Optimising the Selective Leaching and Recovery of Cobalt, Lanthanum, and Strontium for Recycling End-of-Life Solid Oxide Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples’ Preparation and Characterization
2.2. Ultrasound-Assisted Selective Leaching
2.3. Recovery of Co, La, and Sr via Chemical Precipitation
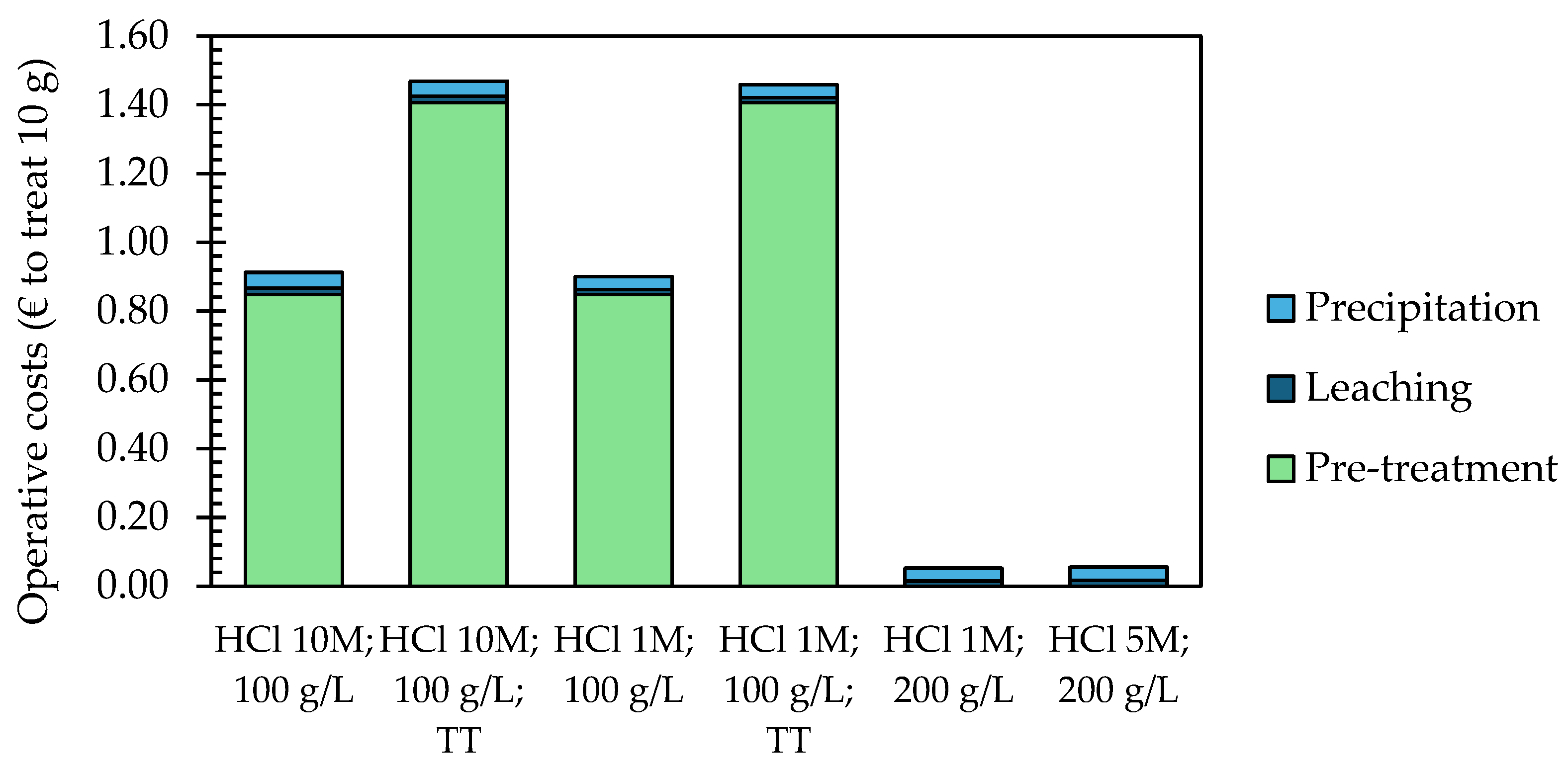
2.4. Preliminary Economic Analysis
3. Results
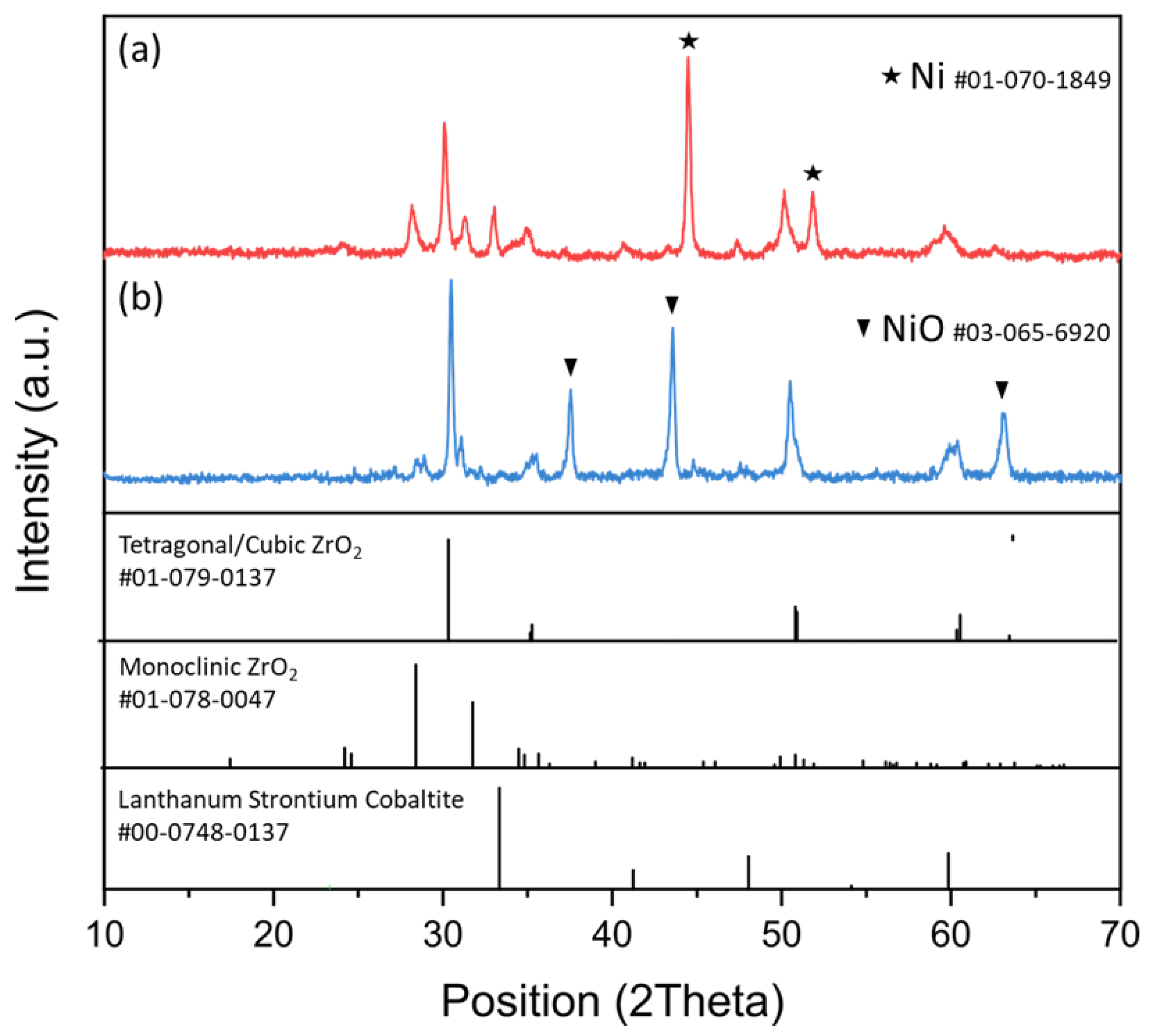
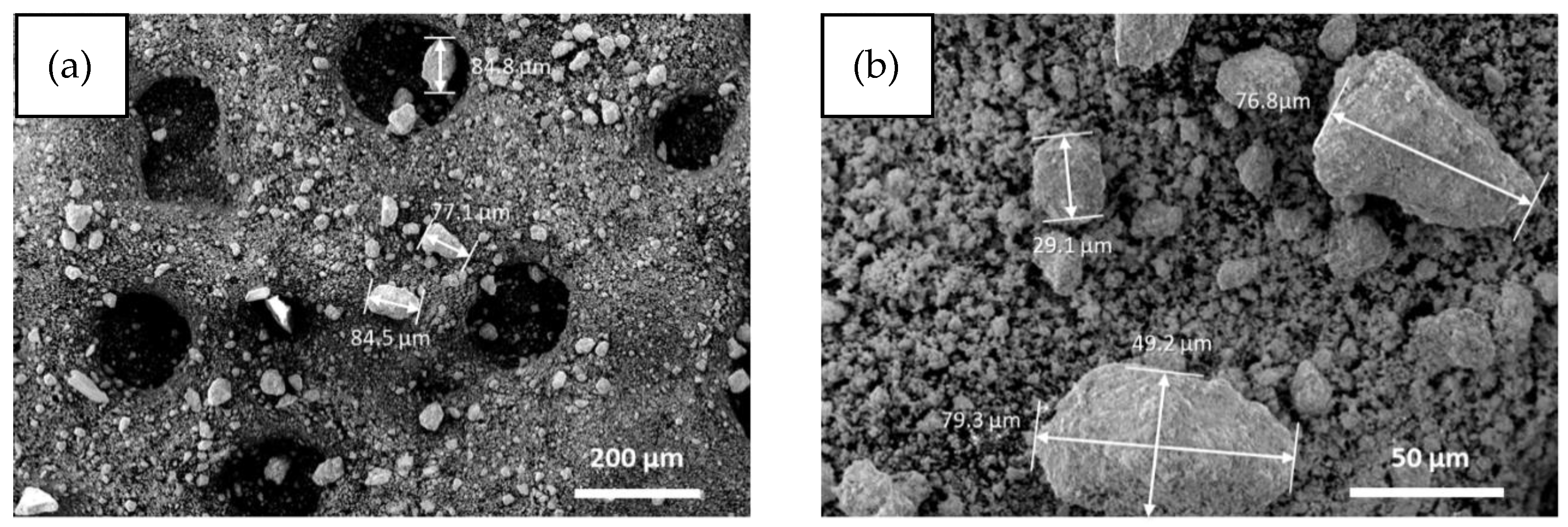
3.1. Characterization of SOC Milled Powder (Pre- and Post-Thermal Treatment)
3.2. Ultrasound Selective Leaching
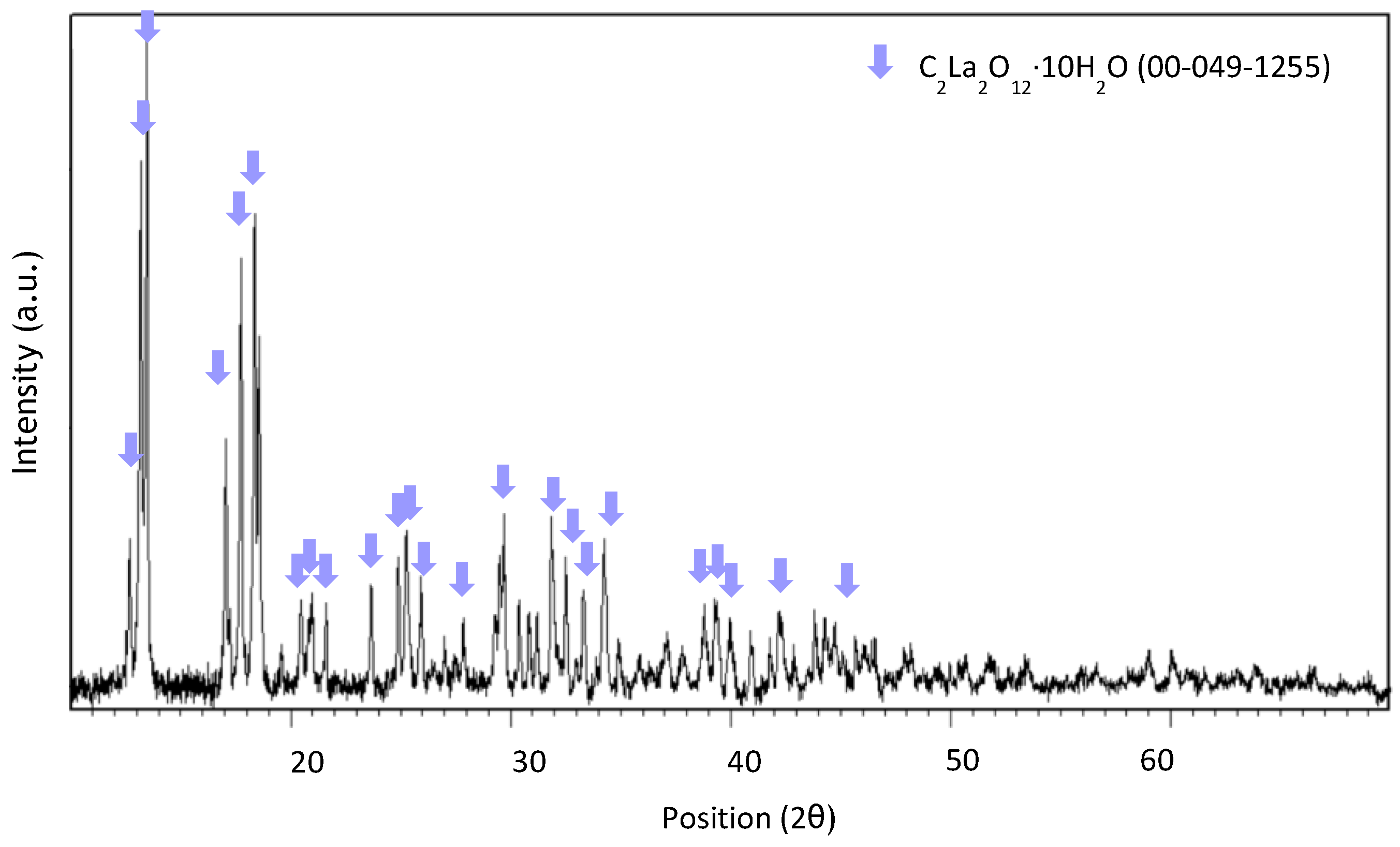
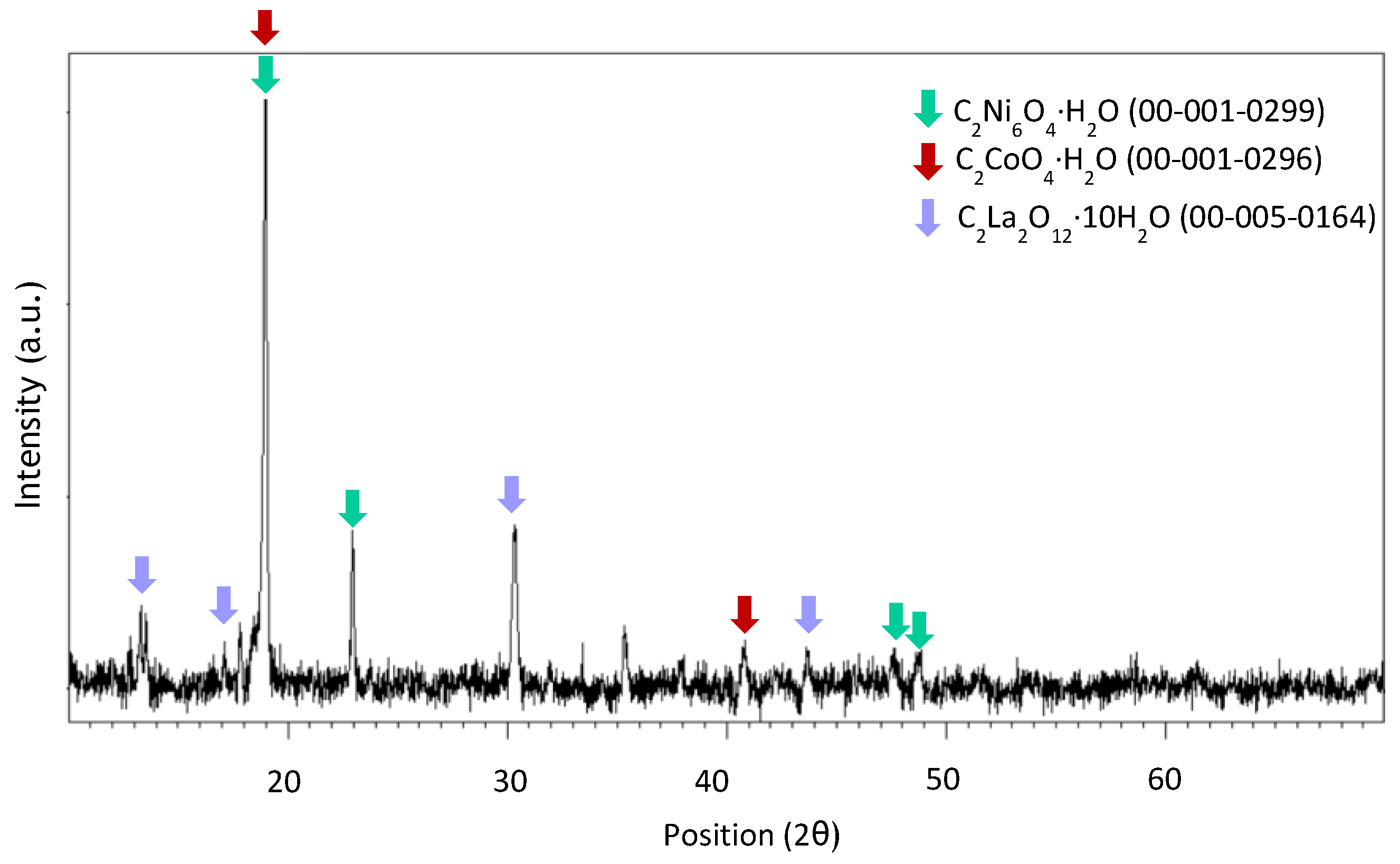
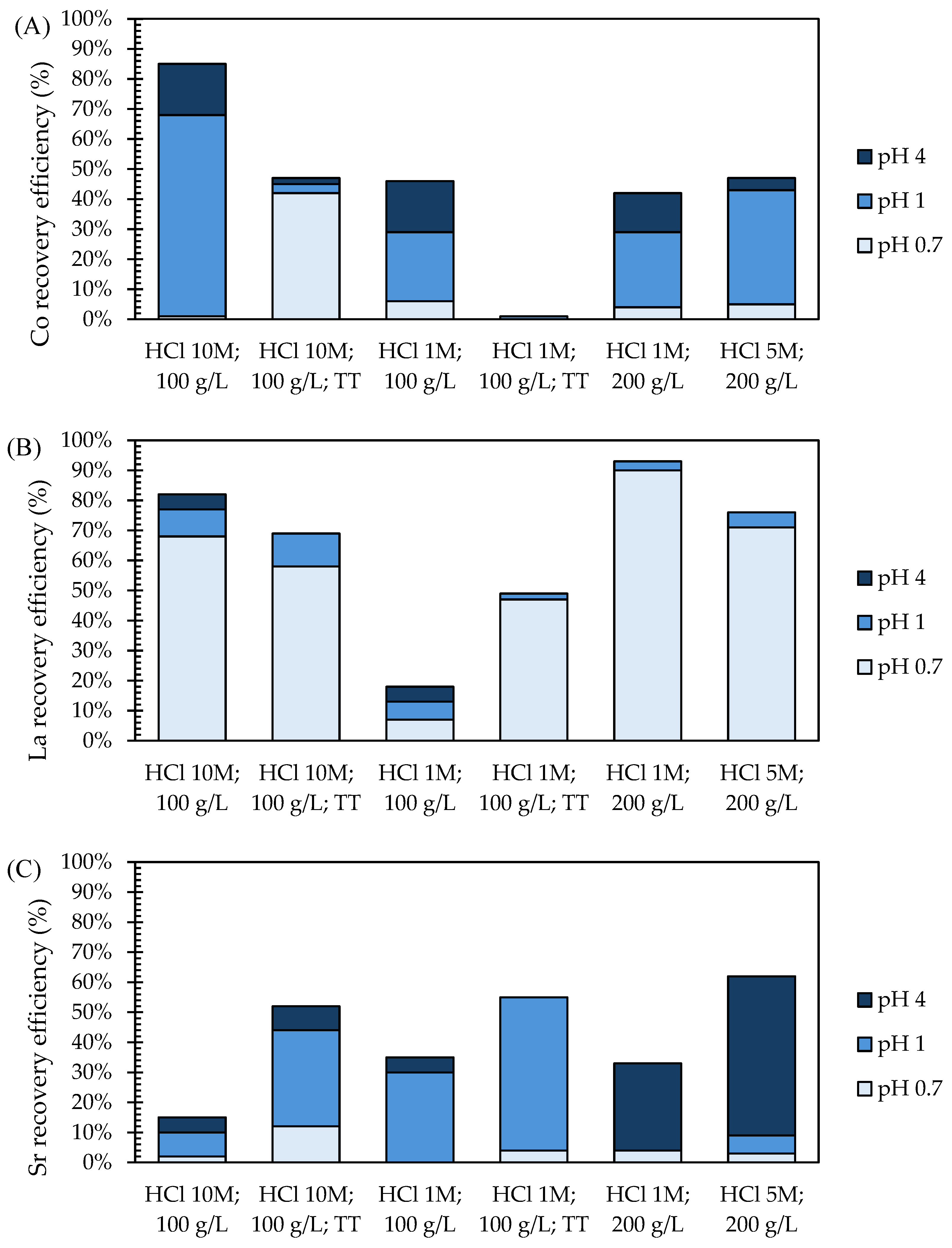
3.3. Recovery via Chemical Precipitation
3.4. Preliminary Economic Assessment
4. Conclusions
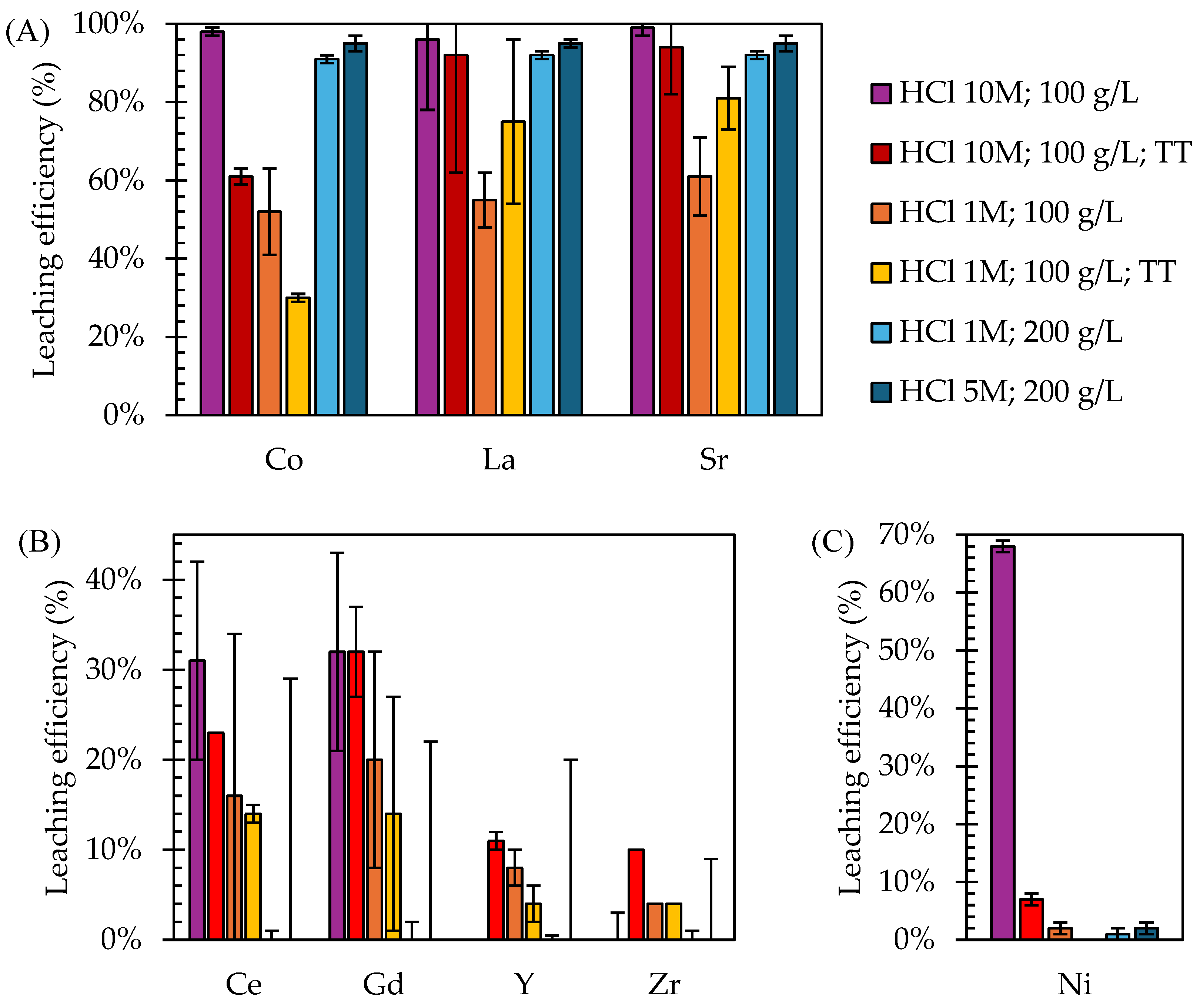
- Leaching tests on ball-milled SOC samples with 10 M HCl and a 100 g/L solid-to-liquid (S/L) ratio achieved the highest efficiencies for Co (98%), La (96%), and Sr (99%) but also for Ni (68%), limiting selectivity.
- The thermal pre-treatment of milled powders re-oxidized metallic Ni into NiO, in-creasing selectivity by reducing Ni leaching by 90%. However, this also resulted in a reduction in Co leaching efficiency by 42% and La and Sr by 9%.
- Leaching tests with a lower concentration of 1 M HCl reduced Ni leaching by 97% in the milled SOC samples without thermal pre-treatment but also decreased the leaching of target metals by up to 60%. For SOC samples oxidized by thermal pre-treatment, Ni leaching was completely avoided during 1 M HCl leaching, while the leaching efficiencies for La, Co, and Sr were 75%, 30%, and 81%, respectively.
- The direct leaching of EoL SOCs (not subjected to any milling and thermal treatment) employing 200 g/L of HCl obtained minimal Ni leaching and 95% of Co, La, and Sr when 5 M HCl was applied and 91–92% of Co, La, and Sr with 1 M HCl.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EoL | End of life |
| GHGs | Greenhouse gasses |
| LSC | Strontium-doped lanthanum-cobalt oxides |
| SOCs | Solid oxide cells |
| YSZ | Yttria-stabilized Zirconia |
Appendix A


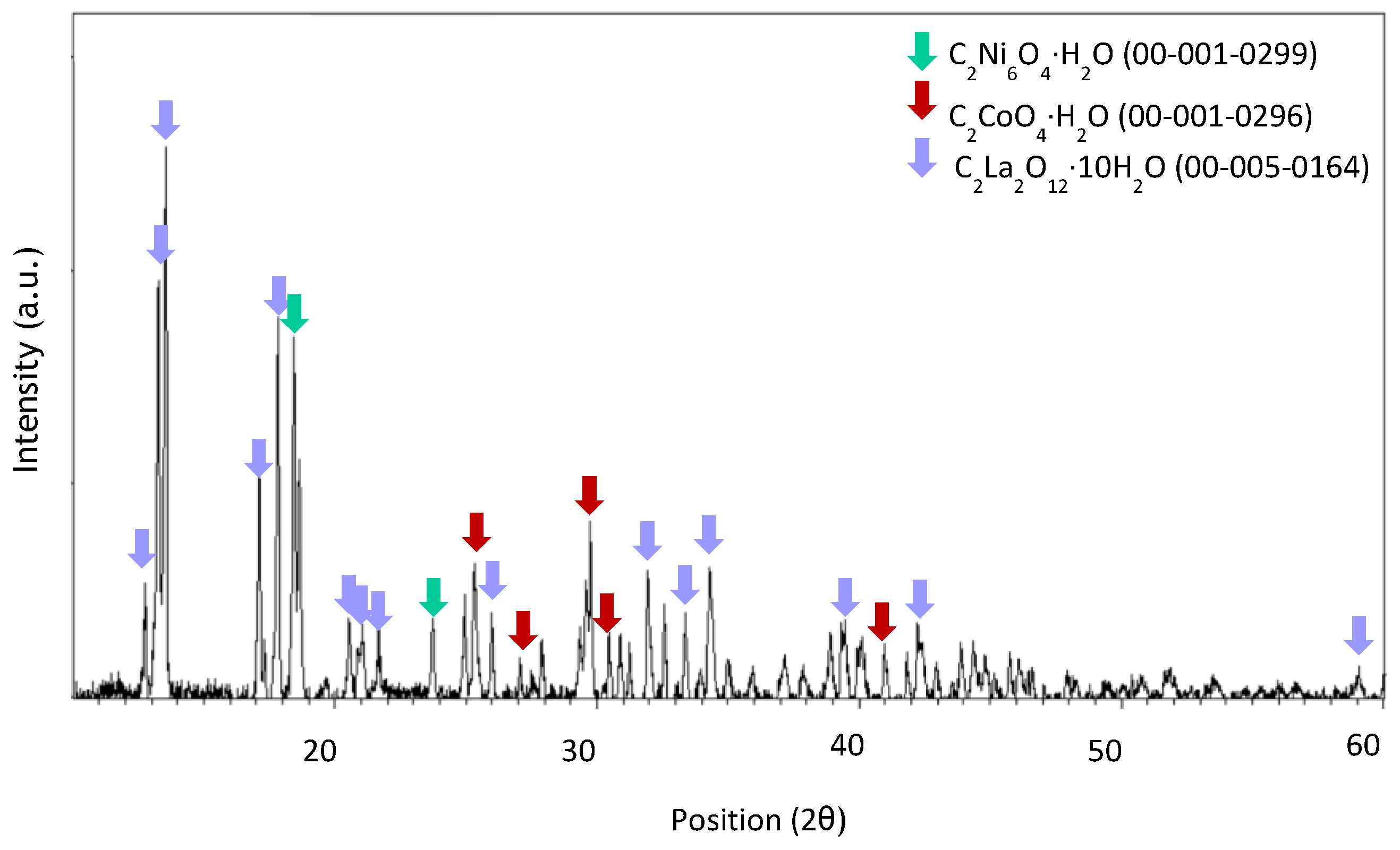
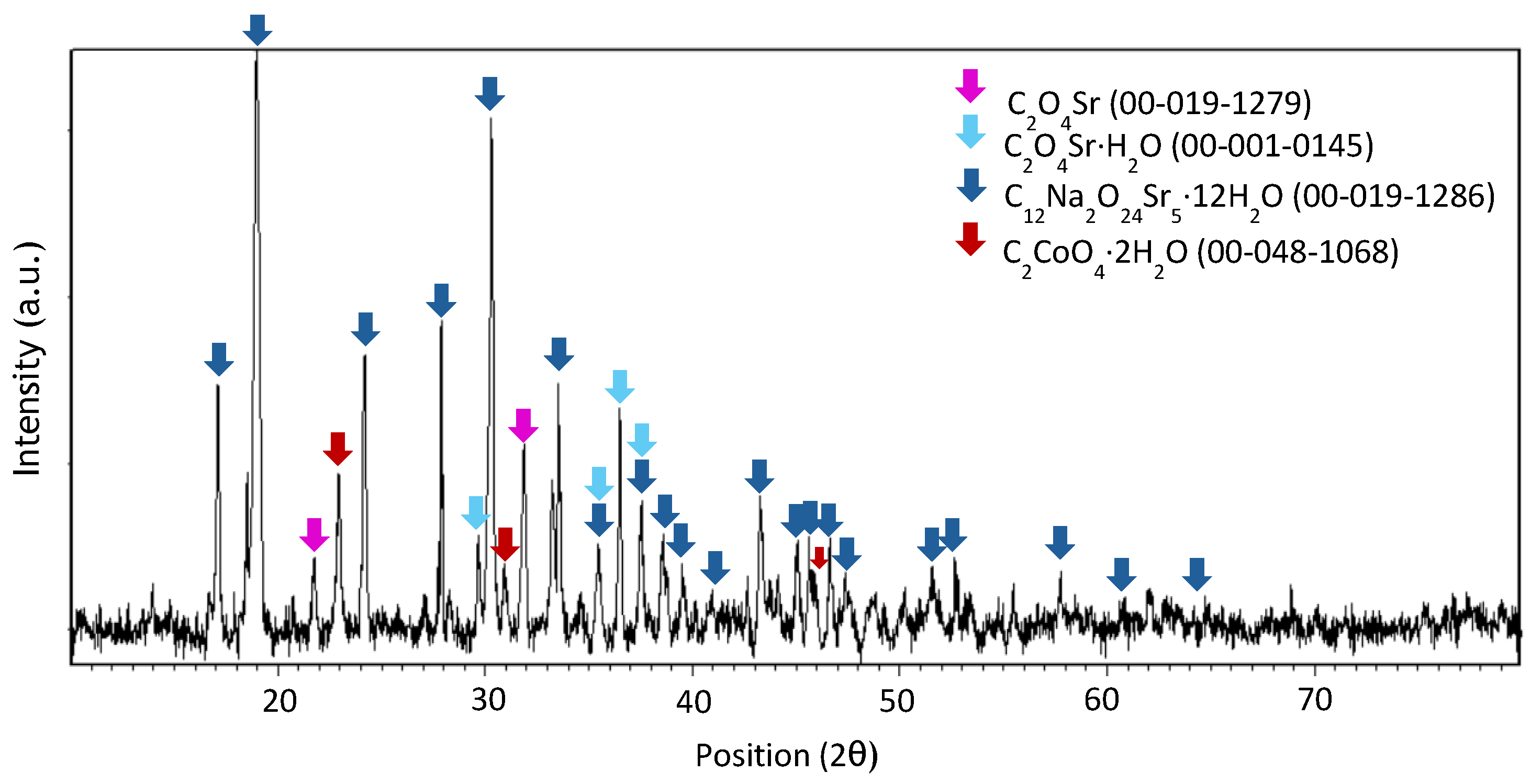
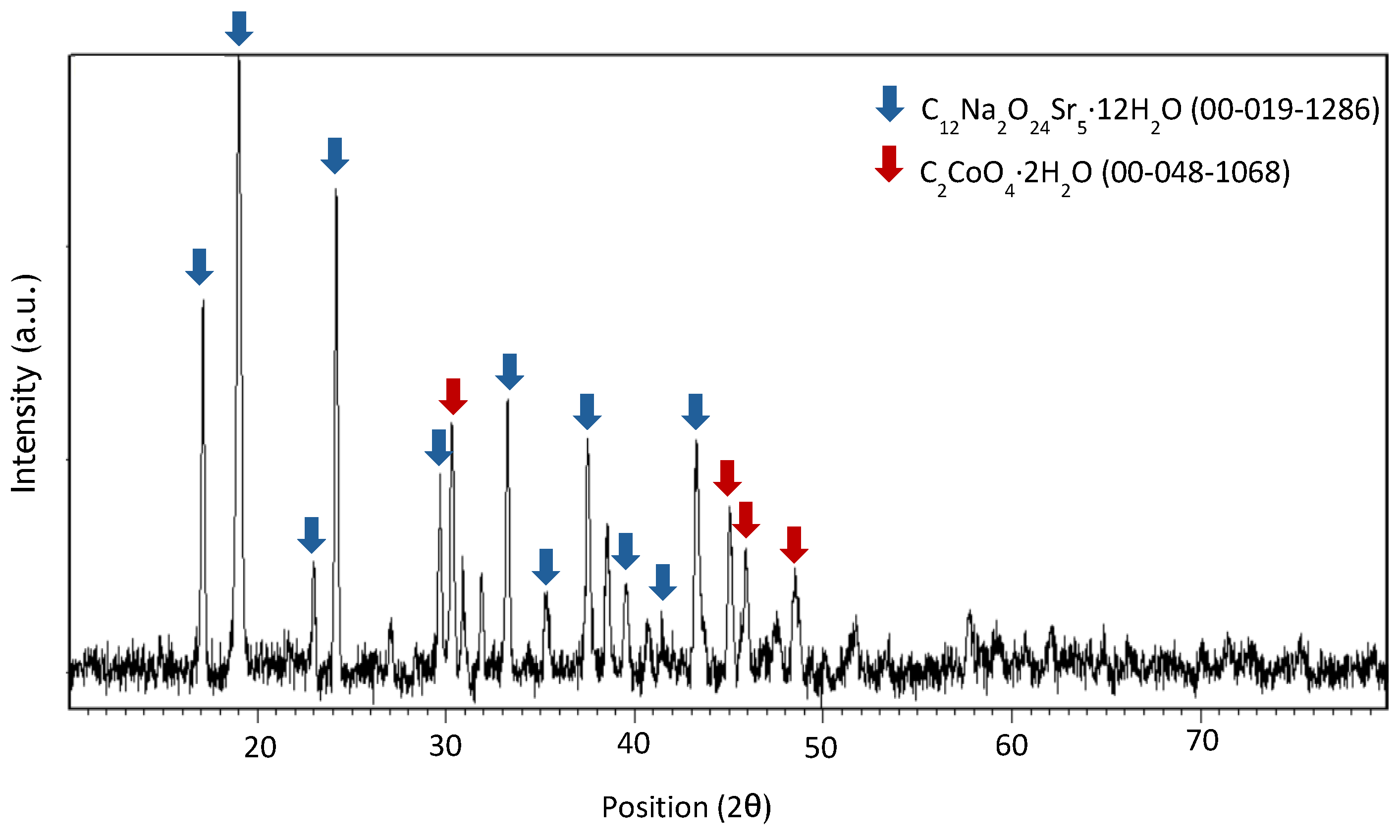
References
- Subotić, V.; Napporn, T.W. Nanostructured Metal Oxides for High-Performance Solid Oxide Fuel Cells (SOFCs). In Metal Oxide-Based Nanostructured Electrocatalysts for Fuel Cells, Electrolyzers, and Metal-Air Batteries; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–261. [Google Scholar]
- Tsai, S.-Y.; Fung, K.-Z. Progress and Prospects of Intermsediate-Temperature Solid Oxide Fuel Cells. In Energy Storage and Conversion Materials: Properties, Methods, and Applications; CRC Press: Boca Raton, FL, USA, 2023; pp. 265–276. [Google Scholar] [CrossRef]
- Smith, L.; Ibn-Mohammed, T.; Yang, F.; Reaney, I.M.; Sinclair, D.C.; Koh, S.C.L. Comparative Environmental Profile Assessments of Commercial and Novel Material Structures for Solid Oxide Fuel Cells. Appl. Energy 2019, 235, 1300–1313. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, Y.; Song, L.; Lei, T. Environmental Impact Assessment of Solid Oxide Fuel Cell Power Generation System Based on Life Cycle Assessment—A Case Study in China. Sustainability 2024, 16, 3863. [Google Scholar] [CrossRef]
- Du, Y.; Cheekatamarla, P. SOFC’s Bumpy Road and Hopeful Future-A Case Study. In Proceedings of the ECS Transactions, Kyoto, Japan, 8–13 September 2019; Volume 91, pp. 179–186. [Google Scholar]
- Yatoo, M.A.; Habib, F.; Malik, A.H.; Qazi, M.J.; Ahmad, S.; Ganayee, M.A.; Ahmad, Z. Solid-Oxide Fuel Cells: A Critical Review of Materials for Cell Components. MRS Commun. 2023, 13, 378–384. [Google Scholar] [CrossRef]
- Gil-Muñoz, G.; Alcañiz-Monge, J. Examining the Effect of Zirconium Doping in Lanthanum Nickelate Perovskites on Their Performance as Catalysts for Dry Methane Reforming. J. Environ. Chem. Eng. 2025, 13, 115387. [Google Scholar] [CrossRef]
- Han, M.; Zhang, Y. Ceramic Materials for Solid Oxide Fuel Cell. Kuei Suan Jen Hsueh Pao/J. Chin. Ceram. Soc. 2017, 45, 1548–1554. [Google Scholar] [CrossRef]
- Wang, Y.; Song, M. Redox Stability Optimization in Anode-Supported Solid Oxide Fuel Cells. Materials 2024, 17, 3257. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, N.; Zhang, H.; Xue, Q.; Zhang, J.; Huang, X. Research Progress in Zirconium-Based Electrolyte Applied in Solid Oxide Fuel Cell. Xiyou Jinshu/Chin. J. Rare Met. 2017, 41, 437–444. [Google Scholar] [CrossRef]
- Predoana, L.; Malič, B.; Petrescu, S.; Preda, S.; Zaharescu, M. Influence of the Precursors on the La0.5Sr0.5CoO3 Formation by Water-Based Sol-Gel Method. Rev. Roum. Chim. 2018, 63, 711–718. [Google Scholar]
- Qiang, J.; Wang, D.; Hui, S. Synthesis of La1-XSrxCoO3-Δand Its REDOX Performance in Air. J. Environ. Chem. Eng. 2022, 10, 108794. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions-Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; European Commission: Brussels, Belgium, 2020.
- Campos-Carriedo, F.; Iribarren, D.; Calvo-Rodríguez, F.; García-Díaz, Á.; Dufour, J. Methodological and Practical Lessons Learned from Exploring the Material Criticality of Two Hydrogen-Related Products. Resour. Conserv. Recycl. 2024, 206, 107614. [Google Scholar] [CrossRef]
- Mishra, S.; Panda, S.; Akcil, A. Environmental and Social Impacts of Recycling Critical Raw Materials. In Critical Materials and Sustainability Transition; CRC Press: Boca Raton, FL, USA, 2023; pp. 86–101. [Google Scholar]
- Sultana, I.; Chen, Y.; Huang, S.; Rahman, M.M. Recycled Value-Added Circular Energy Materials for New Battery Application: Recycling Strategies, Challenges, and Sustainability-a Comprehensive Review. J. Environ. Chem. Eng. 2022, 10, 108728. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. End of Life of Fuel Cells and Hydrogen Products: From Technologies to Strategies. Int. J. Hydrogen Energy 2019, 44, 20965–20977. [Google Scholar] [CrossRef]
- Sarner, S.; Schreiber, A.; Menzler, N.H.; Guillon, O.; Sarner, S.; Menzler, N.H.; Guillon, O.; Schreiber, A. Recycling Strategies for Solid Oxide Cells. Adv. Energy Mater. 2022, 12, 2201805. [Google Scholar] [CrossRef]
- Dragan, M. Closing the Loop: Solid Oxide Fuel and Electrolysis Cells Materials for a Net-Zero Economy. Materials 2024, 17, 6113. [Google Scholar] [CrossRef]
- Sarner, S.; Menzler, N.H.; Malzbender, J.; Hilger, M.; Sebold, D.; Weber, A.; Guillon, O. Towards a Scalable Recycling Process for Ceramics in Fuel-Electrode-Supported Solid Oxide Cells. Green Chem. 2025, 27, 2252–2262. [Google Scholar] [CrossRef]
- Saffirio, S.; Pylypko, S.; Fiorot, S.; Schiavi, I.; Fiore, S.; Santarelli, M.; Ferrero, D.; Smeacetto, F.; Fiorilli, S. Hydrothermally-Assisted Recovery of Yttria- Stabilized Zirconia (YSZ) from End-of-Life Solid Oxide Cells. Sustain. Mater. Technol. 2022, 33, e00473. [Google Scholar] [CrossRef]
- Yenesew, G.T.; Quarez, E.; Salle, A.L.G.L.; Nicollet, C.; Joubert, O. Recycling and Characterization of End-of-Life Solid Oxide Fuel/Electrolyzer Ceramic Material Cell Components. Resour. Conserv. Recycl. 2023, 190, 106809. [Google Scholar] [CrossRef]
- Kaiser, C.; Buchwald, T.; Peuker, U.A. Ultrasonic Decoating as a New Recycling Path to Separate Oxygen Side Layers of Solid Oxide Cells. Green Chem. 2023, 26, 960–967. [Google Scholar] [CrossRef]
- Mori, M.; Gramc, J.; Hojkar, D.; Lotrič, A.; Smeacetto, F.; Fiorilli, S.; Fiore, S.; Stropnik, R. New Life Cycle Inventories for End-of-Life Solid Oxide Cells Based on Novel Recycling Processes for Critical Solid Oxide Cell Materials. Int. J. Hydrogen Energy 2024, 104, 635–650. [Google Scholar] [CrossRef]
- Saffirio, S.; Anelli, S.; Pylypko, S.; Rath, M.K.; Smeacetto, F.; Fiorilli, S. Recycling and Reuse of Ceramic Materials from Components of Waste Solid Oxide Cells (SOCs). Ceram. Int. 2024, 50, 34472–34477. [Google Scholar] [CrossRef]
- Sutama, D.K.; Prasetya, A.; Petrus, H.T.B.M.; Astuti, W. Recovery of Cobalt and Molybdenum from Consumed Catalyst Using Hydrochloric Acid. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 21 September 2021; Volume 882. [Google Scholar]
- Peeters, N.; Binnemans, K.; Riaño, S. Recovery of Cobalt from Lithium-Ion Battery Cathode Material by Combining Solvoleaching and Solvent Extraction. Green Chem. 2022, 24, 2839–2852. [Google Scholar] [CrossRef]
- Mitterdorfer, A.; Gauckler, L.J. La2Zr2O7 Formation and Oxygen Reduction Kinetics of the La0.85Sr0.15MnyO3, O2(g)|YSZ System. Solid State Ion. 1998, 111, 185–218. [Google Scholar] [CrossRef]
- Mas, A.B.; Fiore, S.; Fiorilli, S.; Smeacetto, F.; Santarelli, M.; Schiavi, I. Analysis of Lanthanum and Cobalt Leaching Aimed at Effective Recycling Strategies of Solid Oxide Cells. Sustainability 2022, 14, 3335. [Google Scholar] [CrossRef]
- Ecoinvent Ecoinvent v3.8-Ecoinvent. Available online: https://ecoinvent.org/the-ecoinvent-database/data-releases/ecoinvent-3-8/ (accessed on 8 March 2023).
- Eurostat Electricity Prices for Non-Household Consumers-Bi-Annual Data (from 2007 Onwards). Available online: https://ec.europa.eu/eurostat/databrowser/view/nrg_pc_205/default/table?lang=en&category=nrg.nrg_price.nrg_pc (accessed on 29 July 2024).
- Wang, R.C.; Lin, Y.C.; Wu, S.H. A Novel Recovery Process of Metal Values from the Cathode Active Materials of the Lithium-Ion Secondary Batteries. Hydrometallurgy 2009, 99, 194–201. [Google Scholar] [CrossRef]
- Guzolu, J.S.; Gharabaghi, M.; Mobin, M.; Alilo, H. Extraction of Li and Co from Li-Ion Batteries by Chemical Methods. J. Inst. Eng. Ser. D 2017, 98, 43–48. [Google Scholar] [CrossRef]
- Lie, J.; Lin, Y.C.; Liu, J.C. Process Intensification for Valuable Metals Leaching from Spent NiMH Batteries. Chem. Eng. Process.-Process Intensif. 2021, 167, 108507. [Google Scholar] [CrossRef]
- Tang, Y.C.; Wang, J.Z.; Chou, C.M.; Shen, Y.H. Material and Waste Flow Analysis for Environmental and Economic Impact Assessment of Inorganic Acid Leaching Routes for Spent Lithium Batteries’ Cathode Scraps. Batteries 2023, 9, 207. [Google Scholar] [CrossRef]
- Pichugina, N.M.; Kutepov, A.M.; Gorichev, I.G.; Izotov, A.D.; Zaitsev, B.E. Dissolution Kinetics of Nickel(II) and Nickel(III) Oxides in Acid Media. Transl. Teor. Osn. Khimicheskoi Tekhnologii 2002, 36, 533–543. [Google Scholar]
- Wang, Y.; Chang, X.; Chen, M.; Qin, W.; Han, J. Effective Extraction of Nickel and Cobalt from Sintered Nickel Alloy via Reduction Roasting and Leaching. Miner. Eng. 2023, 203, 108336. [Google Scholar] [CrossRef]
- Mir, S.; Shukla, N.; Dhawan, N. Investigation of Microwave and Thermal Processing of Electrode Material of End-of-Life Ni-MH Battery. JOM 2021, 73, 951–961. [Google Scholar] [CrossRef]
- Wang, Y.W.; Qin, W.Q.; Han, J.W. Efficient Nickel Extraction from Nickel Matte by Combined Atmospheric-Oxygen Pressure Acid Leaching: Thermodynamic Analysis and Sulfur Conversion Mechanism. Miner. Eng. 2024, 207, 108577. [Google Scholar] [CrossRef]
- Gualandris, F.; Simonsen, S.B.; Wagner, J.B.; Sanna, S.; Muto, S.; Kuhn, L.T. In Situ TEM Analysis of a Symmetric Solid Oxide Cell in Oxygen and Vacuum–Cation Diffusion Observations. ECS Trans. 2017, 75, 123–133. [Google Scholar] [CrossRef]
- Balázs Illés, I.; Kékesi, T. Extraction of Pure Co, Ni, Mn, and Fe Compounds from Spent Li-Ion Batteries by Reductive Leaching and Combined Oxidative Precipitation in Chloride Media. Miner. Eng. 2023, 201, 108169. [Google Scholar] [CrossRef]
- Gerold, E.; Luidold, S.; Antrekowitsch, H. Selective Precipitation of Metal Oxalates from Lithium Ion Battery Leach Solutions. Metals 2020, 10, 1435. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, Z.; Kuru, Y.; Ma, W.; Tuller, H.L.; Yildiz, B. Electronic Activation of Cathode Superlattices at Elevated Temperatures-Source of Markedly Accelerated Oxygen Reduction Kinetics. Adv. Energy Mater. 2013, 3, 1221–1229. [Google Scholar] [CrossRef]
- Zhao, T.; Ji, R.; Meng, Y. The Role of Precipitant in the Preparation of Lithium-Rich Manganese-Based Cathode Materials. Chem. Phys. Lett. 2019, 730, 354–360. [Google Scholar] [CrossRef]
- Ajiboye, A.E.; Dzwiniel, T.L. Sequential Recovery of Critical Metals from Leached Liquor of Processed Spent Lithium-Ion Batteries. Batteries 2023, 9, 549. [Google Scholar] [CrossRef]
- Meshram, P.; Virolainen, S.; Abhilash; Sainio, T. Solvent Extraction for Separation of 99.9% Pure Cobalt and Recovery of Li, Ni, Fe, Cu, Al from Spent LIBs. Metals 2022, 12, 1056. [Google Scholar] [CrossRef]
- Banda, R.; Jeon, H.S.; Lee, M.S. Solvent Extraction Separation of La from Chloride Solution Containing Pr and Nd with Cyanex 272. Hydrometallurgy 2012, 121–124, 74–80. [Google Scholar] [CrossRef]
- Free, M.L. Hydrometallurgy; Springer Nature: Dordrecht, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Shahid, M.; Ashoka Sahadevan, S.; Ramani, V.; Sankarasubramanian, S. Recommended Practices for the Electrochemical Recovery of Cobalt from Lithium Cobalt Oxide: A Case Study of the Choline Chloride: Ethylene Glycol Deep Eutectic Solvent. ChemSusChem 2024, 18, e202401205. [Google Scholar] [CrossRef]
- Peeters, N.; Binnemans, K.; Riaño, S. Solvometallurgical Recovery of Cobalt from Lithium-Ion Battery Cathode Materials Using Deep-Eutectic Solvents. Green Chem. 2020, 22, 4210–4221. [Google Scholar] [CrossRef]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating Organic Acids as Alternative Leaching Reagents for Metal Recovery from Lithium Ion Batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Gerold, E.; Schinnerl, C.; Antrekowitsch, H. Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries. Recycling 2022, 7, 4. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, M.; Saffirio, S.; Smeacetto, F.; Fiorilli, S.; Fiore, S. Optimising the Selective Leaching and Recovery of Cobalt, Lanthanum, and Strontium for Recycling End-of-Life Solid Oxide Cells. Batteries 2025, 11, 124. https://doi.org/10.3390/batteries11040124
Bruno M, Saffirio S, Smeacetto F, Fiorilli S, Fiore S. Optimising the Selective Leaching and Recovery of Cobalt, Lanthanum, and Strontium for Recycling End-of-Life Solid Oxide Cells. Batteries. 2025; 11(4):124. https://doi.org/10.3390/batteries11040124
Chicago/Turabian StyleBruno, Martina, Sofia Saffirio, Federico Smeacetto, Sonia Fiorilli, and Silvia Fiore. 2025. "Optimising the Selective Leaching and Recovery of Cobalt, Lanthanum, and Strontium for Recycling End-of-Life Solid Oxide Cells" Batteries 11, no. 4: 124. https://doi.org/10.3390/batteries11040124
APA StyleBruno, M., Saffirio, S., Smeacetto, F., Fiorilli, S., & Fiore, S. (2025). Optimising the Selective Leaching and Recovery of Cobalt, Lanthanum, and Strontium for Recycling End-of-Life Solid Oxide Cells. Batteries, 11(4), 124. https://doi.org/10.3390/batteries11040124








