Na4Fe3(PO4)2(P2O7)@C/Ti3C2Tx Hybrid Cathode Materials with Enhanced Performances for Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
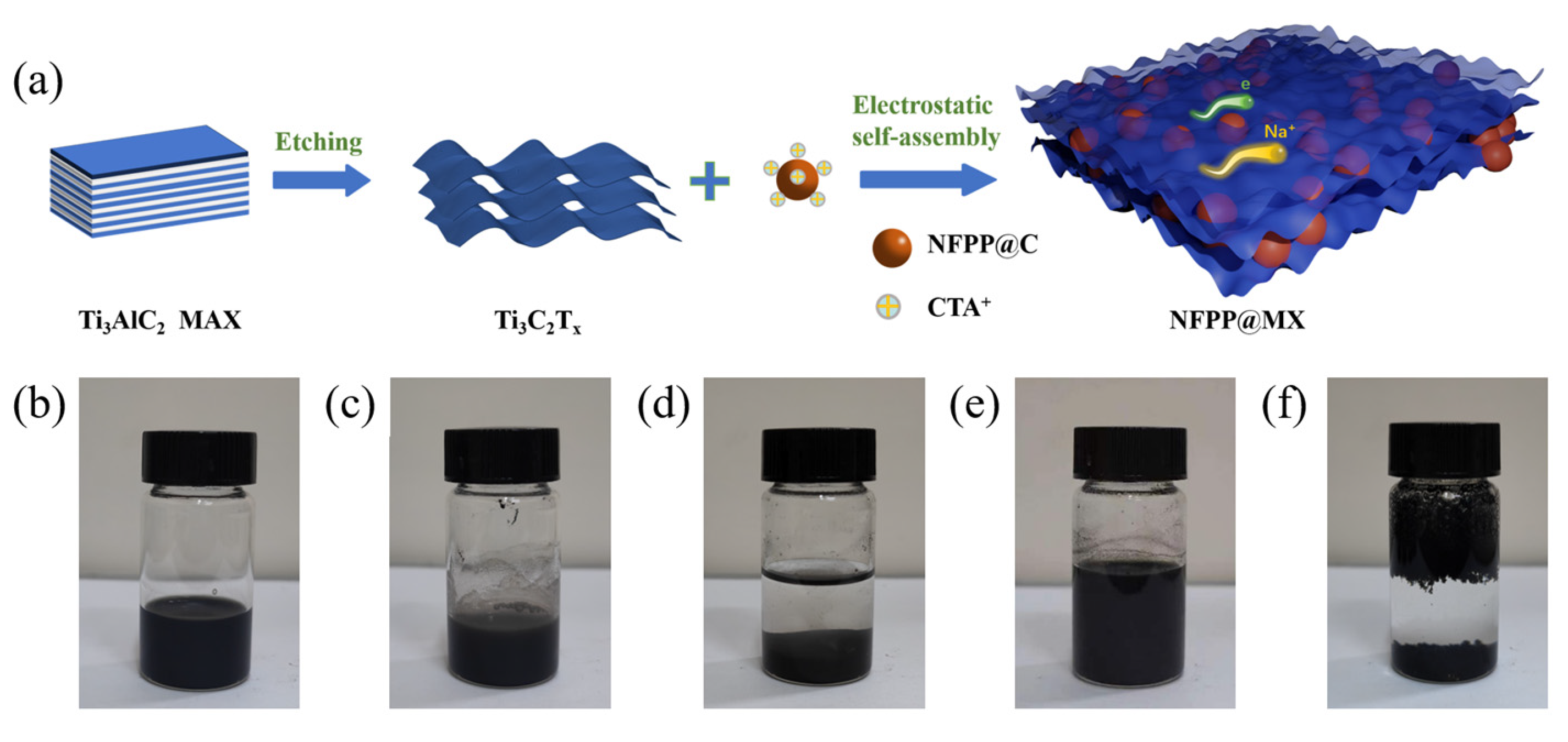
2.1. Synthesis of Materials
2.2. Material Characterization
2.3. Electrochemical Test
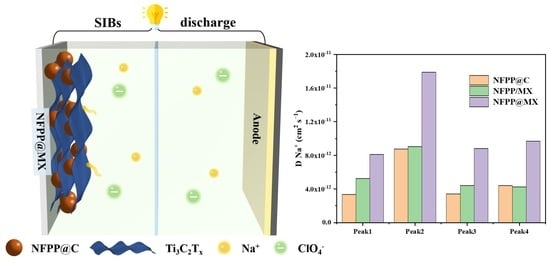
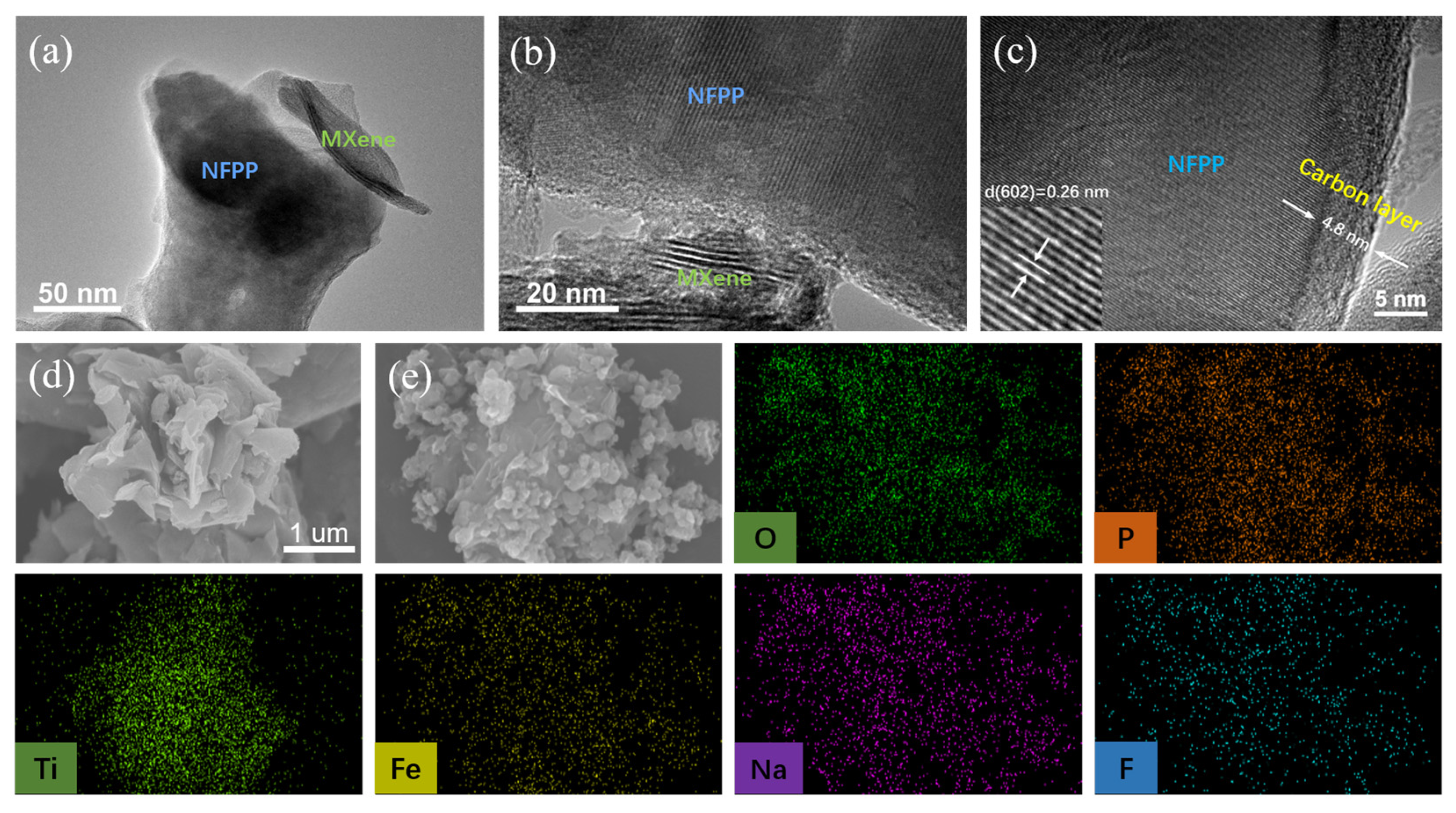
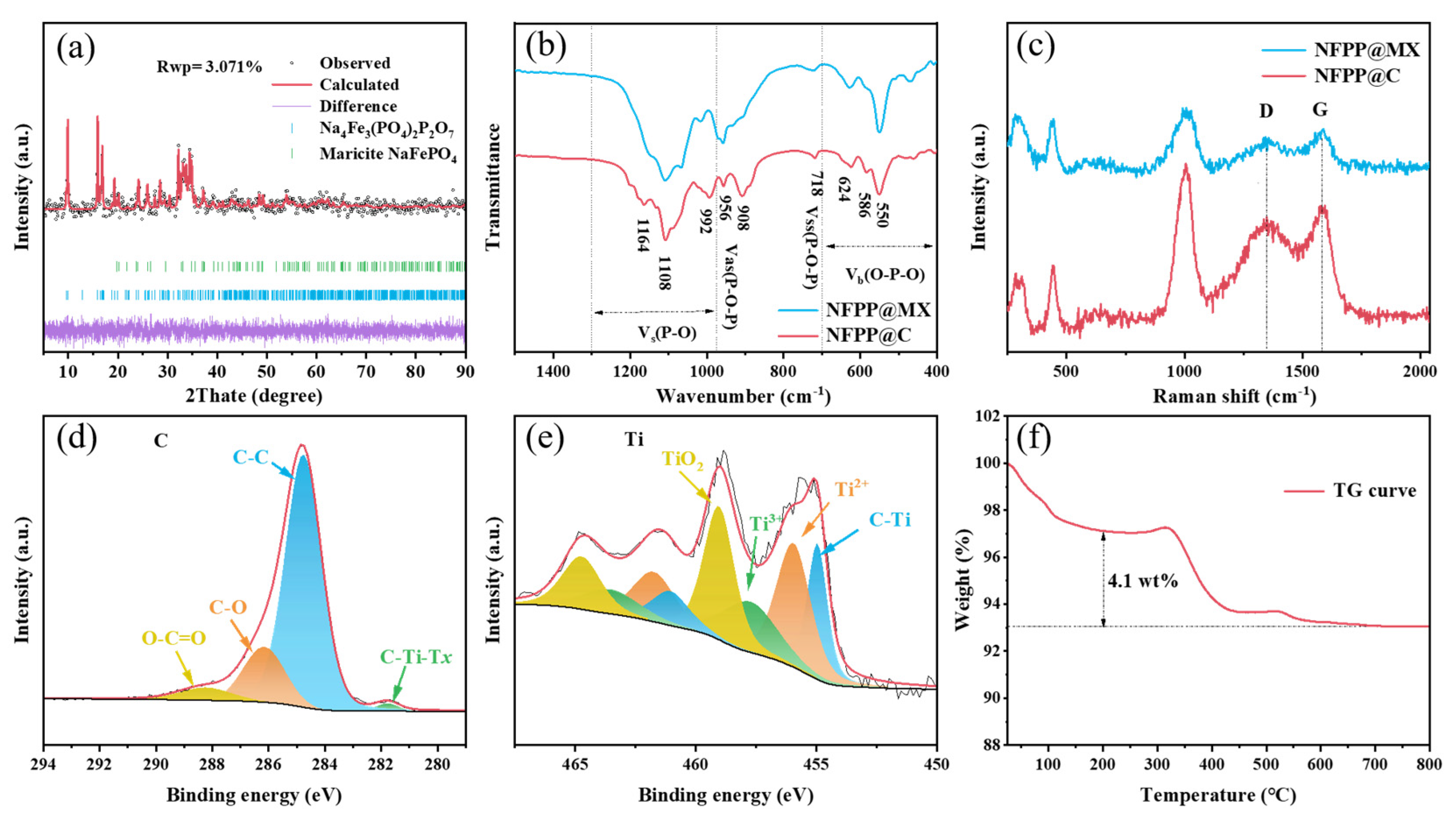
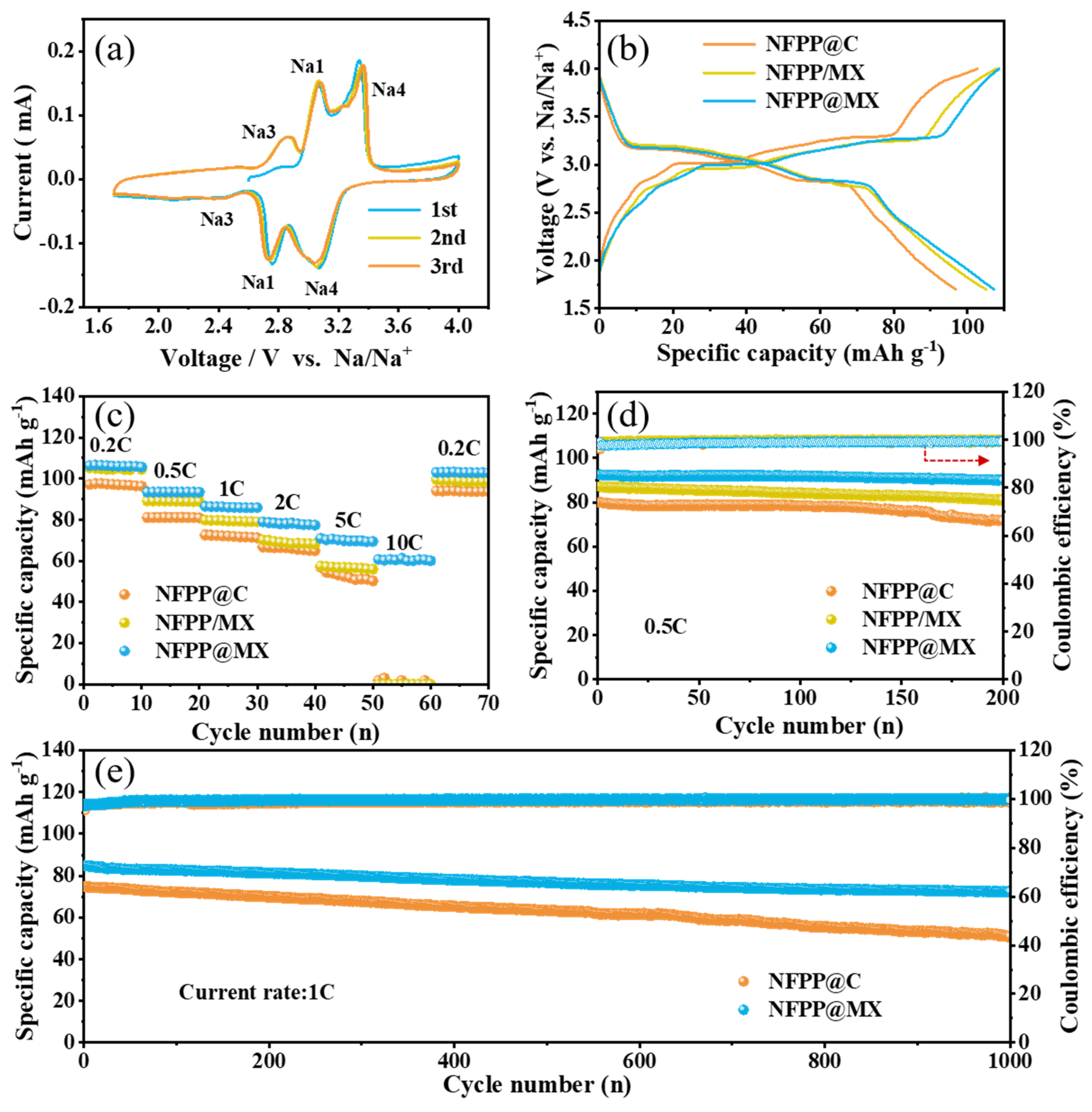
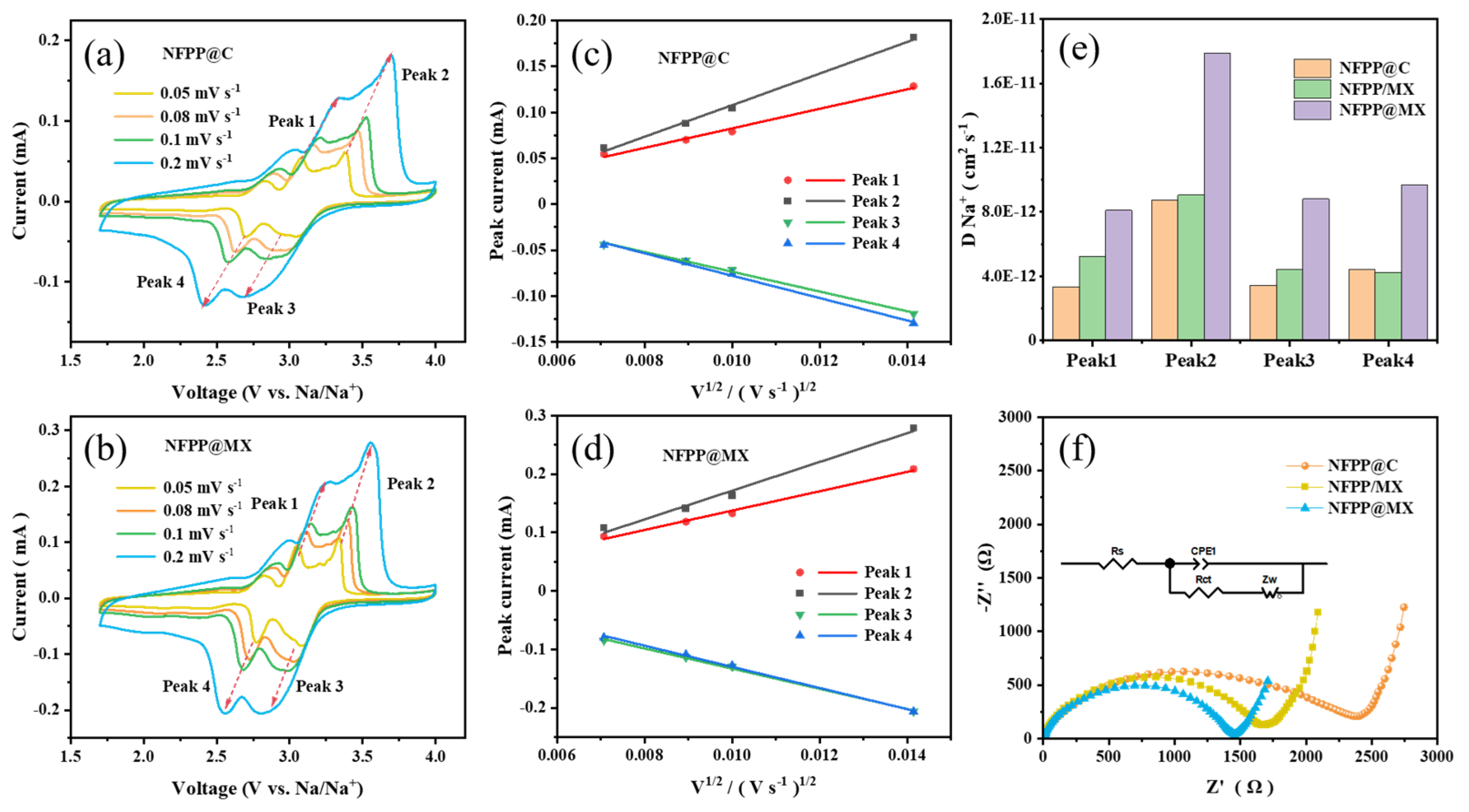
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ayerbe, E.; Berecibar, M.; Clark, S.; Franco, A.A.; Ruhland, J. Digitalization of Battery Manufacturing: Current Status, Challenges, and Opportunities. Adv. Energy Mater. 2022, 12, 2102696. [Google Scholar] [CrossRef]
- Fichtner, M.; Edström, K.; Ayerbe, E.; Berecibar, M.; Bhowmik, A.; Castelli, I.E.; Clark, S.; Dominko, R.; Erakca, M.; Franco, A.A.; et al. Rechargeable Batteries of the Future—The State of the Art from a BATTERY 2030+ Perspective. Adv. Energy Mater. 2022, 12, 2102904. [Google Scholar] [CrossRef]
- Frith, J.T.; Lacey, M.J.; Ulissi, U. A non-academic perspective on the future of lithium-based batteries. Nat. Commun. 2023, 14, 420. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hua, W.; Xiao, J.; Cortie, D.; Chen, W.; Wang, E.; Hu, Z.; Gu, Q.; Wang, X.; Indris, S.; et al. NASICON-type air-stable and all-climate cathode for sodium-ion batteries with low cost and high-power density. Nat. Commun. 2019, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Konarov, A.; Myung, S.-T.; Sun, Y.-K. Cathode Materials for Future Electric Vehicles and Energy Storage Systems. ACS Energy Lett. 2017, 2, 703–708. [Google Scholar] [CrossRef]
- Vincent, M.; Kumar, S.S.; Kowalski, D. Pseudocapacitive vs diffusion controlled charge storage in Fe2O3 nanosheet Na-ion battery. Electrochim. Acta 2023, 469, 143161. [Google Scholar] [CrossRef]
- Rubio, S.; Maça, R.R.; Aragón, M.J.; Cabello, M.; Castillo-Rodríguez, M.; Lavela, P.; Tirado, J.L.; Etacheri, V.; Ortiz, G.F. Superior electrochemical performance of TiO2 sodium-ion battery anodes in diglyme-based electrolyte solution. J. Power Sources 2019, 432, 82–91. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, L.; Zhao, S.; Liu, Y.; Yang, Y.; Yu, H.; Lee, S.; Lee, G.-H.; Kang, Y.-M.; Liu, R.; et al. Tuning local chemistry of P2 layered-oxide cathode for high energy and long cycles of sodium-ion battery. Nat. Commun. 2021, 12, 2256. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Liu, Q.; Wang, J.; Chou, S.; Liu, H.; Dou, S. Prussian Blue Analogues for Sodium-Ion Batteries: Past, Present, and Future. Adv. Mater. 2022, 34, 2108384. [Google Scholar] [CrossRef]
- Hou, P.; Yin, J.; Lu, X.; Li, J.; Zhao, Y.; Xu, X. A stable layered P3/P2 and spinel intergrowth nanocomposite as a long-life and high-rate cathode for sodium-ion batteries. Nanoscale 2018, 10, 6671–6677. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.-R.; Wang, P.-F.; Gong, Y.; Zhang, J.; Yu, X.; Gu, L.; OuYang, C.; Yin, Y.-X.; Hu, E.; Yang, X.-Q.; et al. Designing Air-Stable O3-Type Cathode Materials by Combined Structure Modulation for Na-Ion Batteries. J. Am. Chem. Soc. 2017, 139, 8440–8443. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, W.; Zhou, C.; Yang, L.; Wang, H.; Gao, Q.; Zhu, M. N-doped carbon encapsulated CoMoO4 nanorods as long-cycle life anode for sodium-ion batteries. J. Colloid. Interface Sci. 2020, 576, 176–185. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Wei, C.; Fu, X.-Y.; Chen, Z.-Y.; Yan, B.; Sun, P.-P.; Chang, K.-J.; Yang, X.-L. Ternary Ni-based Prussian blue analogue with superior sodium storage performance induced by synergistic effect of Co and Fe. Carbon. Energy 2021, 3, 827–839. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Hu, Z.; Zhao, L.; Wu, C.; Peleckis, G.; Gu, Q.; Wang, J.-Z.; Liu, H.K.; Dou, S.X.; et al. Ice-Assisted Synthesis of Highly Crystallized Prussian Blue Analogues for All-Climate and Long-Calendar-Life Sodium Ion Batteries. Nano Lett. 2022, 22, 1302–13102. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Ou, M.; Yi, H.; Sun, X.; Zhang, Y.; Zhang, B.; Ding, Y.; Wang, F.; Gu, S.; López, C.A.; et al. Defect-free-induced Na+ disordering in electrode materials. Energy Environ. Sci. 2021, 14, 3130–3140. [Google Scholar] [CrossRef]
- Nose, M.; Nakayama, H.; Nobuhara, K.; Yamaguchi, H.; Nakanishi, S.; Iba, H. Na4Co3(PO4)2P2O7: A novel storage material for sodium-ion batteries. J. Power Sources 2013, 234, 175–179. [Google Scholar] [CrossRef]
- Zhu, C.; Song, K.; van Aken, P.A.; Maier, J.; Yu, Y. Carbon-Coated Na3V2(PO4)3 Embedded in Porous Carbon Matrix: An Ultrafast Na-Storage Cathode with the Potential of Outperforming Li Cathodes. Nano Lett. 2014, 14, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zeng, L.; Wang, J.; Li, W.; Pan, F.; Yu, Y. A carbon coated NASICON structure material embedded in porous carbon enabling superior sodium storage performance: NaTi2(PO4)3 as an example. Nanoscale 2015, 7, 14723–14729. [Google Scholar] [CrossRef]
- Langrock, A.; Xu, Y.; Liu, Y.; Ehrman, S.; Manivannan, A.; Wang, C. Carbon coated hollow Na2FePO4F spheres for Na-ion battery cathodes. J. Power Sources 2013, 223, 62–67. [Google Scholar] [CrossRef]
- Wang, P.-F.; You, Y.; Yin, Y.-X.; Guo, Y.-G. Layered Oxide Cathodes for Sodium-Ion Batteries: Phase Transition, Air Stability, and Performance. Adv. Energy Mater. 2018, 8, 1701912. [Google Scholar] [CrossRef]
- Ge, P.; Li, S.; Shuai, H.; Xu, W.; Tian, Y.; Yang, L.; Zou, G.; Hou, H.; Ji, X. Ultrafast Sodium Full Batteries Derived from X—Fe (X = Co, Ni, Mn) Prussian Blue Analogs. Adv. Mater. 2019, 31, 1806092. [Google Scholar] [CrossRef]
- Xu, J.; Gu, E.; Zhang, Z.; Xu, Z.; Xu, Y.; Du, Y.; Zhu, X.; Zhou, X. Fabrication of porous Na3V2(PO4)3/reduced graphene oxide hollow spheres with enhanced sodium storage performance. J. Colloid. Interface Sci. 2020, 567, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, L.; Li, X.; Qiu, D.; Qiu, S.; Zhou, Q.; Deng, W.; Lu, X.; Yang, Z.; Qiu, M.; et al. Carbon enhanced nucleophilicity of Na3V2(PO4)3: A general approach for dendrite-free zinc metal anodes. J. Energy Chem. 2024, 91, 203–212. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, S. 1D Nanostructured Na7V4(P2O7)4(PO4) as High-Potential and Superior-Performance Cathode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 9111–9117. [Google Scholar] [CrossRef] [PubMed]
- Gezović, A.; Vujković, M.J.; Milović, M.; Grudić, V.; Dominko, R.; Mentus, S. Recent developments of Na4M3(PO4)2(P2O7) as the cathode material for alkaline-ion rechargeable batteries: Challenges and outlook. Energy Storage Mater. 2021, 37, 243–273. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, C.; Chen, L.; Fu, C.; Zeng, Y.; Wang, Q.; Cao, Y.; Chen, Z. “One stone two birds” design for hollow spherical Na4Fe3(PO4)2P2O7/C cathode enabled high-performance sodium-ion batteries from iron rust. EcoMat 2023, 5, e12393. [Google Scholar] [CrossRef]
- Pu, X.; Wang, H.; Yuan, T.; Cao, S.; Liu, S.; Xu, L.; Yang, H.; Ai, X.; Chen, Z.; Cao, Y. Na4Fe3(PO4)2P2O7/C nanospheres as low-cost, high-performance cathode material for sodium-ion batteries. Energy Storage Mater. 2019, 22, 330–336. [Google Scholar] [CrossRef]
- Cao, Y.; Xia, X.; Liu, Y.; Wang, N.; Zhang, J.; Zhao, D.; Xia, Y. Scalable synthesizing nanospherical Na4Fe3(PO4)2(P2O7) growing on MCNTs as a high-performance cathode material for sodium-ion batteries. J. Power Sources 2020, 461, 228130. [Google Scholar] [CrossRef]
- Kim, H.; Park, I.; Seo, D.-H.; Lee, S.; Kim, S.-W.; Kwon, W.J.; Park, Y.-U.; Kim, C.S.; Jeon, S.; Kang, K. New Iron-Based Mixed-Polyanion Cathodes for Lithium and Sodium Rechargeable Batteries: Combined First Principles Calculations and Experimental Study. J. Am. Chem. Soc. 2012, 134, 10369–10372. [Google Scholar] [CrossRef]
- Wu, X.; Zhong, G.; Yang, Y. Sol-gel synthesis of Na4Fe3(PO4)2(P2O7)/C nanocomposite for sodium ion batteries and new insights into microstructural evolution during sodium extraction. J. Power Sources 2016, 327, 666–674. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Zhang, Y.; Li, X.; Xu, Q.; Liu, H.; Ma, Z.-F. Polyvinylpyrrolidone assisted synthesized ultra-small Na4Fe3(PO4)2(P2O7) particles embedded in 1D carbon nanoribbons with enhanced room and low temperature sodium storage performance. J. Power Sources 2021, 498, 229907. [Google Scholar] [CrossRef]
- Ma, X.; Wu, X.; Shen, P. Rational Design of Na4Fe3(PO4)2(P2O7) Nanoparticles Embedded in Graphene: Toward Fast Sodium Storage Through the Pseudocapacitive Effect. ACS Appl. Energy Mater. 2018, 1, 6268–6278. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Z.; Chen, H.; Pu, X.; Chen, Z. MXene-based materials for multivalent metal-ion batteries. Batteries 2023, 9, 174. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Q.; Xu, M.; Zang, B.; Wang, Y.; Xu, B. Cu-Modified Ti3C2Cl2 MXene with Zincophilic and Hydrophobic Characteristics as a Protective Coating for Highly Stable Zn Anode. Adv. Funct. Mater. 2023, 33, 2213416. [Google Scholar] [CrossRef]
- Xu, W.; Liao, X.; Xu, W.; Zhao, K.; Yao, G.; Wu, Q. Ion Selective and Water Resistant Cellulose Nanofiber/MXene Membrane Enabled Cycling Zn Anode at High Currents. Adv. Energy Mater. 2023, 13, 2300283. [Google Scholar] [CrossRef]
- Xu, E.; Zhang, Y.; Wang, H.; Zhu, Z.; Quan, J.; Chang, Y.; Li, P.; Yu, D.; Jiang, Y. Ultrafast kinetics net electrode assembled via MoSe2/MXene heterojunction for high-performance sodium-ion batteries. Chem. Eng. J. 2020, 385, 123839. [Google Scholar] [CrossRef]
- Yue, L.; Wang, J.; Li, M.; Qin, J.; Cao, M. Conductive Ti3C2Tx networks to optimize Na3V2O2(PO4)2F cathodes for improved rate capability and low-temperature operation. Dalton Trans. 2023, 52, 4717–4727. [Google Scholar] [CrossRef]
- Chun, J.; Wang, X.; Wei, C.; Wang, Z.; Zhang, Y.; Feng, J. Flexible and free-supporting Prussian blue analogs/MXene film for high-performance sodium-ion batteries. J. Power Sources 2023, 576, 233165. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, C.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Flexible and Free-Standing Ti3C2Tx MXene@Zn Paper for Dendrite-Free Aqueous Zinc Metal Batteries and Nonaqueous Lithium Metal Batteries. ACS Nano 2019, 13, 11676–11685. [Google Scholar] [CrossRef]
- Zhao, A.; Yuan, T.; Li, P.; Liu, C.; Cong, H.; Pu, X.; Chen, Z.; Ai, X.; Yang, H.; Cao, Y. A novel Fe-defect induced pure-phase Na4Fe2.91(PO4)2P2O7 cathode material with high capacity and ultra-long lifetime for low-cost sodium-ion batteries. Nano Energy 2022, 91, 106680. [Google Scholar] [CrossRef]
- Gezović, A.; Milović, M.; Bajuk-Bogdanović, D.; Grudić, V.; Dominko, R.; Mentus, S.; Vujković, M.J. An effective approach to reaching the theoretical capacity of a low-cost and environmentally friendly Na4Fe3(PO4)2(P2O7) cathode for Na-ion batteries. Electrochim. Acta 2024, 476, 143718. [Google Scholar] [CrossRef]
- Wei, C.; Fei, H.; An, Y.; Zhang, Y.; Feng, J. Crumpled Ti3C2Tx (MXene) nanosheet encapsulated LiMn2O4 for high performance lithium-ion batteries. Electrochim. Acta 2019, 309, 362–370. [Google Scholar] [CrossRef]
- Kim, J.; Seo, D.-H.; Kim, H.; Park, I.; Yoo, J.-K.; Jung, S.-K.; Park, Y.-U.; Goddard Iii, W.A.; Kang, K. Unexpected discovery of low-cost maricite NaFePO4 as a high-performance electrode for Na-ion batteries. Energy Environ. Sci. 2015, 8, 540–545. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, Y.; Zhang, J.; Pu, X.; Ai, X.; Chen, Z.; Yang, H.; Cao, Y. 3D graphene decorated Na4Fe3(PO4)2(P2O7) microspheres as low-cost and high-performance cathode materials for sodium-ion batteries. Nano Energy 2019, 56, 160–168. [Google Scholar] [CrossRef]
- Ma, X.; Pan, Z.; Wu, X.; Shen, P.K. Na4Fe3(PO4)2(P2O7)@NaFePO4@C core-double-shell architectures on carbon cloth: A high-rate, ultrastable, and flexible cathode for sodium ion batteries. Chem. Eng. J. 2019, 365, 132–141. [Google Scholar] [CrossRef]
- Shah, S.A.; Habib, T.; Gao, H.; Gao, P.; Sun, W.; Green, M.J.; Radovic, M. Template-free 3D titanium carbide (Ti3C2Tx) MXene particles crumpled by capillary forces. Chem. Commun. 2017, 53, 400–403. [Google Scholar] [CrossRef]
- He, J.; Tao, T.; Yang, F.; Sun, Z.; Huang, H. Phase-manipulated hierarchically core-shell Na3(VO1-xPO4)2F1+2x (0≤x≤1)@ Na3V2(PO4)3 and its synergistic effect with conformally wrapped reduced graphene oxide framework towards high-performance cathode for sodium-ion batteries. Mater. Today Phys. 2022, 27, 100813. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Z.; Huang, J.; Deng, W.; Li, X.; Zhang, H.; Wen, Z. Graphene-decorated carbon-coated LiFePO4 nanospheres as a high-performance cathode material for lithium-ion batteries. Carbon 2018, 127, 149–157. [Google Scholar] [CrossRef]
- Pu, X.; Zhao, D.; Fu, C.; Chen, Z.; Cao, S.; Wang, C.; Cao, Y. Understanding and Calibration of Charge Storage Mechanism in Cyclic Voltammetry Curves. Angew. Chem. Int. Ed. 2021, 60, 21310–21318. [Google Scholar] [CrossRef]
- Das, S.R.; Majumder, S.B.; Katiyar, R.S. Kinetic analysis of the Li+ ion intercalation behavior of solution derived nano-crystalline lithium manganate thin films. J. Power Sources 2005, 139, 261–268. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.; Jiang, M.; Zhang, J. Electrochemical properties of mixed-phosphates Nax+2Fex+1(PO4)x(P2O7) with different ratios of PO43−/P2O74−. J. Alloys Compd. 2021, 870, 159382. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, Y.; Xu, H.-H.; Huang, Y.-H. High-performance porous nanoscaled LiMn2O4 prepared by polymer-assisted sol–gel method. Electrochim. Acta 2013, 106, 63–68. [Google Scholar] [CrossRef]
- Wang, S.-B.; Ran, Q.; Yao, R.-Q.; Shi, H.; Wen, Z.; Zhao, M.; Lang, X.-Y.; Jiang, Q. Lamella-nanostructured eutectic zinc–aluminum alloys as reversible and dendrite-free anodes for aqueous rechargeable batteries. Nat. Commun. 2020, 11, 1634. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.; Kate, R.; Chothe, U.; Chalwadi, P.; Shingare, J.; Kulkarni, M.; Kalubarme, R.; Kale, B. Highly Stable MWCNT@NVP Composite as a Cathode Material for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 34651–34661. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, K.; Shen, S.; Liu, Z.; Xu, J.; Yang, L.; Zhao, L. Realizing efficient sodium storage property with NASICON-type Na2VTi(PO4)3 modified by nitrogen and sulfur dual-doped carbon layer for sodium ion batteries. J. Alloys Compd. 2021, 856, 157992. [Google Scholar] [CrossRef]
- Li, J.; Wang, R.; Zhao, W.; Hou, X.; Paillard, E.; Ning, D.; Li, C.; Wang, J.; Xiao, Y.; Winter, M.; et al. A high-voltage symmetric sodium ion battery using sodium vanadium pyrophosphate with superior power density and long lifespan. J. Power Sources 2021, 507, 230183. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, A.; Shi, D.; Chen, P.; Li, Z.; Tu, Q.; Liu, D.; Zhang, X.; Lu, J.; Jiang, Y.; Yang, Z.; et al. Na4Fe3(PO4)2(P2O7)@C/Ti3C2Tx Hybrid Cathode Materials with Enhanced Performances for Sodium-Ion Batteries. Batteries 2024, 10, 121. https://doi.org/10.3390/batteries10040121
Xiang A, Shi D, Chen P, Li Z, Tu Q, Liu D, Zhang X, Lu J, Jiang Y, Yang Z, et al. Na4Fe3(PO4)2(P2O7)@C/Ti3C2Tx Hybrid Cathode Materials with Enhanced Performances for Sodium-Ion Batteries. Batteries. 2024; 10(4):121. https://doi.org/10.3390/batteries10040121
Chicago/Turabian StyleXiang, Ao, Deyou Shi, Peng Chen, Zhongjun Li, Quan Tu, Dahui Liu, Xiangguang Zhang, Jun Lu, Yan Jiang, Ze Yang, and et al. 2024. "Na4Fe3(PO4)2(P2O7)@C/Ti3C2Tx Hybrid Cathode Materials with Enhanced Performances for Sodium-Ion Batteries" Batteries 10, no. 4: 121. https://doi.org/10.3390/batteries10040121
APA StyleXiang, A., Shi, D., Chen, P., Li, Z., Tu, Q., Liu, D., Zhang, X., Lu, J., Jiang, Y., Yang, Z., & Hu, P. (2024). Na4Fe3(PO4)2(P2O7)@C/Ti3C2Tx Hybrid Cathode Materials with Enhanced Performances for Sodium-Ion Batteries. Batteries, 10(4), 121. https://doi.org/10.3390/batteries10040121