Abstract
Lithium-ion polymer batteries, also known as lithium-polymer, abbreviated Li-po, are one of the main research topics nowadays in the field of energy storage. This review focuses on the use of the phosphorus containing compounds in Li-po batteries, such as polyphosphonates and polyphosphazenes. Li-po batteries are mini-devices, capable of providing power for any portable gadget. From a constructive point of view, Li-po batteries contain an anode (carbon), a cathode (metal oxide), and a polymer electrolyte, which could be liquid electrolytes or solid electrolytes. In general, a divider is used to keep the anode and cathode from touching each other directly. Since liquid electrolytes have a generally high ionic conductivity, they are frequently employed in Li-ion batteries. In the last decade, the research in this field has also focused on solving safety issues, such as the leakage of electrolytes and risk of ignition due to volatile and flammable organic solvents. The research topics in the field of Li-po remain focused on solving safety problems and improving performance.
1. Introduction
The field of energy production and energy storage is of great interest nowadays, especially for green energy sources, a big challenge for the future. Among others, lithium-ion polymer batteries are a main research subject in the area of energy storage. These batteries are mini-devices capable of providing power for any portable gadget. The classic Li-ion batteries contain an anode (carbon), a cathode (metal oxide), and an electrolyte (low viscosity liquid). The used electrolyte has a significant influence on the cell capacity, operating temperature range, safety issues, and the charge–discharge cycle of the obtained batteries. In classic batteries, the liquid used as an electrolyte is usually a highly flammable substance, increasing the risks of ignition. The use of less flammable electrolytes is a main task for obtaining safer batteries [1]. The suppression of electrolyte flammability could be achieved by using flame retardants. One option was the use of phosphate additives, for instance, trimethyl phosphate (TMP).
TMP is a flame retardant compound widely used in plastics. Although the risk of flammability is decreased, in the last decades researchers have proved that such compounds could react to the anode. This will affect the stability range in order to create high-performance formulations for lithium-ion batteries. Among liquid electrolytes and inorganic solid electrolytes (dry or solvent-free electrolytes), polymer electrolytes have many benefits and advantages, such as greater safety features and an increased resilience to changes in the volume of the electrodes throughout the charge/discharge process [2].
As already mentioned, two different classes of polymer electrolytes were used for Li-po batteries: solid polymer electrolytes (SPEs) and gel polymer electrolytes (GPEs). Each of them has benefits and disadvantages. For example, flexibility, light weight, and ease of processing are all benefits of SPEs. The disadvantages are connected to charge–discharge cycles and also to safety issues. The traditional polymer matrix for SPEs (Figure 1) has been the subject of extensive research [1,2,3,4,5,6,7].
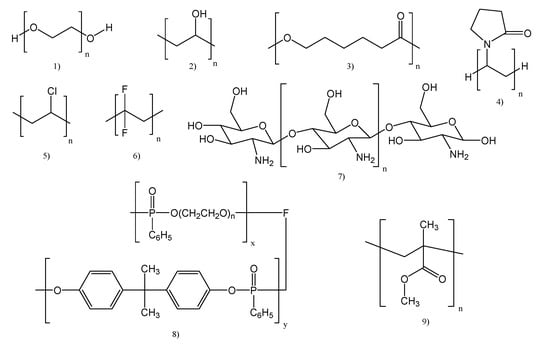
Figure 1.
Chemical structures of some polymers used for polymer electrolytes: (1) Poly (ethylene oxide) (PEO), (2) Poly(vinyl alcohol) (PVA), (3) Poly(3-caprolactone) (PCL), (4) Poly(vinylpyrrolidone) (PVP), (5) Poly(vinyl chloride) (PVC), (6) Poly(vinylidene fluoride) (PVDF), (7) Chitosan, (8) Poly(phosponates) (PPA) and (9) Poly(methyl methacrylate) (PMMA).
One of the main issues in the field of batteries is the risk of ignition. It is well known that hydrogen radicals could cause the combustion. While phosphorus atoms from phosphonates act as trapping agents for the hydrogen radicals, as a consequence, low molar mass phosphonate could be used as flame retardants in electrolytes.
2. Polyphosphonates as Polymer Electrolytes
2.1. Solid Polymer Electrolytes
Since the 1980s, new ionic conductive materials known as solid polymer electrolytes (SPEs) have been obtained [3]. Inorganic materials such as conductive ceramics and glasses are examples of solid electrolytes. To obtain safer lithium-ion batteries with high performance, several requirements for the polymer formulations are necessary. Therefore, in order to be used for Li-ion batteries, the polymer electrolytes should be able to ensure high conductivities and high transfer numbers. The identification of a suitable solid polymer electrolyte is essential for obtaining a completely solid-state, high-power lithium-ion battery. The polymer electrolyte, used between the composite cathode and the anode, serves as both a separator and electrolyte. The performance of lithium polymer batteries is strongly influenced by the polymer membrane.
A polymer electrolyte used for lithium polymer batteries should have a high conductivity, considerable transference number of Li+, strong mechanical properties, broad electrochemical stability window, and also high thermal and chemical stability. Further, all of those required properties of the polymer electrolytes, necessary for their application for lithium polymer batteries, will be described.
- (1)
- High conductivity of ions.
To promote ion mobility and ion movement, the polymer electrolyte should be first of all a good ionic conductor. The required conductivities are in general higher than 10−4 S cm−1 at room temperature. Nevertheless, a quick charge process is made possible also from the high conductivity.
- (2)
- Considerable transference number of Li+.
A high Li+ transference number makes it feasible to reduce the electrolyte concentration polarization throughout the charge/discharge operation, which leads to higher power density. The Li+ transference number in an electrolyte system needs to be as close to one as possible. It can be considerably raised by reducing the mobility of anions. Anions’ mobility can reportedly be reduced by anchoring them to the polymer’s backbone or by adding receptors for anions to electrolytes that only interact with them.
- (3)
- Strong mechanical properties.
The most crucial aspect that must be considered is the mechanical strength of a polymer electrolyte. Elasticity is required in order to allow relaxation under stress during the production, cell assembly, storage, and use, rather than becoming brittle like some ceramics.
- (4)
- Broad electrochemical stability window and high thermal and chemical stability.
The polymer electrolytes should not react with the anode, cathode, cell separator, and all the other materials involved. The polymer electrolytes should have an electrochemical window of up 4–5 V vs. Li/Li+, to be compatible with both electrode materials. The electrochemical window represents the difference between the potential of the oxidation reaction and the potential of the reduction reaction. To boost the dimensional stability of electrolyte membranes, some practical methods have been developed, including crosslinking, adding inorganic fillers, and providing physical support with a polyolefin membrane. Such materials contain salt that is dissolved in a polymer matrix.
The most common electrolytes are aprotic solvents, like cyclic carbonates, and dissolved lithium salts, because they provide superior electrochemical performance over a broad temperature range. The trade-off with aprotic solvents is that the cell voltage and cathode performance suffer as a result of this wide temperature performance. Sulfur-containing solvents (like sulfolane), for example, show an electrochemical window of 6.1 V. They are the most resilient. Although ethyl methyl carbonate (EM) has a narrower electrochemical window (4.0 V) than sulfolane, it offers better ionic conductivity. In general, aprotic organic solvents with lithium salts generally have ionic conductivities between 10−2 and 10−3 S cm−1. The ideal SPE would be a solid material with strong ionic conductivity and a widespread electrochemical voltage window. For instance, PEO-based compounds are also of great interest for applications as SPEs [8,9] because the compounds obtained by the complexation of polyethylene oxide (PEO) with alkali metal salts showed high ionic conductivities [4]. Theoretical models were considered to describe the ion transport mechanisms, and also the physical and chemical characteristics of the electrolyte/electrode interface, etc. Many reports from the scientific literature focused on the area of using PEO-based derivatives for solid-state lithium-ion batteries, as the work of Homann et al., Changmin et al., and Xiuhong et al. [10,11,12,13] report.
PEO-based solid-state electrolytes have garnered more attention in recent years for use in lithium-ion batteries because of their increased safety features, high energy density, and enhanced potential window. Because PEO is a semi-crystalline polymer with a high degree of crystallinity at room temperature, PEO-based derivatives have a few disadvantages as well. These disadvantages include a relatively low Li-ion transport rate at room temperature and low ionic conductivity, as well as a high degree of temperature fluctuation in ionic conductivity. Their applications are, therefore, often carried out at higher temperatures. On the other hand, PEO-based electrolytes can also be used at room temperature for energy storage if their crystallinity is reduced. It was found that the increase in the lithium salt concentration led to a decrease in PEO crystallinity [13].
One of the most promising candidates to be used as polymer matrices is PEO and its derivatives. PEO can easily solvate cations and efficiently coordinate with them to create homogenous solutions. By coordinating with the lithium, the PEO matrix dissolves the lithium salts. Other polymers already studied for such applications are shown in Figure 1 (PEO, PVA, PVC, PPA, PMMA, and so on). Many novel lithium salts have been investigated, and solid polymer electrolytes have been enriched with inorganic fillers for increasing their ionic conductivity. Some of those lithium salts are illustrated in Figure 2.
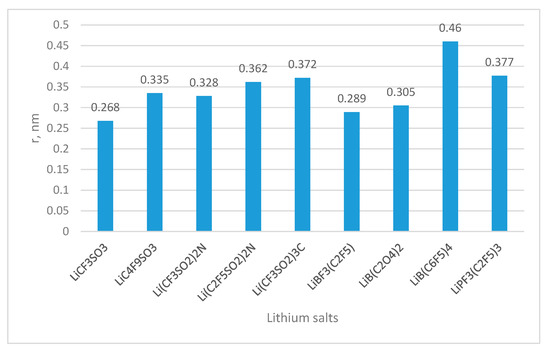
Figure 2.
Examples of inorganic lithium salts used for batteries (Ionic radii r are calculated from van der Waals volumes of the ion models [in encyclopedia of electrochemical power sources, 2009].
The counter anion type controls the capacity of the lithium salts to dissociate. In agreement with Coulomb’s law, bigger anions, with a delocalized electronic charge, will be easily dissolved in dipolar aprotic solvents. On the other hand, in agreement with Stokes’ law, bigger anions have less mobility. Therefore, the requirements for lithium salts, as mentioned for solvents, are incompatible with the dissociation ability and ionic mobility requirements. Two factors influence significantly the electrolytic conductivity of the lithium salts: dissociation ability and mobility. Lithium bis(trifluoromethylsulfonyl)imide (LiN(SO2CF3)2, and lithium bis(perfluoroethylsulfonyl)imide (LiN(SO2CF2CF3)2, LiBETI) are two examples of the lithium imide salts. These salts with large and “weak-coordinate” anions are frequently employed as plasticized salts for polymer, while they contain flexible anions which will decrease the recrystallization kinetics of polymer electrolytes. Their conductivities at room temperature are two to three orders of magnitude higher than those of LiClO4 in PEO-based electrolytes [8]. In addition to the solvent, lithium salt also contributes significantly to the formation of solid electrolyte interphase (SEI). It was found that LiBF4 and LiB(C2O4)2 provide inferior SEI, but the combination of EC and LiPF6 provides better SEI on the graphite negative electrode. Li(CF3SO2)2N corrodes the positive electrode represented by the aluminum current collector, whereas LiMFn salts provide a stable passivation coating containing fluorine. Such solid polymer electrolytes showed an ionic conductivity still below 10−5 S cm−1.
The polymer matrix used for obtaining the solid polymer electrolytes must first be able to dissolve or complex lithium ions. The salt’s lattice energy and the host polymer’s dielectric constant should both be reasonably high to promote the dissociation of organic salts in polymer hosts. Lithium salts are able to dissolve in polymers containing heteroelements or groups like –O–, =O, –S–, –N–, –P–, C=O, and C=N, and to form polymer–salt complexes. Ionic conductivity is influenced by the amount of mobile ions. The actual number of mobile ions, or free ions, depends on the degree of salt dissociation in the polymer host. Due to segmental chain mobility, the amorphous areas above the glass transition temperature (Tg) in dry solid polymer electrolytes allowed for ion movement. Tg is the temperature at which the polymer undergoes a phase transition from a glassy solid to a rubbery material. It is influenced by the chemical structure and the molecular weight of the used polymer and by the salt concentration. Amorphous phases are generally favorable for Li+ conduction, but at the same time they decrease the mechanical stability of the entire system [10]. Lithium ions are found in the polymer at appropriate coordination sites: –O–, –C=N, –N–R, etc. (such as in polyethylene oxide, polyacrylonitrile or polyamide, etc.). Free volumes are created by the polymer chains’ ongoing local segmental motion. Lithium ions migrate through these free volumes under the action of an electric field, either by jumping from one polymeric chain to another or by moving from one coordination site to new sites along the chains. In addition to the amorphous area, it was discovered that, beyond the glass transition temperature (Tg), the ionic conductivity in ordered polymers may be even higher in the crystalline phase than it is in the comparable amorphous material. A plausible interpretation for the ionic conductivity observed in highly crystalline polymer electrolytes suggests that Li+ ions migrate rapidly between adjacent units, akin to inorganic crystals, resulting in the polymer matrix remaining essentially stationary. Additionally, the Li+ ion is being transported down the tunnels as a result of the defects and cavities that occurred in the crystalline phase [9]. For example, PEO chains tend to form cylindrical tunnels in the crystalline phase of the polymer, where the Li+ cations are situated and coordinated by ether oxygen atoms. Without the assistance of segmental motion, the Li+ ions can move along these cylindrical tunnels in the inter-chain space. The ionic conductivity of these stoichiometric crystalline complexes can increase in up to two orders of magnitude by substituting a small percentage of the XF6 ions with very different monovalent ions (shape and size), as N(SO2CF3)2.
Future batteries with enhanced energy density, safety, and processability will rely heavily on solid-state polymer electrolytes (solvent-free electrolytes). New polymer electrolytes containing phosphorous, in both the +3 and +5 oxidation states, are promising compounds to be used in the field of batteries and energy storage. Different copolymers with flame-retardant phosphonate units, ion-conductive cyclocarbonate moieties, and lithium salts were combined to obtain solid polymer electrolytes (SPEs). The ionic conductivity of those copolymers at room temperature is around 10−5 S cm−1. This is similar to the conductivity of other solid polymeric electrolytes (for example based on ethylene oxide). Furthermore, compared to the homopolymer blends of similar composition, the copolymer electrolytes are more stable in a broad electrochemical window (0.5–4.5 V vs. Li+/Li) and also at temperatures higher than 120 °C. This is due to the copolymer’s better lithium salt solubility.
During the last decades, different organophosphorus compounds like phosphoramidic derivatives [14], phosphates, phosphonates [15], phosphine derivatives, and phosphonium salts have been developed and used for their fire-retardancy properties [16,17]. Such compounds simultaneously suppress flames in both the gas phase and the condensed phase. Trimethyl phosphate (TMP), triethyl phosphate (TEP), diethyl ethyl-phosphonate (DEEP), 2-(triphenylphosphoranylidene) succinic anhydride and ethyl phosphate–polyethylene glycol based copolymer have been developed as low-molecular-weight alkylphosphine flame retardants and the corresponding liquid electrolytes [16]. Extra SEI film-forming additives are primarily needed to reduce the interfacial side effects of these small molecular alkylphosphonates because they are liquid and are likely to be involved in interfacial side reactions. In contrast, the advantages of polymeric flame retardants include their high melting points, superior thermal and electrochemical stability, and lack of volatility or leakage. These properties are crucial for the stability of the electrochemical interface, particularly for solid polymer electrolytes [17]. Properties such as high acidity, corrosion resistance, fire retardancy, good adhesion, complexation, and ion exchange capacity lead to a constantly increasing demand for employing phosphorus compounds in solid polymer electrolytes. Studies on polyphosphoesters are continuously evolving in light of the diversification and characterization of the polymer family as well as their use in a variety of fields. Polyphosphoesters (Figure 3) are structurally flexible polymers that are biodegradable through hydrolysis as well as enzymatic digestion when used in physiological contexts. Their backbone consists of repeated phosphoester linkages [18].
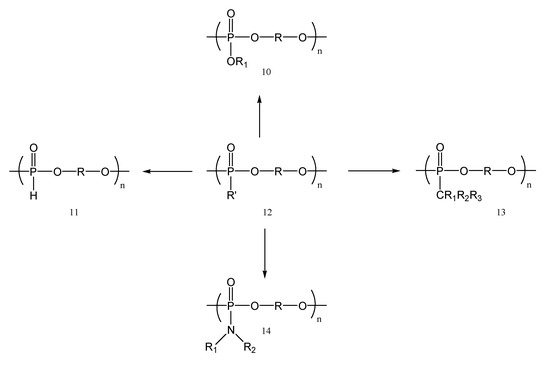
Figure 3.
Chemical structures of polyphosphoesters: polyphosphate (10), polyphosphite (11), polyphosphoester (12), polyphosphonate (13) and polyphosphoramidate (14).
It is not easy to obtain a solid polymer electrolyte with good flame-retardant properties and also with high lithium-ion transportation capabilities. Still, an example is phosphates-based copolymer c-PPO, synthesized by copolymerizing oligo(ethylene glycol) ether methacrylate (OEGEMA) with dimethyl-p-vinyl benzyl phosphonate (DMpVBnP) [19]. Benzyl-phosphonates derivatives were synthesized with higher yields and at lower reaction time also by a green method, using ultrasounds [20]. To create c-PPO/LP30 GPE, the c-PPO polymer films were mixed with 1M LiPF6 in EC/DMC (1:1 wt.%). The obtained GPE proved a strong ionic conductivity of 1.5 mS cm−1 at 25 °C and showed capacity retentions of 94% (Figure 4).
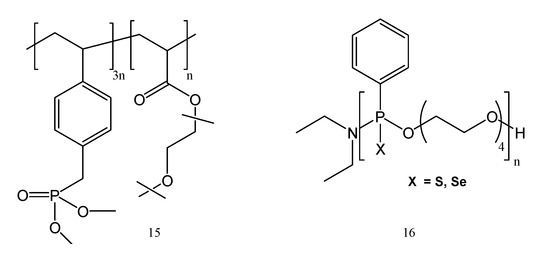
Figure 4.
Examples of flame-retardant polymers containing phosphorus: c-PPO (15) and tetraEG-S and tetraEG-Se (16).
To have a strong flame-retardant effect in c-PPO, the DMpVBnP moiety was used, resulting in nonflammable c-PPO/LP30 GPE. Further, polyethylene glycol (PEG) was copolymerized with silicon and phosphorus-containing monomers, for obtaining a solid electrolyte. These silicon/phosphorus polymers proved very good thermal stability and also flame resistance [21,22]. At a mass ratio of 10:1 LiTFSI:DKP, the maximum conductivity at 25 °C was 2.98 × 10−5 S cm−1. This is due to the quantity and mobility of free lithium ions, because both are the primary factors influencing the conductivity of electrolyte membranes. The free lithium ions in the electrolyte progressively rose as the amount of lithium salt increased, which also led to a rise in the effective carrier and ion conductivity. The LiFePO4/Li all-solid-state battery made with this solid electrolyte presents a promising application opportunity for high-safety lithium-ion batteries, with a specific capacity of 129.2 mAh/g at 0.2 C after 100 cycles. The incorporation of phosphonate groups in PEO polymers has made it possible to investigate cation binding in each polyphosphonate by using 31P NMR and 1H NMR spectroscopic titrations. The outcomes demonstrated that positive cooperativity-binding behavior is followed during interactions with Li+. The polyphosphonate containing the Se (Figure 4) showed high conductivity at and above room temperature, and at the same time had higher Tg. These results call for additional research into these modular polyphosphonates, to comprehend how modifications to their functions (the presence of additional oxygen, boranes, or transition–metal complexes and various length PEO backbone) [23] could alter their capacity for cation binding and ionic conductivity. Each of these might have an impact on cation binding and open up fascinating new possibilities and new directions for SPE development. By curing under UV light, solid polymer electrolyte films containing lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and polyphosphoester copolymers were obtained. The amount of salt in the electrolyte affects how much crystallinity is present in the copolymers caused by the PEG section. The polyphosphoester copolymers, when used as solid polymer electrolytes (SPEs), showed an ionic conductivity of 2 × 10−4 S cm−1 at 70 °C. Compared to PEO’s transference number of 0.17, lithium has a transference value of 0.26. The polyphosphoesters also possess high flame retardancy, according to the measurements performed with pyrolysis flow combustion calorimetry (PCFC) or micro-calorimetry. The solid-state lithium cell Li/polymer electrolyte/LFP battery proved a coulombic efficiency of more than 98% over 100 cycles at 70 °C and specific capacity retention up to 80% [24].
Copolymers containing fluorine and phosphorus are also used as SPEs. Phosphinate groups give the electrolytes remarkable fire-resistance qualities, while fluorinated groups provide excellent thermal, (electro-)chemical, and mechanical stabilities. Copolymers based on vinyl dimethyl phosphonate and vinylidene fluoride (Figure 5), poly-VDF-co-VDMP (19) were synthesized by the radical copolymerization of vinylidene fluoride (VDF, 17) with vinyl dimethylphosphonate (VDMP, 18) [25].
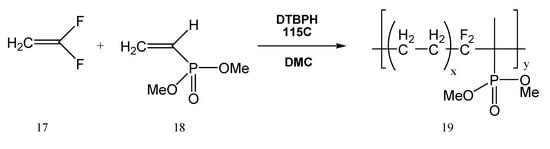
Figure 5.
Synthesis of copolymers based on vinyl dimethyl phosphonate and vinylidene fluoride.
Applying the advantages of UV curing, those polymers and copolymers could be therefore synthesized by a green chemistry method. In the literature are several examples of the synthesis of such copolymers. For example, the copolymerization obtained in the work of Bai et al. [25] takes place in the presence of 2,5-dimethyl-2,5-di(tert-butylperoxy) hexane (DTBPH, a peroxide compound) at 115 °C in dimethyl carbonate (DMC) [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46], as shown in Figure 5. In our previous work, UV curing was also used for obtaining different copolymers starting from vinyl dimethylphosphonate (18) by radical polymerization [47]. The UV light was used as a photoinitiator.
The thermal stability and the degree of crystallinity (χ) of the poly(VDF-co-VDMP) copolymers were higher when the vinylidene fluoride content was increased. On the other hand, when the content of vinyl dimethylphosphonate increased, the copolymers molar mass decreased. SPEs were then obtained from a mixture of poly(VDF-co-VDMP) and bis(trifluoromethanesulfonyl)imide (LiTFSI) dissolved in acetonitrile. The degree of crystallinity of VDF units was not affected by the addition of LiTFSI. In the sample with molar ratio dimethylphosphonate units, Li+ ions 1:1 showed relatively good conductivity; 1.9 × 10−2 mS cm−1 at 20 °C and 0.2 mS cm−1 at 60 °C. In this case, the electrochemical stability window was between 0 and 5.5 V, versus Li/Li+. An increased ionic mobility was observed by adding small amounts of plasticizer, while at the same time the electrochemical, mechanical stability, and flame retardant properties were the same. To improve the flame resistance of the PEG600 (20) chain even more, silicon- and phosphorus-containing DOPO (21) and KH560 (22) monomers were added. The synthesis of silicon- and phosphorus-containing DKP polymer is shown in Figure 6.
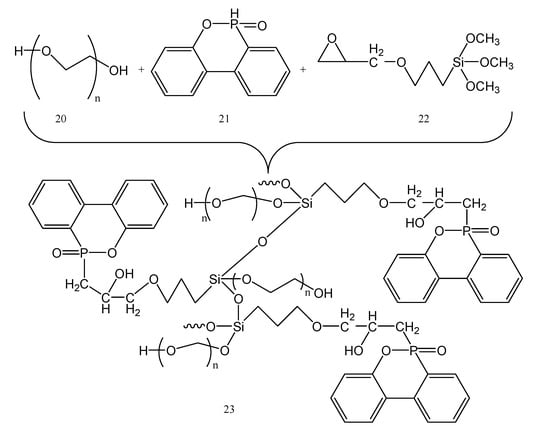
Figure 6.
Synthesis of silicon- and phosphorus-containing DKP polymer (23) starting from PEG 600 (20).
The added chemical link increased the matrix’s stability to produce even higher flame-retardant properties. The hydroxyl groups from the structure of PEG 600 (20) can polymerize with the methoxy group of 22, which then further can react with the DOPO’s active P-H bonds [27]. The DKP/LiTFSI electrolyte exhibits strong ionic conductivity (mainly due to the Si-O core of 23). At a mass ratio of 10:1 LiTFSI:DKP, the obtained conductivity was 2.98 × 10−5 S cm−1 at 25 °C. As the temperature increases, the conductivity increases even more and respects the Arrhenius equation. The performances of a LiFePO4/Li battery containing DKP/LiTFSI solid electrolytes showed the discharge capacities after 10 cycles at 60 °C, as follows: 142.0, 133.2, 126.6 and 115.5 mAh/g, for current densities of 0.1 C, 0.2, 0.5, and 1 C (1 C = 170 mA/g). At 0.1 C current density, the solid-state battery produced a specific capacity of 134.5 mAh/g. After six charge–discharge cycles, its reversible capacity stabilized, and the coulombic efficiency increased and was very close to 100% for DKP/LiTFSI electrolyte-assembled LiFePO4/Li solid-state battery [22].
High-temperature stability, amorphous nature, and flame retardancy are important properties and characteristics which recommend the incorporation of phosphorus into polymer chains to be used for Li-po. Different polymers containing phosphorus used for solid-state batteries were also synthesized by ring-opening polymerization (ROP) (Figure 7) [28] and by polycondensation (Figure 8) [17].
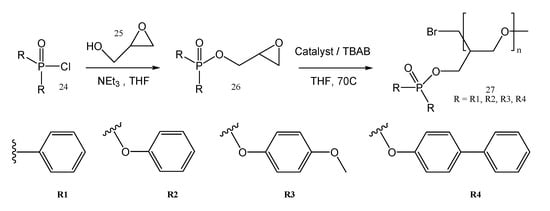
Figure 7.
ROP synthesis and structures of the obtained phosphorus containing polyethers.
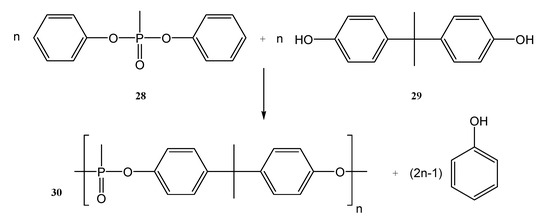
Figure 8.
Synthesis of PBMP (30).
The polyethers 27 presented in Figure 7, are stable, amorphous, and solid at room temperature (except R3). The solid polymer electrolyte containing R2 and having a 40% LiTFSI content had the maximum conductivity of 2.9 × 10−4 S cm−1 at 80 °C from the polymers synthesized as shown in Figure 7. It can be used as solid polymer electrolytes in lithium-ion batteries, with an improved flame retardancy also. Poly(bis(4-phenoxyl)propane methylphosphonate) (PBMP, 30) synthesized by the polycondensation of diphenyl methylphosphonate (DPMP, 28) with bisphenol A (BPA, 29) is another example of a strong flame retardant used for energy storage applications (Figure 8) [17].
Poly (bis(4-phenoxyl) propane methylphosphonate) (PBMP), synthesized by polycondensation (Figure 8), was used to obtain new materials with a high conductivity, good ion transport properties, and also good flame retardancy. The PEO/PBMP blend with 3% phosphorus content showed a LOI (limiting oxygen index) value of 25.6%. The PEO/PBMP mix has a strong electrochemical stability window of 4.0 V vs. Li+/Li, and an improved ionic conductivity at room temperature (1.25 × 10−5 S cm−1). In addition, there are no significant side effects on battery performance and on charge/discharging behavior and cycling capability [17]. When LiTFSI was used at concentrations higher than 25%, it tended to aggregate. The aggregation decreases the ionic conductivity. The charge/discharge plateau was 3.5 V vs. Li+/Li and showed an initial specific capacity of 100 mAh/g, which is enhanced to 150 mAh/g after 20 cycles. From the 20th to the 100th cycle, the discharge-specific capacity remained around 150 mAh/g [17].
The homopolymerization of monomer dimethyl(methacryloyloxy)methyl phosphonate (MAPC1, 32) and monomer(2-Oxo-1,3-dioxolan-4-yl)methyl methacrylate (MACyCB, 31), allowed the obtaining of homopolymer poly(MAPC1), with an apparent Mn of 61 × 103 g mol−1 and a polydispersity of 1.9 and, respectively, of poly(MACyCB) with a molar mass of Mn of 67 × 103 g mol−1 and a polydispersity of 2.60 [29]. SPE membranes were prepared either from copolymer or a mix of homopolymers with the same ratio of both comonomers (MAPC1 and MACyCB) (Figure 9).
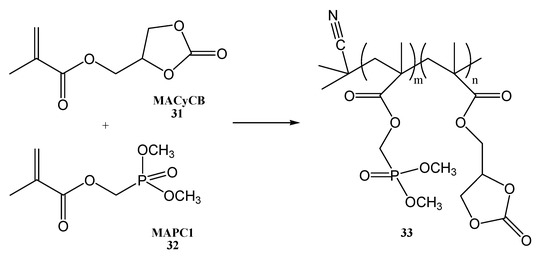
Figure 9.
Synthesis of poly(MAPC1-r-MAcy CB) copolymers (33).
MAPC1 (31) and MACyCB (32) are interesting monomers for applications in solid polymer electrolytes because they have the fire-retardant properties of phosphonate and also of ionic conductivity. The homopolymers poly(MAPC1) and poly(MACyCB), and also 1:1 mol copolymer of MAPC1 and MACyCB, loaded with LiTFSI at a same molar ratio of 1:1, showed that the poly(MAPC1) is stable at a low voltage (1.5 V against Li+/Li) and at a high voltage (over 5 V vs. Li+/Li) as well. On the other hand, the cyclocarbonate-containing poly(MACyCB) polymer is only stable between 1.8 and 3.5 V vs. Li+/Li. This suggests that the inclusion of MAPC1 in the copolymer will improve the electrochemical stability of MACyCB. The SPE membranes based on the poly(MAPC1-r-MAcy CB) showed better LiTFSI solubilization in comparison with the analogous homopolymer-based SPEs. This will lead to obtaining a higher ionic conductivity, based on the polymer architecture [29].
The plasticizers (TMPO) and the crosslinker (TAMEPO) with a phosphate core attached, and with an oligo(ethylene oxide) structure (Figure 10), increased the electrochemical stability and thermal resistance of the obtained materials [30].
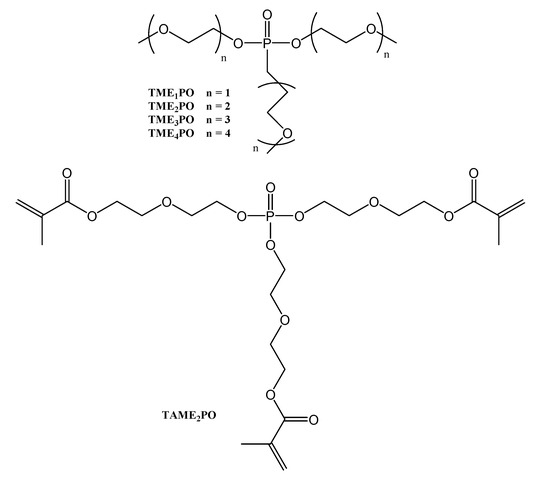
Figure 10.
Structure of the used plasticizers (TMPO) and crosslinker (TAMEPO) containing a phosphate core.
SPEs were prepared by radical polymerization. The reaction mixture contained the crosslinker (TAME2PO), the plasticizers (TMPO), and a lithium salt. The crosslinking has provided enough mechanical stability (tensile strength of up to 1.2 MPa). The ionic conductivities increased as the EO content was lower, and the plasticizer concentration was increased. The optimal ratio was EO]/[Li] 15:1. LiN(SO2CF3)2 lithium salt, leading to better results than LiSO3CF3. At room temperature, the highest ionic conductivity was around 5 × 10−4 S cm−1 for the SPE containing 80 wt% TME3PO and LiN(SO2CF3)2. The electrochemical stability window was above 5.0 V (vs. Li+/Li), resulting in a lithium plating/stripping efficiency of 75% [30].
With a molar ratio of DPOX:DDOE 1:1 (Figure 11), the self-extinguishable property of a solvent-free cross-linked poly(oxetane)-based electrolyte films is derived from the photo-initiated polymerization of a compound containing a dimethyl phosphate ester group (DPOX) and 1,9-bis(3-ethyl-3-oxcetanyl)-2,5, 8-trioxanonane (DDOE).
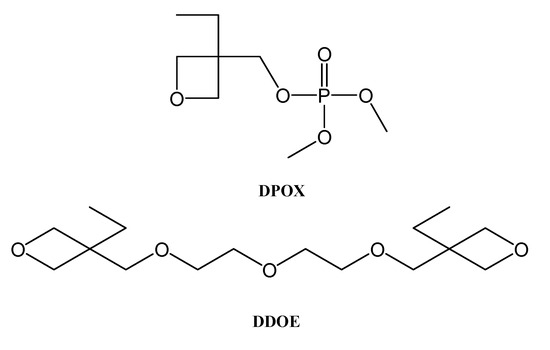
Figure 11.
Structures of oxetane derivatives DPOX and DDOE.
The conductivity strongly depends on the used ratio and on the nature of lithium salts. For the DPOX:DDOE 1:1 (LiX)n the conductivity at 353 K was, as follows:
−5.15 μS cm−1 when X = BF4 and n = 1
−56.1 μS cm−1 when X = TFSI and n = 1
−0.122 mS cm−1 when X = TFSI and n = 2 [31]
2.2. Gel Polyphosphonate Electrolytes
Gel polymer electrolytes (GPEs) are a result of attempts to increase the ionic conductivity of solid polymer electrolytes (SPEs), usually by adding a low amount of plasticizer or solvent to SPEs. In general, the ionic conductivity of GPEs is two orders of magnitude higher than the one of SPEs and can reach 10−3 S cm−1. The increase in ionic conductivity was observed together with a decrease in mechanical strength. GPEs’ mechanical strength, transport characteristics, and electrochemical properties have all been significantly improved by the addition of inorganic fillers. To increase the ionic conductivity even more, different methods were employed during the last decades, including changing the polymer matrix, looking for new kinds of lithium salts, and adding inorganic fillers [32]. Especially in the last two decades, significant progress has been achieved in the field of electrochemical energy storage. Due to their many uses (including fixed power grid storage, electrical vehicles, and portable electronic gadgets), lithium-ion batteries (LIBs) are gaining a lot of attention, as was previously highlighted [33,34]. As power LIBs with a higher energy density continue to be developed, safety concerns remain a serious concern and are becoming more stringent [35,36]. The utilization of conventional organic liquid electrolyte, which comprises volatile carbonate solvents with a low flash point, is widely recognized to increase the danger of leakage and combustion, as previously mentioned. It is possible to argue that the flammable electrolyte is the primary element influencing overall thermal stability. Organic phosphorus-containing compounds have been used with organic carbonates to develop flame retardants or even nonflammable electrolytes [40]. Wang et al. [16] obtained a concentrated electrolyte by using trimethyl phosphate (TMP) as flame retardant. This leads to a very stable charge/discharge cycling, with high cell performance. Cao et al. [41] also proved the increased stability of those systems by using nonflammable electrolytes with high salt-to-solvent ratios in lithium-ion batteries. Zhang et al. [42] reported an interesting approach by formulating a nonflammable electrolyte mainly based on triethyl phosphate (TEP) and ethylene carbonate.
Using gel polymer electrolytes resulted in significant advancements. In comparison with typical liquid electrolytes, gel polymer electrolytes (GPEs) [43,44] and solid-state electrolytes [45,46] are highly chosen due to their wide electrochemical window and capability to tackle the safety issue by minimizing flammability and boosting stability. It has been established that environmentally acceptable and efficient fire-retardant polymer electrolytes are materials that offer safer operating conditions for lithium-ion batteries. The safe and non-flammable properties of phosphorus-based polymer electrolytes were used already in lithium-ion batteries [43,44,45,46,47,48,49,50], but their ionic conductivity is generally low at room temperature (around 10−5–10−8 S cm−1). In GPEs, the liquid electrolyte is immobilized in a polymeric matrix. This will significantly decrease the risk of leakage. As a consequence, the nonflammable GPEs became the most important alternative for the fabrication of advanced LIBs [32,51,52].
Tris(2-(acryloyloxy)ethyl)phosphate cross-linking gel polymer electrolytes were synthesized by Kim et al. [53,54] and used in lithium-ion batteries [52]. There must be a minimum of two polymerizable functional vinyl groups present in the cross-linkers. Allyl alcohol, triethylene glycol dimethacrylate (TEGDMA), 1,4-butanediol dimethacrylate (BDDMA), and pentaerythritol tetraacrylate (PET4A) are a few examples of cross-linkers utilized to generate GPEs for lithium-ion batteries [32]. Then, in situ heat polymerization (at 75 °C) was used to create the appropriate cross-linking gel copolymer electrolytes. Therefore, alkenyl phosphate molecules could be added to the polymer chain. The GPEs containing TEGDMA showed elastic and soft properties, with higher conductivity. At the same time the GPEs containing BDDMA or PET4A were more rigid materials and showed lower conductivity. It was observed that the TEGDMA-based GPEs showed higher performances. Therefore, the copolymerization process could improve the mechanical properties (elasticity and leakage) and also the ionic conductivity of GPEs [32,34].
Alkenyl compounds containing phosphorus, with different ester structures (as AEDEP and MADBP, Figure 12), should match different monomers to form GPEs with higher performance.
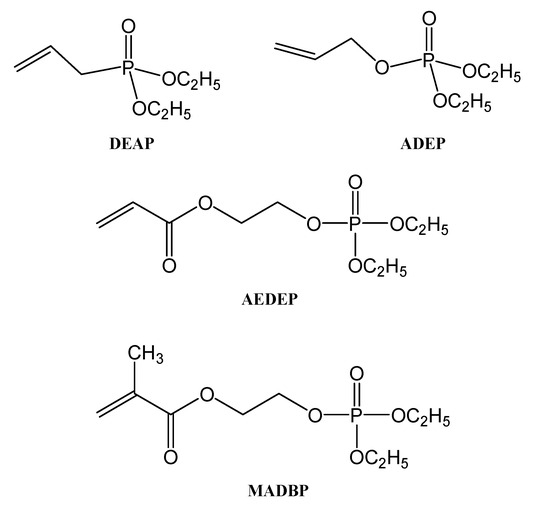
Figure 12.
The structure of alkenyl phosphonates/phosphates monomers.
The monomer ratio is important and can influence the properties of the obtained GPE. The best GPEs in this case were AEDEP—EA (ethyl acrylate)—TEGDMA and MADBP—MMA (methyl methacrylate)—TEGDMA, both with the monomer’s ratio of 3:3:2. The MADBP—MMA—TEGDMA gel polymer electrolyte obtained by radical polymerization (Figure 13) showed a conductivity around 7.88 mS cm−1.
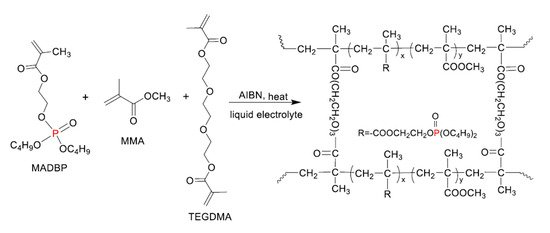
Figure 13.
The synthesis of MADBP gel polymer electrolyte (GPE).
The presence of the polar groups on the structure of the polymer matrix of the obtained GPE increases its solubility. As a result, a homogenous gel polymer electrolyte with acceptable mechanical qualities will be obtained. Metal–organic frameworks containing phosphorus and also polyphosphonates are present in the network structures formed usually by the chemically cross-linkable constituents [32,55,56]. Such compounds are suitable for different applications, including their use as corrosion inhibitors. The polymer network, containing 90% electrolyte, is responsible for high ionic conductivity, and therefore it can be used also for Li-ion batteries. By washing the liquid electrolyte from GPE, one may produce the porous network of the polymer matrix. The polymer matrix’s surface and cross-section revealed a homogeneous layered porosity distribution, which contributes to its strong ionic conductivity, high surface area, high lithium-ion transport channel, and good liquid-electrolyte absorbability [32]. All of these properties and characteristics make those GPEs containing MADBP very promising alternatives for applications in Li-ion batteries. The GPEs containing MADBP, in addition to their good stability, high conductivity, and good electrochemical stability, also showed significant flame retardant properties.
The DEAP and ADEP have both low homopolymerization ability because they are relatively easy to split into stable allyl radicals during the radical thermal polymerization process, and as a consequence, low-molecular-weight homopolymers were obtained (below 5000 g/mol). On the other hand, in the case of AEDEP it is not easy to obtain an intramolecular chain transfer reaction, and consequently this monomer can form homopolymers with molecular weight higher than 30,000 g/mol. The polymerization capability of DEAP, ADEP, and AEDEP (Figure 12) was improved significantly when they were copolymerized with methyl methacrylate (MMA) and styrene monomers. The molecular weights of the obtained copolymers of DEAP and ADEP with MMA were much higher than their corresponding homopolymers (Table 1) [32].

Table 1.
The molecular weights (Mn, Mw) and polydispersity (PDI = Mw/Mn) of the homopolymers and copolymers based on DEAP, ADEP, and AEDEP monomers.
Due in large part to the coordination interaction between MADBP and lithium ions as well as the loosely porous polymer-matrix structure, the Li cell based on the polyphosphonate gel electrolyte demonstrated good charge–discharge cycle performance and high efficiency [32].
3. Polyphosphazenes Used as Polymer Electrolytes
The structure of phosphazenes contains a double bond between a phosphorus atom and a nitrogen atom. Phosphazenes can be found in the following configurations: small molecules, different cyclic species, high molar mass polymers with –P=N– repeating units, or linear oligomers with a ring made up of alternating phosphorus and nitrogen atoms. The polymerization of substituted phosphazene leads to the synthesis of polyphosphazenes. Because of the extremely high torsional mobility of the P-N phosphazene link, polyphosphazenes exhibit very beneficial features for their use as polymer electrolytes, such as low glass transition temperature Tg, which is frequently as low as −100 °C. The properties of polyphosphazenes are mostly influenced by the type of side groups that are attached through P-C, P-O, or P-N bonds. Although the P-C bonds are hydrolytically stable, it is challenging to incorporate them into polyphosphazene backbone. Moreover, the P-O bond exhibits hydrolytic stability. For instance, poly-phosphazene is hydrophobic and chemically stable due to the fluorinated alkoxy side groups [57].
3.1. Solid Polyphosphazene Electrolytes
In 1984, Allock et al. [57,58,59] proved that polyphosphazene-based electrolytes have good ionic conductivity and high electrochemical stability. Therefore, such polymer electrolytes could be a good candidate for applications in Li-ion batteries. Furthermore, this polymer has a strong fire resistance due to the phosphorus atom from its structure [60]. Diethylene glycol monomethyl ether, found in the literature also as 2-(2-(2-methoxyethoxy)ethoxy) ethanol, was obtained by using the previously synthesized poly(dichlorophosphazene) with ionic conducting units, such as diethylene glycol (Figure 14 and Figure 15), to create poly[bis(2-(2-methoxyethoxy)ethoxy)phosphazene] (MEEP).
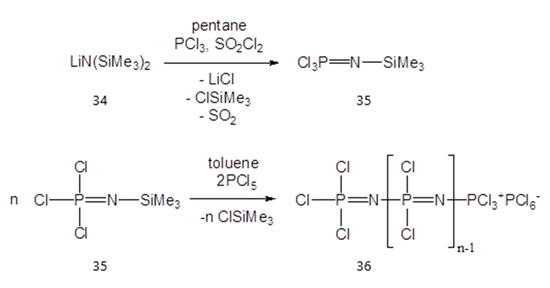
Figure 14.
The chemical route to obtain the precursor polymer (36).
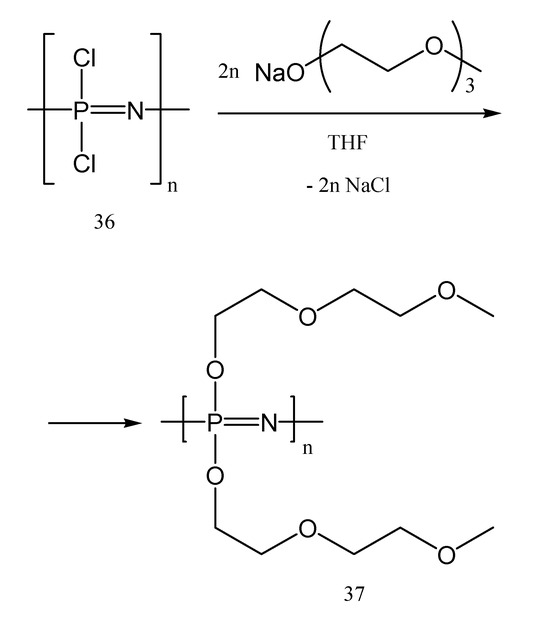
Figure 15.
The synthesis of poly[bis(2-(2-methoxyethoxy)ethoxy)phosphazene] MEEP (37).
MEEP is the most commonly used polyphosphazene for energy storage. In comparison with PEO-based polymers combined with lithium salts as polymer electrolytes in batteries, MEEP (37) showed three to four orders of magnitude greater ionic conductivity. The explanation is that the existence of etheric oxygen bonds in the MEEP matrix allows the salts to dissociate. It was believed that the nitrogen atoms in the backbone may dissolve the cation and induce dissociation as a result. The flexibility of the side chains and backbone of polyphosphazenes is another benefit, as evidenced by their structures in Figure 16 (polymers 38 and 39), which provide also a low glass-transition temperature (Tg = −84 °C). This is an advantage for their use in Li-po batteries, while it allows a faster ion migration than in the case of rigid polymers. MEEP (37), one of the most used compounds for Li-po batteries, belongs to the polymer structures 38.
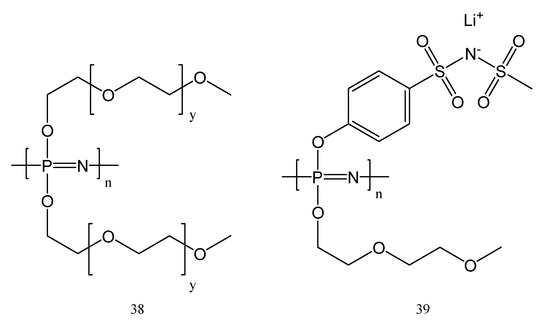
Figure 16.
Ion conducting polyphosphazene electrolytes: polyphosphazene with varying ethyleneoxy chain length (38, y = 0–15) and single-ion polyphosphazene (39).
Such compounds could be involved in different chemical syntheses and processes as exposure to ultrasounds, γ-irradiation, UV curing, transesterification, sol-gel process, syntheses of heterocyclic compounds containing phosphorus and nitrogen (as phosphazenes), and so on [25,47,61,62,63,64,65,66,67,68,69,70]. MEEP, which is an amorphous polymer, has one of the highest conductivities from the class of unplasticized polymer electrolyte systems [71]. The polyphosphazene electrolyte membranes containing linear polymers with repeating -(N=PR2)- units, grafted with ethylene oxide chains R(OCH2CH2)3OCH3, and in addition, containing LiTFSI and LiBOB lithium salts, dissolved in EC/DMC, showed the following conductivities at room temperature: 1.2 × 10−4 S cm−1 in the case of LiTFSI and 2.6 × 10−4 S cm−1 for LiBOB [72]. Several charge/discharge tests were performed in different conditions for MEEP/LiBOB/EC/DMC systems. Without any evidence of lithium-related short circuits, those studies revealed a consistent cycling behavior with over 1300 cycles and capacities greater than 110 mAh/g [73,74]. The ionic conductivity was shown to rise with a chain length of up to eight ethyleneoxy units (CH2CH2O). When it contained more than eight ethyleneoxy units, the gel crystallized, and as a consequence, the conductivity was affected [71]. Polymers containing cyclic ether side groups attached to the polyphosphazene backbone were synthesized (Figure 17) and their mixtures with LiTFSI (at different salt concentrations) were studied as polymer electrolytes [75].
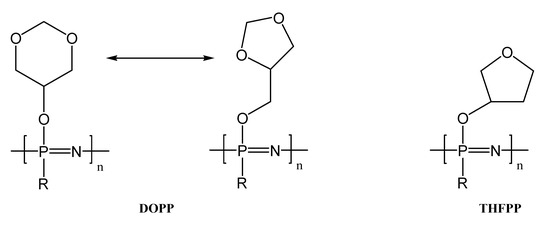
Figure 17.
Structures of DOPP and THFPP.
Both, poly[(1,3-dioxane-5-oxy) (1,3-dioxolane-4-methoxy) phosphazene] DOPP and 5-hydroxy-1,3-dioxane poly[bis(2-tetrahydro-3-furanoxy)phosphazene] THFPP, have low glass transition temperatures (Tg) and thermal stability only up to 200 °C. The LiTFSI salt dissolution takes place under the dissociation of the ions at low concentrations. When the salt concentration increased, less dissociation occurred. Therefore, the whole LiTFSI system settles down into the polymer side chain substituent. The maximum lithium-ion conductivities were obtained at a temperature of 60 °C, as follows:
- -
- for DOPP (12:1) 2.81 × 10−6 S cm−1
- -
- for THFPP (6:1 at 60 °C) 9.03·10−7 S cm−1
Due to lithium’s greatest electrode potential, the conductivity of lithium salts in SPEs, particularly MEEP, have been thoroughly explored in recent decades for applications in storage energy devices [75]. The flexibility of the polymer chains affects partly the conduction of ions in a polymer electrolyte. Hence, flexible macromolecules have better ionic conductivities than rigid polymers because they permit faster ion migration. The glass transition temperature (Tg) is also correlated with the polymer chain’s flexibility [71].
Another example of phosphazene polymer electrolytes synthesis is the obtaining of poly(phosphazene)s (MEEPP) containing 2-(2-methoxyethoxy)ethoxy and 2-(phenoxy)ethoxy units, with 2,4,6-tris[bis(methoxymethyl)amino]-1,3,5-triazine (CYMEL) as a cross-linking agent [76]. MEEP has good ionic conductivity, a flexible polymer backbone, and good fire-retardant properties. Cross-linking is a practical method to obtain membranes with adequate mechanical strength: poly(phosphazene) MEEP with 2-(2-methoxyethoxy)ethoxy and 2-(phenoxy)ethoxy units are mixed with 2,4,6-tris[bis(methoxymethyl)amino]-1,3,5-triazine (CYMEL) in DMSO. The used molar ratio of the phenyl group, CYMEL, was 3:1. The resulted solution was then stirred at 135 °C. After 30 min, the lithium salt LiTFSI was added under stirring to the solution and mixed further until a homogeneous mixture was obtained [76].
An important class of phosphazenes used for obtaining membranes is represented by the polyphosphazenes containing chlorine. Poly(dichlorophosphazene) is one example. It could be synthesized in the first step by the ring-opening polymerization of hexachlorophosphazene with sulfamic acid (H3NSO3) as a catalyst. The chemical process between poly(dichlorophosphazene), diethylene glycol monomethyl ether sodium salts and 2-(phenoxy) ethanol yields polyphosphazene 43 with 2-(2-methoxyethoxy)ethoxy, and 2-(phenoxy)ethoxy units (Figure 18). The amount of the 2-(phenoxy)ethoxy unit should be around 6–18 mol %. Otherwise, a content higher than 18 mol % leads to a decrease in lithium-ion mobility [76].
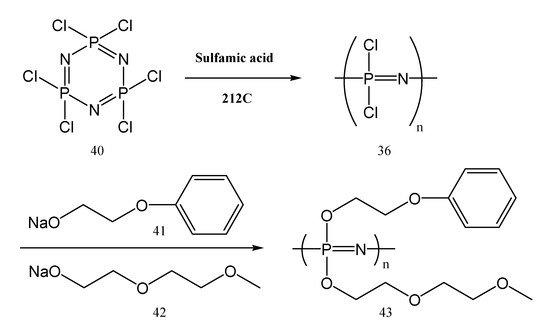
Figure 18.
The synthesis of phenoxy-containing polyphosphazenes (43) used as polymer electrolytes.
The work of Schmohl et al. [77] demonstrated that mixed substituted polyphosphazenes possess the electrochemical properties and the requirements to be used as polymer electrolytes together with lithium metal anodes [77,78,79,80,81]. Therefore, two series of mixed polyphosphazenes MEE-co-OBF3Li5P and MEE-co-OBF3Li(10)P were synthesized with different concentrations of the anionic oxytrifluoroborate groups (5% and 10%). The oxytrifluoroborate groups were further neutralized by an additional number of lithium cations. Then, around 1 g of the polymer (44) dried at 60 °C under vacuum for one week was dissolved in THF (Figure 19). Benzophenone (10%) and lithium salt (15%) were then added to the polymer solution under stirring.
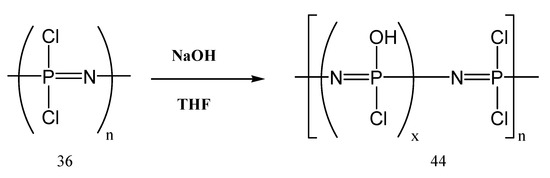
Figure 19.
The synthesis of poly-hydroxophosphazene’s precursor.
The resulting mixture was dried under a vacuum at 60 °C for more 24 h, to completely remove THF. For obtaining thin membranes of the salt-in-polymer electrolyte, a suitable amount of the dried mixture was pressed between two solid foils. Membranes with a thickness of 200–300 μm were obtained. The last step of this procedure is the cross-linking process under UV-irradiation for 12 min. In the same way, polymer electrolyte membranes were obtained also with different lithium salts (for example 5/10 mol % -OBF3Li substituted polymers with 15 wt% LiBOB, LiTFSI, and LiFSI) [77].
3.2. Gel Polyphosphazene Electrolytes
Due to their high flame retardancy [77,78], polyphosphazenes were used also as polymer gel electrolytes in Li-ion batteries. MEEP, when used in Li-po batteries, showed a high ion conductivity (2.4 mS cm−1) at 25 °C and stable long-term charge/discharge cycling ability against lithium metal anodes (1350 charge–discharge cycles at 0.5 °C [73,74,79,80,81]. While the surface morphology and the SEI (solid electrolyte interphase) property of the obtained GPEs remain still unclear, further research is necessary to clarify completely the interfacial behavior. Jankowsky et al. [72,73,74] have previously synthesized MEEP and obtained salt-in-polymer membranes. Gel polymer electrolytes were prepared by soaking a liquid salt solution containing 0.7 M LiPF6 in ethylene carbonate–dimethyl carbonate (EC/DMC 1:1) in a salt-free cross-linked MEEP membrane [26,82,83]. Two different cells were studied and presented in the scientific literature:
- (a)
- the two-electrode cell for in situ monitoring of lithium deposition/dissolution by an optical microscope;
- (b)
- a three-electrode cell (Swagelok cell, Figure 20) for electrochemical analysis by cell cycling and EIS (electrochemical impedance spectroscopy) measurements.
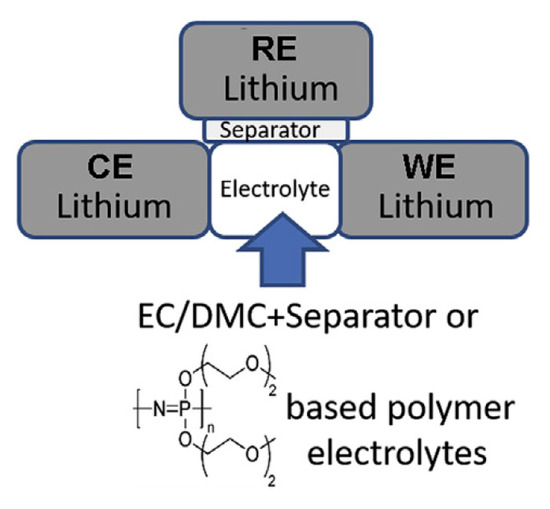 Figure 20. Configuration of Swagelok cell.
Figure 20. Configuration of Swagelok cell.
The Swagelok cell is symmetrical. It contains three electrodes (the third one is a Li+/Li electrode). Lithium metal foil was used for working and for counter electrodes. Because lithium metal and other components in the cell are extremely sensitive to moisture, both types of assembly cells were conducted in a dry room. In comparison to the dry MEEP base membrane, the polymeric gel electrolyte EC/DMC + MEEP/LiPF6 is a more intriguing solution. Once more, the lithium WE’s time-dependent sequence of over potential pulses was observed during galvanostatic pulses that alternated between anodic and cathodic conditions at a rate of ±0.1 mA cm−2. The initial step in the lithium dissolving process for the MEEP/LiPF6 gel electrolyte begins with an instantaneous increase in overpotential to 60 mV [26].
While poly[bis(2-(2-methoxyethoxy)ethoxy)-polyphosphazene] (MEEP) was proved to be a good polymer electrolyte [80,81,82,83,84,85,86,87,88,89,90], Schmohl et al. [77] reported the synthesis of polyphosphazene derivatives boron and fluorine containing, used for application as both, solid and gel polymer electrolyte. Poly[bis 2(2-methoxyethoxy)ethoxy-co-oxytrifluoroborat lithium phosphazenes, abbreviated MEE-co-OBF3Li(5)P and MEE-co-OBF3Li(10)P, was successfully used also as GPE. The synthesized polymers were mixed with lithium salt, ethylene carbonate–dimethyl carbonate (EC/DMC) 1:1, and benzophenone. Then, the obtained mixture lead to obtaining the GPE. Further, the gel polymer electrolyte membranes occurred after cross-linking using UV-irradiation for 12 min. After each minute, during the UV cross-linking process, an intermediate cooling by dry ice is necessary. The obtained MEEP/LiBOB gel polymer proved enhanced charge–discharge cycles and showed high stability of the Li/MEEP interface [73,79].
4. Other Phosphorus Containing Polymers Used as Polymer Electrolytes
4.1. Solid Polymer Electrolytes
An example of solid polymer electrolytes used in Li-po batteries, other than the previously mentioned ones, is the product obtained by the polymerization of butyl bis(hydroxymethyl)phosphine oxide 46 [84].
The butyl bis(hydroxy methyl)phosphine oxide 46 is first obtained from the precursor 45 in a three-step synthesis (Figure 21); with BuI in methanol at 70 °C, and then with an excess of trimethylamine (NEt3) at 60 °C, and finally with H2O2 in methanol. Product 46 was obtained at a 1:1 ratio with a secondary product 47, and it was further copolymerized with different dibromo monomers such as 48–50, to yield the polyethers 51–53, respectively, via nucleophilic substitution reactions (Figure 22).
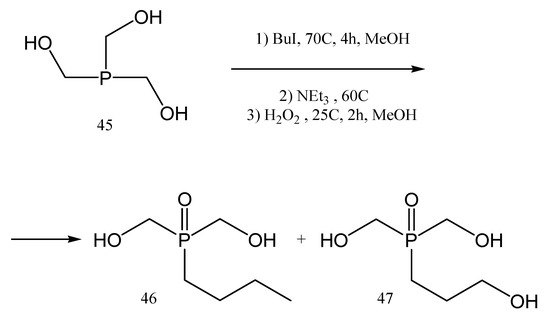
Figure 21.
Synthesis of butyl bis(hydroxymethyl)phosphine oxide 46.
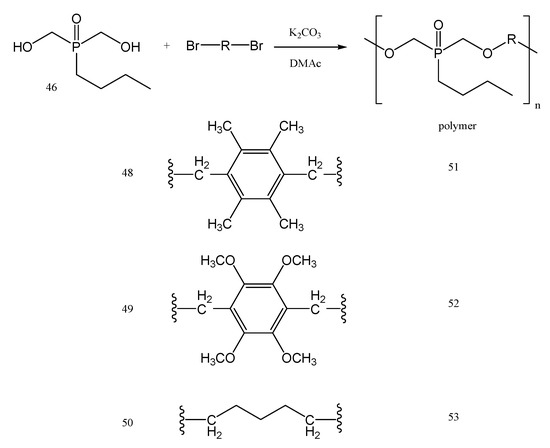
Figure 22.
The synthesis of phoshorus containing polyethers 51–53.
The polymerization reaction was carried out in two steps, in the presence of K2CO3 as a base in N,N–dimethylacetamide (DMAc). Toluene was added and then an azeotropic distillation was necessary until the water was completely removed [85]. Further, by increasing the temperature up to 160◦C, the solid polyethers 51 and 52, and also the liquid polyether 53, were all obtained. The polyethers 51–53 showed good solubility in CHCl3, THF, and DMSO [80]. The main properties of polymers 51–53 used as solid polymer electrolytes in Li-po batteries are shown in Table 2 (number average molar mass Mn, weight average molar mass Mw, polydispersity PDI, decomposition temperature Td and glass transition temperature Tg).

Table 2.
Properties of polyethers 51–53.
The solid polymers 51 and 52 were involved in the preparation of the solid polymer electrolytes SPE1 and SPE2 by using lithium bis(trifluoromethanesulfonyl) imide (LiTFSI) (10, 20, 30, and 40 wt%). The polymers 51 and 52 and THE lithium salt were dissolved together in THF at 25 °C and kept in those conditions for 12 h. After the solvent evaporation, the product was dried under vacuum to obtain the SPEs. The thermal stability increased for SPE1 and SPE2 in comparison with the polymers 51 and 52. Moreover, the SPE1 and SPE2 showed higher thermal stability when the lithium salt content was increased (from 10 wt% of LiTFSI to 40 wt% of LiTFSI, Table 3).

Table 3.
Properties of polymer electrolytes SPE1 (containing the polymer 51) and SPE2 (containing the polymer 52).
As a result, when the lithium salt was added, the glass transition temperatures (Tg) of SPE1 and SPE2 likewise climbed steadily, rising from 10% to 40%. The efficient coordination of the polymer chains with lithium ion may be the cause of this. Table 3 [84] also lists the conductivities (σ) and the ionic capacities at 30 °C and 80 °C of SPE1 with 10–40 wt% of LiTFSI (SPE1 10–40%) and SPE2 with 10–40 wt% LiTFSI (SPE2 10–40%).
4.2. Gel Polymer Electrolytes
In addition to the previously mentioned polyphosphonates and polyphosphazenes, phosphates could also be used for obtaining gel polymer electrolytes (GPEs). One example is tri(2-(2-methoxyethoxy)ethyl)phosphate (56). It is suitable to be used for energy storage [86] and it could be synthesized by the chemical process described in Figure 23, starting from phosphoryl chloride 54, also called phosphorus oxychloride (Cl3P=O).
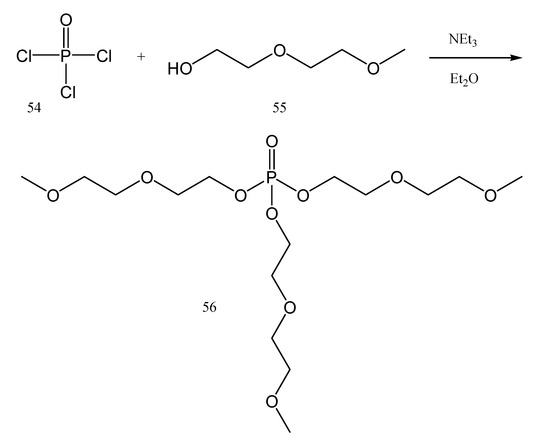
Figure 23.
The synthesis of phosphate 56 starting from Cl3P = O (54).
The obtained tri(2-(2-methoxyethoxy)ethyl)phosphate 56 is a non-volatile compound at room temperature and has low flammability in comparison with propylene carbonate. One important characteristic for a compound to be used as an additive for GPEs is low volatility. But it should be taken into account that above 180 °C, tri(2-(2-methoxyethoxy)ethyl)phosphate (56) decomposes. The GPEs could be prepared in this case by using lithium bis(trifluoromethylsulfonyl)imide (57) as the lithium salt (Figure 24).
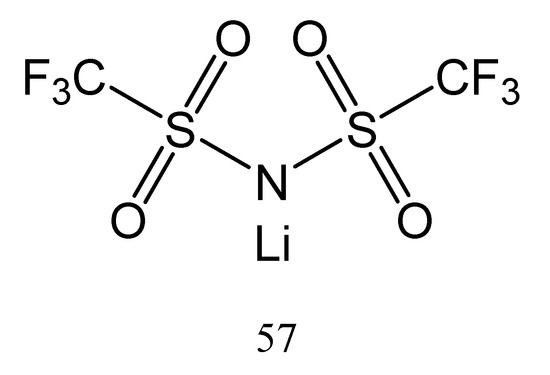
Figure 24.
The structure of lithium bis(trifluoromethylsulfonyl)imide (57).
The conductivities of phosphorous containing gels were slightly lower than those obtained with propylene carbonate, but the risk of flammability was reduced significantly. Therefore, using the phosphate GPEs has the advantage of fire retardancy, but the conductivity, and as a consequence the performances, decreased a little. On the other hand, the glass transition temperatures are similar (Tg from −80 °C to −90 °C) for the GPEs obtained either with tri(2-(2-methoxyethoxy)ethyl)phosphate or with propylene carbonate [86].
5. Conclusions
The major contribution of the present review is to describe the use of different phosphorus containing polymer electrolytes as materials for Li-po batteries. Among those materials used in the last decade for energy storage are PEO-based SPEs [8,9,10,11,12,13,23,24], or polymers such as MEEP [71,72,73,74,75,76]. Both the dry polymer (solid polymer electrolytes) as well as the gel polymer electrolytes showed a high conductivity. Those are the two main directions for applications in the field of Li-po batteries and energy storage. In addition to their high conductivities, such polymers offer also better charge–discharge cycles (as faster charging and higher stability). The MEEP-based polymer electrolytes enhance the solid electrolyte interphase (SEI) formation on lithium anodes in comparison to the liquid solutions of LiPF6 in EC/DMC. The GPE based on MEEP leads to the obtaining of a membrane with high conductivity. Through a gelification process using polar aprotic liquids, which significantly increases ionic mobility, the polymer electrolytes are often transformed into polymer gels [21,80,87,88,89,90,91,92,93,94,95,96]. The application of polymers with immobilized anions (polyanions) is anticipated to yield an extra enhancement. In this instance, the mobile lithium-ions balance the charge. One lithium-ion transport and a transference number of one are anticipated outcomes of this approach. When polyphosphazenes were used, it was shown to be successful and promising for many applications in the field of Li-po batteries, to introduce boron trifluoride (BF3) as the anionic species to the polyphosphazene backbone [75,76,77,78,79,80,81]. Recently, a few approaches were developed for an even higher increase in the lithium conductivity of the modified MEEP, such as introducing a higher amount of substituted anion groups such as –OBF3Li or lithium salts into the polymer structure. Those MEEP modifications also increased the Li+ ions mobility [77]. As proved by the current state-of-the-art field of energy storage, it is expected that the use of phosphorus containing polymers (polyphosphonates, polyphosphazenes, polyethers and polyesters phosphorus containing, and so on) for Li-po batteries will increase in the future, due to their significant advantages as high conductivities, high stability, lower flammability, and better charge–discharge cycle.
Author Contributions
Conceptualization, G.I., N.P. and L.M.; methodology, G.I. and V.S.; software, N.V. and P.M.; validation, G.I., N.P. and L.M.; formal analysis, V.S.; investigation, G.I.; resources, N.V.; data curation, P.M. and V.S.; writing—original draft preparation, N.P. and L.M.; writing—review and editing, G.I. and V.S.; funding acquisition, G.I., N.V. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support of research and development contract 6 PFE from University of Life Sciences ‘‘King Michael I’’ from Timisoara.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, S.S.; Xu, K.; Jow, T.R. A Thermal Stabilizer for LiPF6-Based Electrolytes of Li Ion Cells. Electrochem. Sol. State Lett. 2022, 5, A206. [Google Scholar] [CrossRef]
- Manuel, S.A.; Nahm, K.S. Review on composite polymer electrolytes for lithium batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Liu, D.F.; Nie, J.; Guan, W.C.; Duan, H.Q.; Zhuo, L.M. Characterizations of a branched ester-type lithium imide in poly(ethylene oxide)-based polymer electrolytes. Solid State Ion. 2004, 167, 131–136. [Google Scholar] [CrossRef]
- Gray, F.M.; Armand, M. Energy Storage Systems for Electronics; Osaka, T., Datta, M., Eds.; Gordon and Breach: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Heitner, K.L. The search for the better polymer electrolyte. J. Power Sources 2000, 89, 128–131. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Henderson, W.; Villano, P.; Berrettoni, M.; Passerini, S. PEO-LiN (SO2CF2CF3)2 Polymer Electrolytes: I. XRD, DSC, and Ionic Conductivity Characterization. J. Electrochem. Soc. 2001, 148, A1171–A1178. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, B.; Shen, M.; Hu, B.; Chen, Q. Polymer chain diffusion and Li+ hopping of poly(ethylene oxide)/LiAsF6 crystalline polymer electrolytes as studied by solid state NMR and ac impedance. Solid State Ion. 2014, 255, 74–79. [Google Scholar] [CrossRef]
- Homann, G.; Stolz, L.; Nair, J.; Cekic Laskovic, I.; Winter, M.; Kasnatscheew, J. Poly(Ethylene Oxide)-based Electrolyte for Solid-State-Lithium-Batteries with High Voltage Positive Electrodes: Evaluating the Role of Electrolyte Oxidation in Rapid Cell Failure. Sci. Rep. 2020, 10, 4390. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yu, M. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Storage Mat. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Shi, C.; Hamann, T.; Takeuchi, S.; Alexander, G.V.; Nolan, A.M.; Limpert, M.; Fu, Z.; O’Neill, J.; Godbey, G.; Dura, J.A.; et al. 3D Asymmetric Bilayer Garnet-Hybridized High-Energy-Density Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2023, 15, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, Y.; Li, K.; Yang, Z.; Hu, X.; Liu, Y.; Zhang, Z. Advancements in Performance Optimization of Electrospun Polyethylene Oxide-Based Solid-State Electrolytes for Lithium-Ion Batteries. Polymers 2023, 15, 3727. [Google Scholar] [CrossRef]
- Petric, M.; Crisan, L.; Crisan, M.; Micle, A.; Maranescu, B.; Ilia, G. Synthesis and QSRR Study for a Series of Phosphoramidic Acid Derivatives. Heteroat. Chem. 2013, 24, 138–145. [Google Scholar] [CrossRef]
- Drehe, M.; Simulescu, V.; Ilia, G. Progress in the development of flame retardants. Rev. Chem. Eng. 2008, 24, 263–302. [Google Scholar]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Watanabe, E.; Takada, K.; Tateyama, Y.; Yamada, A. Fire-extinguishing organic electrolytes for safe batteries. Nat. Energy 2018, 3, 22–29. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Gao, S.; Cai, S.; Wang, Q.; Liu, J.; Liu, Z. A novel polyphosphonate flame-retardant additive towards safety-reinforced all-solid-state polymer electrolyte. Mat. Chem. Phys. 2020, 239, 122014. [Google Scholar] [CrossRef]
- Kaczorowska, M.A.; Cooper, H.J. Characterization of polyphosphoesters by fourier transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 2009, 20, 2238–2247. [Google Scholar] [CrossRef]
- Jia, H.; Onishi, H.; Wagner, R.; Winter, M.; Cekic-Laskovic, I. Intrinsically Safe Gel Polymer Electrolyte Comprising Flame-Retarding Polymer Matrix for Lithium Ion Battery Application. ACS Appl. Mater. Interfaces 2018, 10, 42348–42355. [Google Scholar] [CrossRef]
- Song, B.; Yang, S.; Hong, Y.; Zhang, G.; Jin, L.; Hu, D. Synthesis and bioactivity of fluorine compounds containing isoxazolylamino and phosphonate groups. J. Fluor. Chem. 2005, 126, 1419–1424. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Xingang, L.; Chuhong, Z. A Novel Silicon/Phosphorus Co-Flame Retardant Polymer Electrolyte for High-Safety All-Solid-State Lithium Ion Batteries. Polymers 2020, 12, 2937. [Google Scholar]
- Tosch, R.T.; Popov, I.; Stanford, V.L.; Lucius, A.R.; Foulger, S.H.; Gray, G.M. Polyphosphonates as ionic conducting polymers. J. Polym. Sci. 2021, 59, 139–145. [Google Scholar] [CrossRef]
- Jorge, L.; Olmedo-Martínez, L.M.; Raphael, R.; Gregorio, G.-G.; Luca, P.; Maria, F.; Agurtzane, M.; Itxaso, C.; Alejando, J.M.; Philippe, L.; et al. Flame retardant polyphosphoester copolymers as solid polymer electrolyte for lithium batteries. Polym. Chem. 2021, 12, 3441–3450. [Google Scholar]
- Lu, B.; Mohammad, W.; Guillaume, D.; Bruno, A.; Jean-François, G. Solid Polymer Electrolytes from Copolymers Based on Vinyl Dimethyl Phosphonate and Vinylidene Fluoride. Macromol. Chem. Phys. 2020, 222, 2000389. [Google Scholar] [CrossRef]
- He, X.; Schmohl, S.; Wiemhöfer, H.-D. Comparative study of interfacial behavior for polyphosphazene based polymer electrolytes and LiPF6 in EC/DMC against lithium metal anodes. Polym. Test. 2019, 76, 505–512. [Google Scholar] [CrossRef]
- Han, P.; Zhu, Y.; Liu, J. An all-solid-state lithium ion battery electrolyte membrane fabricated by hot-pressing method. J. Power Sources 2015, 284, 459–465. [Google Scholar] [CrossRef]
- Babu, H.V.; Muralidharan, K. Polyethers with phosphate pendant groups by monomer activated anionic ring opening polymerization: Syntheses, characterization and their lithium-ion conductivities. Polymer 2014, 55, 83–94. [Google Scholar] [CrossRef]
- Benoît, N.; Fernand, G.; Vincent, F.; Jean-François, G. Solid Polymer Electrolytes Based on Phosphonate and Cyclocarbonate Units for Safer Full Solid State Lithium Metal Batteries. Macromol. Chem. Phys. 2022, 223, 2200152. [Google Scholar]
- Dan, H.; Dong, W.K.; Ji, S.P.; Song, Y.C.; Yongku, K. Electrochemical properties of semi-interpenetrating polymer network solid polymer electrolytes based on multi-armed oligo(ethyleneoxy) phosphate. J. Power Sources 2013, 244, 170–176. [Google Scholar]
- Ryo, S.; Hiromori, T. Fire-retardant solid polymer electrolyte films prepared from oxetane derivative with dimethyl phosphate ester group. J. Power Sources 2012, 202, 369–373. [Google Scholar]
- Zheng, J.; Yang, Y.; Feng, X.; Li, X.; Zhen, X.; Chen, W.; Zhao, Y. The polymerization capability of alkenyl phosphates and application as gel copolymer electrolytes for lithium ion batteries with high flame-retardancy. React. Funct. Polym. 2020, 149, 104535. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sust. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Huang, F. Analysis on safety problems of dynamic lithium-ion batteries and review on prevention and control technologies. Mod. Chem. Indus. 2019, 39, 7–10. [Google Scholar]
- Abada, S.; Marlair, G.; Lecocq, A.; Petit, M.; Sauvant-Moynot, V.; Huet, F. Safety focused modeling of lithium-ion batteries: A review. J. Power Sources 2016, 306, 178–192. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Tris(2,2,2-trifluoroethyl) phosphite as a co-solvent for nonflammable electrolytes in Li-ion batteries. J. Power Sources 2003, 116, 166–179. [Google Scholar] [CrossRef]
- Zeng, G.; An, Y.; Xiong, S.; Feng, J. Nonflammable fluorinated carbonate electrolyte with high salt-to-solvent ratios enables stable silicon-based anode for next-generation lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 23229–23235. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, F.; Liu, L.; Xiao, S.; Chang, Z.; Wu, Y. Composite of a nonwoven fabric with poly(vinylidene fluoride) as a gel membrane of high safety for lithium ion battery. Energy Environ. Sci. 2013, 6, 618–624. [Google Scholar] [CrossRef]
- Hess, A.; Barber, G.; Chen, C.; Mallouk, T.E.; Allcock, H.R. Organophosphates as solvents for electrolytes in electrochemical devices. ACS Appl. Mater. Interfaces 2013, 5, 13029–13034. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Murugesan, V.; Han, K.S.; Jiang, X.; Cao, Y.; Xiao, L.; Ai, X.; Yang, H.; Zhang, J.-G.; Sushko, M.L.; et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 2018, 3, 674–681. [Google Scholar] [CrossRef]
- Cao, X.; Xu, Y.; Zhang, L.; Engelhard, M.H.; Zhong, L.; Pen, X.; Jia, H.; Liu, B.; Niu, C.; Mathews, B.E.; et al. Nonflammable electrolytes for lithium ion batteries enabled by ultraconformal passivation interphases. ACS Energy Lett. 2019, 4, 2529–2534. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, N.; Ju, J.; Li, Z.; Kang, W.; Cheng, B. Novel configuration of heat-resistant gel polymer electrolyte with electrospun poly (vinylidene fluoride-cohexafluoropropylene) and poly-m-phenyleneisophthalamide composite separator for high-safety lithium-ion battery. Mater. Lett. 2019, 236, 101–105. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Cao, H.; Zhao, L.; Huang, Y.; Song, A.; Lin, Y.; Li, X.; Wang, M. A novel porous gel polymer electrolyte based on poly(acrylonitrile-polyhedral oligomeric silsesquioxane) with high performances for lithium ion batteries. J. Membr. Sci. 2018, 545, 140–149. [Google Scholar] [CrossRef]
- Hu, J.; Wang, W.; Zhou, B.; Feng, Y.; Xie, X.; Xue, Z. Poly(ethylene oxide)-based composite polymer electrolytes embedding with ionic bond modified nanoparticles for all-solid-state lithium-ion battery. J. Membr. Sci. 2019, 575, 200–208. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.; Fan, L.-Z.; Nan, C.-W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Macarie, L.; Pekař, M.; Simulescu, V.; Plesu, N.; Iliescu, S.; Ilia, G.; Tara-Lunga-Mihali, M. Properties in aqueous solution of homo- and copolymers of vinylphosphonic acid derivatives obtained by UV-curing. Macromol. Res. 2017, 25, 214–221. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.Y.; Kim, H.S.; Cho, H.N. Ionic conductivity of polymer electrolytes based on phosphate and polyether copolymers. Solid State Ion. 1999, 116, 63–71. [Google Scholar] [CrossRef]
- Dixon, B.G.; Morris, R.S.; Dallek, S. Non-flammable polyphosphonate electrolytes. J. Power Sources 2004, 138, 274–276. [Google Scholar] [CrossRef]
- Scott Morris, R.; Dixon, B.G. A novel approach for development of improved polymer electrolytes for lithium batteries. J. Power Sources 2003, 119, 487–491. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, H.Q.; Yen, H.-J. Strategic structural design of a gel polymer electrolyte toward a high efficiency lithium-ion battery. ACS Appl. Energy Mater. 2019, 2, 3937–3971. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kang, Y.; Shim, H.; Kim, D.; Song, H.-K.; Kim, D.-W. Effect of the crosslinking agent on cycling performances of lithium-ion polymer cells assembled by in situ chemical cross-linking with tris(2-(acryloyloxy)ethyl) phosphate. J. Power Sources 2009, 189, 809–813. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Y.; Feng, X.; Chen, W.; Zhao, Y. Novel safer phosphonate-based gel polymer electrolytes for sodium-ion batteries with excellent cycling performance. J. Mater. Chem. A 2018, 6, 6559–6564. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, X.; Duan, Y.; Chen, L.; Zhang, X.; Feng, X.; Chen, W.; Zhao, Y. Stable cross-linked gel terpolymer electrolyte containing methyl phosphonate for sodium ion batteries. J. Membr. Sci. 2019, 583, 163–170. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A.; Mracec, M.; Ilia, G.; Maranescu, V.; Simon, Z.; Mracec, M. Lamellar Co2+ vinylphosphonate metal organic framework. PM3 semi-empirical analysis of structural properties. Rev. Roum. Chim. 2011, 56, 473–482. [Google Scholar]
- Zheng, J.; Li, X.; Yu, Y.; Zhen, X.; Song, Y.; Feng, X.; Zhao, Y. Cross-linking copolymers of acrylates gel electrolytes with high conductivity for lithium-ion batteries. J. Solid State Electrochem. 2014, 18, 2013–2018. [Google Scholar] [CrossRef]
- Allcock, H.R.; Steely, L.; Singh, A.; Hindenlang, M.D. Hydrophobic and Superhydrophobic Polyphosphazene. J. Adhesion Sci. Technol. 2009, 23, 435–445. [Google Scholar] [CrossRef]
- Blonsky, P.M.; Shriver, D.F.; Austin, P.; Allcock, H.R. Polyphosphazene solid electrolytes. J. Am. Chem. Soc. 1984, 106, 6854–6855. [Google Scholar] [CrossRef]
- Blonsky, P.M.; Shriver, D.F.; Austin, P.; Allcock, H.R. Complex formation and ionic conductivity of polyphosphazene solid electrolytes. Solid State Ion. 1986, 18–19, 258–264. [Google Scholar] [CrossRef]
- Kurachi, Y.; Okuyama, T.; Oohasi, T. Synthesis and properties of urethane foams having a N3P3 ring compound. J. Mater. Sci. 1989, 24, 2671–2764. [Google Scholar] [CrossRef]
- Nelson, C.J.; Coggio, W.D.; Allcock, H.R. Ultraviolet radiation-induced crosslinking of poly[bis(2-(2-methoxyethoxy)ethoxy)phosphazene]. Chem. Mat. 1991, 3, 786–787. [Google Scholar] [CrossRef]
- Bennett, J.L.; Dembek, A.A.; Allcock, H.R.; Heyen, B.J.; Shriver, D.F. Radiation Crosslinking of Poly[bis(2-(2-methoxyethoxy)ethoxy)]phosphazene: Effect on Solid State Ionic Conductivity. Chem. Mat. 1989, 1, 14–16. [Google Scholar] [CrossRef]
- Allcock, H.R.; Chang, Y.; Welna, D.T. Ionic conductivity of covalently interconnected polyphosphazene–silicate hybrid networks. Solid State Ion. 2006, 177, 569–572. [Google Scholar] [CrossRef]
- Mutin, P.H.; Guerrero, G.; Alauzun, J.G. Sol–gel processing of phosphonate-based organic–inorganic hybrid materials. J. Ceram. Soc. Jpn. 2015, 123, 709–713. [Google Scholar] [CrossRef]
- Ilia, G.; Simulescu, V.; Mak, C.A.; Crasmareanu, E. The use of transesterification method for obtaining phosphorus-containing polymers. Adv. Polymer Technol. 2014, 33, 21437. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Ribot, F.; In, M. Molecular Design of Sol-Gel Derived Hybrid Organic-Inorganic Nanocomposites. J. Sol-Gel Sci. Technol. 2000, 19, 31–38. [Google Scholar] [CrossRef]
- Mehring, M.; Lafond, V.; Mutin, P.H.; Vioux, A. New Sol-Gel Routes to Organic-Inorganic Hybrid Materials: Modification of Metal Alkoxide by Phosphonic or Phosphinic Acids. J. Sol-Gel Sci. Technol. 2003, 26, 99–102. [Google Scholar] [CrossRef]
- Gheonea, R.; Crasmareanu, E.; Plesu, N.; Sauca, S.; Simulescu, V.; Ilia, G. New hybrid materials synthesized with different dyes by sol-gel method. Adv. Mater. Sci. Eng. 2017, 2017, 4537039. [Google Scholar] [CrossRef]
- Guerrero, G.; Mutin, P.H.; Vioux, A. Mixed Nonhydrolytic/Hydrolytic Sol−Gel Routes to Novel Metal Oxide/Phosphonate Hybrids. Chem. Mater. 2000, 12, 1268–1272. [Google Scholar] [CrossRef]
- Hiller, M.M.; Joost, M.; Gores, H.J.; Passerini, S.; Wiemhöfer, H.D. The influence of interface polarization on the determination of lithium transference numbers of salt in polyethylene oxide electrolytes. Electrochim. Acta 2013, 114, 21–29. [Google Scholar] [CrossRef]
- David Kim, Y.L. Ion Conduction Mechanisms in Polymer Electrolytes for Lithium Batteries and Fuel Cells, and Crystal Engineering of Cyclophosphazenes. In Dissertation in Chemistry; The Pennsylvania State University: State College, PA, USA, 2010. [Google Scholar]
- Jankowsky, S.; Hiller, M.M.; Wiemhöfer, H.-D. Preparation and electrochemical performance of polyphosphazene based salt-in-polymer electrolyte membranes for lithium ion batteries. J. Power Sources 2014, 253, 256–262. [Google Scholar] [CrossRef]
- Jankowsky, S.; Hiller, M.M.; Fromm, O.; Winter, M.; Wiemhöfer, H.D. Enhanced Lithium-Ion Transport in Polyphosphazene based Gel Polymer Electrolytes. Electrochim. Acta 2015, 155, 364–371. [Google Scholar] [CrossRef]
- Jankowsky, S.; Hiller, M.M.; Stolina, R.; Wiemhöfer, H.D. Performance of polyphosphazene based gel polymer electrolytes in combination with lithium metal anodes. J. Power Sources 2015, 273, 574–579. [Google Scholar] [CrossRef]
- Fiedler, C.; Luerssen, B.; Luchtb, B.; Janek, J. Synthesis and characterization of polyphosphazene electrolytes including cyclic ether side groups. J. Power Sources 2018, 384, 165–171. [Google Scholar] [CrossRef]
- Tsao, C.-H.; Ueda, M.; Kuo, P.-L. Synthesis and Characterization of Polymer Electrolytes Based on Cross-linked Phenoxy-Containing Polyphosphazenes. J. Pol. Sci. Part A Polym. Chem. 2016, 54, 352–358. [Google Scholar] [CrossRef]
- Schmohl, S.; He, X.; Wiemhöfer, H.-D. Boron Trifluoride Anionic Side Groups in Polyphosphazene Based Polymer Electrolyte with Enhanced Interfacial Stability in Lithium Batteries. Polymers 2018, 10, 1350. [Google Scholar] [CrossRef]
- Hu, Y.; Fan, W.; Wang, Q.; Kawamora, T.; Koseki, N.; Akutsu, Y.; Tamura, M. Study on gas phase long-lived radicals in combustion of polyurethane modified by phosphazene. Prog. Nat. Sci. 1999, 9, 103–108. [Google Scholar]
- Feng, S.W.; Shi, D.Y.; Liu, F.; Zheng, L.P.; Nie, J.; Feng, W.F.; Huang, X.J.; Armand, M.; Zhou, Z.B. Single lithium-ion conducting polymer electrolytes based on poly (4-styrenesulfonyl)(trifluoromethanesulfonyl)imide anions. Electrochim. Acta 2013, 93, 254–263. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, X.; Xia, X.; Xu, R.; Zhang, S.; Wu, J.; Liang, Y.; Gu, C.; Tu, J. A newly designed composite gel polymer electrolyte based on poly (vinylidene fluoride-hexafluoropropylene)(pvdf-hfp) for enhanced solid-state lithium-sulfur batteries. Chem. A Euro. J. 2017, 23, 15203–15209. [Google Scholar] [CrossRef]
- Allcock, H.R.; Welna, D.T.; Maher, A.E. Single ion conductors–polyphosphazenes with sulfonimide functional groups. Solid State Ion. 2006, 177, 741–747. [Google Scholar] [CrossRef]
- Wang, B. Development of a One-Pot in Situ Synthesis of Poly(dichlorophosphazene) from PCl. Macromolecules 2005, 38, 643–645. [Google Scholar] [CrossRef]
- Wang, B.; Rivard, E.; Manners, I. A New High-Yield Synthesis of Cl3P=NSiMe3, a Monomeric Precursor for the Controlled Preparation of High Molecular Weight Polyphosphazenes. Inorg. Chem. 2002, 41, 1690–1691. [Google Scholar] [CrossRef]
- Babu, H.V.; Srinivas, B.; Praveen, K.; Naik, K.; Muralidharan, K. Polymerization behaviour of butyl bis(hydroxymethyl)phosphine oxide: Phosphorus containing polyethers for Li-ion conductivity. J. Chem. Sci. 2015, 127, 635–641. [Google Scholar] [CrossRef]
- Chen, X.-T.; Sun, H.; Tang, X.-D.; Wang, C.-Y. Synthesis and properties of novel phosphorus-containing poly(ether ether ketone ketone)s. J. Appl. Polym. Sci. 2008, 110, 1304. [Google Scholar] [CrossRef]
- Morford, R.V.; Kellam III, E.C.; Hofmann, M.A.; Baldwin, R.; Allcock, H.R. A fire-resistant organophosphorus gel polymer electrolyte additive for use in rechargeable lithium batteries. Solid State Ion. 2000, 133, 171–177. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Girard, G.M.; Yunis, R.; MacFarlane, D.R.; Mecerreyes, D.; Bhattacharyya, A.J.; Howlett, P.C.; Forsyth, M. Preparation and characterization of gel polymer electrolytes using poly (ionic liquids) and high lithium salt concentration ionic liquids. J. Mater. Chem. 2017, A5, 23844–23852. [Google Scholar] [CrossRef]
- Zuo, X.; Ma, X.; Wu, J.; Deng, X.; Xiao, X.; Liu, J.; Nan, J. Self-supporting ethyl cellulose/poly (vinylidene fluoride) blended gel polymer electrolyte for 5 v high-voltage lithium-ion batteries. Electrochim. Acta 2018, 271, 582–590. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Shao, H. Co-polymerization and blending based peo/pmma/p (vdf-hfp) gel polymer electrolyte for rechargeable lithium metal batteries. J. Membr. Sci. 2018, 547, 1–10. [Google Scholar] [CrossRef]
- Lv, P.; Xie, S.; Sun, Q.; Chen, X.; He, Y. Flame-Retardant Solid Polymer Electrolyte Based on Phosphorus-Containing Polyurethane Acrylate/Succinonitrile for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 7199–7209. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Pric, D. Fire Retardant Materials; Woodhead Publishing Limited: Sawston, UK, 2001; p. 128. [Google Scholar]
- Liu, W.; Zhi, H.; Yu, X. Recent progress in phosphorus based anode materials for lithium/sodium ion batteries. Energy Storage Mat. 2019, 16, 290–332. [Google Scholar] [CrossRef]
- Baboukani, A.R.; Khakpour, I.; Adelowo, E.; Drozd, V.; Shang, W.; Wang, C. High-performance red phosphorus-sulfurized polyacrylonitrile composite by electrostatic spray deposition for lithium-ion batteries. Electrochim. Acta 2020, 345, 136227. [Google Scholar] [CrossRef]
- Allcock, H.R. Chemistry and Applications of Polyphosphazenes; Wiley-Interscience: New Jersey, NJ, USA, 2003. [Google Scholar]
- Winter, R.; Brodd, J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Bieker, G.; Winter, M.; Bieker, P. Electrochemical in situ investigations of SEI and dendrite formation on the lithium metal anode. Phys. Chem. Chem. Phys. 2015, 17, 8670–8679. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).