Nanoporous Carbon Coatings Direct Li Electrodeposition Morphology and Performance in Li Metal Anode Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. NPC Deposition and Characterization
2.2. Coin Cell Fabrication and Testing
2.3. Characterization of Li Electrodeposits
3. Results
3.1. Theoretical Li Capacity with NPC Coating Mass/Volume Included
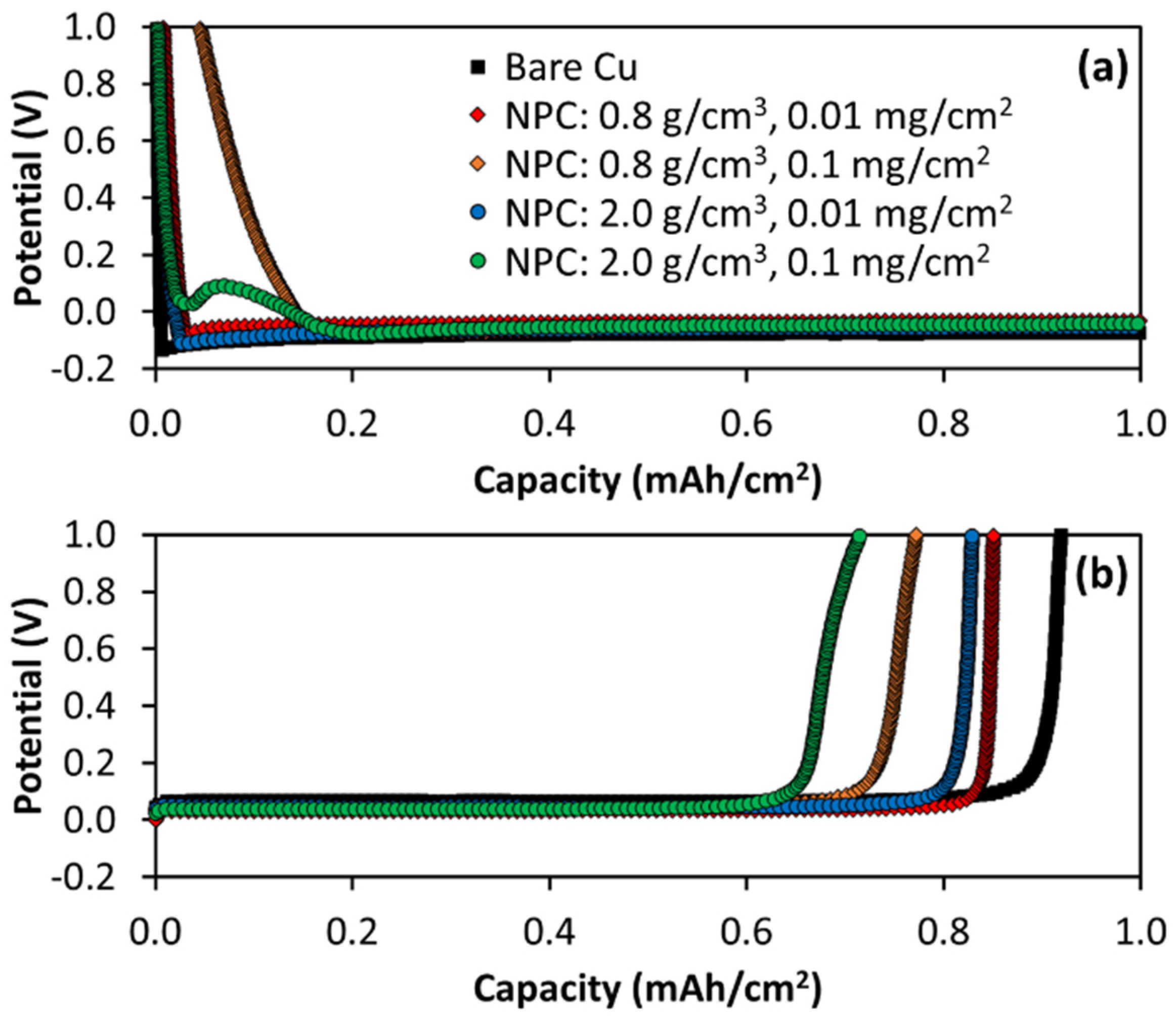
3.2. First Cycle Li Electrodeposition on NPC-Coated Cu
3.3. First Cycle Li Electrodeposition Morphology
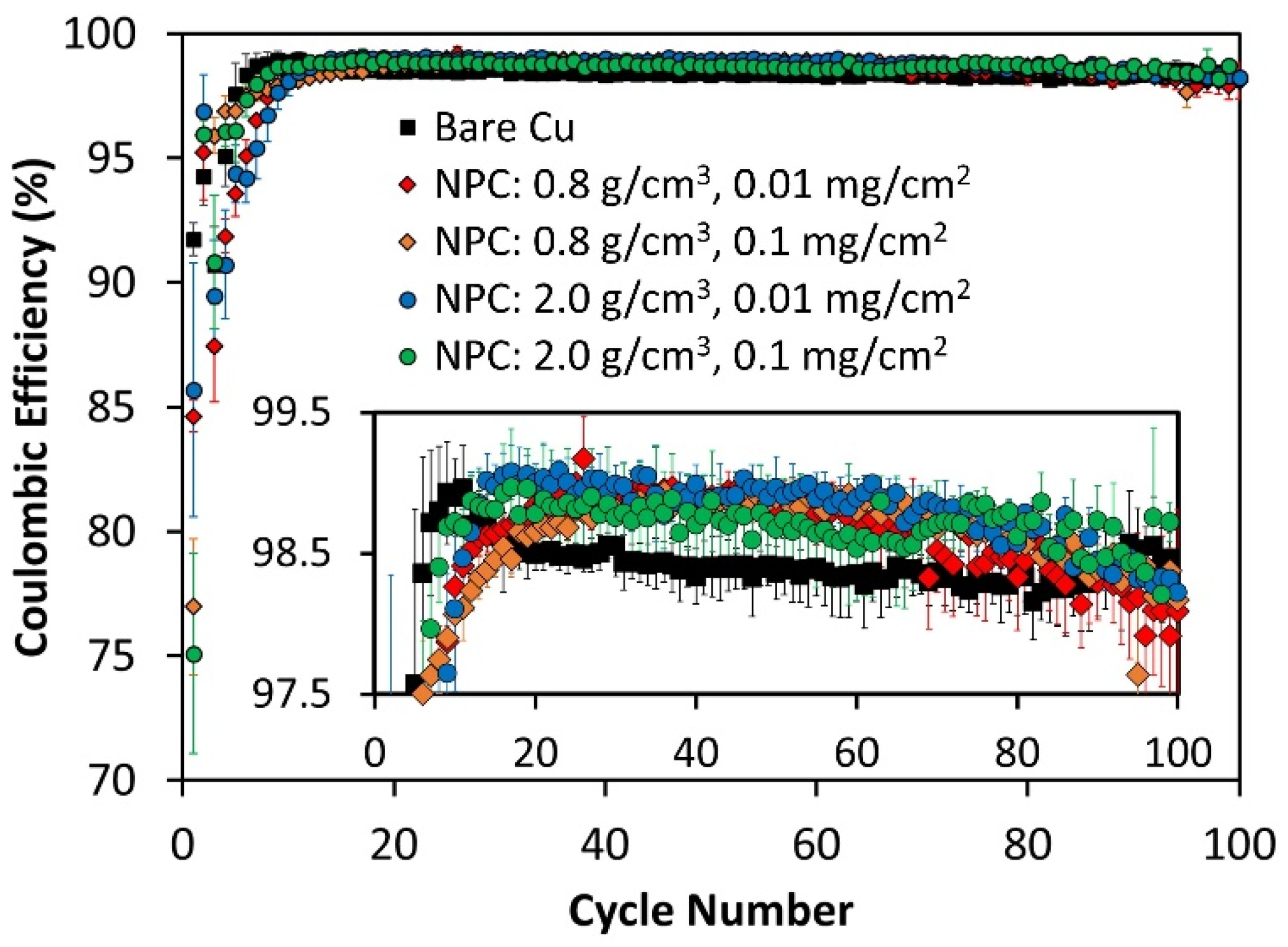
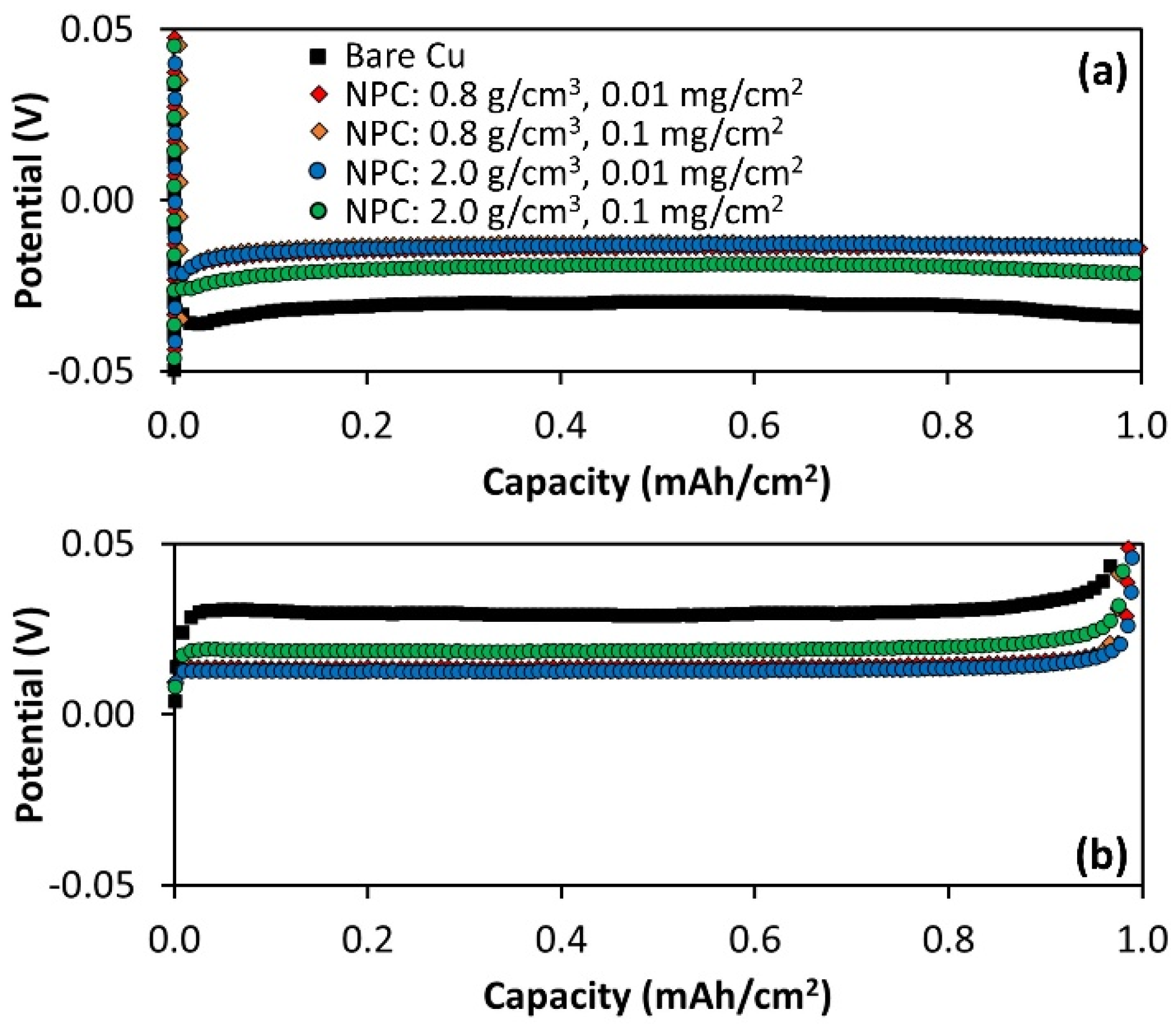
3.4. Li Metal Cycling and Calendar Aging on NPC-Coated Cu
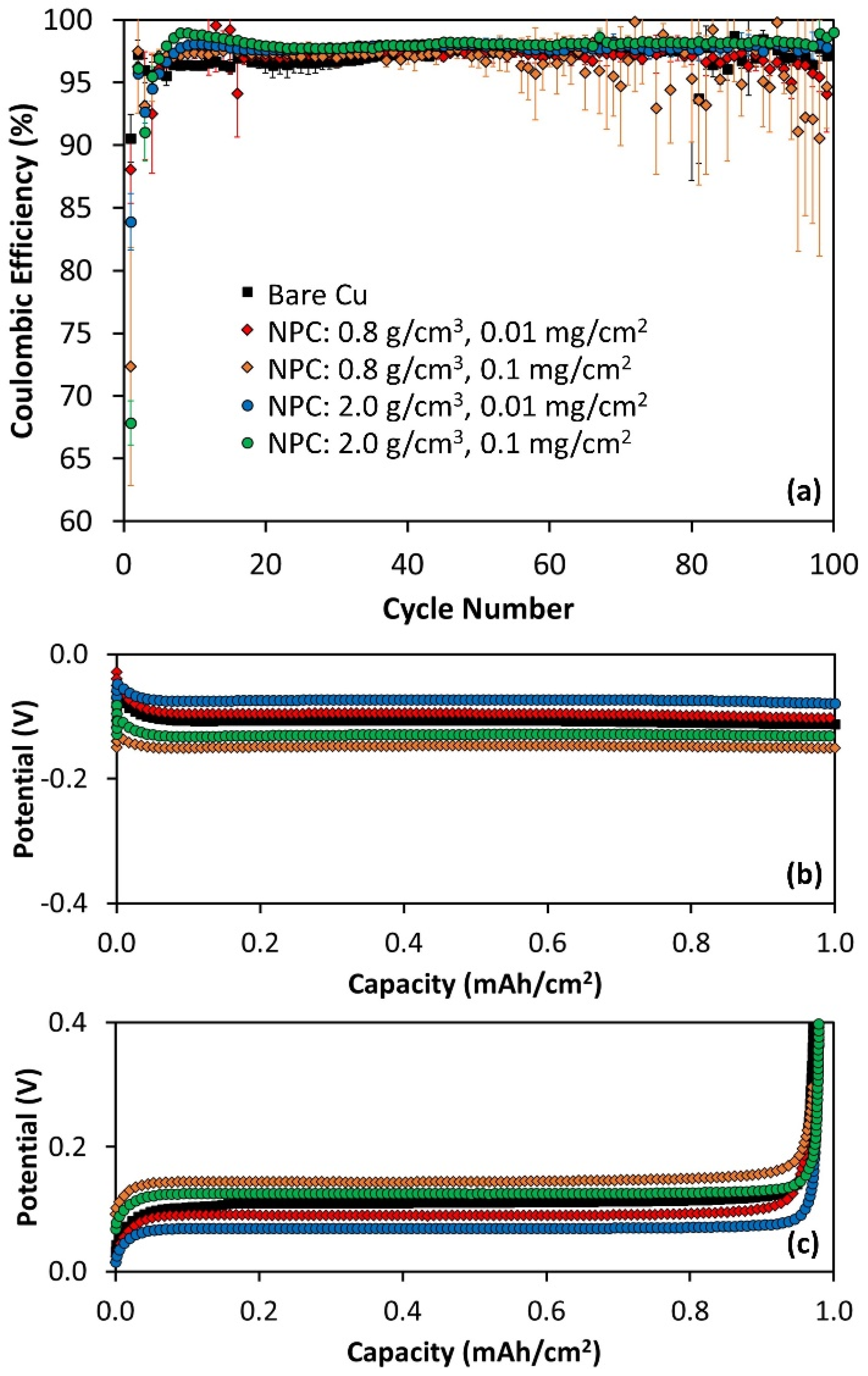
3.5. Higher Current Li Cycling on Bare and NPC-Coated Cu
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, B.D.; Zheng, J.; Ren, X.; Xu, W.; Zhang, J.G. Accurate determination of Coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 2018, 8, 1702097. [Google Scholar] [CrossRef]
- Li, L.; Basu, S.; Wang, Y.; Chen, Z.; Hundekar, P.; Wang, B.; Shi, J.; Shi, Y.; Narayanan, S.; Koratkar, N. Self-heating–induced healing of lithium dendrites. Science 2018, 359, 1513–1516. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Chen, X.; Zhang, J.; Shao, Y.; Engelhard, M.H.; Zhang, Y.; Blake, T.A.; Graff, G.L.; Liu, X. Effects of cesium cations in lithium deposition via self-healing electrostatic shield mechanism. J. Phys. Chem. C 2014, 118, 4043–4049. [Google Scholar] [CrossRef]
- Choi, J.-W.; Cheruvally, G.; Kim, D.-S.; Ahn, J.-H.; Kim, K.-W.; Ahn, H.-J. Rechargeable lithium/sulfur battery with liquid electrolytes containing toluene as additive. J. Power Sources 2008, 183, 441–445. [Google Scholar] [CrossRef]
- Jozwiuk, A.; Berkes, B.B.; Weiß, T.; Sommer, H.; Janek, J.; Brezesinski, T. The critical role of lithium nitrate in the gas evolution of lithium–sulfur batteries. Energy Environ. Sci. 2016, 9, 2603–2608. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Han, H.-B.; Zhou, S.-S.; Zhang, D.-J.; Feng, S.-W.; Li, L.-F.; Liu, K.; Feng, W.-F.; Nie, J.; Li, H.; Huang, X.-J. Lithium bis (fluorosulfonyl) imide (LiFSI) as conducting salt for nonaqueous liquid electrolytes for lithium-ion batteries: Physicochemical and electrochemical properties. J. Power Sources 2011, 196, 3623–3632. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Chen, X.; Zhang, J.; Engelhard, M.H.; Zhang, Y.; Johnson, B.R.; Crum, J.V.; Blake, T.A.; Liu, X. Effects of carbonate solvents and lithium salts on morphology and coulombic efficiency of lithium electrode. J. Electrochem. Soc. 2013, 160, A1894–A1901. [Google Scholar] [CrossRef]
- Naoi, K.; Mori, M.; Naruoka, Y.; Lamanna, W.M.; Atanasoski, R. The Surface Film Formed on a Lithium Metal Electrode in a New Imide Electrolyte, Lithium Bis (perfluoroethylsulfonylimide) [LiN(C2F5SO2)2]. J. Electrochem. Soc. 1999, 146, 462–469. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.-G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef]
- Takada, K. Progress and prospective of solid-state lithium batteries. Acta Mater. 2013, 61, 759–770. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Hallinan, D.T., Jr.; Balsara, N.P. Polymer electrolytes. Annu. Rev. Mater. Res. 2013, 43, 503–525. [Google Scholar] [CrossRef]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.-H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P. Inorganic solid-state electrolytes for lithium batteries: Mechanisms and properties governing ion conduction. Chem. Rev. 2015, 116, 140–162. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Xin, L.; Liu, Y.; Yang, F.; Stach, E.A.; Xie, J. Making Li-metal electrodes rechargeable by controlling the dendrite growth direction. Nature Energy 2017, 2, 17083. [Google Scholar] [CrossRef]
- Ma, L.; Fu, C.; Li, L.; Mayilvahanan, K.S.; Watkins, T.; Perdue, B.R.; Zavadil, K.R.; Helms, B.A. Nanoporous Polymer Films with High Cation Transference Number Stabilize Lithium Metal Anodes in Light-Weight Batteries for Electrified Transportation. Nano Lett. 2019, 19, 1387–1394. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Kovalev, I.; Schock, R.; Kumaresan, K.; Xu, J.; Affinito, J. High energy rechargeable Li-S cells for EV application: Status, remaining problems and solutions. Ecs Trans. 2010, 25, 23–34. [Google Scholar] [CrossRef]
- Wilkinson, D.P.; Blom, H.; Brandt, K.; Wainwright, D. Effects of physical constraints on Li cyclability. J. Power Sources 1991, 36, 517–527. [Google Scholar] [CrossRef]
- Harrison, K.L.; Zavadil, K.R.; Hahn, N.T.; Meng, X.; Elam, J.W.; Leenheer, A.; Zhang, J.-G.; Jungjohann, K.L. Lithium self-discharge and its prevention: Direct visualization through in situ electrochemical scanning transmission electron microscopy. ACS Nano 2017, 11, 11194–11205. [Google Scholar] [CrossRef]
- Cao, Y.; Meng, X.; Elam, J.W. Atomic Layer Deposition of LixAlyS Solid-State Electrolytes for Stabilizing Lithium-Metal Anodes. ChemElectroChem 2016, 3, 858–863. [Google Scholar] [CrossRef]
- Kozen, A.C.; Lin, C.-F.; Pearse, A.J.; Schroeder, M.A.; Han, X.; Hu, L.; Lee, S.-B.; Rubloff, G.W.; Noked, M. Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano 2015, 9, 5884–5892. [Google Scholar] [CrossRef] [PubMed]
- Kazyak, E.; Wood, K.N.; Dasgupta, N.P. Improved cycle life and stability of lithium metal anodes through ultrathin atomic layer deposition surface treatments. Chem. Mater. 2015, 27, 6457–6462. [Google Scholar] [CrossRef]
- Aetukuri, N.B.; Kitajima, S.; Jung, E.; Thompson, L.E.; Virwani, K.; Reich, M.L.; Kunze, M.; Schneider, M.; Schmidbauer, W.; Wilcke, W.W. Flexible Ion-Conducting Composite Membranes for Lithium Batteries. Adv. Energy Mater. 2015, 5, 1500265. [Google Scholar] [CrossRef]
- Hao, X.; Zhu, J.; Jiang, X.; Wu, H.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Ultrastrong Polyoxyzole Nanofiber Membranes for Dendrite-Proof and Heat-Resistant Battery Separators. Nano Lett. 2016, 16, 2981–2987. [Google Scholar] [CrossRef]
- Kang, I.S.; Lee, Y.-S.; Kim, D.-W. Improved cycling stability of lithium electrodes in rechargeable lithium batteries. J. Electrochem. Soc. 2014, 161, A53–A57. [Google Scholar] [CrossRef]
- Li, F.S.; Wu, Y.S.; Chou, J.; Winter, M.; Wu, N.L. A mechanically robust and highly ion-conductive polymer-blend coating for high-power and long-life lithium-ion battery anodes. Adv. Mater. 2015, 27, 130–137. [Google Scholar] [CrossRef]
- Yan, K.; Lee, H.-W.; Gao, T.; Zheng, G.; Yao, H.; Wang, H.; Lu, Z.; Zhou, Y.; Liang, Z.; Liu, Z. Ultrathin two-dimensional atomic crystals as stable interfacial layer for improvement of lithium metal anode. Nano Lett. 2014, 14, 6016–6022. [Google Scholar] [CrossRef]
- Merrill, L.C.; Long, D.M.; Small, K.A.; Jungjohann, K.L.; Leung, K.; Bassett, K.L.; Harrison, K.L. Role of coatings as artificial solid electrolyte interphases on lithium metal self-discharge. J. Phys. Chem. C 2022, 126, 17490–17501. [Google Scholar] [CrossRef]
- Bassett, K.L.; Small, K.A.; Long, D.M.; Merrill, L.C.; Warren, B.; Harrison, K.L. Interfacial pressure improves calendar aging of lithium metal anodes. Front. Batter. Electrochem. 2023, 2, 1292639. [Google Scholar] [CrossRef]
- Zheng, G.; Lee, S.W.; Liang, Z.; Lee, H.-W.; Yan, K.; Yao, H.; Wang, H.; Li, W.; Chu, S.; Cui, Y. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 2014, 9, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Arie, A.A.; Lee, J.K. Electrochemical characteristics of lithium metal anodes with diamond like carbon film coating layer. Diam. Relat. Mater. 2011, 20, 403–408. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Bai, W.; Tang, H.; Shi, S.; Wang, X.; Gu, C.; Tu, J. Magnetron sputtering amorphous carbon coatings on metallic lithium: Towards promising anodes for lithium secondary batteries. J. Power Sources 2014, 266, 43–50. [Google Scholar] [CrossRef]
- Liu, W.; Xia, Y.; Wang, W.; Wang, Y.; Jin, J.; Chen, Y.; Paek, E.; Mitlin, D. Pristine or highly defective? Understanding the role of graphene structure for stable lithium metal plating. Adv. Energy Mater. 2019, 9, 1802918. [Google Scholar] [CrossRef]
- Huang, S.; Tang, L.; Najafabadi, H.S.; Chen, S.; Ren, Z. A highly flexible semi-tubular carbon film for stable lithium metal anodes in high-performance batteries. Nano Energy 2017, 38, 504–509. [Google Scholar] [CrossRef]
- Bai, M.; Xie, K.; Yuan, K.; Zhang, K.; Li, N.; Shen, C.; Lai, Y.; Vajtai, R.; Ajayan, P.; Wei, B. A scalable approach to dendrite-free lithium anodes via spontaneous reduction of spray-coated graphene oxide layers. Adv. Mater. 2018, 30, 1801213. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Abbas, S.A.; Kaisar, N.; Wu, S.H.; Chen, H.-A.; Boopathi, K.M.; Singh, M.; Fang, J.; Pao, C.-W.; Chu, C.-W. Mitigating Metal Dendrite Formation in Lithium–Sulfur Batteries via Morphology-Tunable Graphene Oxide Interfaces. ACS Appl. Mater. Interfaces 2018, 11, 2060–2070. [Google Scholar] [CrossRef]
- Hong, B.; Fan, H.; Cheng, X.-B.; Yan, X.; Hong, S.; Dong, Q.; Gao, C.; Zhang, Z.; Lai, Y.; Zhang, Q. Spatially uniform deposition of lithium metal in 3D Janus hosts. Energy Storage Mater. 2019, 16, 259–266. [Google Scholar] [CrossRef]
- Yun, Q.; He, Y.B.; Lv, W.; Zhao, Y.; Li, B.; Kang, F.; Yang, Q.H. Chemical dealloying derived 3D porous current collector for Li metal anodes. Adv. Mater. 2016, 28, 6932–6939. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.; Guan, Y.; Zhang, J.; Ai, F.; Zhang, W.; Xiang, Y.; Vijayan, S.; Li, G.; Huang, Y. ZnO/carbon framework derived from metal-organic frameworks as a stable host for lithium metal anodes. Energy Storage Mater. 2018, 11, 191–196. [Google Scholar] [CrossRef]
- Ye, H.; Xin, S.; Yin, Y.-X.; Li, J.-Y.; Guo, Y.-G.; Wan, L.-J. Stable Li plating/stripping electrochemistry realized by a hybrid Li reservoir in spherical carbon granules with 3D conducting skeletons. J. Am. Chem. Soc. 2017, 139, 5916–5922. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yu, C.; Li, S.; Guo, W.; Zhao, Y.; Dong, Q.; Lin, X.; Song, Z.; Tan, X.; Wang, C. Ultrahigh-Capacity and Long-Life Lithium–Metal Batteries Enabled by Engineering Carbon Nanofiber–Stabilized Graphene Aerogel Film Host. Small 2018, 14, 1803310. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-W.; Wang, Z.-Y.; Liu, S.; Li, G.-R.; Gao, X.-P. Uniform lithium plating within 3D Cu foam enabled by Ag nanoparticles. Electrochim. Acta 2021, 379, 138152. [Google Scholar] [CrossRef]
- Sun, Z.; Jin, S.; Jin, H.; Du, Z.; Zhu, Y.; Cao, A.; Ji, H.; Wan, L.J. Robust Expandable Carbon Nanotube Scaffold for Ultrahigh-Capacity Lithium-Metal Anodes. Adv. Mater. 2018, 30, 1800884. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, W.; Song, X.; Wang, W.; Hou, Z.; Zhao, X.; Deng, K.; Ju, H.; Sun, Y.; Zhao, Y. Thermally reduced graphene paper with fast Li ion diffusion for stable Li metal anode. Electrochim. Acta 2019, 294, 413–422. [Google Scholar] [CrossRef]
- Huang, G.; Han, J.; Zhang, F.; Wang, Z.; Kashani, H.; Watanabe, K.; Chen, M. Lithiophilic 3D Nanoporous Nitrogen-Doped Graphene for Dendrite-Free and Ultrahigh-Rate Lithium-Metal Anodes. Adv. Mater. 2019, 31, 1805334. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Liang, Z.; Lee, H.-W.; Sun, J.; Wang, H.; Yan, K.; Xie, J.; Cui, Y. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 2016, 11, 626–632. [Google Scholar] [CrossRef]
- Deng, W.; Zhu, W.; Zhou, X.; Liu, Z. Graphene nested porous carbon current collector for lithium metal anode with ultrahigh areal capacity. Energy Storage Mater. 2018, 15, 266–273. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Tong, Y.; Qiu, J.; Liu, S.; Zhang, S.; Guan, Z.; Xu, B.; Wang, Z.; Chen, L. Lithium plating and stripping on carbon nanotube sponge. Nano Lett. 2018, 19, 494–499. [Google Scholar] [CrossRef]
- Cui, J.; Yao, S.; Ihsan-Ul-Haq, M.; Wu, J.; Kim, J.K. Correlation between Li plating behavior and surface characteristics of carbon matrix toward stable Li metal anodes. Adv. Energy Mater. 2019, 9, 1802777. [Google Scholar] [CrossRef]
- Song, Q.; Yan, H.; Liu, K.; Xie, K.; Li, W.; Gai, W.; Chen, G.; Li, H.; Shen, C.; Fu, Q. Vertically Grown Edge-Rich Graphene Nanosheets for Spatial Control of Li Nucleation. Adv. Energy Mater. 2018, 8, 1800564. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Li, Y.; Liu, Y.; Lin, D.; Zhu, C.; Chen, G.; Yang, A.; Yan, K.; Chen, H. Wrinkled graphene cages as hosts for high-capacity Li metal anodes shown by cryogenic electron microscopy. Nano Lett. 2019, 19, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, R.; Hu, Z.; Ye, S.; Zeng, S.; Yao, Y.; Li, S.; Yu, Y. Regulating lithium nucleation via CNTs modifying carbon cloth film for stable Li metal anode. Small 2019, 15, 1803734. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.-R.; Hou, T.-Z.; Li, B.-Q.; Cheng, X.-B.; Zhang, R.; Zhang, Q. Lithiophilicity chemistry of heteroatom-doped carbon to guide uniform lithium nucleation in lithium metal anodes. Sci. Adv. 2019, 5, eaau7728. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, D.; Zhao, J.; Lu, Z.; Liu, Y.; Liu, C.; Lu, Y.; Wang, H.; Yan, K.; Tao, X. Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. Proc. Natl. Acad. Sci. USA 2016, 113, 2862–2867. [Google Scholar] [CrossRef]
- Merrill, L.C.; Gannon, R.N.; Jungjohann, K.L.; Randolph, S.J.; Goriparti, S.; Zavadil, K.R.; Johnson, D.C.; Harrison, K.L. Evaluation of lithium metal anode volumetric expansion through laser plasma focused ion beam cross-sectional imaging. J. Electrochem. Soc. 2023, 170, 080527. [Google Scholar] [CrossRef]
- Siegal, M.; Overmyer, D.; Kottenstette, R.; Tallant, D.; Yelton, W. Nanoporous-carbon films for microsensor preconcentrators. Appl. Phys. Lett. 2002, 80, 3940–3942. [Google Scholar] [CrossRef]
- Siegal, M.; Yelton, W.; Overmyer, D.; Provencio, P. Nanoporous carbon films for gas microsensors. Langmuir 2004, 20, 1194–1198. [Google Scholar] [CrossRef]
- Siegal, M.P.; Yelton, W.G.; Perdue, B.R.; Gallis, D.F.S.; Schwarz, H.L. Nanoporous-carbon as a potential host material for reversible Mg ion intercalation. J. Electrochem. Soc. 2016, 163, A1030–A1035. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Harrison, K.L.; Wolak, M.; Siegal, M.P. Nanoporous Carbon as an Anode Material for Li-Ion Batteries. U.S. Patent 10,784,511, 22 September 2020. [Google Scholar]
- Harrison, K.L.; Siegal, M.; Wolak, M.; Cuillier, P. Nanoporous Carbon as Host Material for Sodium. U.S. Patent 11,355,747, 7 June 2022. [Google Scholar]
- Merrill, L.C.; Rosenberg, S.G.; Jungjohann, K.L.; Harrison, K.L. Uncovering the relationship between aging and cycling on lithium metal battery self-discharge. ACS Appl. Energy Mater. 2021, 4, 7589–7598. [Google Scholar] [CrossRef]
- Kang, H.; Kang, H.; Lyu, M.; Cho, E. A review of recent developments in the design of electrolytes and solid electrolyte interphase for lithium metal batteries. EcoMat 2024, 6, e12498. [Google Scholar] [CrossRef]
- Zhang, W.; Sayavong, P.; Xiao, X.; Oyakhire, S.T.; Shuchi, S.B.; Vilá, R.A.; Boyle, D.T.; Kim, S.C.; Kim, M.S.; Holmes, S.E. Recovery of isolated lithium through discharged state calendar ageing. Nature 2024, 626, 306–312. [Google Scholar] [CrossRef] [PubMed]



| NPC Mass Density (g/cm3) | NPC Coating Mass Loading (mg/cm2) | Thickness NPC (μm) | NPC Thickness Compared to Theoretical Li (%) | NPC Mass Compared to Theoretical Li (%) | Theoretical Capacity Including Li and NPC (mAh/cm3) | Theoretical Capacity Including Li and NPC (mAh/g) |
|---|---|---|---|---|---|---|
| N/A | N/A | 0 | 0 | 0 | 2061 | 3860 |
| 0.8 | 0.01 | 0.125 | 3 | 4 | 2009 | 3717 |
| 0.8 | 0.1 | 1.25 | 26 | 39 | 1639 | 2785 |
| 2.0 | 0.01 | 0.05 | 1 | 4 | 2040 | 3717 |
| 2.0 | 0.1 | 0.5 | 10 | 39 | 1869 | 2785 |
| NPC Condition | 1st Cycle CE |
|---|---|
| No NPC | 91.7 ± 0.7 |
| NPC: 0.8 g/cm3, 0.01 mg/cm2 | 84.6 ± 0.6 |
| NPC: 0.8 g/cm3, 0.1 mg/cm2 | 77.0 ± 2.7 |
| NPC: 2.0 g/cm3, 0.01 mg/cm2 | 85.7 ± 5.1 |
| NPC: 2.0 g/cm3, 0.1 mg/cm2 | 75.1 ± 4.0 |
| Bare Cu | NPC-Coated Cu 0.8 g/cm3 0.01 mg/cm2 | NPC-Coated Cu 0.8 g/cm3 0.1 mg/cm2 | NPC-Coated Cu 2.0 g/cm3 0.01 mg/cm2 | NPC-Coated Cu 2.0 g/cm3 0.1 mg/cm2 | |
|---|---|---|---|---|---|
| cycling 0.5 mA/cm2 | CE is generally lower than NPC samples | CE is higher than Cu in middle cycles | CE is higher than Cu in middle cycles | CE is higher than Cu in most cycles | CE is higher than Cu in most cycles |
| cycling 2.0 mA/cm2 | CE is lower than NPC early cycles | CE slightly higher than Cu ~50 cycles | CE slightly higher than Cu ~50 cycles | Cu is higher than Cu ~50 cycles | Cu is higher than Cu ~50 cycles |
| calendar aging | CE is similar to or lower than NPC | CE is higher than Cu in most cycles | CE is higher than Cu in most cycles | CE is similar to Cu in most cycles | CE is similar to Cu in most cycles |
| ηcycle50 0.5 mA/cm2 | higher than NPC | lower than Cu | lower than Cu | lower than Cu | lower than Cu |
| ηcycle50 2.0 mA/cm2 | varies relative to NPC thickness | lower than Cu | higher than Cu | lower than Cu | higher than Cu |
| ηcycle50 aging | higher than NPC | lower than Cu | lower than Cu | lower than Cu | lower than Cu |
| morphology | irregular | long strands | thick strands | strands, blobs | blobs |
| nucleation density | higher than NPC | low | very low | low | low |
| nucleation/growth | on Cu | on top of NPC | below NPC on Cu | below NPC on Cu | below NPC on Cu |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrison, K.L.; Goriparti, S.; Long, D.M.; Martin, R.I.; Warren, B.; Merrill, L.C.; Wolak, M.A.; Sananes, A.; Siegal, M.P. Nanoporous Carbon Coatings Direct Li Electrodeposition Morphology and Performance in Li Metal Anode Batteries. Batteries 2025, 11, 10. https://doi.org/10.3390/batteries11010010
Harrison KL, Goriparti S, Long DM, Martin RI, Warren B, Merrill LC, Wolak MA, Sananes A, Siegal MP. Nanoporous Carbon Coatings Direct Li Electrodeposition Morphology and Performance in Li Metal Anode Batteries. Batteries. 2025; 11(1):10. https://doi.org/10.3390/batteries11010010
Chicago/Turabian StyleHarrison, Katharine L., Subrahmanyam Goriparti, Daniel M. Long, Rachel I. Martin, Benjamin Warren, Laura C. Merrill, Matthaeus A. Wolak, Alexander Sananes, and Michael P. Siegal. 2025. "Nanoporous Carbon Coatings Direct Li Electrodeposition Morphology and Performance in Li Metal Anode Batteries" Batteries 11, no. 1: 10. https://doi.org/10.3390/batteries11010010
APA StyleHarrison, K. L., Goriparti, S., Long, D. M., Martin, R. I., Warren, B., Merrill, L. C., Wolak, M. A., Sananes, A., & Siegal, M. P. (2025). Nanoporous Carbon Coatings Direct Li Electrodeposition Morphology and Performance in Li Metal Anode Batteries. Batteries, 11(1), 10. https://doi.org/10.3390/batteries11010010







