Experimental and Modeling Study of Arc Fault Induced Thermal Runaway in Prismatic Lithium-Ion Batteries
Abstract
1. Introduction
2. Arc Experiment of Batteries
2.1. Battery Selection
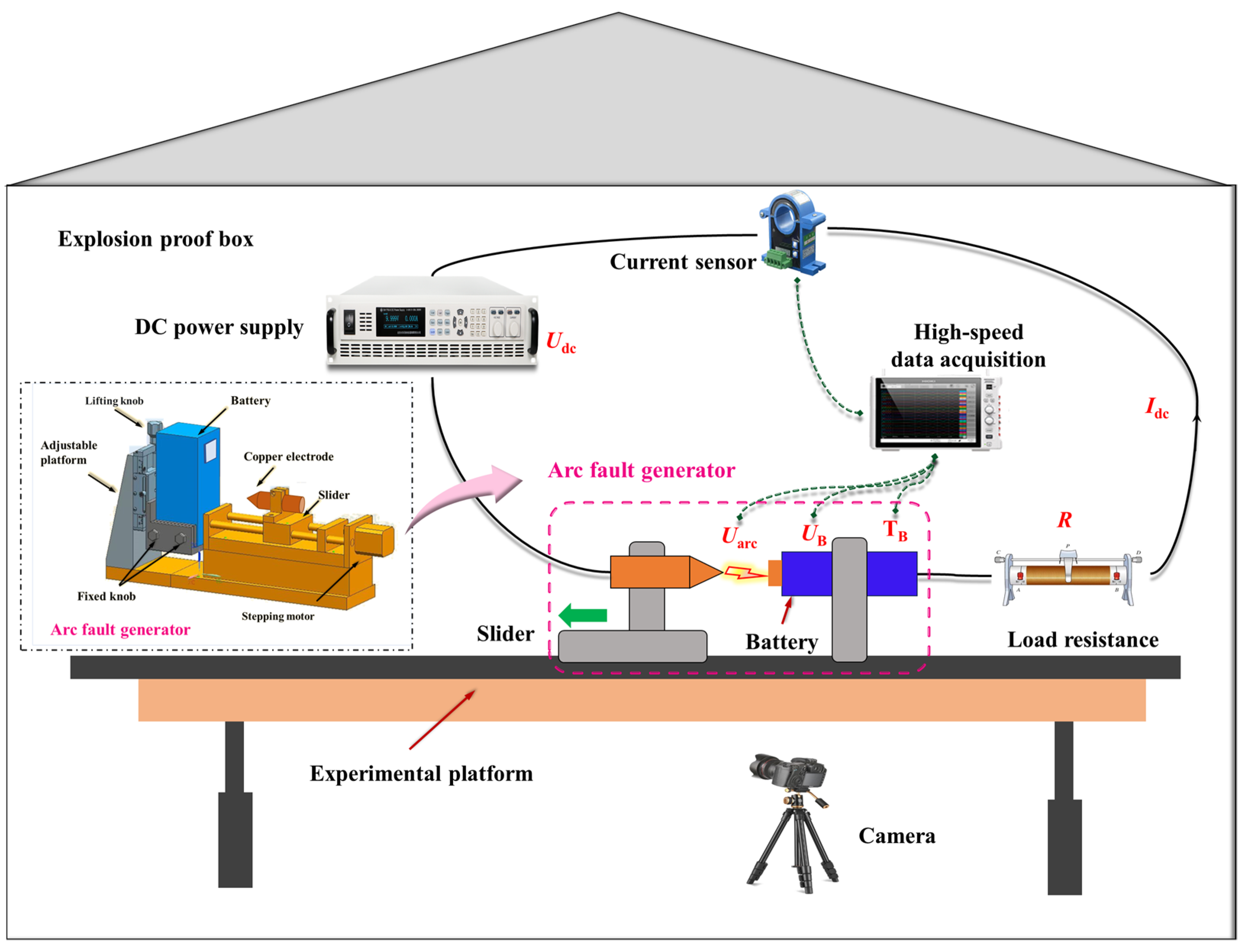
2.2. Experimental Platform
2.2.1. Main Circuit and Measurement Equipment
2.2.2. Experiment Procedure
- Circuit connection:
- 2.
- Parameter settings:
- (1)
- Circuit parameters: power supply voltage Udc = 200–300 V, load resistance R = 10–20 Ω.
- (2)
- AFG parameters: The stepper motor is controlled by a computer. The separation gap L = 1 mm between movable electrode and battery.
- (3)
- Test subject: Battery top cover, battery with 100% state of charge (SOC).
- (4)
- Measurement equipment parameters: The sampling frequency of the high-speed data acquisition system is 1 kHz, recording real-time data of battery voltage UB, arc voltage Uarc, circuit current Idc, and temperature TB. A high-definition camera is used to monitor the entire experiment process. A type K-thermocouple is utilized to capture changes in battery temperature TB caused by the evolution of the arc during the experiment.
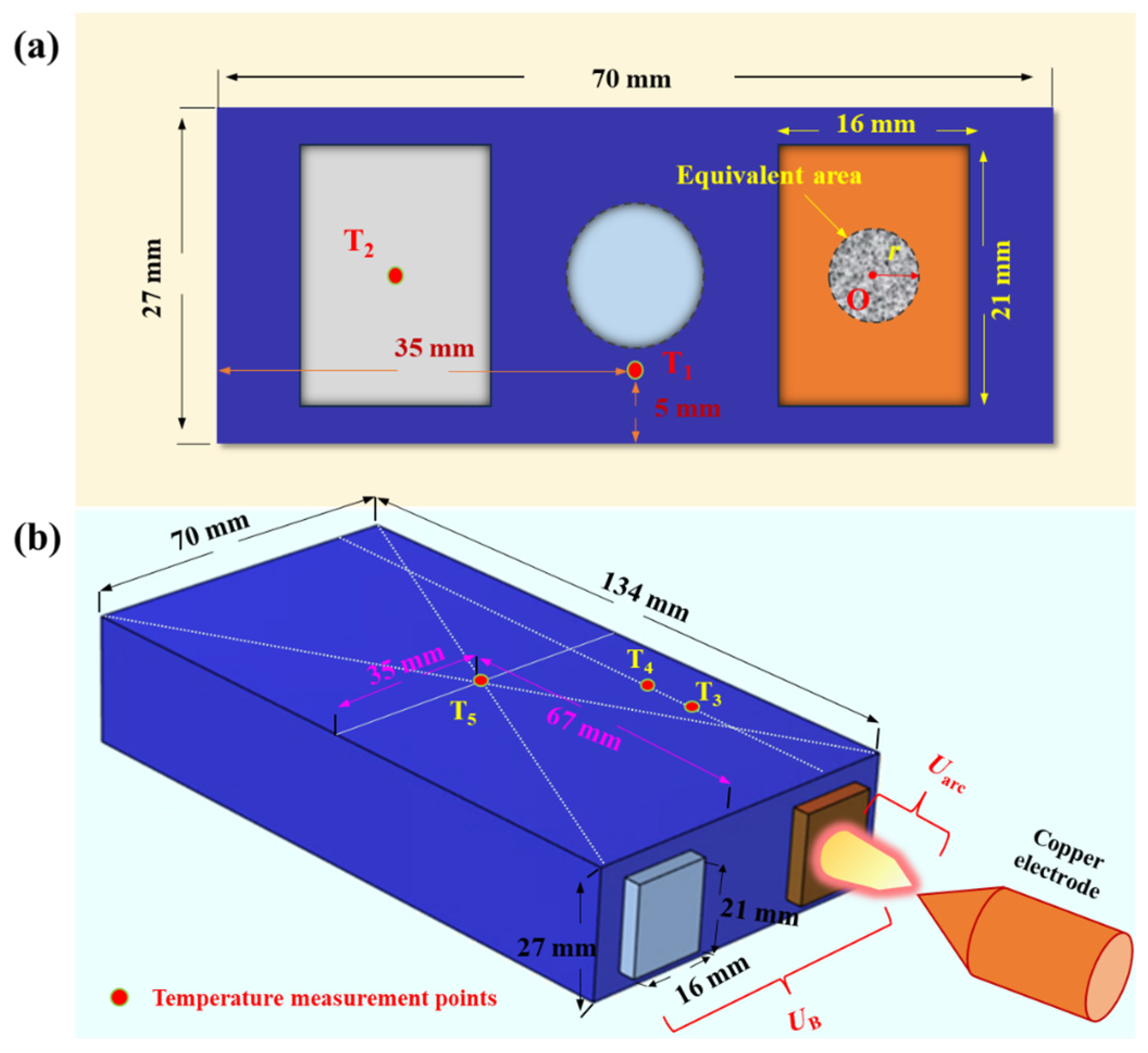
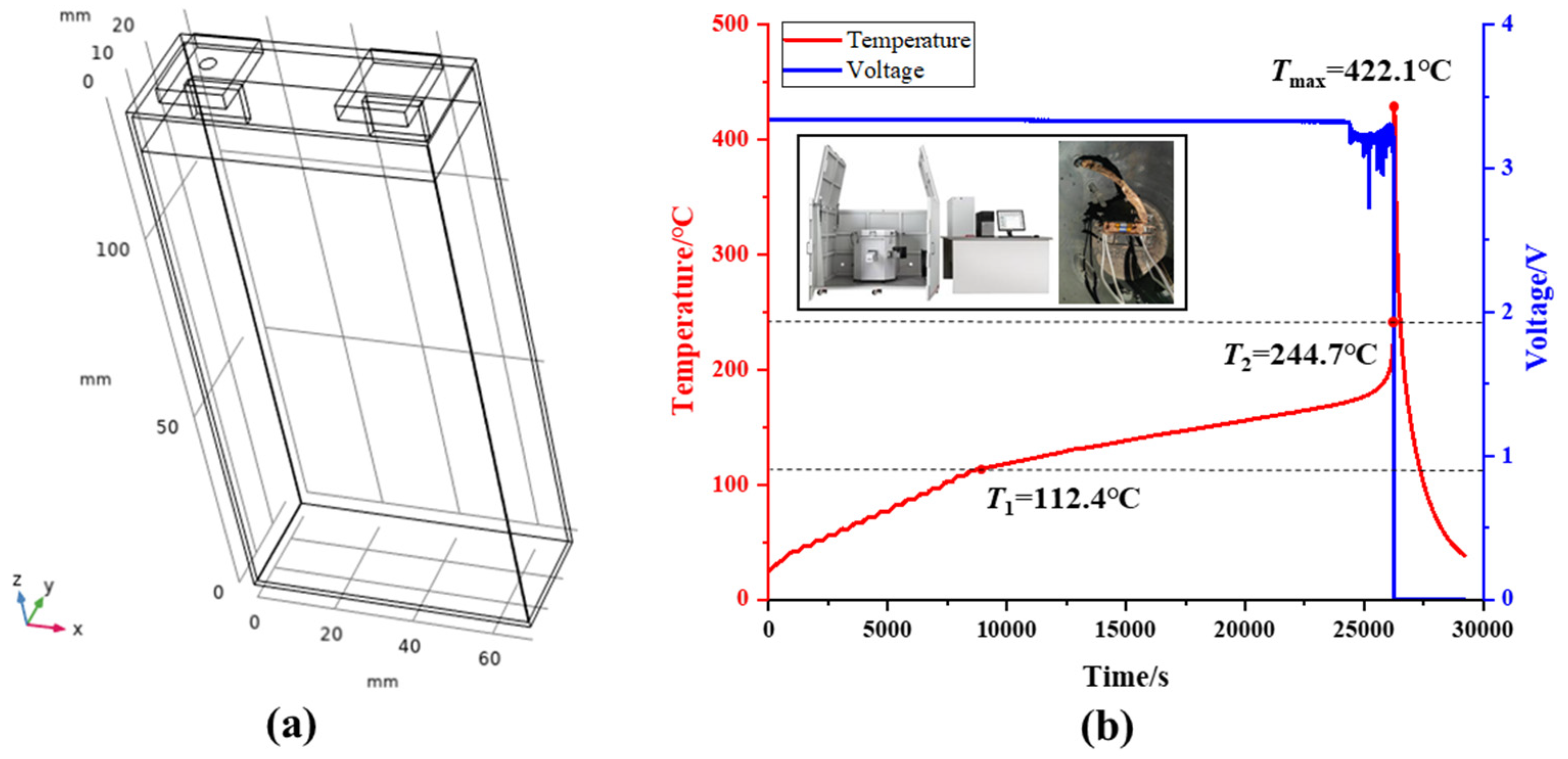
- Battery top cover: 2 predefined temperature points (T1 and T2). T1 is located 8.5 mm below the center point of the top cover surface, and T2 is at the center point of the positive electrode terminal surface, as shown in Figure 2a.
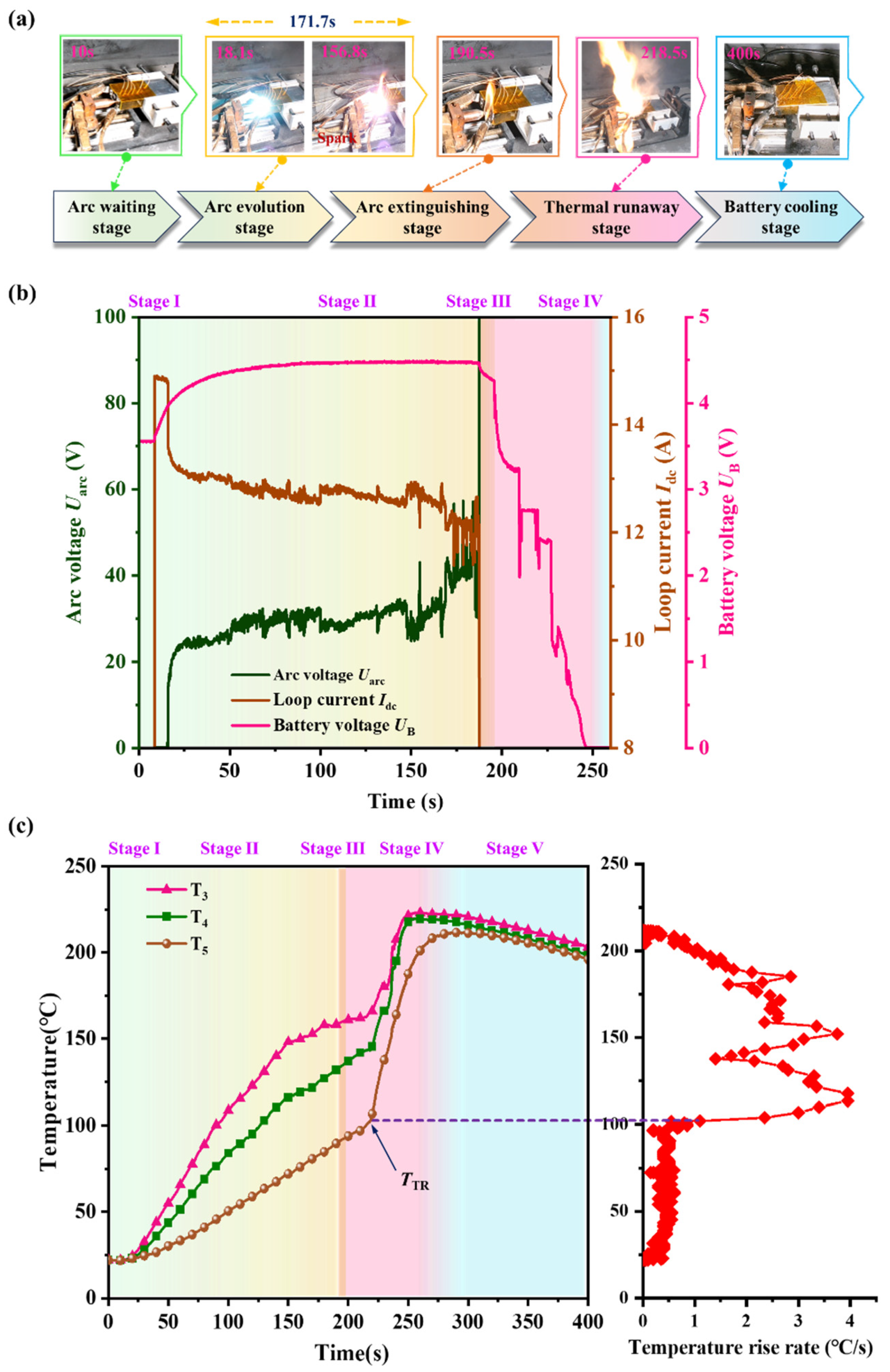
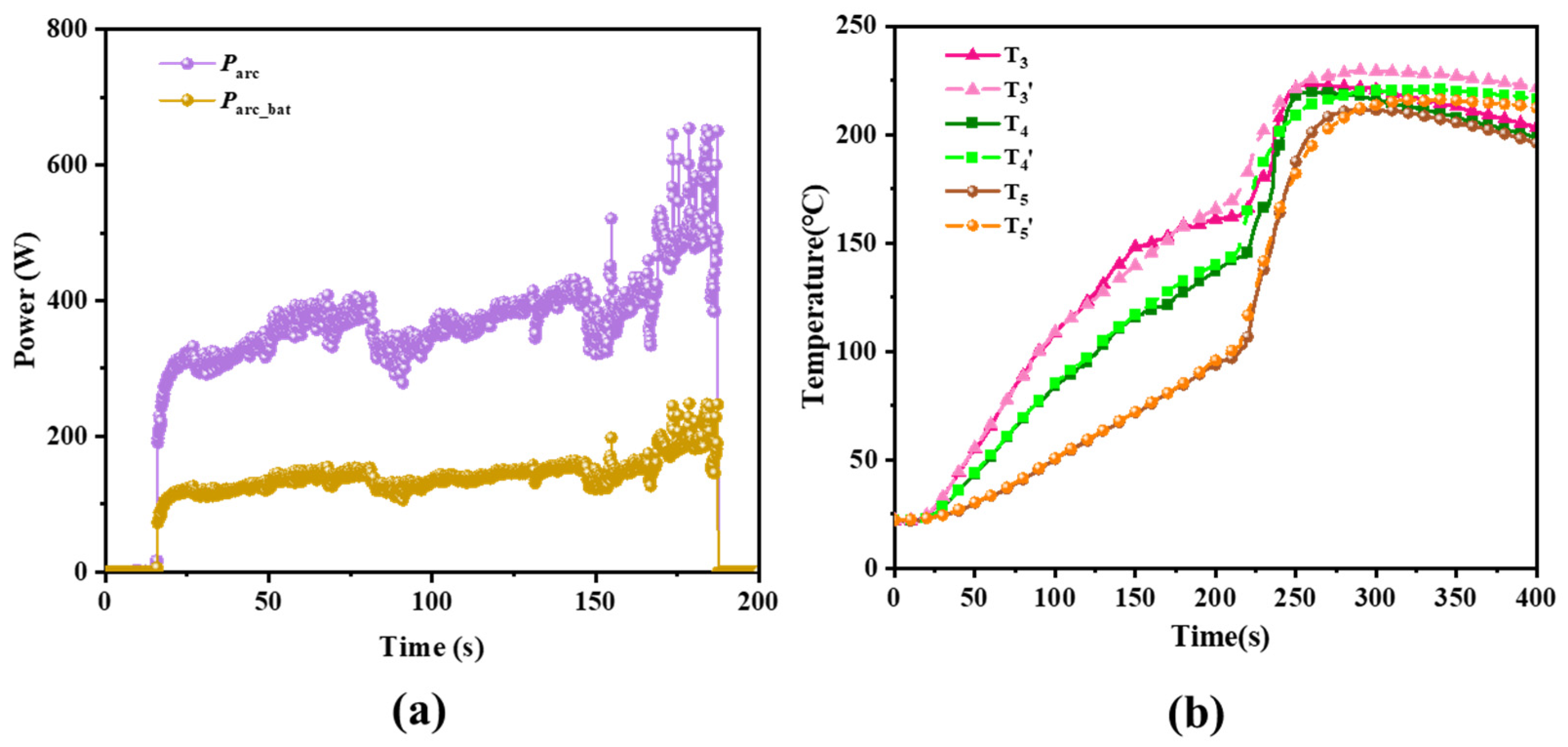
- Battery: The battery is equipped with 3 predefined temperature points (T3, T4, and T5) located near the negative electrode, as depicted in Figure 2b. Among these points, T3, T4, and the center point O of the negative electrode terminal are aligned on the same axis. T3 is positioned 3 cm from the top cover, T4 is 4 cm from the top cover, and T5 is located at the center of the larger face of battery, as shown in Figure 2b. In this study, the critical temperatures for T5, defined as the thermal transition rate (TTR), encompass gradual temperature increases and sharp temperature rises, indicating TR of the battery. The threshold for determining TTR is set at 1 °C/s [20].
- 3.
- Experimental procedure:
2.3. Results and Analysis
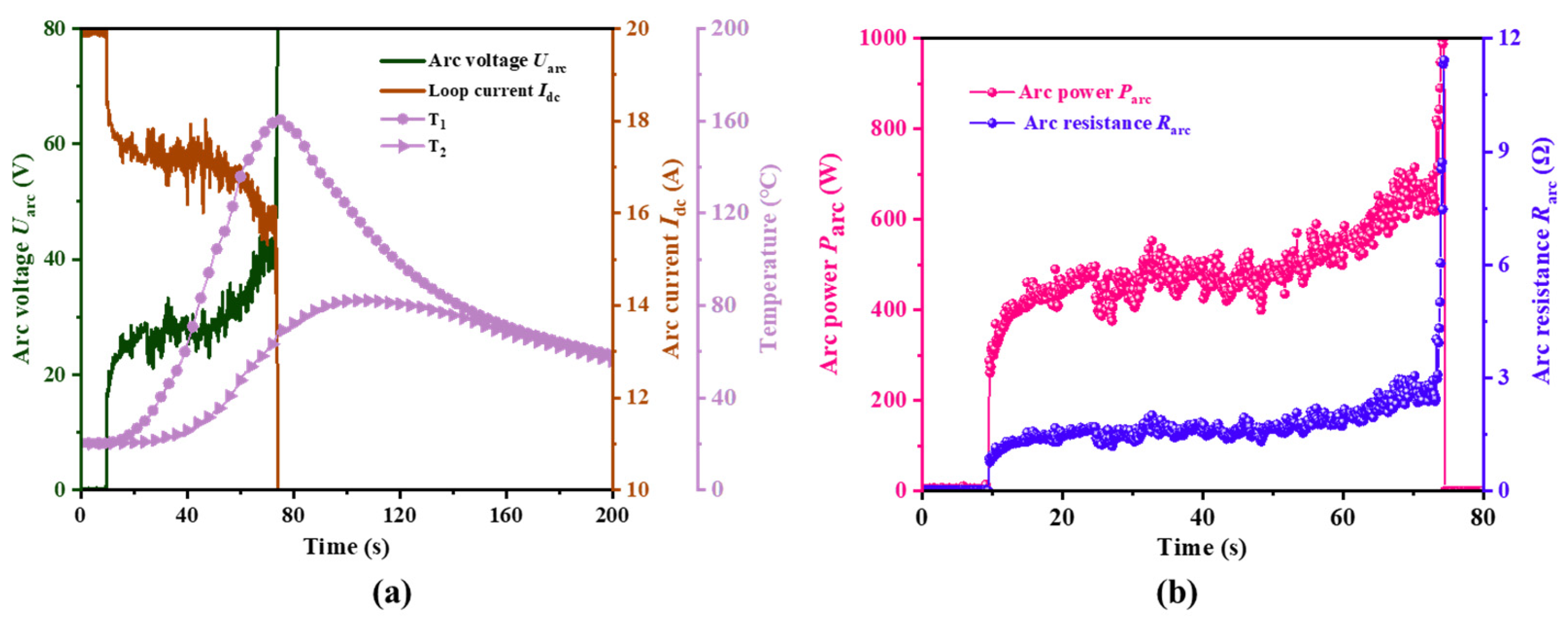
2.3.1. Arc Experiment on the Negative Terminal of Battery Top Cover
2.3.2. Arc Experiment on the Negative Terminal of Battery
3. The Model of Arc-Induced TR in Batteries
3.1. The Principle of Equivalent Heat Source of an Arc
- (1)
- Maintain the overall structure of the arc model and simplify the battery TR model, focusing on capturing the evolutionary characteristics of the arc itself.
- (2)
- Maintain the structure of the battery TR model and simplify the arc model, emphasizing the impact of the arc on inducing thermal hazards in the battery.
3.1.1. Equivalent Area Sarc
3.1.2. Equivalent Power Parc_bat
3.2. Model Establishment
3.2.1. Geometric Description
3.2.2. Determination of Power Transfer Coefficient k
3.2.3. Control Equations, Heat Generation, and Heat Transfer of 3D TR Model
3.3. Model Establishment
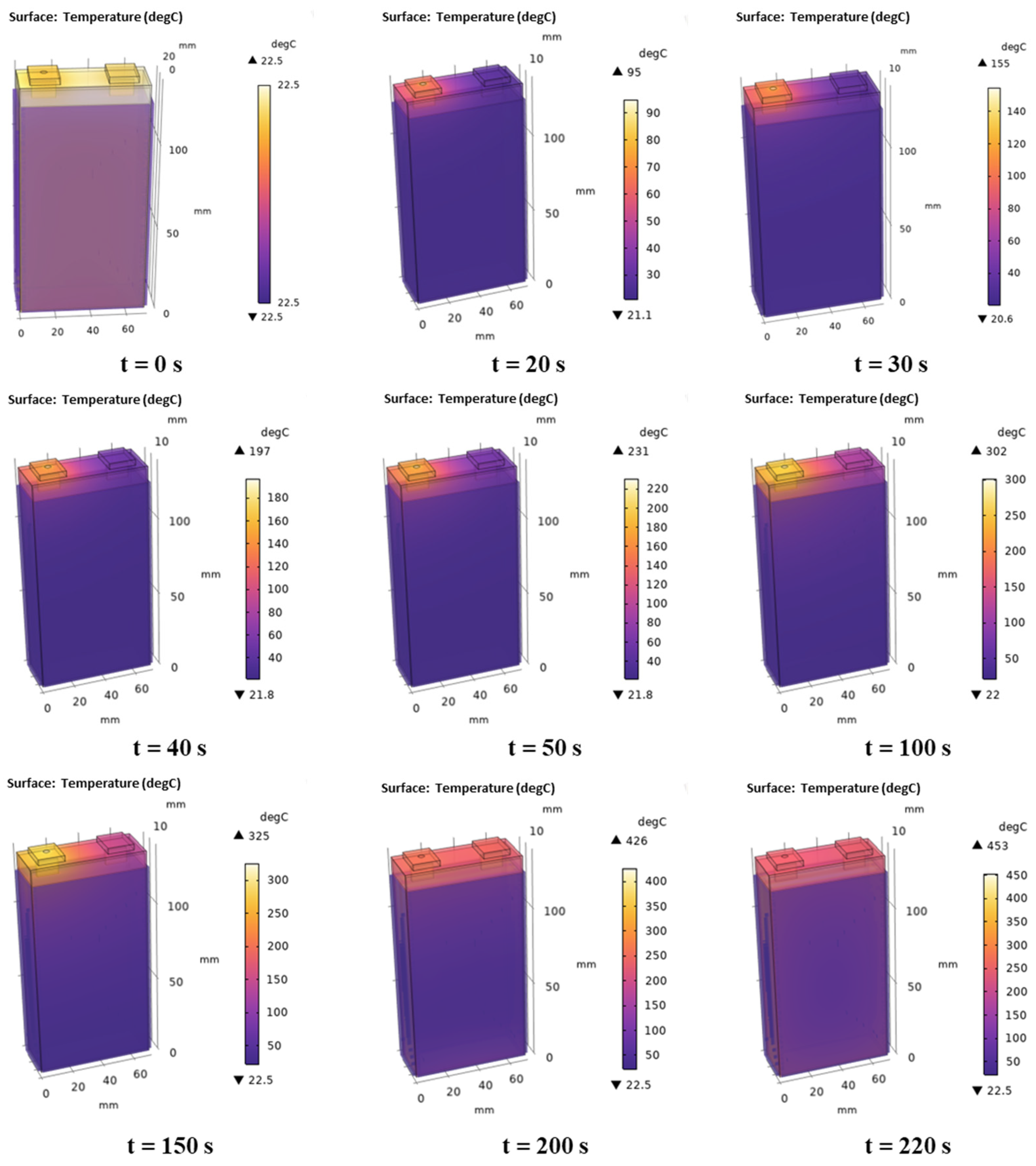
3.4. Model Analysis
4. Conclusions
- (1)
- A specialized experimental platform was established to study arc faults in battery systems, simulating the generation, evolution, and catastrophic effects of arcs at the negative terminal of the battery. During the experiments, it was observed that the minimum arc voltage was approximately 15.8 V, and the stable arc voltage ranged between 20–30 V. Under experimental conditions of 300 V and 20 Ω, the arc lasted for 171.7 s, ultimately leading to TR of the battery. This indicates that arcs are a novel factor contributing to TR in batteries.
- (2)
- This paper employs the equivalent arc heat source method to simplify arc simulation, streamline the computation process, and enhance the coupling simulation between the arc and the battery. Initially, key parameters of the arc heat source equivalent method were determined based on experiments and models involving the battery cover: power transfer coefficient k = 0.38, equivalent radius r = 1.86 mm, and equivalent power Parc_bat = 0.38 × Parc. Subsequently, using COMSOL software and experimental data from arc tests on battery, a coupled 3D model was developed to successfully simulate the process of arc-induced TR in batteries. The research results indicate that there is a time difference of 3.5 s between simulation and experimental TR at the center of the battery surface, with a RMSE = 5.8 °C in temperature profiles. This demonstrates that the simulation model exhibits high accuracy.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wassiliadis, N.; Steinsträter, M.; Schreiber, M.; Rosner, P.; Nicoletti, L.; Schmid, F.; Ank, M.; Teichert, O.; Wildfeuer, L.; Schneider, J.; et al. Quantifying the state of the art of electric powertrains in battery electric vehicles: Range, efficiency, and lifetime from component to system level of the Volkswagen ID.3. eTransportation 2022, 12, 100167. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Shang, Z.; Zhang, Y.; Xu, S. A review on comprehensive recycling of spent power lithium-ion battery in China. eTransportation 2022, 11, 100155. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wang, J. In-depth analysis on thermal hazards related research trends about lithium-ion batteries: A bibliometric study. J. Energy Storage 2021, 35, 102253. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Ren, D.; Ouyang, M.; Lu, L.; Han, X. Thermal Runaway Triggered by Plated Lithium on the Anode after Fast Charging. ACS Appl. Mater. Interfaces 2019, 11, 46839–46850. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.A.; Milojevic, Z.; Wise, M.S.; Ahmeid, M.; Attidekou, P.S.; Mrozik, W.; Dickmann, N.A.; Restuccia, F.; Lambert, S.M.; Das, P.K. Thermal and mechanical abuse of electric vehicle pouch cell modules. Appl. Therm. Eng. 2021, 189, 116623. [Google Scholar] [CrossRef]
- Nie, B.; Dong, Y.; Chang, L. The evolution of thermal runaway parameters of lithium-ion batteries under different abuse conditions: A review. J. Energy Storage 2024, 96, 112624. [Google Scholar] [CrossRef]
- Xavier, L.S.; Amorim, W.C.S.; Cupertino, A.F.; Mendes, V.F.; do Boaventura, W.C.; Pereira, H.A. Power converters for battery energy storage systems connected to medium voltage systems: A comprehensive review. BMC Energy 2019, 1, 7. [Google Scholar] [CrossRef]
- Xu, W.; Wu, X.; Li, Y.; Wang, H.; Lu, L.; Ouyang, M. A comprehensive review of DC arc faults and their mechanisms, detection, early warning strategies, and protection in battery systems. Renew. Sustain. Energy Rev. 2023, 186, 113674. [Google Scholar] [CrossRef]
- Yoon, Y.-H. Safety Analysis of Smart Grid Lines According to DC Arc Generation. J. Electr. Eng. Technol. 2023, 18, 697–703. [Google Scholar] [CrossRef]
- Augeard, A.; Singo, T.; Desprez, P.; Abbaoui, M.H. Contribution to the study of electric arcs in lithium-ion batteries. IEEE Trans. Compon. Packag. Manuf. Technol. 2016, 6, 1066–1076. [Google Scholar] [CrossRef]
- Augeard, A.; Singo, T.; Desprez, P.; Abbaoui, M.H. Numerical methods for detecting DC arc fault in lithium-ion batteries. In Proceedings of the 2015 IEEE 61st Holm Conference on Electrical Contacts (Holm), San Diego, CA, USA, 11–14 October 2015; pp. 39–46. [Google Scholar]
- Guo, L.; Ke Xb, T.Y.S. Design of arc fault detection method and test system for new energy automobiles. Insul. Mater. 2018, 51, 74–79. [Google Scholar]
- Jie, D.; Baohui, C.; Jiazheng, L.; Tiannian, Z.; Chuanping, W. Thermal runaway and combustion characteristics, risk and hazard evaluation of lithium-iron phosphate battery under different thermal runaway triggering modes. Appl. Energy 2024, 368, 123451. [Google Scholar] [CrossRef]
- Lystianingrum, V.; Priyadi, A.; Negara, I.M.Y. Lessons learned from large-scale lithium-ion battery energy storage systems incidents: A mini review. Process Saf. Prog. 2023, 42, 348–355. [Google Scholar] [CrossRef]
- Dong, C.; Gao, B.; Li, Y.; Wu, X. Experimental and Model Analysis of the Thermal and Electrical Phenomenon of Arc Faults on the Electrode Pole of Lithium-Ion Batteries. Batteries 2024, 10, 127. [Google Scholar] [CrossRef]
- Park, Y.; Song, T. Plasma arc simulation of high voltage circuit breaker with a hybrid 2D/3D model. In Proceedings of the 2022 6th International Conference on Electric Power Equipment-Switching Technology (ICEPE-ST), Seoul, Republic of Korea, 15–18 March 2022; pp. 190–193. [Google Scholar]
- Jin, C.; Sun, Y.; Wang, H.; Lai, X.; Wang, S.; Chen, S.; Rui, X.; Zheng, Y.; Feng, X.; Wang, H. Model and experiments to investigate thermal runaway characterization of lithium-ion batteries induced by external heating method. J. Power Sources 2021, 504, 230065. [Google Scholar] [CrossRef]
- Johnson, J.; Schoenwald, D.; Kuszmaul, S.; Strauch, J.; Bower, W. Creating dynamic equivalent PV circuit models with impedance spectroscopy for arc fault modeling. In Proceedings of the 2011 37th IEEE Photovoltaic Specialists Conference, Seattle, WA, USA, 19–24 June 2011; pp. 002328–002333. [Google Scholar]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Liu, X.; Jin, C.; Zhang, F.; Xu, C. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Liu, K.; Yang, F.; Wang, S.; Gao, B.; Xu, C. The research on the heat source characteristics and the equivalent heat source of the arc in gaps. Int. J. Heat Mass Transf. 2018, 124, 177–189. [Google Scholar] [CrossRef]
- Yang, F.; Liu, K.; Wang, S.; Gao, B.; Ai, S.; Zheng, X.; Le, Y.; Uilah, I. A thermal-stress field calculation method based on the equivalent heat source for the dielectric fitting under discharging. Appl. Therm. Eng. 2018, 138, 183–196. [Google Scholar] [CrossRef]
- Wang, H.; Du, Z.; Rui, X.; Wang, S.; Jin, C.; He, L.; Zhang, F.; Wang, Q.; Feng, X. A comparative analysis on thermal runaway behavior of Li (NixCoyMnz) O2 battery with different nickel contents at cell and module level. J. Hazard. Mater. 2020, 393, 122361. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Anwar, S.; Casa, J.; Hasnain, M.; Ahmed, H.; Sezer, H. Modeling Thermal Runaway in Prismatic Lithium-Ion Batteries. In Proceedings of the ASME 2023 International Mechanical Engineering Congress and Exposition, New Orleans, LA, USA, 29 October–2 November 2023; p. V010T011A048. [Google Scholar]





| Battery Cell | Specification |
|---|---|
| Cathode material | LiFePO4 |
| Anode material | graphite |
| Nominal capacity (Ah) | 20 |
| Nominal voltage (V) | 3.2 |
| Dimensions L × W × H (mm) | 70 × 27 × 134 |
| Top cover dimensions(mm) | 70 × 27 × 4 |
| Weight (kg) | 0.42 |
| Equipment | Parameters |
|---|---|
| DC power supply (V) | 0–1500 |
| Hall current sensor (A) | 0–25 |
| Load resistance (Ω) | 0–30 |
| High-speed data acquisition (MS/s) | 0–10 |
| Camera (dpi) | 2048 × 1080 |
| Arc fault generator | UL1699B |
| Thermocouple | K-type |
| Component Name | Material | ρ [kg·m−3] | Cp [J·kg−1·K−1] | λ [W·m−1·K−1] |
|---|---|---|---|---|
| Battery shell | Al | 2700 | 880 | 158 |
| Battery core | Al | 2700 | 880 | 158 |
| Battery cover | User-defined | 1900 | 981 | λx = λz = 29 λy = 0.20 |
| Positive terminal | Al | 2700 | 880 | 237 |
| Negative terminal | Cu | 8960 | 385 | 400 |
| Air space | Air | 0.3–1 | ~1 | 3–8 |
| Parameters | Material | Thickness [mm] | Heat Transfer Coefficient [W·m−¹·K−¹] | Heat Dissipation Coefficient [W·m−²·K−¹] |
|---|---|---|---|---|
| Heat conduction | Insulating layer between the equivalent heat source and the battery shell | 0.05 | 0.023 | / |
| Insulating layer between the front face of the cell and the battery shell | 0.1 | 0.023 | / | |
| Insulating layer between the side face of the cell and the battery shell | 0.5 | 0.023 | / | |
| Insulating layer between the rear face of the cell and the battery shell | 0.2 | 0.023 | / | |
| Convective heat transfer coefficient | Equivalent heat source | / | / | 18 |
| The top cover and terminal | / | / | 18 | |
| The circum of the shell | / | / | 20 | |
| The bottom of the shell | / | / | 20 |
| Parameters | T3 | T3′ | T4 | T4′ | T5 | T5′ |
|---|---|---|---|---|---|---|
| tarc_TR/s | 201 | 195.8 | 201.8 | 197.7 | 202.5 | 199 |
| Time difference/s | 5.2 | 4.1 | 3.5 | |||
| RMSE before arc extinguishes/°C | 2.86 | 2.21 | 0.55 | |||
| RMSE in the first 300 s/°C | 6.47 | 6.00 | 2.67 | |||
| RMSE in the first 400 s/°C | 9.02 | 8.07 | 5.80 | |||
| Parameters | T3 | T3′ | T4 | T4′ | T5 | T5′ |
|---|---|---|---|---|---|---|
| tarc_TR/s | 179.7 | 174.2 | 180.8 | 177.1 | 182.1 | 179.3 |
| Time difference/s | 5.5 | 3.7 | 2.9 | |||
| RMSE before arc extinguishes/°C | 3.61 | 3.56 | 2.15 | |||
| RMSE in the first 300 s/°C | 7.03 | 6.81 | 4.80 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Zhou, K.; Wang, H.; Lu, L.; Gao, B.; Wang, Y.; Li, Y. Experimental and Modeling Study of Arc Fault Induced Thermal Runaway in Prismatic Lithium-Ion Batteries. Batteries 2024, 10, 269. https://doi.org/10.3390/batteries10080269
Xu W, Zhou K, Wang H, Lu L, Gao B, Wang Y, Li Y. Experimental and Modeling Study of Arc Fault Induced Thermal Runaway in Prismatic Lithium-Ion Batteries. Batteries. 2024; 10(8):269. https://doi.org/10.3390/batteries10080269
Chicago/Turabian StyleXu, Wenqiang, Kai Zhou, Hewu Wang, Languang Lu, Bin Gao, Yan Wang, and Yalun Li. 2024. "Experimental and Modeling Study of Arc Fault Induced Thermal Runaway in Prismatic Lithium-Ion Batteries" Batteries 10, no. 8: 269. https://doi.org/10.3390/batteries10080269
APA StyleXu, W., Zhou, K., Wang, H., Lu, L., Gao, B., Wang, Y., & Li, Y. (2024). Experimental and Modeling Study of Arc Fault Induced Thermal Runaway in Prismatic Lithium-Ion Batteries. Batteries, 10(8), 269. https://doi.org/10.3390/batteries10080269








