An Investigation into Electrolytes and Cathodes for Room-Temperature Sodium–Sulfur Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Cell Components
- 1M NaTFSI in DOL/DME 1:1 v/v;
- 1M NaTFSI in TEGDME;
- 1M NaFSI in DOL/DME 1:1 v/v;
- 1M NaFSI in TEGDME;
- 1M NaCF3SO3 in DOL/DME 1:1 v/v;
- 1M NaCF3SO3 in TEGDME.
2.2. Methods
3. Results
3.1. Results of Electrochemical Testing
3.2. Results of Material Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, K.; Zhao, F.; Hao, H.; Liu, Z. Selection of Li-ion battery technologies for electric vehicles under China’s new energy vehicle credit regulation. Energy Procedia 2019, 158, 3038–3044. [Google Scholar] [CrossRef]
- Najera, J.; Arribas, J.R.; de Castro, R.M.; Nunez, C.S. Semi-empirical ageing model for LFP and NMC Li-ion battery chemistries. J. Energy Storage A 2023, 72, 108016. [Google Scholar] [CrossRef]
- Sanad, M.M.S.; Meselhy, N.K.; El-Boraey, H.A. Surface protection of NMC811 cathode material via ZnSnO3 perovskite film for enhanced electrochemical performance in rechargeable Li-ion batteries. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 672, 131748. [Google Scholar] [CrossRef]
- Minnetti, L.; Marangon, V.; Andreotti, P.; Staffolani, A.; Nobili, F.; Hassoun, J. Reciprocal irreversibility compensation of LiNi0.2Co0.2Al0.1Mn0.45O2 cathode and silicon oxide anode in new Li-ion battery. Electrochim. Acta 2023, 452, 142263. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, N.Q.; Thai, D.; Tieu, T.D.; Tran, V.; Le, M.L.P. Enabling stable and high-rate of an olivine-type cathode LiFePO4 for Li-ion batteries by using graphene nanoribbons as conductive agent. Adv. Nat. Sci.-Nanosci. Nanotechnol. 2023, 14, 015009. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chiang, C.L.; Wu, X.H.; Tang, Y.L.; Zeng, G.F.; Zhou, S.Y.; Zhang, B.D.; Zhang, H.T.; Yan, Y.W.; Liu, T.T.; et al. Prolonged lifespan of initial-anode-free lithium-metal battery by pre-lithiation in Li-rich Li2Ni0.5Mn1.5O4 spinel cathode. Chem. Sci. 2023, 14, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Kwon, H.; Lim, S.; Jeong, T.; Jung, H.C.; Kim, Y.I.; Han, D.W. Unexplored orthorhombic LiMn1-XTiXO2 cathode materials with a stable atomic site occupancy and phase transition. Energy Fuels 2023, 37, 1404–1413. [Google Scholar] [CrossRef]
- Lin, K.; Xu, X.; Qin, X.; Liu, M.; Zhao, L.; Yang, Z.; Liu, Q.; Ye, Y.; Chen, G.; Kang, F.; et al. Batteries with high energy density realized by symbiotic anode and prelithiated cathode. Nano-Micro Lett. 2022, 14, 149. [Google Scholar] [CrossRef]
- Kang, J.; Atwair, M.; Nam, I.; Lee, C.J. Experimental and numerical investigation on effects of thickness of NCM622 cathode in Li-ion batteries for high energy and power density. Energy 2022, 263, 125801. [Google Scholar] [CrossRef]
- Singh, S.K.; Dutta, D.P.; Gupta, H.; Srivastava, N.; Mishra, R.; Meghnani, D.; Tiwari, R.K.; Patel, A.; Tiwari, A.; Singh, R.K. Electrochemical investigation of double layer surface-functionalized Li-NMC cathode with nano-composite gel polymer electrolyte for Li-battery applications. Electrochim. Acta 2022, 435, 141328. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, C.; Chi, Z.; Ke, F.; Yang, Y.; Wang, A.; Wang, W.; Miao, L. How far away are lithium-sulfur batteries from commercialization? Frontiers 2019, 7, 00123. [Google Scholar] [CrossRef]
- Fei, Y.; Li, G. Unveiling the pivotal parameters for advancing high energy density in lithium-sulfur batteries: A comprehensive review. Adv. Funct. Mater. 2024, 34, 2312550. [Google Scholar] [CrossRef]
- Zhao, M.; Peng, H.-J.; Li, B.-Q.; Huang, J.-Q. Kinetic promoters for sulfur cathodes in lithium-sulfur batteries. Acc. Chem. Res. 2024, 57, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Brieske, D.M.; Warnecke, A.; Sauer, D.U. Modeling the volumetric expansion of the lithium-sulfur battery considering charge and discharge profiles. Energy Storage Mater. 2023, 55, 289–300. [Google Scholar] [CrossRef]
- Shaibani, M.; Mirshekarloo, M.S.; Singh, R.; Easton, C.D.; Cooray, M.C.D.; Eshraghi, N.; Abendroth, T.; Dörfler, S.; Holger Althues, H.; Kaskel, S.; et al. Expansion-tolerant architectures for stable cycling of ultrahigh-loading sulfur cathodes in lithium-sulfur batteries. Sci. Adv. 2020, 6, eaay2757. [Google Scholar] [CrossRef]
- Grabe, S.; Dent, M.; Zhang, T.; Tennison, S.; Lekakou, C. A physicochemical model-based digital twin of Li–S batteries to elucidate the effects of cathode microstructure and evaluate different microstructures. J. Power Sources 2023, 580, 233470. [Google Scholar] [CrossRef]
- He, G.; Evers, S.; Liang, X.; Cuisinier, M.; Garsuch, A.; Nazar, L.F. Tailoring porosity in carbon nanospheres for lithium–sulfur battery cathodes. ACS Nano 2013, 7, 10920–10930. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.X.; Fan, K.B.; Zhang, Z.Q.; Cai, L.L.; Zhu, Z.H.; Huang, H.Z.; Zhang, Q.S.; Sun, L.; Zhou, Z.H.; Wang, L.; et al. Cobalt hydroxide decorating N-doped hollow carbon sphere as a new sulfur host to improve long cycle stability in lithium-sulfur batteries. Ioniczangs 2024, online. [Google Scholar] [CrossRef]
- Lu, W.; Wang, L.; Han, C.; Chao, Y.; Xu, C.; Zhu, J.; Tian, Y.; Wang, Z.; Cui, X. MoP quantum dots based multifunctional efficient electrocatalyst for stable and long-life flexible lithium-sulfur batteries. J. Colloid Interface Sci. 2024, 661, 83–90. [Google Scholar] [CrossRef]
- Lu, X.; Meng, Y.S.; Wang, X.; Xiao, M.J.; Xu, Y.S.; Zhu, F.L. Preparation of NiCo2S4/carbon hollow sphere for long cycle lithium sulfur batteries. J. Mater. Sci. Mater. Electron. 2024, 35, 83. [Google Scholar] [CrossRef]
- Zang, J.; An, T.; Dong, Y.; Fang, X.; Zheng, M.; Dong, Q.; Zheng, N. Hollow-in-hollow carbon spheres with hollow foam-like cores for lithium–sulfur batteries. Nano Res. 2015, 8, 2663–2675. [Google Scholar] [CrossRef]
- Li, B.; Xie, M.; Yia, G.; Zhang, C. Biomass-derived activated carbon/sulfur composites as cathode electrodes for Li–S batteries by reducing the oxygen content. RSC Adv. 2020, 10, 2823–2829. [Google Scholar] [CrossRef]
- Moreno, N.; Caballero, A.; Hernán, L.; Morales, J. Lithium–sulfur batteries with activated carbons derived from olive stones. Carbon 2014, 70, 241–248. [Google Scholar] [CrossRef]
- Pozio, A.; Di Carli, M.; Aurora, A.; Falconieri, M.; Seta, L.D.; Prosini, P.P. Hard carbons for use as electrodes in Li-S and Li-ion batteries. Nanomaterials 2022, 12, 1349. [Google Scholar] [CrossRef]
- Lama, F.L.; Marangon, V.; Caballero, A.; Morales, J.; Hassoun, J. Diffusional features of a lithium-sulfur battery exploiting highly microporous activated carbon. ChemSusChem 2023, 16, e202202095. [Google Scholar] [CrossRef]
- Bora, M.; Bhattacharjya, D.; Saikia, B.K. Coal-derived activated carbon for electrochemical energy storage: Status on supercapacitor, Li-ion battery, and Li–S battery applications. Energy Fuels 2021, 35, 18285–18307. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, M.; Lin, Z.; Li, N.; Liu, Y.; Zhao, B.; Pang, H.; Cao, J.; Hea, P.; Shi, Y. Activated carbon with ultrahigh specific surface area synthesized from natural plant material for lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 15889–15896. [Google Scholar] [CrossRef]
- Tonoya, T.; Ando, H.; Takeichi, N.; Senoh, H.; Kojima, T.; Hinago, H.; Matsui, Y.; Ishikawa, M. Capacity decay mechanism of lithium–sulfur batteries using a microporous activated carbon–sulfur composite as the cathode material. J. Phys. Chem. C 2023, 127, 10038–10044. [Google Scholar] [CrossRef]
- Fields, R.; Lei, C.; Markoulidis, F.; Lekakou, C. The composite supercapacitor. Energy Technol. 2016, 4, 517–525. [Google Scholar] [CrossRef]
- Lei, C.; Fields, R.; Wilson, P.; Lekakou, C.; Amini, N.; Tennison, S.; Perry, J.; Gosso, M.; Martorana, B. Development and evaluation of a composite supercapacitor-based 12 V transient start–stop power system for vehicles: Modelling, design and fabrication scaling up. Proc. Inst. Mech. Eng. Part A 2021, 235, 914–927. [Google Scholar] [CrossRef]
- Markoulidis, F.; Lei, C.; Lekakou, C. Investigations of activated carbon fabric-based supercapacitors with different interlayers via experiments and modelling of electrochemical processes of different timescales. Electrochim. Acta 2017, 249, 122–134. [Google Scholar] [CrossRef]
- Elazari, R.; Salitra, G.; Garsuch, A.; Panchenko, A.; Aurbach, D. Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li-S batteries. Adv. Mater. 2011, 23, 5641–5644. [Google Scholar] [CrossRef]
- Swiderska-Mocek, A.; Rudnicka, E. Lithium–sulphur battery with activated carbon cloth-sulphur cathode and ionic liquid as electrolyte. J. Power Sources 2015, 273, 162–167. [Google Scholar] [CrossRef]
- He, N.; Zhong, L.; Xiao, M.; Wang, S.; Han, D.; Meng, Y. Foldable and high sulfur loading 3D carbon electrode for high-performance Li-S battery application. Sci. Rep. 2016, 6, 33871. [Google Scholar] [CrossRef]
- Brehm, W.; Marangon, V.; Panda, J.; Thorat, S.B.; del Rio Castillo, A.E.; Bonaccorso, F.; Pellegrini, V.; Hassoun, J. A Lithium–sulfur battery using binder-free graphene-coated aluminum current collector. Energy Fuels 2022, 36, 9321–9328. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Song, N.; He, J.; Li, X. Graphene and its derivatives in lithium–sulfur batteries. Mater. Today Energy 2018, 9, 319–335. [Google Scholar] [CrossRef]
- Yang, T.; Xia, J.; Piao, Z.; Yang, L.; Zhang, S.; Xing, Y.; Zhou, G. Graphene-based materials for flexible lithium–sulfur batteries. ACS Nano 2021, 15, 13901–13923. [Google Scholar] [CrossRef]
- Tian, J.; Xing, F.; Gao, Q. Graphene-based nanomaterials as the cathode for lithium-sulfur batteries. Molecules 2021, 26, 2507. [Google Scholar] [CrossRef]
- Kim, H.; Lim, H.-D.; Kima, J.; Kang, K. Graphene for advanced Li/S and Li/air batteries. J. Mater. Chem. A 2014, 2, 33–47. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. J. ACS 2011, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Reece, R.; Lekakou, C.; Smith, P.A.; Grilli, R.; Trapalis, C. Sulphur-linked graphitic and graphene oxide platelet-based electrodes for electrochemical double layer capacitors. J. Alloys Compd. 2019, 792, 582–593. [Google Scholar] [CrossRef]
- Baboo, J.P.; Babar, S.; Kale, D.; Lekakou, C.; Laudone, G.M. Designing a graphene coating-based supercapacitor with lithium ion electrolyte: An experimental and computational study via multiscale modeling. Nanomaterials 2021, 11, 2899. [Google Scholar] [CrossRef]
- Vermisoglou, E.C.; Giannakopoulou, T.; Romanos, G.; Boukos, N.; Psycharis, V.; Lei, C.; Lekakou, C.; Petridis, D.; Trapalis, C. Graphene-based materials via benzidine-assisted exfoliation and reduction of graphite oxide and their electrochemical properties. Appl. Surf. Sci. 2017, 392, 244–255. [Google Scholar] [CrossRef]
- Doñoro, A.; Muñoz-Mauricio, A.; Etacheri, V. High-Performance lithium sulfur batteries based on multidimensional graphene-CNT-nanosulfur hybrid cathodes. Batteries 2021, 7, 26. [Google Scholar] [CrossRef]
- Zheng, M.; Chi, Y.; Hu, Q.; Tang, H.; Jiang, X.; Zhang, L.; Zhang, S.; Pang, H.; Xu, Q. Carbon nanotube-based materials for lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 17204–17241. [Google Scholar] [CrossRef]
- Yahalom, N.; Snarski, L.; Maity, A.; Bendikov, T.; Leskes, M.; Weissman, H.; Rybtchinski, B. Durable lithium–sulfur batteries based on a composite carbon nanotube cathode. ACS Appl. Energy Mater. 2023, 6, 4511–4519. [Google Scholar] [CrossRef]
- Yoshie, Y.; Hori, K.; Mae, T.; Noda, S. High-energy-density Li–S battery with positive electrode of lithium polysulfides held by carbon nanotube sponge. Carbon 2021, 182, 32–41. [Google Scholar] [CrossRef]
- Zhu, S.; Sheng, J.; Chen, Y.; Ni, J.; Li, Y. Carbon nanotubes for flexible batteries: Recent progress and future perspective. Natl. Sci. Rev. 2021, 8, nwaa261. [Google Scholar] [CrossRef]
- Ma, L.; Zhuang, H.L.; Wei, S.; Hendrickson, K.E.; Kim, M.S.; Cohn, G.; Hennig, R.G.; Archer, L.A. Enhanced Li–S batteries using amine-functionalized carbon nanotubes in the cathode. ACS Nano 2016, 10, 1050–1059. [Google Scholar] [CrossRef]
- Murugesh, A.K.; Uthayanan, A.; Lekakou, C. Electrophoresis and orientation of multiple wall carbon nanotubes in polymer solution. Appl. Phys. A 2010, 100, 135–144. [Google Scholar] [CrossRef]
- Yu, B.; Chen, Y.; Wang, Z.; Chen, D.; Wang, X.; Zhang, W.; He, J.; He, W. 1T-MoS2 nanotubes wrapped with N-doped graphene as highly-efficient absorbent and electrocatalyst for Li–S batteries. J. Power Sources 2020, 447, 227364. [Google Scholar] [CrossRef]
- Li, M.; Sami, I.; Yang, J.; Li, J.; Kumar, R.V.; Chhowalla, M. Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium–sulfur batteries. Nat. Energy 2023, 8, 84–93. [Google Scholar] [CrossRef]
- Hojaji, E.; Andritsos, E.I.; Li, Z.; Chhowalla, M.; Lekakou, C.; Cai, Q. DFT Simulation-based design of 1T-MoS2 cathode hosts for Li-S batteries and experimental evaluation. Int. J. Mol. Sci. 2022, 23, 15608. [Google Scholar] [CrossRef] [PubMed]
- Andritsos, E.I.; Lekakou, C.; Cai, Q. Single-atom catalysts as promising cathode materials for lithium–sulfur batteries. J. Phys. Chem. C 2021, 125, 18108–18118. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, S.; Wang, T.; Yang, S.-Z.; Johannessen, B.; Chen, H.; Liu, C.; Ye, Y.; Wu, Y.; Peng, Y.; et al. Theoretical calculation guided design of single-atom catalysts toward fast kinetic and long-life Li–S batteries. Nano Lett. 2020, 20, 1252–1261. [Google Scholar] [CrossRef]
- Wang, K.; Liu, S.; Shu, Z.; Zheng, Q.; Zheng, M.; Dong, Q. Single-atom site catalysis in Li–S batteries. Phys. Chem. Chem. Phys. 2023, 25, 25942–25960. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Liang, J.; Ye, S.; Zhang, Q.; Liu, J. Fundamental, application and opportunities of single atom catalysts for Li-S batteries. Energy Storage Mater. 2023, 55, 322–355. [Google Scholar] [CrossRef]
- Sun, X.; Qiu, Y.; Jiang, B.; Chen, Z.; Zhao, C.; Zhou, H.; Yang, L.; Fan, L.; Zhang, Y.; Zhang, N. Isolated Fe-Co heteronuclear diatomic sites as efficient bifunctional catalysts for high-performance lithium-sulfur batteries. Nat. Commun. 2023, 14, 291. [Google Scholar] [CrossRef]
- Grabe, S.; Dent, M.; Babar, S.; Zhang, T.; Tennison, S.; Watts, J.F.; Lekakou, C. Investigation and Determination of Electrochemical Reaction Kinetics in Lithium-Sulfur Batteries with Electrolyte LiTFSI in DOL/DME. J. Electrochem. Soc. 2023, 170, 020527. [Google Scholar] [CrossRef]
- Li, H.; Lampkin, J.; Garcia-Araez, N. Facilitating charge reactions in Al-S batteries with redox mediators. ChemSusChem 2021, 14, 3139–3146. [Google Scholar] [CrossRef]
- Klimpel, M.; Kovalenko, M.V.; Kravchyk, K.V. Advances and challenges of aluminum–sulfur batteries. Commun. Chem. 2022, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Q.; Zhang, R.; Li, Y.; Ma, Y.; Huo, H.; Gao, Y.; Zuo, P.; Wang, J.; Yin, G. Achieving high-energy-density magnesium/sulfur battery via a passivation-free Mg-Li alloy anode. Energy Storage Mater. 2022, 50, 380–386. [Google Scholar] [CrossRef]
- Li, Z.; Vinayan, B.P.; Diemant, T.; Behm, R.J.; Fichtner, M.; Zhao-Karger, Z. Rechargeable calcium–sulfur batteries enabled by an efficient borate-based electrolyte. Small 2020, 16, 2001806. [Google Scholar] [CrossRef]
- Nikiforidis, G.; van de Sanden, M.C.M.; Tsampas, M.N. High and intermediate temperature sodium–sulfur batteries for energy storage: Development, challenges and perspectives. RSC Adv. 2019, 9, 5649–5673. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, B.; Shi, Y.; Jiang, R.; Zhang, H. Research on wide-temperature rechargeable sodium-sulfur batteries: Features, challenges and solutions. Materials 2023, 16, 4263. [Google Scholar] [CrossRef] [PubMed]
- Vudata, S.P.; Bhattacharyya, D. Thermal management of a high temperature sodium sulphur battery stack. Int. J. Heat and Mass Transf. 2021, 181, 122025. [Google Scholar] [CrossRef]
- Antonano, H.C.; Panganiban, J.M.F.; Yu, J.V.T.; Castro, M.T.; Ocon, J.D. Multiphysics modeling of high temperature planar sodium-sulfur batteries. Chem. Eng. Trans. 2022, 94, 1093–1098. [Google Scholar]
- Hueso, K.; Armand, M.; Rojo, T. High temperature sodium batteries: Status, challenges and future trends. Energy Environ. Sci. 2013, 6, 734–749. [Google Scholar] [CrossRef]
- Dent, M.; Jakubczyk, E.; Zhang, T.; Lekakou, C. Kinetics of sulphur dissolution in lithium–sulphur batteries. J. Phys. Energy 2022, 4, 024001. [Google Scholar] [CrossRef]
- Li, G.; Li, Z.; Zhang, B.; Lin, Z. Developments of electrolyte systems for lithium–sulfur batteries: A review. Front. Energy Res. 2015, 3, 00005. [Google Scholar] [CrossRef]
- Gao, J.; Lowe, M.A.; Kiya, Y.; Abruña, H.D. Effects of liquid electrolytes on the charge–discharge performance of rechargeable lithium/sulfur batteries: Electrochemical and in-situ X-ray absorption spectroscopic studies. J. Phys. Chem. C 2011, 115, 25132–25137. [Google Scholar] [CrossRef]
- Yim, T.; Park, M.-S.; Yu, J.-S.; Kim, K.J.; Im, K.Y.; Kim, J.-H.; Jeong, G.; Jo, Y.N.; Woo, S.-G.; Kang, K.S.; et al. Effect of chemical reactivity of polysulfide toward carbonate-based electrolyte on the electrochemical performance of Li–S batteries. Electrochim. Acta 2013, 107, 454–460. [Google Scholar] [CrossRef]
- He, M.; Li, X.; Yang, X.; Wang, C.; Zheng, M.L.; Li, R.; Zuo, P.; Yin, G.; Sun, X. Realizing solid-phase reaction in Li–S batteries via localized high-concentration carbonate electrolyte. Adv. Energy Mater. 2021, 11, 2101004. [Google Scholar] [CrossRef]
- Rafie, A.; Kim, J.W.; Sarode, K.K.; Kalra, V. A review on the use of carbonate-based electrolytes in Li-S batteries: A comprehensive approach enabling solid-solid direct conversion reaction. Energy Storage Mater. 2022, 50, 197–224. [Google Scholar] [CrossRef]
- Li, X.; Banis, M.; Lushington, A.; Yang, X.; Sun, Q.; Zhao, Y.; Liu, C.; Li, Q.; Wang, B.; Xiao, W.; et al. A high-energy sulfur cathode in carbonate electrolyte by eliminating polysulfides via solid-phase lithium-sulfur transformation. Nat. Commun. 2018, 9, 4509. [Google Scholar] [CrossRef]
- Syali, M.S.; Kumar, D.; Mishra, K.; Kanchan, D.K. Recent advances in electrolytes for room-temperature sodium-sulfur batteries: A review. Energy Storage Mater. 2020, 31, 352–372. [Google Scholar] [CrossRef]
- Wei, S.; Xu, S.; Agrawral, A.; Choudhury, S.; Lu, Y.; Tu, Z.; Ma, L.; Archer, L.A. A stable room-temperature sodium–sulfur battery. Nat. Comm. 2016, 7, 11722. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Y.; Bai, Z.; Fang, Z.; Zhang, X.; Xu, Z.; Ding, Y.; Xu, X.; Du, Y.; Dou, S.; et al. High-performance room-temperature sodium–sulfur battery enabled by electrocatalytic sodium polysulfides full conversion. Energy Environ. Sci. 2020, 13, 562–570. [Google Scholar] [CrossRef]
- Chen, P.; Wang, C.; Wang, T. Review and prospects for room-temperature sodium-sulfur batteries. Mater. Res. Lett. 2022, 10, 691–719. [Google Scholar] [CrossRef]
- Wang, L.; Wang, T.; Peng, L.; Wang, Y.; Zhang, M.; Zhou, J.; Chen, M.; Cao, J.; Fei, H.; Duan, X.; et al. The promises, challenges and pathways to room-temperature sodium-sulfur batteries. Natl. Sci. Rev. 2022, 9, nwab050. [Google Scholar] [CrossRef]
- Adeoye, H.A.; Dent, M.; Watts, J.F.; Tennison, S.; Lekakou, C. Solubility and dissolution kinetics of sulfur and sulfides in electrolyte solvents for lithium–sulfur and sodium–sulfur batteries. J. Chem. Phys. 2023, 158, 064702. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.; Markoulidis, F.; Lekakou, C.; Laudone, C.M. Design of porous carbons for supercapacitor applications for different organic solvent-electrolytes. C 2021, 7, 15. [Google Scholar] [CrossRef]
- Markoulidis, F.; Bates, J.; Lekakou, C.; Slade, R.; Laudone, G.M. Supercapacitors with lithium-ion electrolyte: An experimental study and design of the activated carbon electrodes via modelling and simulations. Carbon 2020, 164, 422–434. [Google Scholar] [CrossRef]
- Lasetta, K.; Baboo, J.P.; Lekakou, C. Modeling and simulations of the sulfur infiltration in activated carbon fabrics during composite cathode fabrication for lithium-sulfur batteries. J. Compos. Sci. 2021, 5, 65. [Google Scholar] [CrossRef]
- Grabe, S.; Baboo, J.P.; Tennison, S.; Zhang, T.; Lekakou, C.; Andritsos, E.I.; Cai, Q.; Downes, S.; Hinder, S.; Watts, J.F. Sulfur infiltration and allotrope formation in porous cathode hosts for lithium-sulfur batteries. AIChE J. 2022, 68, e17638. [Google Scholar] [CrossRef]
- Li, Y.; Fu, K.; Chen, C.; Luo, W.; Gao, T.; Xu, S.; Dai, J.; Pastel, G.; Wang, Y.; Liu, B.; et al. Enabling high-areal-capacity lithium–sulfur batteries: Designing anisotropic and low-tortuosity porous architectures. ACS Nano 2017, 11, 4801–4807. [Google Scholar] [CrossRef]
- Feng, S.; Singh, R.K.; Fu, Y.; Li, Z.; Wang, Y.; Bao, J.; Xu, Z.; Li, G.; Anderson, C.; Shi, L.; et al. Low-tortuous and dense single-particle-layer electrode for high-energy lithium-sulfur batteries. Energy Environ. Sci. 2022, 15, 3842–3853. [Google Scholar] [CrossRef]
- Babar, S.; Lekakou, C. Molecular modeling of electrolyte and polysulfide ions for lithium-sulfur batteries. Ionics 2021, 27, 635–642. [Google Scholar] [CrossRef]
- Sdanghi, G.; Schaefer, S.; Maranzana, G.; Celzard, A.; Fierro, V. Application of the modified Dubinin-Astakhov equation for a better understanding of high-pressure hydrogen adsorption on activated carbons. Int. J. Hydrog. Energy 2020, 45, 25912–25926. [Google Scholar] [CrossRef]
- Kannan, D.R.R.; Terala, P.K.; Moss, P.L.; Weatherspoon, M.H. Analysis of the separator thickness and porosity on the performance of lithium-ion batteries. Int. J. Electrochem. 2018, 2018, 1925708. [Google Scholar]
- Zhou, Y.-T.; Yang, J.; Liang, H.-Q.; Pi, J.-K.; Zhang, C.; Xu, Z.-K. Sandwich-structured composite separators with an anisotropic pore architecture for highly safe Li-ion batteries. Compos. Commun. 2018, 8, 46–51. [Google Scholar] [CrossRef]
- Jang, J.; Oh, J.; Jeong, H.; Kang, W.; Jo, C. A review of functional separators for lithium metal battery applications. Materials 2020, 13, 4625. [Google Scholar] [CrossRef]
- Baboo, J.P.; Jakubczyk, E.; Yatoo, M.A.; Phillips, M.; Grabe, S.; Dent, M.; Hinder, S.J.; Watts, J.F.; Lekakou, C. Investigating battery-supercapacitor material hybrid configurations in energy storage device cycling at 0.1 to 10C rate. J. Power Sources 2023, 561, 232762. [Google Scholar] [CrossRef]
- Baboo, J.P.; Yatoo, M.A.; Dent, M.; Hojaji Najafabadi, E.; Lekakou, C.; Slade, R.; Hinder, S.J.; Watts, J.F. Exploring different binders for a LiFePO4 battery, battery testing, modeling and simulations. Energies 2022, 15, 2332. [Google Scholar] [CrossRef]
- Dent, M.; Grabe, S.; Lekakou, C. The challenge of electrolyte impregnation in the fabrication and operation of Li-ion and Li-S batteries. Batter. Supercaps 2024, 7, e202300327. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, S.; Kim, K.; Zhang, H.; Liu, X.; Yan, C.; Xia, H. A novel one-step reaction sodium-sulfur battery with high areal sulfur loading on hierarchical porous carbon fiber. Carbon Energy 2021, 3, 440–448. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, L.; Wang, Y.; Zhu, Z.; Chou, S.-L. The future for room-temperature sodium–sulfur batteries: From persisting issues to promising solutions and practical applications. Adv. Funct. Mater. 2022, 32, 2205622. [Google Scholar] [CrossRef]
- Kumar, D.; Rajouria, S.K.; Kuhar, S.B.; Kanchan, D.K. Progress and prospects of sodium-sulfur batteries: A review. Solid State Ion. 2017, 312, 8–16. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, D.; Qin, X.; Lin, K.; Kang, F.; Li, B.; Shanmukaraj, D.; Rojo, T.; Armand, M.; Wang, G. A room-temperature sodium–sulfur battery with high capacity and stable cycling performance. Nat. Commun. 2018, 9, 3870. [Google Scholar] [CrossRef]
- Pham, T.D.; Bin Faheem, A.; Kim, J.; Oh, H.M.; Lee, K.-K. Practical high-voltage lithium metal batteries enabled by tuning the solvation structure in weakly solvating electrolyte. Small 2022, 18, 2107492. [Google Scholar] [CrossRef]
- Safari, M.; Kwok, C.Y.; Nazar, L.F. Transport properties of polysulfide species in lithium–sulfur battery electrolytes: Coupling of experiment and theory. ACS Cent. Sci. 2016, 2, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Metelmann, H.; Raiß, C.; Dürr, A.K.; Janek, J.; Adelhelm, P. Thermodynamics and cell chemistry of room temperature sodium/sulfur cells with liquid and liquid/solid electrolyte. J. Power Sources 2013, 243, 758–765. [Google Scholar] [CrossRef]
- Kim, K.-M.; Hur, J.-W.; Jung, S.; Kang, A.-S. Electrochemical characteristics of activated carbon/Ppy electrode combined with P(VdF-co-HFP)/PVP for EDLC. Electrochim. Acta 2004, 50, 863–872. [Google Scholar] [CrossRef]
- Kim, I.; Park, J.-Y.; Kim, C.H.; Park, J.-W.; Ahn, J.-P.; Ahn, J.-H.; Kim, K.-W.; Ahn, H.-J. Sodium polysulfides during charge/discharge of the room-temperature Na/S battery using TEGDME electrolyte. J. Electrochem. Soc. 2016, 163, A611–A616. [Google Scholar] [CrossRef]
- Liu, H.; Lai, W.-H.; Lei, Y.; Yang, H.; Wang, N.; Chou, S.; Liu, H.K.; Dou, S.X.; Wang, Y.-X. Electrolytes/interphases: Enabling distinguishable sulfur redox processes in room-temperature sodium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2103304. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High sulfur loading and capacity retention in bilayer garnet sulfurized-polyacrylonitrile/lithium-metal batteries with gel polymer electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Hwang, T.H.; Jung, D.S.; Kim, J.-S.; Kim, B.G.; Choi, J.W. One-dimensional carbon–sulfur composite fibers for Na–S rechargeable batteries operating at room temperature. Nano Lett. 2013, 13, 4532–4538. [Google Scholar] [CrossRef]

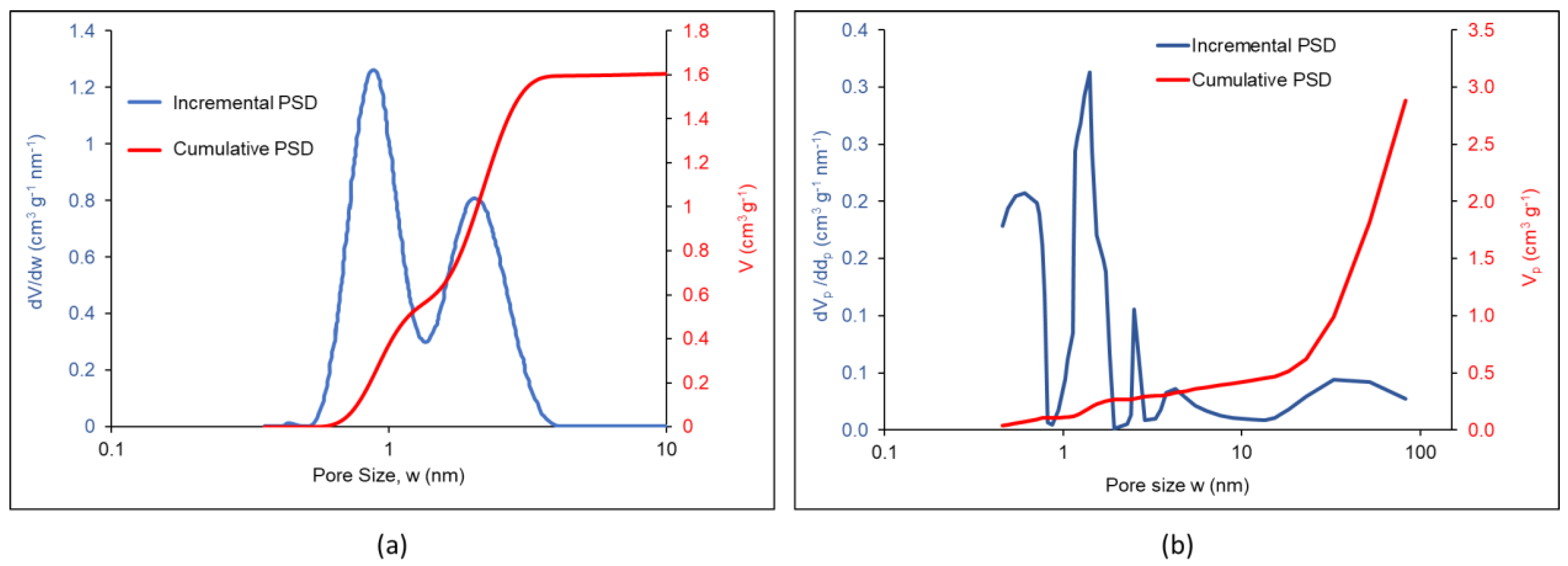
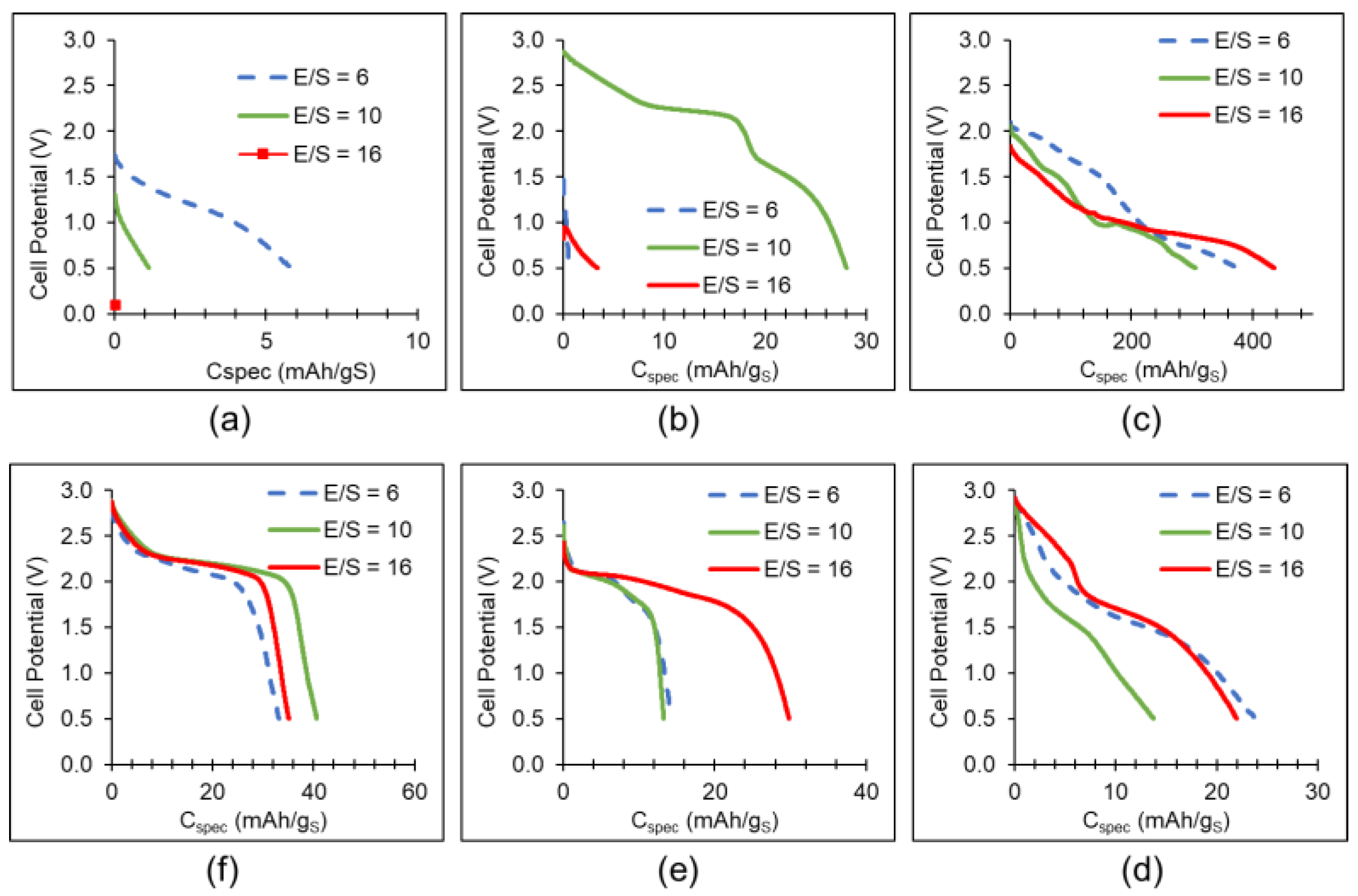
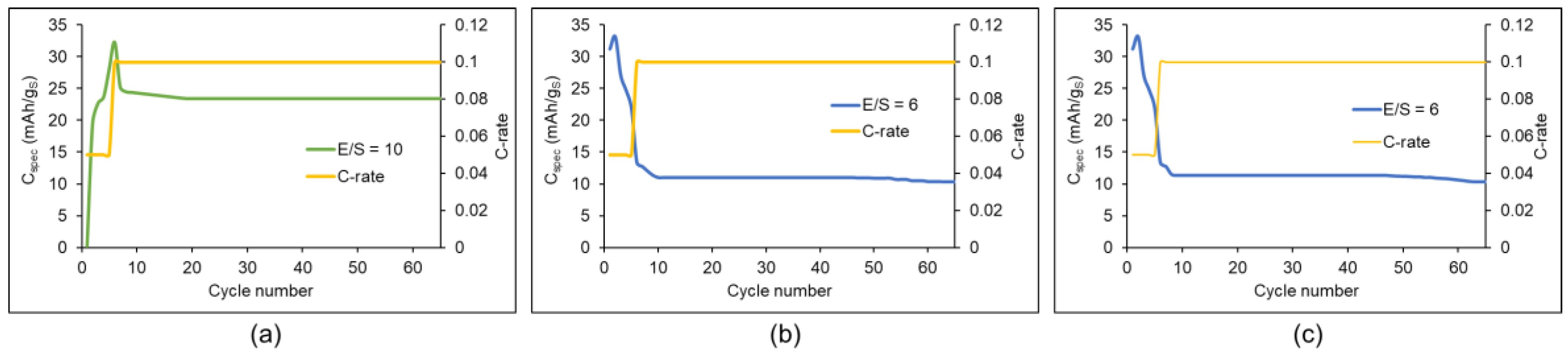
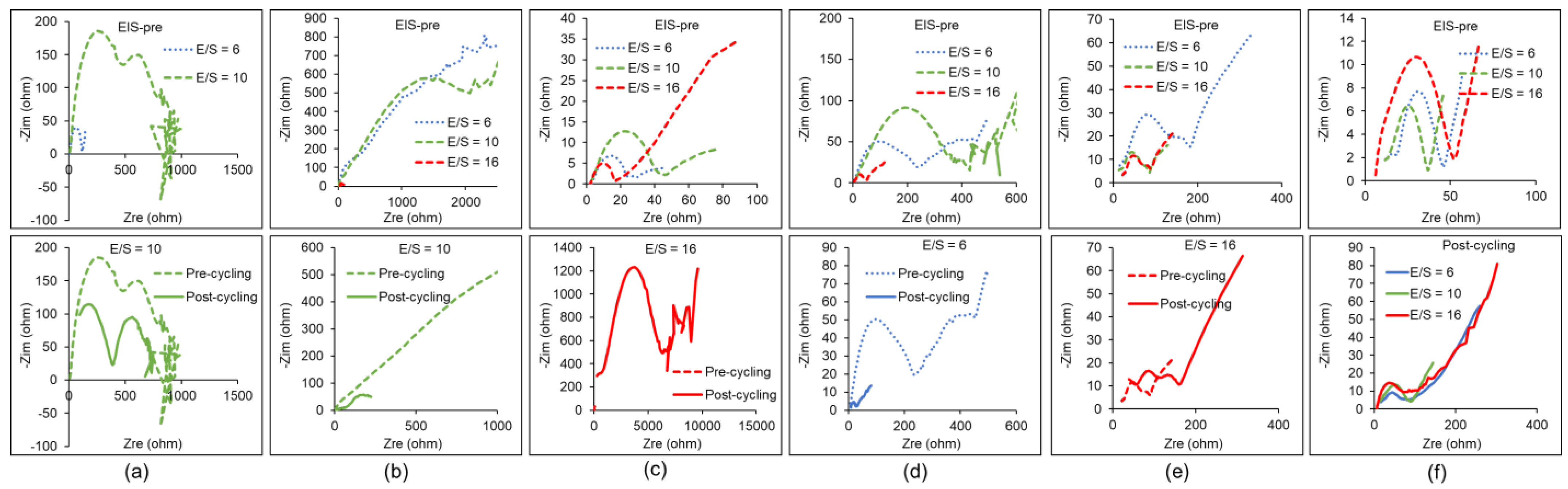
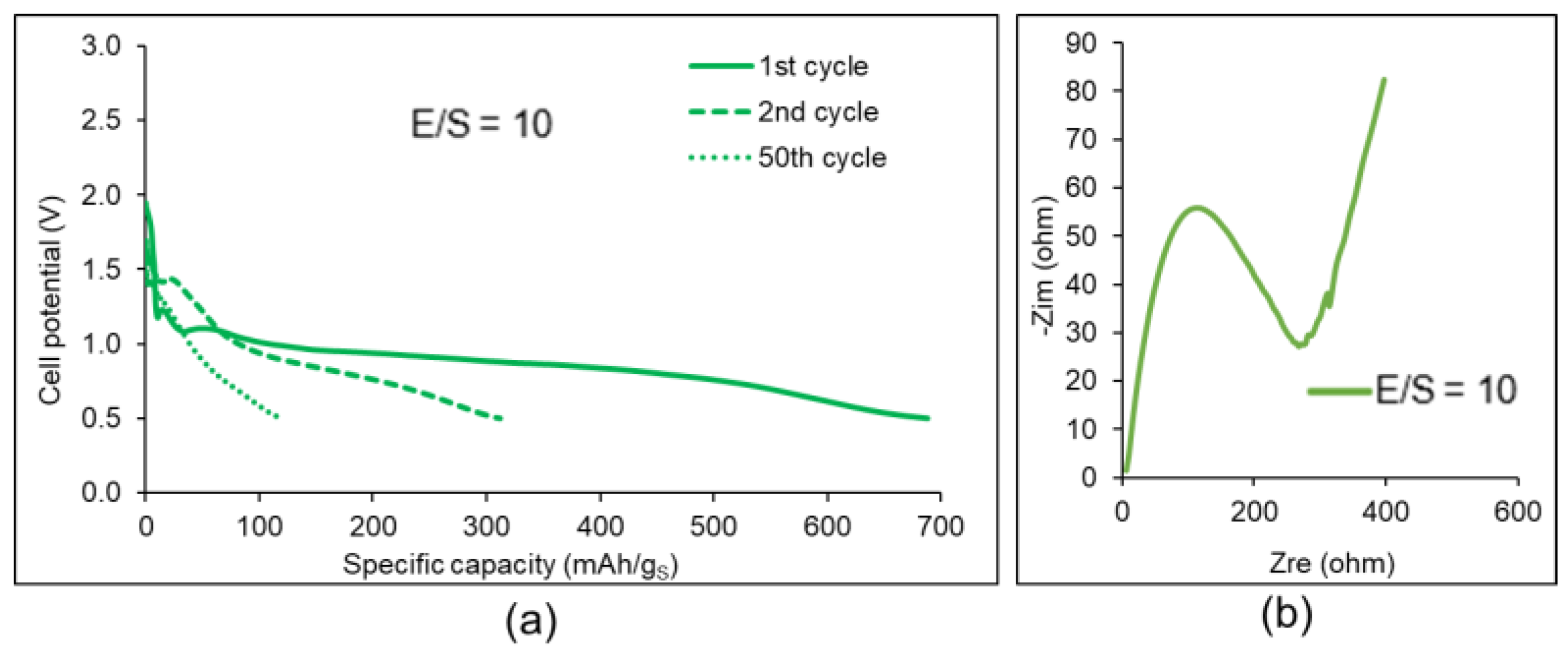
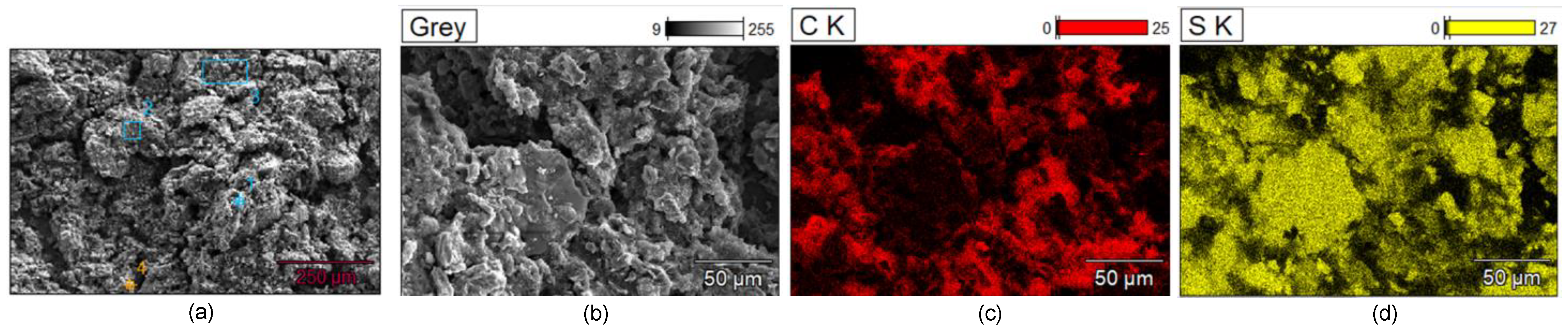
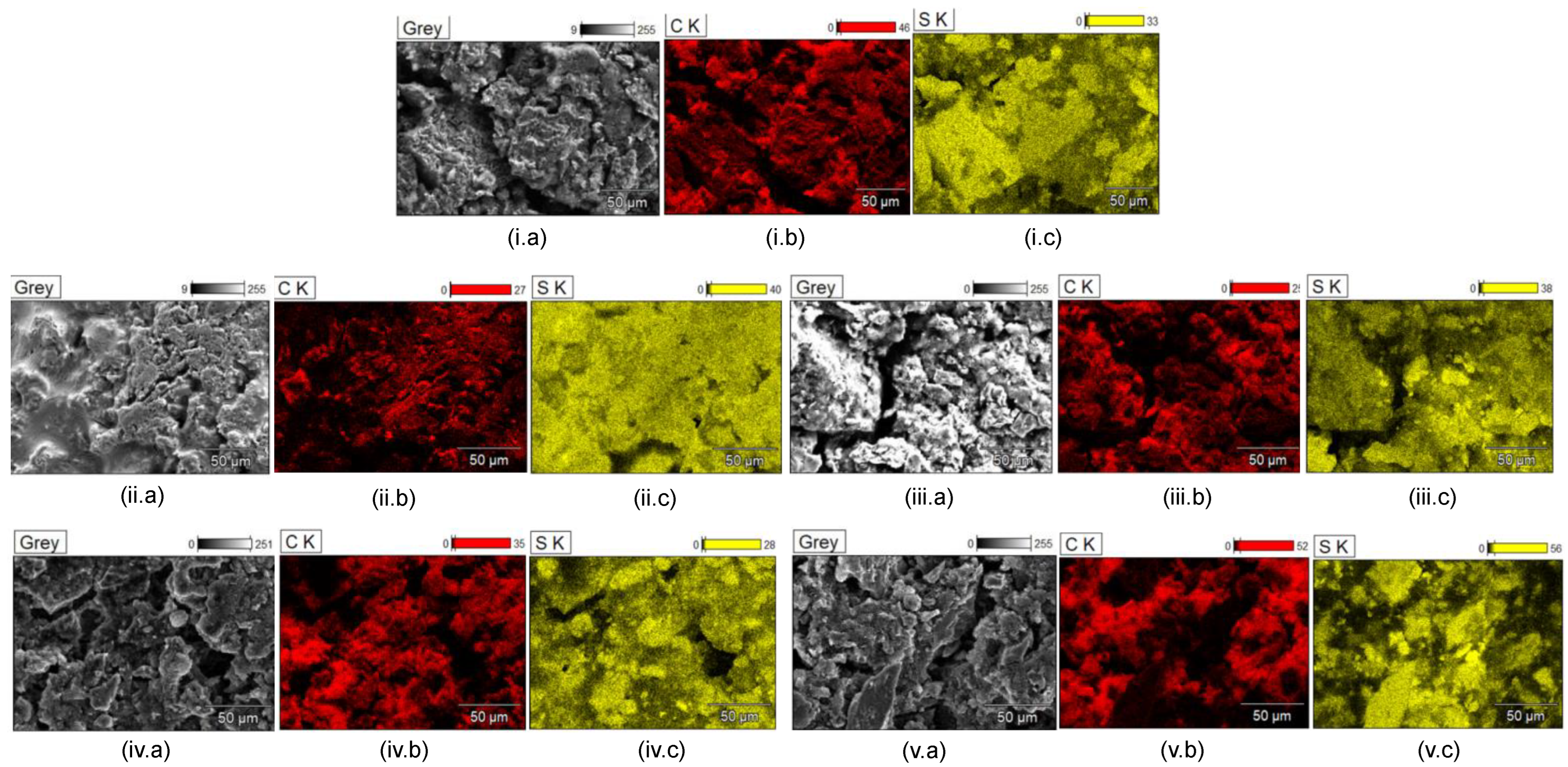

| Factor | Li+ | Na+ | K+ | Mg2+ | Ca2+ | Al3+ |
|---|---|---|---|---|---|---|
| Mem+ + m e−↔Me Eo (V) vs. H+/H2 | −3.04 | −2.71 | −2.93 | −2.37 | −2.87 | −1.66 |
| Cspec (Ah kg−1) | 1168 | 687 | 487 | 952 | 744 | 1072 |
| Espec (Wh kg−1) | 2340 | 1587 | 1232 | 1875 | 1838 | 1350 |
| Expansion (%) | 73 | 160 | 280 | 31 | 73 | 34 |
| Mem+ size (nm) | 0.18 | 0.23 | 0.30 | 0.17 | 0.23 | 0.14 |
| σMe (106 S m−1) | 11 | 21 | 14 | 23 | 29 | 38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeoye, H.A.; Tennison, S.; Watts, J.F.; Lekakou, C. An Investigation into Electrolytes and Cathodes for Room-Temperature Sodium–Sulfur Batteries. Batteries 2024, 10, 216. https://doi.org/10.3390/batteries10060216
Adeoye HA, Tennison S, Watts JF, Lekakou C. An Investigation into Electrolytes and Cathodes for Room-Temperature Sodium–Sulfur Batteries. Batteries. 2024; 10(6):216. https://doi.org/10.3390/batteries10060216
Chicago/Turabian StyleAdeoye, Hakeem Ademola, Stephen Tennison, John F. Watts, and Constantina Lekakou. 2024. "An Investigation into Electrolytes and Cathodes for Room-Temperature Sodium–Sulfur Batteries" Batteries 10, no. 6: 216. https://doi.org/10.3390/batteries10060216
APA StyleAdeoye, H. A., Tennison, S., Watts, J. F., & Lekakou, C. (2024). An Investigation into Electrolytes and Cathodes for Room-Temperature Sodium–Sulfur Batteries. Batteries, 10(6), 216. https://doi.org/10.3390/batteries10060216







