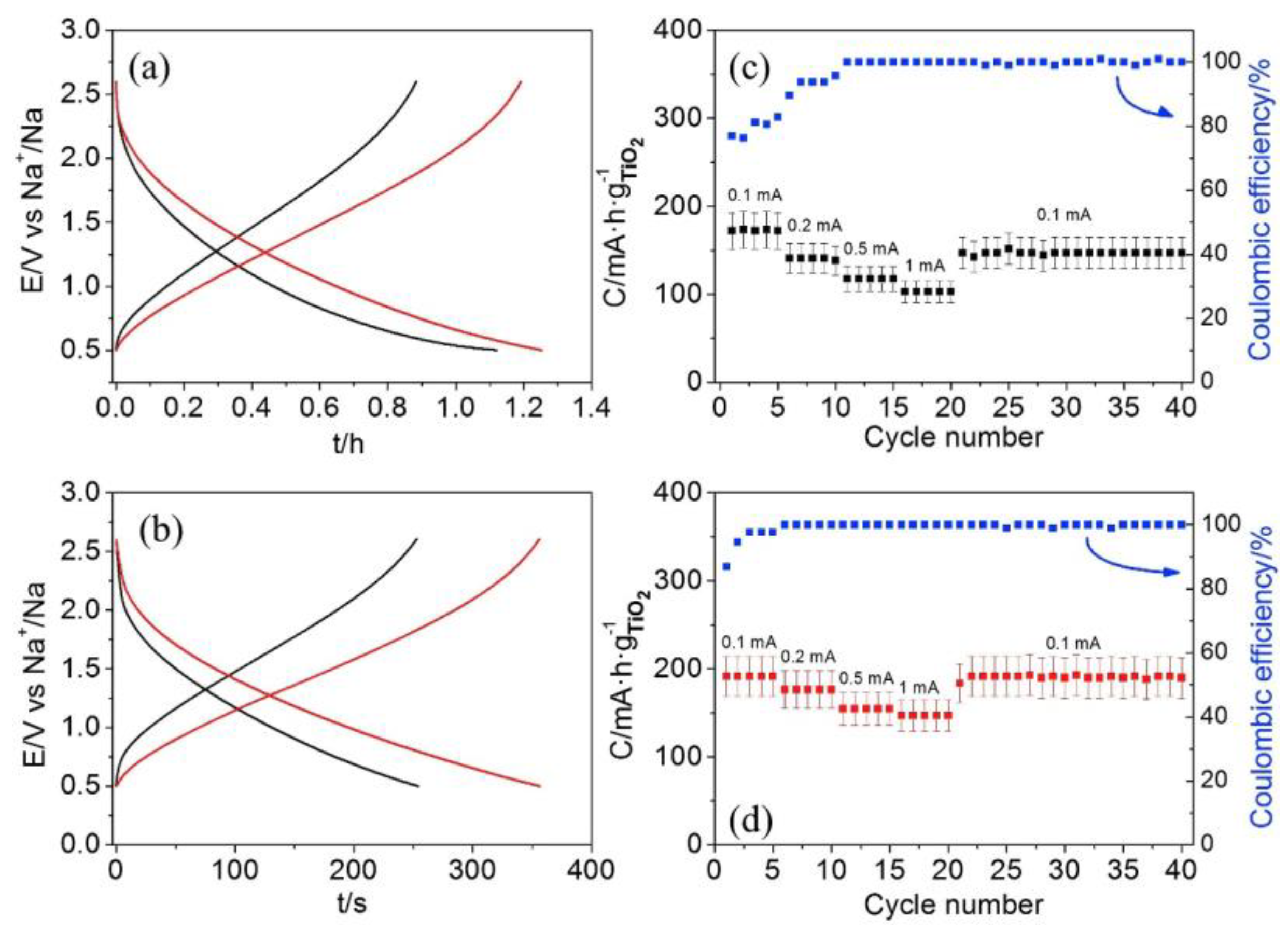

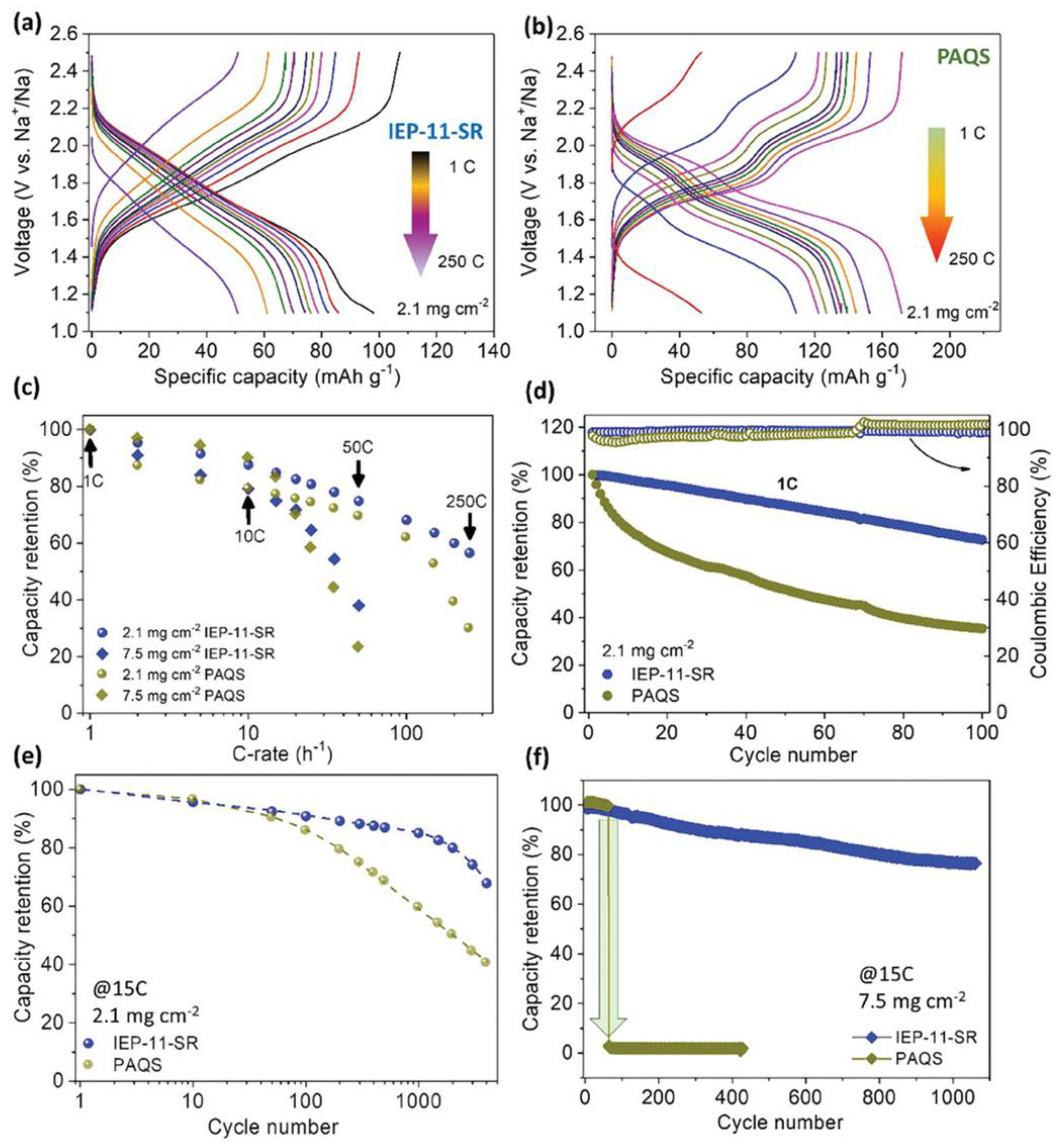

| LIBs | SIBs |
|---|---|
| Larger Li mines are localized in a few countries | Na is more abundant than Li (500 times) and can be extracted from seawater at low cost |
| LIBs require rather high minimum states of charge, raising fire risks in transport | SIBs can be transported fully discharged |
| Anode current collectors are made of Cu, as Li forms alloys with Al at anode potentials | Anode current collector is Al, 3 to 4 times cheaper than copper |
| Low operation temperatures: high risk of fire if operated at high temperature | Higher operational temperatures, without the risk of thermal runaway |
| Relatively slow charge rates and shorter cycle life than SIBs | Faster charging than LIBs with 3 times longer cycle life |
| Ionic Liquid | Tm (°C) | MW (g mol−1) | Decomp. Temperature (°C) | η (cp) at 25 °C | σ (mS cm−1) at 25 °C | ESW (V) |
|---|---|---|---|---|---|---|
| [C4C1im][TFSI] | −4 | 419.36 | 425 [67] | 48.8 | 3.41 | 3.41 |
| [C4C1im][FSI] | - | 319.35 | - | 34.8 | 1.04 | 4.7 |
| [C4C1im][SCN] | −47 | 197.30 | <300 [67] | 35.9 | 8.98 (30 °C) | - |
| [C4C1im][BF4] | −83 | 226.02 | 400 [31] | 103 | 3.15 | 4.9 |
| [C4C1im][PF6] | −8 | 284.18 | 420 [67] | 310 | 1.92 | 4.0 |
| [C4C1im][DCA] | −6 | 205.26 | - | 28 | 9.53 | - |
| [C2C1im][TFSI] | −17 | 391.3 | 420 | 39.4 (20 °C) | 6.63 (20 °C) | 4.7 |
| [C2C1im][FSI] | −23 | 291.3 | - | - | 6.176 | 4.54 |
| [C2C1im][BF4] | 15 | 197.97 | ∼415 [67] | ∼42 [81] | ∼15 [81] | 6.9 [73] |
| [C2C1im][DCA] | −21 | 177.21 | - | 16.8 | 25.3 | 3.0 |
| [C2C1im][SCN] | −6 | 169.25 | <300 [67] | 24.7 | 17.8 | 3.2 |
| [C2C1im][OTf] | −10 | 260.23 | ∼400 [31] | 24.9 [81] | 8.8 [81] | 4.7 [73] |
| [C4C1pyrr][TFSI] | −18 | 422.41 | ∼400 [65] | 94.4 | 2.0 | 5.3 |
| [C4C1pyrr][FSI] | −19 | 322.39 | - | 5 [81] | ||
| [C4C1pyrr][DCA] | −55 | 208.30 | - | 46 (20 °C) | 3.25 (24 °C) | 3.5 |
| [C3C1pyrr][TFSI] | 12 | 408.4 | 498 [82] | 58.7 | 4.92 (30 °C) | 5.9 |
| [C3C1pyrr][FSI] | −9 | - | - | 5.4 [81] | ||
| [N2,1,1,3][TFSI] | 0 | 396.37 | - | 69 | 2.67 | - |
| [N2,2,2,5][TFSI] | 3 | 452.5 | - | 93.5 | 1.08 | - |
| [N4441][FSI] | 19 | 380.51 | - | 45.5 | 4.33 | 5.7 |
| [N1114][TFSI] | 7 | 396.37 | - | 99.5 | 2.86 | 6.1 |
| Cation | Anion | η (cP) | σ (mS cm−1) | ESW (V) | Td (°C) | Ref |
|---|---|---|---|---|---|---|
| (1-n-butyl-2,3-dimethylimidazolium) (BMMI) | TFSI | 93 | 1.6 | 4.3 | 430 | [107] |
| IL1 | TFSI | 83.5 | 3.26 | 5.14 | 427.5 | [106] |
| IL2/6 | TFSI | 63.0 | 5.26 | 4.98 | 408.6 | |
| FSI | 43.6 | 7.66 | 4.89 | 271.2 | ||
| IL3 | TFSI | 287.1 | 1.27 | 5.04 | 351.9 | |
| IL4 | TFSI | 222.4 | 1.68 | 3.95 | 286.0 | |
| IL5 | TFSI | 533.8 | 1.28 | 4.91 | 163.4 | |
| IL7 | TFSI | 156.0 | 1.94 | 5.3 | 396.2 | |
| IL8/IL11 | TFSI | 98.2 | 2.92 | 5.08 | 390.3 | |
| FSI | 71.8 | 5.07 | 5.02 | 280.4 | ||
| IL9 | TFSI | 312.2 | 1.02 | 5.03 | 410.8 | |
| IL10 | TFSI | 156.2 | 1.92 | 4.96 | 345.9 | |
| IL12 | FSI | 119.7 | 3.81 | 4.82 | 261.2 | |
| IL13 | TFSI | 80.1 | 3.99 | 4.92 | 406.5 | |
| IL14 | TFSI | 291.5 | 1.20 | 4.96 | 346.1 | |
| IL15 | TFSI | 256.5 | 1.76 | 5.17 | 367.7 | |
| IL16 | TFSI | 123.4 | 2.64 | 4.96 | 403.7 | |
| IL17 | TFSI | 375.8 | 1.01 | 4.96 | 395.1 |
| Electrolyte | [C]/M | η/cP | σ/mS cm−1 | Boiling Point */°C | Ref. |
|---|---|---|---|---|---|
| NaClO4/PC | 1 | - | 4.4 | 242 | [146] |
| NaFSI/DME | 3 | - | 9.6 | 80 | [146] |
| NaPF6/PC | 1 | 9 | 5 | 242 | [147] |
| NaTFSI/PC | 1 | 8 | 5 | 242 | [147] |
| NaPF6/PC | 3 | 100 | 2 | 242 | [147] |
| NaTFSI/PC | 3 | 90 | 1.5 | 242 | [147] |
| NaI·3.3NH3 | 7.6 | 9.04 ± 0.02 | 50 | 40 | [146] |
| NaBF4·1.5NH3 | 8.7 | 5.06 ± 0.03 | 70 | 10 | [146] |
| NaBH4·2.5NH3 | 12.3 | 5.54 ± 0.05 | 110 | 18 | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiller-Vallina, P.; Miralles, C.; Parra-Puerto, A.; Gómez, R. Ionic Liquids and Ammoniates as Electrolytes for Advanced Sodium-Based Secondary Batteries. Batteries 2025, 11, 147. https://doi.org/10.3390/batteries11040147
Hiller-Vallina P, Miralles C, Parra-Puerto A, Gómez R. Ionic Liquids and Ammoniates as Electrolytes for Advanced Sodium-Based Secondary Batteries. Batteries. 2025; 11(4):147. https://doi.org/10.3390/batteries11040147
Chicago/Turabian StyleHiller-Vallina, Pablo, Carmen Miralles, Andrés Parra-Puerto, and Roberto Gómez. 2025. "Ionic Liquids and Ammoniates as Electrolytes for Advanced Sodium-Based Secondary Batteries" Batteries 11, no. 4: 147. https://doi.org/10.3390/batteries11040147
APA StyleHiller-Vallina, P., Miralles, C., Parra-Puerto, A., & Gómez, R. (2025). Ionic Liquids and Ammoniates as Electrolytes for Advanced Sodium-Based Secondary Batteries. Batteries, 11(4), 147. https://doi.org/10.3390/batteries11040147






