Study on Thermal Runaway Behavior and Jet Characteristics of a 156 Ah Prismatic Ternary Lithium Battery
Abstract
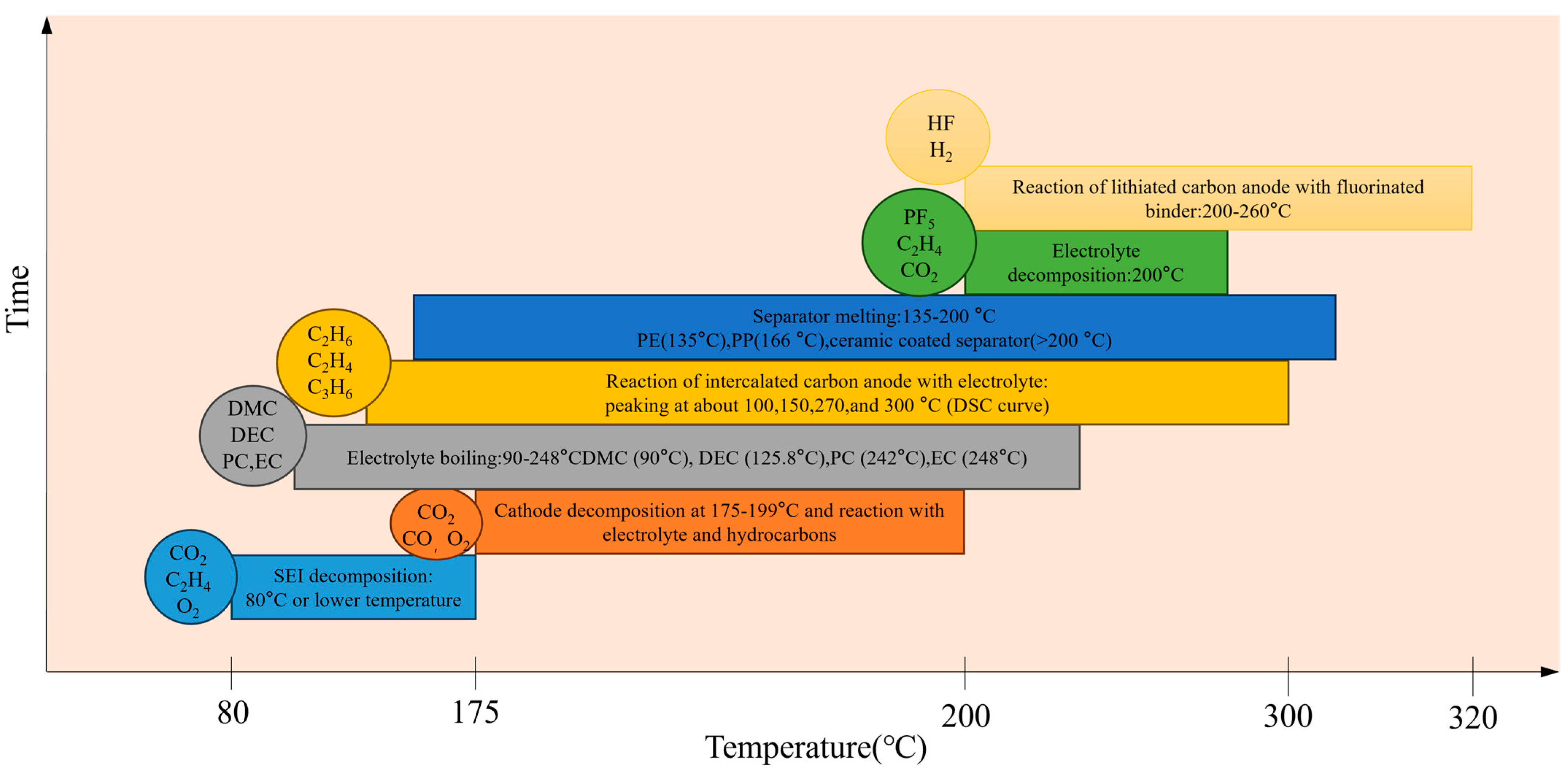
1. Introduction
2. Materials and Methods
2.1. Battery Samples
2.2. Experimental Equipment
2.3. Experimental Procedure
- Preparation: Charge the 156 Ah NCM811 battery to 100% SOC (State of Charge) at 0.1 C using a battery charge-discharge machine (model NEWARE BTS4000-5V30A) and let it rest for 24 h;
- Thermocouple Installation: Fix K-type armored thermocouples to the battery surface using polyimide and Teflon tapes at the temperature monitoring points shown in Figure 2b,c. Special attention should be paid to the thermocouple near the heating plate. First, fix it with a layer of polyimide tape, and then attach two layers of Teflon tape to prevent the thermocouple from coming into direct contact with the heating plate. This ensures that the thermocouple measures the battery surface temperature rather than the surface temperature of the heating plate. Clamp the battery as shown in Figure 2a and use a torque wrench to pre-tighten the fastening bolts to 1 N·m.
- Place the clamped battery in a custom-made sealed pressure vessel with a capacity of 1000 L. The top of the battery should be approximately 800 mm from the top of the experimental chamber. Adjust the positions of the four environmental monitoring thermocouples on all sides (top, bottom, left, and right), and start the camera. Close the pressure vessel door and lock the bolts;
- Nitrogen Purging: Ensure both the intake and exhaust valves are initially closed. At the start of the test, open the exhaust valve and turn on the vacuum pump to evacuate the air inside the chamber. When the pressure drops to 5 Kpa, close the exhaust valve and turn off the vacuum pump. Open the intake valve to fill the chamber with nitrogen until the pressure reaches 101 Kpa, then close the intake valve. Repeat this process three times to reduce the oxygen concentration in the chamber to 0.0025%, ensuring the chamber is filled with 99.75% nitrogen;
- Triggering Thermal Runaway: Turn on the heating plate power supply and maintain the heating plate at a power of 400 W. Monitor the battery voltage data; when the voltage drops to 0 V, immediately turn off the heating plate power supply, indicating that thermal runaway has been triggered;
- Post-Experiment Procedure: After the battery temperature inside the chamber drops to room temperature, open the chamber door, collect the solid substances ejected during thermal runaway, and clean the chamber;
- Repetition: Repeat the above process for a total of three experiments.
3. Experimental Results and Analysis
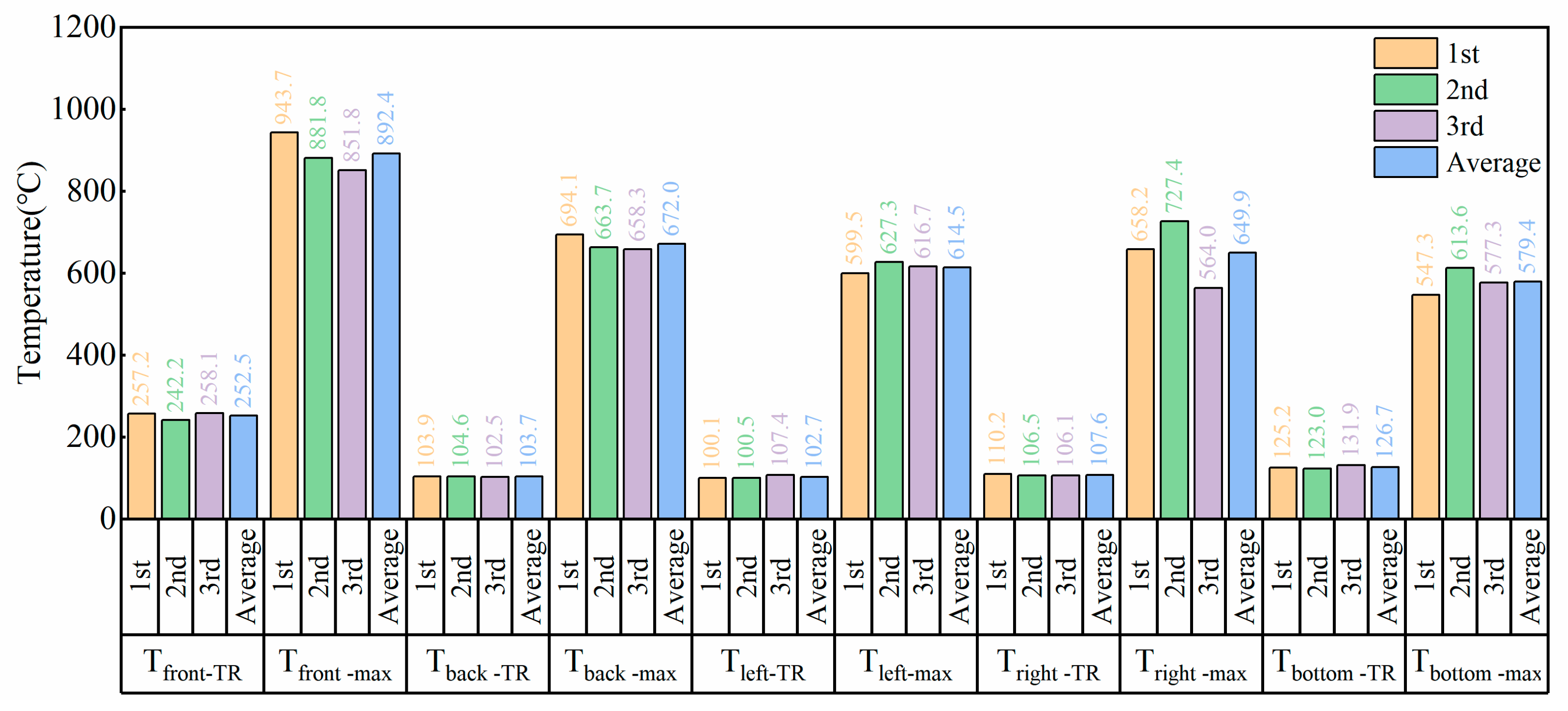
3.1. Battery Surface Temperature and Voltage Changes
- TX-TR: Inflection point temperature at the monitoring point (temperature rise rate greater than 3 °C/s).
- TX-max: Maximum temperature at the monitoring point.
3.2. Battery Thermal Runaway Jet Temperature
3.3. Video and Audio Signals during the Battery Thermal Runaway Ejection Process
3.4. Pressure Changes and Gas Production
- Ultra-High-Speed Ejection Phase (Du): The time from the start of thermal runaway ejection (te) to the moment of maximum ejection rate (tu);
- High-Speed Ejection Phase (Df): The time from the moment of maximum ejection rate (tu) to the moment when the ejection rate drops to zero (tf);
- Slow Ejection Phase (Ds): The time from the moment when the ejection rate drops to zero (tf) to the end of the ejection (ts).
- P is the pressure inside the chamber, in units of Pa;
- P0 is the initial pressure inside the chamber, in units of Pa;
- V is the volume of the experimental chamber, in units of m3;
- n is the amount of gas in the chamber, in units of mol;
- R is the ideal gas constant, with a value of 8.31441 J/(mol·K);
- T0 is the ambient temperature at the start of the experiment, in units of K;
- Tambient-average is the average temperature of the four environmental monitoring points inside the experimental chamber, in units of K.
- nAh represents the molar amount of gas produced per unit capacity, in mol/Ah;
- n represents the total amount of gas produced by the battery, in mol;
- Capacity represents the battery capacity, in Ah.
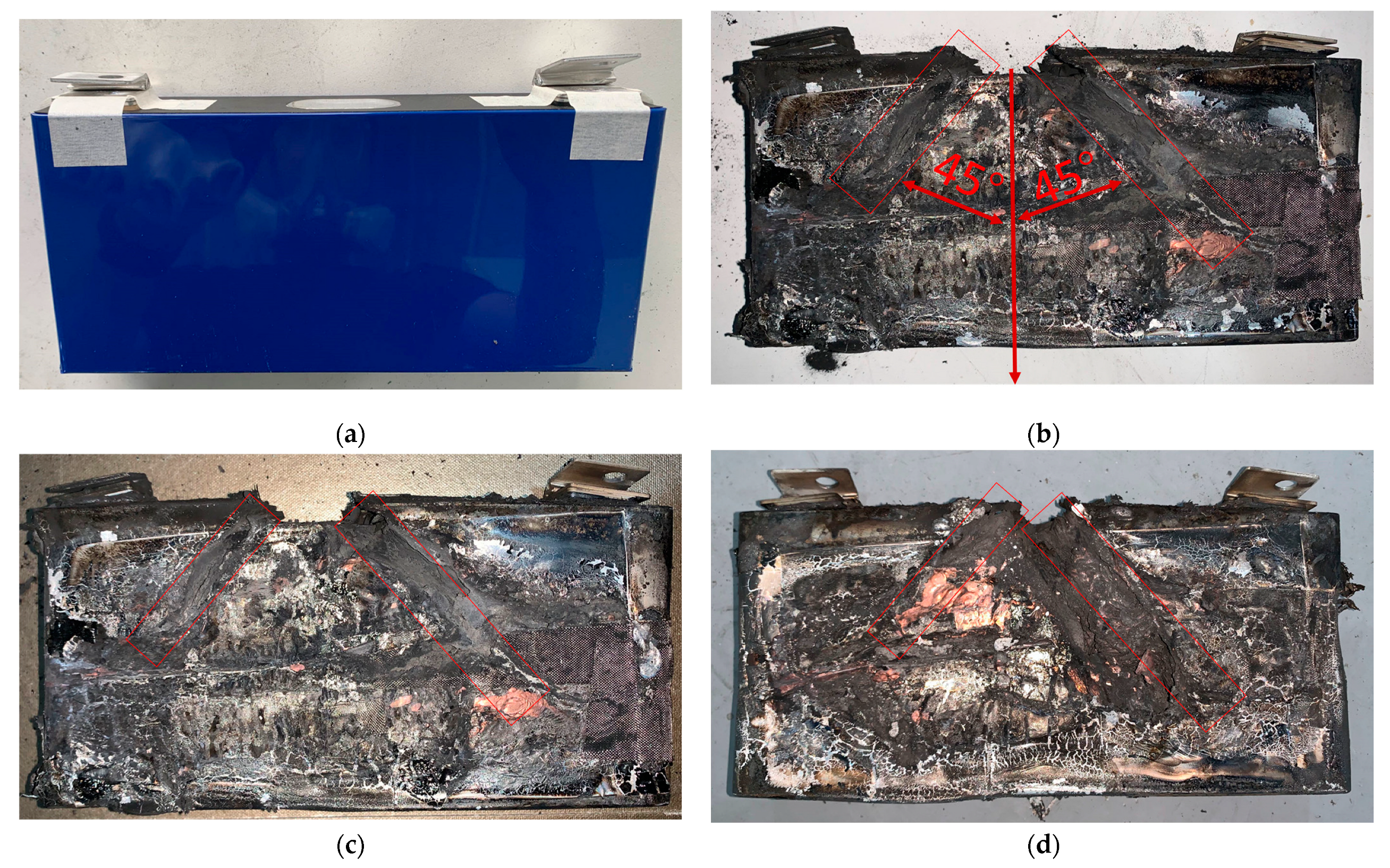
3.5. Battery Morphological Characteristics
- When the Tfront position temperature is below 660.3 °C, the battery casing remains intact, and all the jet is expelled through the safety valve;
- When the Tfront position temperature exceeds 660.3 °C, the battery casing will be damaged, and the jet may be expelled from both the safety valve and the damaged area.
4. Conclusions
- During the thermal runaway of the 811 ternary lithium battery, the front surface temperature of the battery can reach 851.8–943.7 °C, and the back surface temperature can reach 658.3–694.1 °C, both exceeding the melting point of the aluminum casing (660.4 °C). This melting can cause inaccuracies in temperature measurements. The temperatures of the bottom surface and the small side surfaces are lower than the aluminum melting point, and the order of the appearance of thermal runaway temperature inflection points is: Time(Tfront) = Time(Tbottom) = Time(Tleft) < Time(Tright) < Time(Tback). From the perspective of thermal runaway early warning, monitoring the temperature at the bottom surface and the small side surface near the battery anode is more accurate;
- The highest jet temperatures at 50 mm, 150 mm, and 250 mm above the safety valve were 356.9 °C, 302.7 °C, and 216.5 °C, respectively. This indicates that the further from the outlet, the lower the jet temperature. The temperature rise rates at these three points were 77.1 °C/s, 40.9 °C/s, and 26.9 °C/s, respectively, showing that the temperature rise rate is inversely proportional to the distance from the safety valve;
- Based on acoustic and image signals, it was found that there are two intensive sound amplitude stages during the ternary battery ejection process, which are closely related to the number of battery wound cores;
- Under the conditions of this experiment, the average gas production of the battery was 0.089 mol/Ah. Based on the pressure data from the first experiment, one ejection process was observed, which included ultra-high-speed ejection (2 s), high-speed ejection (32 s), and slow ejection (47 s) stages. Combined with the ejection process captured by the acoustic signals (34 s), it indicates that exhaust sounds are produced during the ultra-high-speed and high-speed ejection stages due to high-speed airflow impact, while no exhaust sound is produced during the slow ejection stage. This has significant research value for using acoustic signals to provide early warnings of battery thermal runaway;
- Based on the post-thermal runaway remnants, it was found that the grooves caused by airflow impact are mainly located at ±45°. Therefore, when measuring the jet temperature during thermal runaway, monitoring points should be arranged within the ±45° range.
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.; Meng, J.; Sun, X.; Zhao, P.; Sun, P.; Zheng, B. Experimental Study on Effects of Triggering Modes on Thermal Runaway Characteristics of Lithium-Ion Battery. World Electr. Veh. J. 2023, 14, 270. [Google Scholar] [CrossRef]
- Du, Q.; Fang, Z. Analysis of the Thermal Behavior of a Lithium Cell Undergoing Thermal Runaway. Fluid Dyn. Mater. Process. 2021, 17, 887–898. [Google Scholar] [CrossRef]
- Gao, F.; Yang, K.; Wang, C.; Liu, W.; Zhu, Y. Experimental Study on Thermal Runaway of LiFePO4/C Battery under Heating Condition. IOP Conf. Ser. Earth Environ. Sci. 2020, 546, 042025. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, S.; Wang, B.; Sun, J.; Zhang, Z.; Liu, Y. Study on Thermal Safety of the Overcharged Lithium-Ion Battery. Fire Technol. 2023, 59, 1089–1114. [Google Scholar] [CrossRef]
- Shen, H.; Wang, H.; Li, M.; Li, C.; Zhang, Y.; Li, Y.; Yang, X.; Feng, X.; Ouyang, M. Thermal Runaway Characteristics and Gas Composition Analysis of Lithium-Ion Batteries with Different LFP and NCM Cathode Materials under Inert Atmosphere. Electronics 2023, 12, 1603. [Google Scholar] [CrossRef]
- Lai, Y.; Yang, K.; Liu, H.; Gao, F.; Zhang, M.; Xu, W. A Probe into the Accuracy of Thermal Runaway Simulation Model of Lithium-ion Battery under Adiabatic Condition. IOP Conf. Ser. Earth Environ. Sci. 2021, 680, 012009. [Google Scholar] [CrossRef]
- Li, Q.; Yu, J.; Liu, G.; Ma, X.; Si, W.; Hu, X.; Zhu, G.; Liu, T. Study on the Effectiveness of Water Mist on Suppressing Thermal Runaway in LiFePO4 Batteries. Crystals 2023, 13, 1346. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Zhang, Y. Research and Development of Fire Alarm Detection Device for Lithium Ion Battery Based on Strain Measurement. IOP Conf. Ser. Earth Environ. Sci. 2020, 605, 012005. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, L.; Huang, Z.; Jia, Z.; Qin, P.; Wang, Q. Pressure Effect on the Thermal Runaway Behaviors of Lithium-Ion Battery in Confined Space. Fire Technol. 2023, 59, 1137–1155. [Google Scholar] [CrossRef]
- Available online: https://homeland.house.gov/2024/02/15/subcommittee-chairman-desposito-delivers-opening-statement-in-hearing-on-fire-hazards-lithium-ion-battery-risks/ (accessed on 16 June 2024).
- Miao, H. Research on accurate fire detection & early warning model for lithium-ion battery packs. J. Phys. Conf. Ser. 2024, 2703, 012068. [Google Scholar] [CrossRef]
- Qian, F.; Wang, H.; Li, M.; Cheng, L.; Shen, H.; Wang, J.; Li, Y.; Ouyang, M. Thermal Runaway Vent Gases from High-Capacity Energy Storage LiFePO4 Lithium Iron. Energies 2023, 16, 3485. [Google Scholar] [CrossRef]
- Seyed Saeed, M.; Ziebert, C.; Mousa, M. Thermal Characteristics and Safety Aspects of Lithium-Ion Batteries: An In-Depth Review. Symmetry 2023, 15, 1925. [Google Scholar] [CrossRef]
- Sun, J.; Li, G.; Xie, S.; He, Y. The Influence of Airflow Rate on the Thermal Runaway Propagation Characteristics of Lithium-Ion Batteries in a Low-Pressure Environment. Fire Technol. 2022, 58, 3553–3576. [Google Scholar] [CrossRef]
- Wang, K.; Ouyang, D.; Qian, X.; Yuan, S.; Chang, C.; Zhang, J.; Liu, Y. Early Warning Method and Fire Extinguishing Technology of Lithium-Ion Battery Thermal Runaway: A Review. Energies 2023, 16, 2960. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Wang, Q.; Tian, H. Research on Thermal Out of Control of Lithium Battery in New Energy Vehicles. IOP Conf. Ser. Earth Environ. Sci. 2020, 446, 022038. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.; Zhou, Y.; Huang, H.; Dai, H. Experimental and Modeling Analysis of Thermal Runaway for LiNi0.5Mn0.3Co0.2O2/Graphite Pouch Cell Triggered by Surface Heating. Energies 2024, 17, 826. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Wang, H.; Yuan, D. Study on the Suppression Effect of Cryogenic Cooling on Thermal Runaway of Ternary Lithium-Ion Batteries. Fire 2022, 5, 182. [Google Scholar] [CrossRef]
- Li, J.; Gao, P.; Tong, B.; Cheng, Z.; Cao, M.; Mei, W.; Wang, Q.; Sun, J.; Qin, P. Revealing the mechanism of pack ceiling failure induced by thermal runaway in NCM batteries: A coupled multiphase fluid-structure interaction model for electric vehicles. eTransportation 2024, 20, 100335. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Li, R.; Tao, C.; Chen, Z.; Liu, T.; Li, Y. The experimental investigation of thermal runaway characteristics of lithium battery under different concentrations of heptafluoropropane and air. J. Energy Storage 2024, 84, 110828. [Google Scholar] [CrossRef]
- Xie, H.J.; Sun, J.; Li, J.G.; Zhou, T.; Wei, S.P.; Yi, Z.H. Lithium-Ion Battery Thermal Runaway Electro-Thermal Triggering Method and Toxicity Analysis. IOP Conf. Ser. Earth Environ. Sci. 2021, 701, 012007. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Resendiz, E.G.; Nwophoke, S.M.; Khanna, S.; James, C.; Brown, S.F. Review of gas emissions from lithium-ion battery thermal runaway failure—Considering toxic and flammable compounds. J. Energy Storage 2024, 87, 111288. [Google Scholar] [CrossRef]
- Wei, G.; Huang, R.; Zhang, G.; Jiang, B.; Zhu, J.; Guo, Y.; Han, G.; Wei, X.; Dai, H. A comprehensive insight into the thermal runaway issues in the view of lithium-ion battery intrinsic safety performance and venting gas explosion hazards. Appl. Energy 2023, 349, 121651. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, K.; Niu, J.; Liu, T.; Hu, J. Research on the lower explosion limit of thermal runaway gas in lithium batteries under high-temperature and slight overcharge conditions. J. Energy Storage 2023, 79, 109976. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; De Zhou, C.; Richard, Y.; Wang, J. Experimental Study on the Combustion Characteristics of Primary Lithium Batteries Fire. Fire Technol. 2014, 52, 365–385. [Google Scholar] [CrossRef]
- Jia, L.; Wang, D.; Yin, T.; Li, X.; Li, L.; Dai, Z.; Zheng, L. Experimental Study on Thermal-Induced Runaway in High Nickel Ternary Batteries. ACS Omega 2022, 7, 14562–14570. [Google Scholar] [CrossRef]
- Thomas, F.; Mills, G.; Howe, R.; Zobell, J. Lithium Battery Fires: Implications for Air Medical Transport. Air Med. J. 2012, 31, 242–248. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, M.; Zhu, L.; Hu, C.; Huang, W.; Yao, J.; Yuan, Z.; Xu, C.; Feng, X. Study on Thermal Runaway Behavior of Li-Ion Batteries Using Different Abuse Methods. Batteries 2022, 8, 201. [Google Scholar] [CrossRef]
- Ohneseit, S.; Finster, P.; Floras, C.; Lubenau, N.; Uhlmann, N.; Seifert, H.J.; Ziebert, C. Thermal and Mechanical Safety Assessment of Type 21700 Lithium-Ion Batteries with NMC, NCA and LFP Cathodes—Investigation of Cell Abuse by Means of Accelerating Rate Calorimetry (ARC). Batteries 2023, 9, 237. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, L.; Zhao, J.; Xu, J.; Liu, H.; Chen, M. An Experimental Study on the Thermal Runaway Propagation of Cycling Aged Lithium-Ion Battery Modules. Fire 2024, 7, 119. [Google Scholar] [CrossRef]
- Deng, J.; Chen, B.; Lu, J.; Zhou, T.; Wu, C. Thermal runaway and combustion characteristics, risk and hazard evaluation of lithium-iron phosphate battery under different thermal runaway triggering modes. Appl. Energy 2024, 368, 123451. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, M.; Zhou, X.; Li, L.; Ju, X.; Yang, L. Investigating thermal runaway triggering mechanism of the prismatic lithium iron phosphate battery under thermal abuse. Renew. Energy 2023, 220, 119674. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Han, X.; Wang, Y.; Wang, Y.; Zhang, Y.; Feng, X.; Ouyang, M. An Experimental Study on Thermal Runaway Behavior for High-Capacity Li(Ni0.8Co0.1Mn0.1)O2 Pouch Cells at Different State of Charges. J. Electrochem. Energy Convers. Storage 2021, 18, 021012. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, S.; Li, K.; Zhao, M.; Qiu, Z.; Han, D.; Zhang, G.; Habetler, T.G. Hazard analysis of thermally abused lithium-ion batteries at different state of charges. J. Energy Storage 2019, 27, 101065. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, H.; Zhi, M.; Chen, X.; Lv, P.; He, Y. Thermal characteristics of thermal runaway for pouch lithium-ion battery with different state of charges under various ambient pressures. J. Power Sources 2022. [Google Scholar] [CrossRef]
- Wu, H.; Chen, S.; Hong, Y.; Xu, C.; Zheng, Y.; Jin, C.; Chen, K.; He, Y.; Feng, X.; Wei, X.; et al. Thermal safety boundary of lithium-ion battery at different state of charge. J. Energy Chem. 2023, 91, 59–72. [Google Scholar] [CrossRef]
- Doose, S.; Hahn, A.; Fischer, S.; Müller, J.; Haselrieder, W.; Kwade, A. Comparison of the consequences of state of charge and state of health on the thermal runaway behavior of lithium ion batteries. J. Energy Storage 2023, 62, 106837. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bai, J.; Gao, T.; Mao, N. Heat generation and thermal runaway mechanisms induced by overcharging of aged lithium-ion battery. Appl. Therm. Eng. 2022, 212, 118565. [Google Scholar] [CrossRef]
- Xiao, Y.; Wen, J.; Yao, L.; Zheng, J.; Fang, Z.; Shen, Y. A comprehensive review of the lithium-ion battery state of health prognosis methods combining aging mechanism analysis. J. Energy Storage 2023, 65, 107347. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Terekhov, A.; Warnberg, D.; Zhao, P. Thermal runaway of Li-ion battery with different aging histories. Process Saf. Environ. Prot. 2024, 185, 910–917. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Yang, S.; Xie, Z.; Zhang, F.; Zhao, P. Experimental investigation on thermal runaway suspension with battery health retention. Appl. Therm. Eng. 2023, 225, 120239. [Google Scholar] [CrossRef]
- Jin, C.; Sun, Y.; Wang, H.; Zheng, Y.; Wang, S.; Rui, X.; Xu, C.; Feng, X.; Wang, H.; Ouyang, M. Heating power and heating energy effect on the thermal runaway propagation characteristics of lithium-ion battery module: Experiments and modeling. Appl. Energy 2022, 312, 118760. [Google Scholar] [CrossRef]
- Meng, D.; Wang, X.; Chen, M.; Wang, J. Effects of environmental temperature on the thermal runaway of lithium-ion batteries during charging process. J. Loss Prev. Process Ind. 2023, 83, 105084. [Google Scholar] [CrossRef]
- Parhizi, M.; Ahmed, M.B.; Jain, A. Determination of the core temperature of a Li-ion cell during thermal runaway. J. Power Sources 2017, 370, 27–35. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Q.; Zheng, K.; Qin, J.; Zhou, D.; Wang, J. Impact of Lithium Salts on the Combustion Characteristics of Electrolyte under Diverse Pressures. Energies 2020, 13, 5373. [Google Scholar] [CrossRef]
- Teslenko, V.S.; Drozhzhin, A.P.; Medvedev, N.; Manzhalei, V.I. Initiation of combustion of a gas mixture by an electric explosion of an electrolyte. Combust. Explos. Shock. Waves 2012, 48, 730–733. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Deng, J.; Chen, J.; Ji, X.; Wu, H.; Zhao, J. Experimental study on combustion characteristics of electrolyte pool fire. J. Energy Storage 2024, 93, 112214. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Li, M.; Li, Y.; Li, C.; Zhang, Y.; Chen, S.; Shen, H.; Qian, F.; Feng, X.; et al. Experimental Study on Thermal Runaway Behavior of Lithium-Ion Battery and Analysis of Combustible Limit of Gas Production. Batteries 2022, 8, 250. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, X.; Xu, C.; Jiang, F.; Ouyang, M. Thermal runaway front in failure propagation of long-shape lithium-ion battery. Int. J. Heat Mass Transf. 2021, 182, 121928. [Google Scholar] [CrossRef]
- Ma, Z.; Huo, Q.; Wang, W.; Zhang, T. Voltage-temperature aware thermal runaway alarming framework for electric vehicles via deep learning with attention mechanism in time-frequency domain. Energy 2023, 278, 127747. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, T.; Wang, Q. Experimental study on the influence of different heating methods on thermal runaway of lithium-ion battery. J. Energy Storage 2021, 42, 103063. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Mao, B.; Chen, M.; Huang, Z.; Wang, Q. Experimental study on thermal runaway and fire behaviors of large format lithium iron phosphate battery. Appl. Therm. Eng. 2021, 192, 116949. [Google Scholar] [CrossRef]
- Wang, G.; Ping, P.; Zhang, Y.; Zhao, H.; Lv, H.; Gao, X.; Gao, W.; Kong, D. Modeling thermal runaway propagation of lithium-ion batteries under impacts of ceiling jet fire. Process Saf. Environ. Prot. 2023, 175, 524–540. [Google Scholar] [CrossRef]
- Zhao, R.; Lai, Z.; Li, W.; Ye, M.; Yu, S. Development of a coupled model of heat generation and jet flow of lithium-ion batteries during thermal runaway. J. Energy Storage 2023, 63, 107048. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, H.; Wu, D.; Yang, Y.; Yuan, C.; Li, G. Inhibition effect of inert gas jet on gas and hybrid explosions caused by thermal runaway of lithium-ion battery. J. Loss Prev. Process Ind. 2024, 90, 105336. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, X.; Wang, D.; Li, M.; Wang, B.; Yang, L.; Cao, B. Experimental analysis of lengthwise/transversal thermal characteristics and jet flow of large-format prismatic lithium-ion battery. Appl. Therm. Eng. 2021, 195, 117244. [Google Scholar] [CrossRef]
- Zou, K.; Chen, X.; Ding, Z.; Gu, J.; Lu, S. Jet behavior of prismatic lithium-ion batteries during thermal runaway. Appl. Therm. Eng. 2020, 179, 115745. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Planteu, R.; Krohn, P.; Rasch, B.; Brunnsteiner, B.; Thaler, A.; Hacker, V. Thermal runaway of large automotive Li-ion batteries. RSC Adv. 2018, 8, 40172–40186. [Google Scholar] [CrossRef]
- Su, T.; Lyu, N.; Zhao, Z.; Wang, H.; Jin, Y. Safety warning of lithium-ion battery energy storage station via venting acoustic signal detection for grid application. J. Energy Storage 2021, 38, 102498. [Google Scholar] [CrossRef]
- Kong, D.; Lv, H.; Ping, P.; Wang, G. A review of early warning methods of thermal runaway of lithium ion batteries. J. Energy Storage 2023, 64, 107073. [Google Scholar] [CrossRef]
- Nishant Ranjan Sinha, K.; Ranjan, D.; Raza, M.Q.; Kumar, N.; Kaner, S.; Thakur, A.; Raj, R. In-situ acoustic detection of critical heat flux for controlling thermal runaway in boiling systems. Int. J. Heat Mass Transf. 2019, 138, 135–143. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Liu, J.; Wang, J.; Yan, W. Gas Sensing Technology for the Detection and Early Warning of Battery Thermal Runaway: A Review. Energy Fuels 2022, 36, 6038–6057. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Yan, W.; Liu, J. Investigation of impact pressure during thermal runaway of lithium ion battery in a semi-closed space. Appl. Therm. Eng. 2020, 175, 115429. [Google Scholar] [CrossRef]
- Dubaniewicz, T.H.; Zlochower, I.; Barone, T.; Thomas, R.; Yuan, L. Thermal Runaway Pressures of Iron Phosphate Lithium-Ion Cells as a Function of Free Space within Sealed Enclosures. Min. Metall. Explor. 2020, 38, 539–547. [Google Scholar] [CrossRef]
- Thomas, H.D.; Teresa, L.B.; Connor, B.B.; Richard, A.T. Comparison of thermal runaway pressures within sealed enclosures for nickel manganese cobalt and iron phosphate cathode lithium-ion cells. J. Loss Prev. Process Ind. 2022, 76, 104739. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Li, Y.; Li, Y.; Sun, J.; Zhao, F.; Wang, H.; Wang, Y.; Xu, C.; Feng, X. Thermal runaway propagation behavior and gas production characteristics of NCM622 battery modules at different state of charge. Process Saf. Environ. Prot. 2024, 185, 267–276. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, W.; Li, C.; Ouyang, M. Quantitative analysis of eruption process of abused prismatic Ni-rich automotive batteries based on in-chamber pressure. J. Energy Storage 2020, 31, 101617. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Li, W.; Gao, Z.; Zhang, B.; Ouyang, M. Experimental study on the cell-jet temperatures of abused prismatic Ni-rich automotive batteries under medium and high states of charge. Appl. Therm. Eng. 2022, 202, 117859. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Zhang, Y.; Ouyang, M. Flammability characteristics of the battery vent gas: A case of NCA and LFP lithium-ion batteries during external heating abuse. J. Energy Storage 2019, 24, 100775. [Google Scholar] [CrossRef]
- Gong, Z.; Gu, C.; Sun, J.; Wang, H.; Li, Y.; Zhou, X.; Jia, Y.; Han, D. Experimental study on thermal runaway characteristic and residue of Li(Ni0.8Co0.1Mn0.1)O2 lithium-ion batteries induced by overcharge. J. Energy Storage 2023, 68, 107705. [Google Scholar] [CrossRef]
- Wei, N.; Li, M. Experimental study of thermal runaway process of 256Ah prismatic nickel-rich battery. Front. Energy Res. 2023, 11, 1230429. [Google Scholar] [CrossRef]






| Item | Specification | Condition |
|---|---|---|
| Nominal Capacity | 156 Ah | 25 °C, 52 A(1/3C) DC to 2.8 V |
| Energy | 572 Wh | 25 °C, 52 A(1/3C) DC to 2.8 V |
| Specific Energy | 248.00 Wh/Kg | 25 °C, 52 A(1/3C) DC to 2.8 V |
| Operating voltage | 2.8~4.2 V | −30 °C ≤ T ≤ 55 °C |
| Cathode | Li(Ni0.8Co0.1Mn0.1)O2 | N.A. |
| Anode | Graphite | N.A. |
| Standard Voltage | 3.637 ± 0.01 V | 25 °C, BOL, 40% SOC |
| Operating temperature (Charge) | −20~55 °C | N.A. |
| Operating temperature (discharge) | −30~55 °C | N.A. |
| Cycle life | ≥1500 | 25 °C, 1C/1C, 5–97% SOC, 80% SOH |
| discharge power | ≥2100 W | 25 °C, 50% SOC, 10 s |
| Discharge power density | ≥912 W/Kg | 25 °C, 50% SOC, 10 s |
| SOC | 100% | N.A. |
| Cell weight | 2287 ± 25 g | N.A. |
| Cell dimension | 220 × 102 × 45 mm | N.A. |
| Shell Material | Aluminum alloy | N.A. |
| Experiment | Du (s) | Df (s) | Ds (s) | De (s) | (dP/dt)max (Kpa/s) |
|---|---|---|---|---|---|
| 1st | 2 | 32 | 47 | 81 | 17 |
| 2nd | 3 | 18 | 53 | 74 | 10.8 |
| 3rd | 2 | 17 | 53 | 72 | 14.8 |
| Average | 2.3 | 22.3 | 51 | 75.7 | 14.2 |
| Experiment | P (Pa) | Tambient-average (K) | Tambient-max (K) | n (mol) | nAh (mol/Ah) |
|---|---|---|---|---|---|
| 1st | 143,200 | 321.45 | 465.7 | 13.3 | 0.085 |
| 2nd | 144,889 | 318.87 | 453.2 | 14.4 | 0.092 |
| 3rd | 141,911 | 314.62 | 438.6 | 14.0 | 0.090 |
| Average | 143,333 | 318.31 | 452.5 | 13.9 | 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H. Study on Thermal Runaway Behavior and Jet Characteristics of a 156 Ah Prismatic Ternary Lithium Battery. Batteries 2024, 10, 282. https://doi.org/10.3390/batteries10080282
Zhang H. Study on Thermal Runaway Behavior and Jet Characteristics of a 156 Ah Prismatic Ternary Lithium Battery. Batteries. 2024; 10(8):282. https://doi.org/10.3390/batteries10080282
Chicago/Turabian StyleZhang, Huipeng. 2024. "Study on Thermal Runaway Behavior and Jet Characteristics of a 156 Ah Prismatic Ternary Lithium Battery" Batteries 10, no. 8: 282. https://doi.org/10.3390/batteries10080282
APA StyleZhang, H. (2024). Study on Thermal Runaway Behavior and Jet Characteristics of a 156 Ah Prismatic Ternary Lithium Battery. Batteries, 10(8), 282. https://doi.org/10.3390/batteries10080282





